Abstract
In 2015, the mosquito Aedes albopictus was detected in Rabat, Morocco. This invasive species can be involved in the transmission of more than 25 arboviruses. It is known that each combination of mosquito population and virus genotype leads to a specific interaction that can shape the outcome of infection. Testing the vector competence of local mosquitoes is therefore a prerequisite to assess the risks of emergence. A field-collected strain of Ae. albopictus from Morocco was experimentally infected with dengue (DENV), chikungunya (CHIKV), zika (ZIKV) and yellow fever (YFV) viruses. We found that this species can highly transmit CHIKV and to a lesser extent, DENV, ZIKV and YFV. Viruses can be detected in mosquito saliva at day 3 (CHIKV), day 14 (DENV and YFV), and day 21 (ZIKV) post-infection. These results suggest that the local transmission of these four arboviruses by Ae. albopictus newly introduced in Morocco is a likely scenario.
Trial registration: ClinicalTrials.gov APAFIS#6573-201606l412077987v2.
Author summary
The Asian tiger mosquito Aedes albopictus is responsible for the transmission of several arboviruses such as dengue and chikungunya viruses. In 30 to 40 years, it has extended its geographical distribution in both tropical and temperate regions of all continents. The species was first detected in September 2015, in Rabat, Morocco. Using experimental infections, we demonstrated that Ae. albopictus Morocco are competent to transmit zika and yellow fever viruses in addition to the transmission of dengue and chikungunya viruses. Our results are central to suggest developing the most effective national surveillance program and to designing the most suitable control strategy to avoid the mosquito spreading beyond its point of entry in Morocco.
Introduction
Over the past decades, arboviruses caused acute emergences leading to global pandemics. Dengue viruses (DENV; family Flaviviridae, genus Flavivirus) are responsible for 390 million infections per year including 96 million symptomatic cases [1]. In 2005, chikungunya virus (CHIKV; family Togaviridae, genus Alphavirus) emerged outside Africa producing devastated outbreaks in all continents [2]. While its importance was underestimated, zika virus (ZIKV; family Flaviviridae, genus Flavivirus) hit Brazil in 2015 causing several million cases in the Americas [3] and severe unusual symptoms such as Guillain-Barré syndrome and congenital microcephaly. Despite the availability of an efficient vaccine 17D, yellow fever virus (YFV; family Flaviviridae, genus Flavivirus) continues to cause human fatalities in South America and Sub-Saharan Africa.
All four arboviruses share the same mosquito vectors: Aedes aegypti and Aedes albopictus. Ae. aegypti is an urban mosquito feeding exclusively on humans [4] and Ae. albopictus colonizes a larger range of sites and feeds on both animals and humans [5]. While Ae. aegypti took several centuries to invade most countries in the world [6], Ae. albopictus took only a few decades to establish stable colonies worldwide [7]. Native to Southeast Asia, Ae. albopictus has invaded America, Africa and Europe during the last 40 years [8]. In Europe, it was introduced in 1979 in Albania and then in Italy in 1990. It is now present in 20 European countries [9]. In Africa, Ae. albopictus was first reported in the early 1990s in South Africa [10] and Nigeria [11]. Thereafter, it was described in several West and Central African countries: Cameroon in 2000 [12], Equatorial Guinea in 2003 [13], Gabon in 2007 [14], Central African Republic in 2009 [15], and Republic of Congo in 2011 [16]. More recently, it was detected in Mali [17], Mozambique [18] and São Tomé and Príncipe [19]. In North Africa, Ae. albopictus was detected in Algeria in 2010 [20] then in Morocco in 2015 [21].
Morocco is considered a low prevalent country for mosquito-borne diseases [22]. However, since 1996, the country has faced West Nile virus (WNV) with three epizootic episodes: 1996, 2003 and 2010 [23, 24]. In 2008, a serosurvey of wild birds confirmed the circulation of WNV in native birds [25]. Other arboviruses like Usutu virus and Rift valley fever virus (RVFV) have never been reported despite serological evidence of RVFV antibodies in camels at the border between Morocco and Mauritania [25–27]. Morocco is considered by several reports of the Intergovernmental Panel on Climate Change (IPCC) as a hotspot for climate change with its significant impact for several infectious diseases [28]. The introduction of an invasive species such as Ae. albopictus will likely cause a new public health problem. Moreover, Morocco is a tourist destination with more than 11 million visitors reported in 2017 {http://www.tourisme.gov.ma/fr/tourisme-en-chiffres/chiffres-cles}, increasing the risk of importing arboviral pathogens.
In this work, we evaluate the ability of Ae. albopictus recently introduced in Morocco to transmit CHIKV, DENV, ZIKV and YFV, where the outcome of vector infection depends on specific genotype-by-genotype (G x G) interactions between a vector population and a pathogen lineage [29]. This measure of the vector competence of field-collected mosquitoes helps to assess the risk of arbovirus emergence.
Materials and methods
Ethic statements
Animals were housed in the Institut Pasteur animal facilities accredited by the French Ministry of Agriculture for performing experiments on live rodents. Work on animals was performed in compliance with French and European regulations on care and protection of laboratory animals (EC Directive 2010/63, French Law 2013–118, February 6th, 2013). All experiments were approved by the Ethics Committee #89 and registered under the reference APAFIS#6573-201606l412077987 v2.
