Abstract
Theories in soil biology, such as plant–microbe interactions and microbial cooperation and antagonism, have guided the practice of ecological restoration (ecorestoration). Below‐ground biodiversity (bacteria, fungi, invertebrates, etc.) influences the development of above‐ground biodiversity (vegetation structure). The role of rhizosphere bacteria in plant growth has been largely investigated but the role of phages (bacterial viruses) has received a little attention. Below the ground, phages govern the ecology and evolution of microbial communities by affecting genetic diversity, host fitness, population dynamics, community composition, and nutrient cycling. However, few restoration efforts take into account the interactions between bacteria and phages. Unlike other phages, filamentous phages are highly specific, nonlethal, and influence host fitness in several ways, which make them useful as