Mosquito collections
During the national surveillance plan implemented in 2016 to establish the geographical distribution of Ae. albopictus in Morocco, five ovitraps less than 500 m apart were placed on a street of the Agdal neighborhood in Rabat (33°59'20.9′′ N, 6°51′07.9′′W). Ovitraps were checked for eggs once a week from May to November 2016 and were brought back to the laboratory to be stored in humid chambers (relative humidity of 80%) before being sent to Institut Pasteur in Paris to perform the vector competence studies.
After hatching, larvae were split into pans of 200 individuals and supplied every 2 days with a yeast tablet dissolved in 1L of dechlorinated tap water. All immature stages were reared at 26±1°C. Emerging adults were maintained at 28±1°C with a 16L:8D cycle, 80% relative humidity and supplied with a 10% sucrose solution. Females were fed twice a week on anaesthetized mice (OF1 mice, Charles River laboratories, France). Resulting F2 adults were used for vector competence assays. It should be noted that variations of oral susceptibility to an arbovirus can be considered negligible in fewer than five laboratory generations [30].
Viral strains
CHIKV strain (06.21) was isolated from a patient on La Reunion Island in 2005 [31]. After isolation on Ae. albopictus C6/36 cells, this strain was passaged twice on C6/36 cells and the viral stocks produced were stored at -80°C prior to their use for mosquito oral infections. DENV-2 strain provided by Prof. Leon Rosen, was isolated from a human serum collected in Bangkok (Thailand) in 1974 [32] and had been passed only in different mosquito species (2 times in Ae. albopictus, 2 times in Toxorhynchites amboinensis, and one time in Ae. aegypti) by intrathoracic inoculation. Viral stocks were obtained by inoculating C6/36 cells. ZIKV strain (NC-2014-5132) originally isolated from a patient in April 2014 in New Caledonia was passaged five times on Vero cells; this strain belongs to the same genotype than the ZIKV strains circulating in Brazil in 2015 [33]. Lastly, a YFV strain (S79) belonging to the West African lineage, was isolated from a human case in Senegal in 1979 [34]. YFV-S79 was passaged twice on newborn mice and two times on C6/36 cells.
Mosquito experimental infections
Six to eight batches of 60 7–10 day old females were exposed to an infectious blood meal containing 1.4 mL of washed rabbit erythrocytes and 700 μL of viral suspension. The blood meal was supplemented with ATP as a phagostimulant at a final concentration of 1 mM and provided to mosquitoes at a titer of 107.2 plaque-forming unit (pfu)/mL for ZIKV, 106.5 focus-forming unit (ffu)/mL for YFV and 107 ffu/mL for CHIKV and DENV, using a Hemotek membrane feeding system. Mosquitoes were allowed to feed for 15 min through a piece of pork intestine covering the base of a Hemotek feeder maintained at 37°C. Fully engorged females were transferred in cardboard containers and maintained with 10% sucrose under controlled conditions (28±1°C, relative humidity of 80%, light:dark cycle of 16 h:8 h) for up to 21 days with mosquito analysis at 3, 7, 14 and 21 days post-infection (dpi). For each virus, 21–30 mosquitoes were examined at each dpi.
Infection and dissemination assays
For each mosquito examined, body (abdomen and thorax) and head were tested respectively for infection and dissemination rates at 3, 7, 14 and 21 dpi. For this, each part was ground in 250 μL of Leibovitz L15 medium (Invitrogen, CA, USA) supplemented with 3% FBS, and centrifuged at 10,000×g for 5 min at +4°C. The supernatant was processed for viral titration.
Transmission assays
Mosquitoes examined previously were also tested for viral transmission by collecting saliva using the forced salivation technique [35]. Mosquitoes were anesthetized on ice and legs and wings were removed. The proboscis was then inserted into a pipette tip containing 5 μL of fetal bovine serum (FBS). After 30 min, the tip content was transferred in 45 μL of L15 medium. Saliva was then titrated to estimate the transmission rate.
Viral titration
CHIKV, DENV and YFV were titrated by focus fluorescent assay and ZIKV by plaque forming assay as ZIKV cannot produce distinct viral foci on mosquito cells.
Focus forming assay on C6/36 cells
For mosquitoes challenged with CHIKV, DENV or YFV, saliva, head and body homogenates were titrated by focus fluorescent assay on Ae. albopictus C6/36 cells [36]. Samples were serially diluted and inoculated onto C6/36 cells in 96-well plates. After an incubation of 3 days for CHIKV, and 5 days for YFV and DENV-2 at 28°C, cells were stained using hyper-immune ascetic fluid specific to each virus as the primary antibody (CHIKV: provided by the French National Reference Center for Arbovirus at the Institut Pasteur, YFV: OG5 NB100-64510; Novusbio, CO, USA, and DENV: Ms X Dengue complex MAB 8705, Millipore, MA, USA) and Alexa Fluor 488 goat anti-mouse IgG (Life Technologies, CA, USA) as the secondary antibody. Saliva titers were expressed as ffu/saliva.
Plaque forming assay on Vero cells
For ZIKV, body and head suspensions were serially diluted and inoculated onto monolayers of Vero cells in 96-well plates. Cells were incubated for 7 days at 37°C then stained with a solution of crystal violet (0.2% in 10% formaldehyde and 20% ethanol). Presence of viral particles was assessed by CPE detection. Saliva was titrated on monolayers of Vero cells in 6-well plates incubated 7 days under an agarose overlay. Saliva titers were expressed as pfu/saliva.
Statistical analysis
Means, standard deviations, 95% confidence interval were calculated and statistical analyses were performed using the Stata software (StataCorp LP, Texas, and USA). The effect of virus and dpi on infection, dissemination and transmission rates was evaluated using Fisher’s exact test. The titer of viral particles in mosquito saliva was compared across groups using a Kruskall-Wallis non parametric test. P-values<0.05 were considered statistically significant. Heatmaps were built under R (v 3.3.1) (https://www.R-project.org).
Results
Viral infection
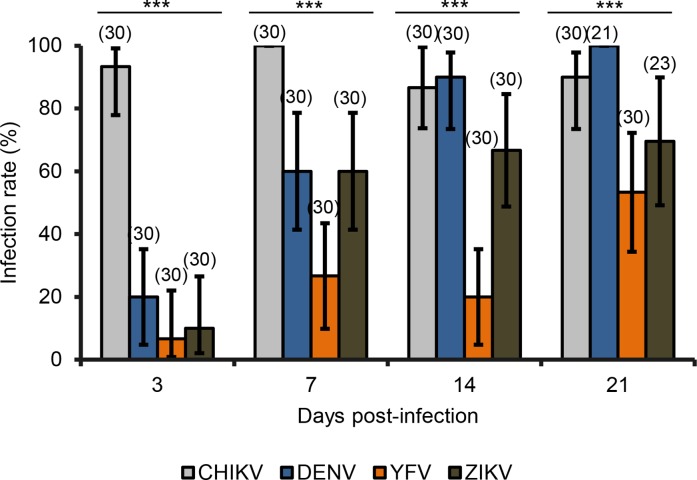
Mosquito females were exposed to four separate infectious blood meals containing CHIKV, DENV, ZIKV or YFV. The first step after the ingestion of the infectious blood meal is the infection of the midgut which is appraised by calculating the infection rate (IR) corresponding to the proportion of mosquitoes with an infected midgut. At 3 dpi, Ae. albopictus Morocco were more infected with CHIKV (Fig 1; Fisher’s exact test: p<10−4, df = 3) with an IR reaching 93% (N = 30) whereas with the 3 other viruses, IRs were lower than 20% (N = 30). At 7 dpi, the IR with CHIKV reached 100% (N = 30) and remained significantly lower with DENV (60%; N = 30), ZIKV (60%; N = 30) and YFV (26.7; N = 30) (Fisher’s exact test: p<10−4, df = 3). At 14 dpi, mosquitoes become more infected with DENV reaching 90% (N = 30) close to CHIKV (86.7%, N = 30) (Fisher’s exact test: p = 0.69, df = 3) but significantly higher than with ZIKV (66.7%, N = 30), and YFV (20%, N = 30) (Fisher’s exact test: p<10−4, df = 30). At 21 dpi, the same pattern was observed: IRs were higher with CHIKV (90%, N = 30) and DENV (100%, N = 21) than with ZIKV (69.6%, N = 23) and YFV (53.3%, N = 30) (Fisher’s exact test: p<10−4, df = 3). IRs with all viruses increased along with dpi except with CHIKV which remained high (>86%) very early from 3 dpi. The lowest IRs were obtained with YFV fluctuating from 6.7% at 3 dpi to 53.3% at 21 dpi.
Fig 1. Infection rates of Ae. albopictus from Morocco with CHIKV, DENV, YFV, and ZIKV.
F2 mosquitoes were orally challenged with CHIKV and DENV at a titer of 107 ffu/mL, YFV at 106.5 ffu/mL and ZIKV at 107.2 pfu/mL. After infection, mosquito bodies were titrated at 3, 7, 14 and 21 days post-infection. Error bars showing the binomial 95% confidence interval (Stata software, StataCorp LP, Texas, and USA). In brackets, the number of mosquitoes examined. ***, P < 10−4.
Viral dissemination
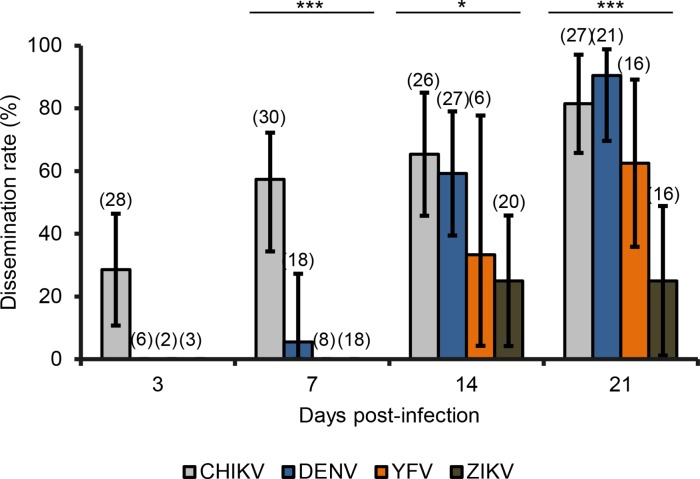
Once the midgut is infected, viral particles can disseminate from the midgut to internal organs and tissues. The dissemination rate (DR) gives the number of mosquitoes with infected heads among mosquitoes with infected midgut. At 3 dpi, only CHIKV was detected in mosquito heads (Fig 2; 28.6%, N = 28). At 7 dpi, DR with CHIKV reached 53.3% (N = 30) and only 5.5% (N = 18) with DENV (Fisher’s exact test: p<10−4, df = 3). At 14 dpi, DRs with CHIKV (65.4%, N = 26) and DENV (59.2%, N = 27) were higher and similar (Fisher’s exact test: p = 0.65, df = 1) compared to YFV (33.3%, N = 6) and ZIKV (25%, N = 20) which were both lower and comparable (Fisher’s exact test: p = 0.69, df = 1). At 21 dpi, DRs for each virus were significantly different (Fisher’s exact test: p<10−4, df = 3) and slightly higher than the DRs at 14 dpi. Viral dissemination started earlier with CHIKV at 3 dpi while it was only at 7 dpi with DENV and 14 dpi with YFV and ZIKV. The lowest DRs were obtained with ZIKV maintained at 25% at 14 and 21 dpi.
Fig 2. Dissemination rates of Ae. albopictus from Morocco with CHIKV, DENV, YFV, and ZIKV.
After infection, mosquito heads were titrated at 3, 7, 14 and 21 days post-infection. Error bars showing the binomial 95% confidence interval (Stata software, StataCorp LP, Texas, and USA). In brackets, the number of mosquitoes examined. *, P < 0.05***, P < 10−4.
Viral transmission
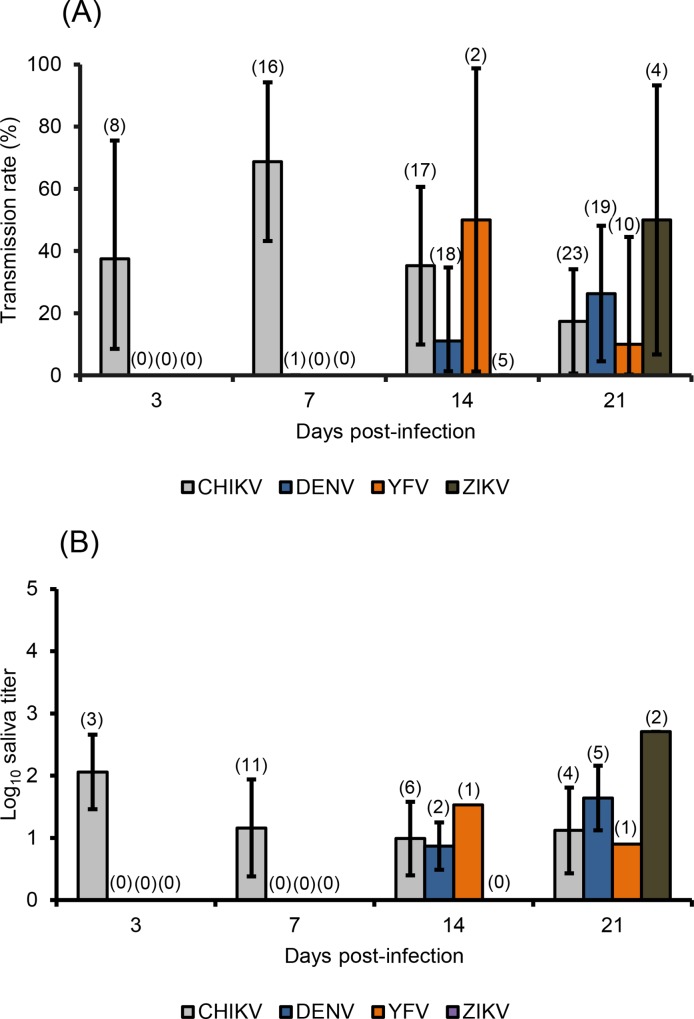
After the virus has spread into the general cavity of the mosquito and infected the salivary glands, the virus must be excreted in saliva for subsequent transmission. The transmission rate (TR) is defined as the proportion of mosquitoes delivering infectious saliva among mosquitoes having disseminated the virus (Fig 3A). At 3 and 7 dpi, viral particles could be detected in saliva of mosquitoes infected with CHIKV, with TRs of 37.5% (N = 8) and 68.7% (N = 16) respectively. At 14 dpi, TR with YFV (50%, N = 2) predominated over TRs with CHIKV (35.3%, N = 17) and DENV (11.1%, N = 18), TR with ZIKV remaining at 0%; no significant difference was observed among all TRs (Fisher’s exact test: p = 0.14, df = 3). At 21 dpi, transmission with ZIKV became detectable with a TR of 50% (N = 4), not significantly different from TRs with DENV (26.3%, N = 19), CHIKV (17.4%, N = 23), and YFV (10%, N = 10) (Fisher’s exact test: p = 0.36, df = 3). Transmission started early at 3 dpi with CHIKV, at 14 dpi with DENV and YFV, and at 21 dpi with ZIKV with respectively, a mean of 2.06±0.60 Log10 ffu/saliva (N = 3), 0.87±0.38 Log10 ffu/saliva (N = 2), 1.53 Log10 ffu/saliva (N = 1), and 2.71±0.01 Log10 pfu/saliva (N = 2) (Fig 3B). No significant difference was detected between all viruses at 14 dpi (Kruskal-Wallis test: p = 0.47, df = 2) and 21 dpi (Kruskal-Wallis test: p = 0.10, df = 3). The highest number of viral particles was detected in saliva of mosquitoes infected with YFV and examined at 21 dpi: TR of 50% (2 among 4 mosquitoes with viral dissemination), 2 females delivering 2.70 Log10 pfu (500) and 2.72 Log10 pfu (530) infectious particles.
Fig 3.
Transmission rates (A) and mean titer of infectious viral particles in saliva (B) of Ae. albopictus from Morocco infected with CHIKV, DENV and YFV, and ZIKV. Saliva was collected from surviving females using the forced salivation technique at 3, 7, 14 and 21 days post-infection. Error bars show the binomial 95% confidence interval in (A) and the standard deviation in (B), both calculated using the Stata software (StataCorp LP, Texas, and USA). In brackets, the number of mosquitoes examined.
Transmission efficiency
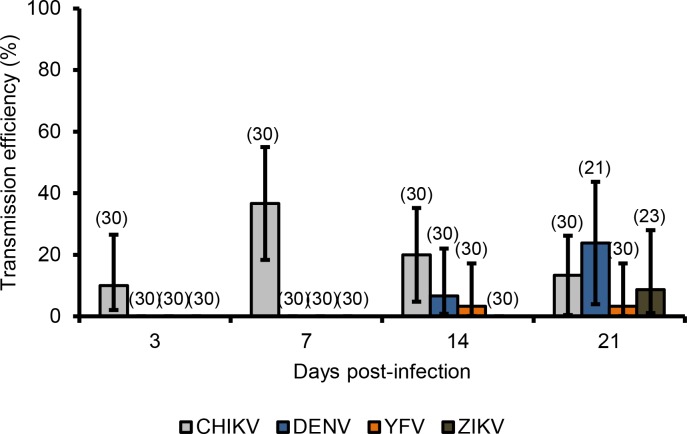
Whereas IR, DR and TR measure the efficiency of the midgut and salivary glands barriers to modulate, respectively, viral dissemination and transmission, the transmission efficiency (TE) gives an overview of transmission potential of mosquitoes tested; it corresponds to the proportion of mosquitoes with infectious saliva among all mosquitoes examined (presenting or not a viral dissemination with infected heads). Fig 4 shows that, the highest TE was detected at 7 dpi with CHIKV, at 21 dpi with DENV, at 14/21 dpi with YFV, and at 21 dpi with ZIKV. Collectively, Ae. albopictus Morocco were more susceptible to CHIKV and secondarily, to DENV, ZIKV and YFV.
Fig 4. Transmission efficiencies of Ae. albopictus from Morocco infected with CHIKV, DENV, YFV, and ZIKV.
Saliva was collected from surviving females at 3, 7, 14 and 21 days post-infection. Transmission efficiency was calculated as the proportion of mosquitoes with infectious saliva among mosquitoes initially tested. Error bars showing the binomial 95% confidence interval (Stata software, StataCorp LP, Texas, and USA). In brackets, the number of mosquitoes examined.
Vector competence
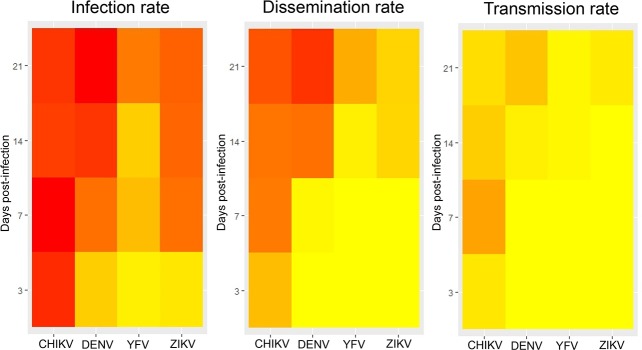
To summarize the vector competence corresponding to the overall ability of a mosquito population to be infected, to ensure the viral dissemination and to transmit the virus, heatmaps were built (Fig 5). Ae. albopictus Morocco were better infected with CHIKV from 3 dpi than with DENV and ZIKV (Fig 5A). Mosquitoes ensured an early dissemination (Fig 5B) and transmission (Fig 5C) with CHIKV (from 3 dpi) than with DENV and ZIKV. The species was less susceptible to YFV. Altogether, vector competence of Ae. albopictus Morocco depends on the virus and the dpi: it is more susceptible to CHIKV and susceptibility increases along with the dpi.
Fig 5. Heatmaps indicating interactions between viruses (DENV, CHIKV, YFV, ZIKV) and days post-infection for infection, dissemination and transmission.
The intensity of red increases with the rates.
Discussion
Using experimental infections, we show that the recently-introduced Ae. albopictus in Morocco were susceptible to all four viruses tested, CHIKV, DENV, YFV and ZIKV. Viral transmission was detected at 3 dpi with CHIKV, 14 dpi with DENV and YFV, and only 21 dpi with ZIKV.
Even if DENV, YFV and ZIKV belong to the same genus, they behave differently in Ae. albopictus mosquitoes. Infection of the midgut increases gradually from 3 dpi: DENV infects more efficiently mosquitoes than YFV and ZIKV, YFV remaining the less successful. Dissemination of DENV from the midgut to the mosquito general cavity started at 7 dpi as observed with most populations of Ae. albopictus [37]; it takes a shorter time with Ae. aegypti, i.e. from 5 dpi [38]. DENV dissemination is more strongly inhibited at early dpi than later meaning that the role of midgut as a barrier is diminished with dpi. Transmission of DENV was observed from 14 dpi suggesting an intrinsic incubation period higher than 7 dpi, likely around 10 dpi [37]. With ZIKV and YFV, dissemination was observed only at 14 dpi, YFV spreading at a higher rate than ZIKV suggestive of a stronger role of the midgut barrier with YFV. Transmission was detected at 14 dpi with YFV as observed with other Ae. albopictus populations [39] and 21 days with ZIKV which is longer than expected [40].
CHIKV presents a different profile. This alphavirus infects, disseminates and is transmitted more intensively and more quickly than the three other viruses. This viral strain presents an amino acid substitution (A226V) in the envelope glycoprotein E1 [31] favoring the viral transmission by Ae. albopictus [41, 42]. Importantly, exposure of infected mosquitoes to lower temperatures (lower than 25°C) compatible to values recorded in Morocco can modulate transmission [37]. It has been demonstrated that Ae. albopictus were able to better transmit CHIKV at a temperature lower than 28°C [43].
These assessments of vector competence of Ae. albopictus from Morocco to CHIKV, DENV, ZIKV and YFV are important for appraising the risk of local transmission. ZIKV shows the longer extrinsic incubation period (EIP) which refers to the time between the uptake of the virus during the blood feeding and the delivery of the virus by vector bite after successful infection and dissemination in the mosquito. If the EIP is longer than the daily survival rate of the mosquito, the risk of transmission is low. By shortening mosquito lifespan, vector control measures reduce disease transmission [44]. However, other factors such as environmental factors, e.g. the temperature, may influence the vector competence [43]. The vector competence and the EIP both contribute to estimating the vector capacity which describes the basic reproductive rate of a pathogen by a vector [44]. A high abundance of the vector [45], increased contacts between the vector and humans (i.e. anthropophily of mosquitoes) [5] and a high proportion of immunologically naïve humans, are also factors that should be considered in estimating the risk of emergence. Introductions of viremic travelers from endemic countries for all these viruses may initiate local transmission and outbreaks. Therefore surveillance of travelers must be reinforced.
Acknowledgments
The authors thank Pei-Shi Yen, Laurence Mousson and Marie Vazeille for technical help. We thank for their support: Ali Bouattour, Said Boubidi, Nabil Haddad, Zoubir Harrat, and Youmna M’Ghirbi. We are grateful to Peter Sahlins for correcting the manuscript. We also warmly thank Tran Minh Nhu Nguyen from the WHO Regional Office for the Eastern Mediterranean.
Data Availability
All relevant data are within the paper.
Funding Statement
This project was funded by the WHO “Vector competence to Zika virus of mosquitoes in the Mediterranean region“ (VEC-ZIKA-MED), grant number WCCPRD5294814 2017/700731. This work was also partially supported by the European Union’s Horizon 2020 Research and Innovation Programme under ZIKAlliance Grant Agreement no. 734548. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–7. 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zouache K, Failloux AB. Insect-pathogen interactions: contribution of viral adaptation to the emergence of vector-borne diseases, the example of chikungunya. Current Opinion in Insect Science. 2015. 10(14–21). [DOI] [PubMed] [Google Scholar]
- 3.Gatherer D, Kohl A. Zika virus: a previously slow pandemic spreads rapidly through the Americas. J Gen Virol. 2016;97(2):269–73. 10.1099/jgv.0.000381 [DOI] [PubMed] [Google Scholar]
- 4.Edman JD, Strickman D, Kittayapong P, Scott TW. Female Aedes aegypti (Diptera: Culicidae) in Thailand rarely feed on sugar. J Med Entomol. 1992;29(6):1035–8. [DOI] [PubMed] [Google Scholar]
- 5.Delatte H, Desvars A, Bouetard A, Bord S, Gimonneau G, Vourc'h G, et al. Blood-feeding behavior of Aedes albopictus, a vector of Chikungunya on La Reunion. Vector Borne Zoonotic Dis. 2010;10(3):249–58. 10.1089/vbz.2009.0026 [DOI] [PubMed] [Google Scholar]
- 6.Powell JR, Tabachnick WJ. History of domestication and spread of Aedes aegypti—a review. Mem Inst Oswaldo Cruz. 2013;108 Suppl 1:11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gratz NG. Critical review of the vector status of Aedes albopictus. Med Vet Entomol. 2004;18(3):215–27. 10.1111/j.0269-283X.2004.00513.x [DOI] [PubMed] [Google Scholar]
- 8.Paupy C, Delatte H, Bagny L, Corbel V, Fontenille D. Aedes albopictus, an arbovirus vector: from the darkness to the light. Microbes and infection / Institut Pasteur. 2009;11(14–15):1177–85. [DOI] [PubMed] [Google Scholar]
- 9.Medlock JM, Hansford KM, Versteirt V, Cull B, Kampen H, Fontenille D, et al. An entomological review of invasive mosquitoes in Europe. Bull Entomol Res. 2015;105(6):637–63. 10.1017/S0007485315000103 [DOI] [PubMed] [Google Scholar]
- 10.Cornel AJ, Hunt RH. Aedes albopictus in Africa? First records of live specimens in imported tires in Cape Town. J Am Mosq Control Assoc. 1991;7(1):107–8. [PubMed] [Google Scholar]
- 11.Savage HM, Ezike VI, Nwankwo AC, Spiegel R, Miller BR. First record of breeding populations of Aedes albopictus in continental Africa: implications for arboviral transmission. J Am Mosq Control Assoc. 1992;8(1):101–3. [PubMed] [Google Scholar]
- 12.Fontenille D, Toto JC. Aedes (Stegomyia) albopictus (Skuse), a potential new Dengue vector in southern Cameroon. Emerg Infect Dis. 2001;7(6):1066–7. 10.3201/eid0706.010631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toto JC, Abaga S, Carnevale P, Simard F. First report of the oriental mosquito Aedes albopictus on the West African island of Bioko, Equatorial Guinea. Med Vet Entomol. 2003;17(3):343–6. [DOI] [PubMed] [Google Scholar]
- 14.Coffinet T, Mourou JR, Pradines B, Toto JC, Jarjaval F, Amalvict R, et al. First record of Aedes albopictus in Gabon. J Am Mosq Control Assoc. 2007;23(4):471–2. 10.2987/5636.1 [DOI] [PubMed] [Google Scholar]
- 15.Diallo M, Laganier R, Nangouma A. First record of Ae. albopictus (Skuse 1894), in Central African Republic. Trop Med Int Health. 2010;15(10):1185–9. 10.1111/j.1365-3156.2010.02594.x [DOI] [PubMed] [Google Scholar]
- 16.Kelvin AA. Outbreak of Chikungunya in the Republic of Congo and the global picture. J Infect Dev Ctries. 2011;5(6):441–4. [PubMed] [Google Scholar]
- 17.Muller GC, Tsabari O, Traore MM, Traore SF, Doumbia S, Kravchenko VD, et al. First record of Aedes albopictus in inland Africa along the River Niger in Bamako and Mopti, Mali. Acta Trop. 2016;162:245–7. 10.1016/j.actatropica.2016.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kampango A, Abilio AP. The Asian tiger hunts in Maputo city—the first confirmed report of Aedes (Stegomyia) albopictus (Skuse, 1895) in Mozambique. Parasit Vectors. 2016;9:76 10.1186/s13071-016-1361-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reis S, Cornel AJ, Melo M, Pereira H, Loiseau C. First record of Aedes albopictus (Skuse 1894) on Sao tome island. Acta Trop. 2017;171:86–9. 10.1016/j.actatropica.2017.03.035 [DOI] [PubMed] [Google Scholar]
- 20.Izri A, Bitam I, Charrel RN. First entomological documentation of Aedes (Stegomyia) albopictus (Skuse, 1894) in Algeria. Clin Microbiol Infect. 2011;17(7):1116–8. 10.1111/j.1469-0691.2010.03443.x [DOI] [PubMed] [Google Scholar]
- 21.Bennouna A, Balenghien T, El Rhaffouli H, Schaffner F, Garros C, Gardes L, et al. First record of Stegomyia albopicta (= Aedes albopictus) in Morocco: a major threat to public health in North Africa? Med Vet Entomol. 2017;31(1):102–6. 10.1111/mve.12194 [DOI] [PubMed] [Google Scholar]
- 22.Failloux AB, Bouattour A, Faraj C, Gunay F, Haddad N, Harrat Z, et al. Surveillance of Arthropod-Borne Viruses and Their Vectors in the Mediterranean and Black Sea Regions Within the MediLabSecure Network. Curr Trop Med Rep. 2017;4(1):27–39. 10.1007/s40475-017-0101-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El Harrak M LGB, Le Gounon P. Isolation of West Nile virus in Morocco [in French]. Virologie. 1997;1:248–9 [Google Scholar]
- 24.Schuffenecker I, Peyrefitte CN, el Harrak M, Murri S, Leblond A, Zeller HG. West Nile virus in Morocco, 2003. Emerg Infect Dis. 2005;11(2):306–9. 10.3201/eid1102.040817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Figuerola J, Baouab RE, Soriguer R, Fassi-Fihri O, Llorente F, Jimenez-Clavero MA. West Nile virus antibodies in wild birds, Morocco, 2008. Emerg Infect Dis. 2009;15(10):1651–3. 10.3201/eid1510.090340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ayari-Fakhfakh E, Ghram A, Bouattour A, Larbi I, Gribaa-Dridi L, Kwiatek O, et al. First serological investigation of peste-des-petits-ruminants and Rift Valley fever in Tunisia. Vet J. 2011;187(3):402–4. 10.1016/j.tvjl.2010.01.007 [DOI] [PubMed] [Google Scholar]
- 27.El-Harrak M, Martin-Folgar R, Llorente F, Fernandez-Pacheco P, Brun A, Figuerola J, et al. Rift Valley and West Nile virus antibodies in camels, North Africa. Emerg Infect Dis. 2011;17(12):2372–4. 10.3201/eid1712.110587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Behnassi M, Kahime K, Boussaa S, Boumezzough A, Messouli M. Infectious diseases and climate vulnerability in Morocco: Governance and adaptation options Examining the Role of Environmental Change on Emerging Infectious Diseases and Pandemics2016. p. 138–62.
- 29.Lambrechts L, Chevillon C, Albright RG, Thaisomboonsuk B, Richardson JH, Jarman RG, et al. Genetic specificity and potential for local adaptation between dengue viruses and mosquito vectors. BMC Evolutionary Biology. 2009;9:160 10.1186/1471-2148-9-160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lambrechts L, Scott TW, Gubler DJ. Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Negl Trop Dis. 2010;4(5):e646 10.1371/journal.pntd.0000646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuffenecker I, Iteman I, Michault A, Murri S, Frangeul L, Vaney MC, et al. Genome microevolution of chikungunya viruses causing the Indian Ocean outbreak. PLoS Med. 2006;3(7):e263 10.1371/journal.pmed.0030263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosen L, Gubler D. The use of mosquitoes to detect and propagate dengue viruses. The American journal of tropical medicine and hygiene. 1974;23(6):1153–60. [DOI] [PubMed] [Google Scholar]
- 33.Dupont-Rouzeyrol M, Diancourt L, Calvez E, Vandenbogaert M, O'Connor O, Teissier A, et al. Zika virus evolution on the edges of the Pacific ocean. Emerg Microbes Infect. 2017;6(12):e111 10.1038/emi.2017.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodhain F, Hannoun C, Jousset FX, Ravisse P. [Isolation of the yellow fever virus in Paris from 2 imported human cases]. Bull Soc Pathol Exot. 1979;72(5–6):411–5. [PubMed] [Google Scholar]
- 35.Dubrulle M, Mousson L, Moutailler S, Vazeille M, Failloux AB. Chikungunya virus and Aedes mosquitoes: saliva is infectious as soon as two days after oral infection. PLoS One. 2009;4(6):e5895 10.1371/journal.pone.0005895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Payne AF, Binduga-Gajewska I, Kauffman EB, Kramer LD. Quantitation of flaviviruses by fluorescent focus assay. J Virol Methods. 2006;134(1–2):183–9. 10.1016/j.jviromet.2006.01.003 [DOI] [PubMed] [Google Scholar]
- 37.Liu Z, Zhang Z, Lai Z, Zhou T, Jia Z, Gu J, et al. Temperature Increase Enhances Aedes albopictus Competence to Transmit Dengue Virus. Front Microbiol. 2017;8:2337 10.3389/fmicb.2017.02337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salazar MI, Richardson JH, Sanchez-Vargas I, Olson KE, Beaty BJ. Dengue virus type 2: replication and tropisms in orally infected Aedes aegypti mosquitoes. BMC Microbiol. 2007;7:9 10.1186/1471-2180-7-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Couto-Lima D, Madec Y, Bersot MI, Campos SS, Motta MA, Santos FBD, et al. Potential risk of re-emergence of urban transmission of Yellow Fever virus in Brazil facilitated by competent Aedes populations. Sci Rep. 2017;7(1):4848 10.1038/s41598-017-05186-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jupille H, Seixas G, Mousson L, Sousa CA, Failloux AB. Zika Virus, a New Threat for Europe? PLoS Negl Trop Dis. 2016;10(8):e0004901 10.1371/journal.pntd.0004901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007;3(12):e201 10.1371/journal.ppat.0030201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vazeille M, Moutailler S, Coudrier D, Rousseaux C, Khun H, Huerre M, et al. Two Chikungunya isolates from the outbreak of La Reunion (Indian Ocean) exhibit different patterns of infection in the mosquito, Aedes albopictus. PLoS One. 2007;2(11):e1168 10.1371/journal.pone.0001168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zouache K, Fontaine A, Vega-Rua A, Mousson L, Thiberge JM, Lourenco-De-Oliveira R, et al. Three-way interactions between mosquito population, viral strain and temperature underlying chikungunya virus transmission potential. Proceedings Biological sciences / The Royal Society. 2014;281(1792). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruckert C, Ebel GD. How Do Virus-Mosquito Interactions Lead to Viral Emergence? Trends Parasitol. 2018;34(4):310–21. 10.1016/j.pt.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsuda Y, Maekawa Y, Ogawa K, Itokawa K, Komagata O, Sasaki T, et al. Biting Density and Distribution of Aedes albopictus during the September 2014 Outbreak of Dengue Fever in Yoyogi Park and the Vicinity of Tokyo Metropolis, Japan. Jpn J Infect Dis. 2016;69(1):1–5. 10.7883/yoken.JJID.2014.576 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.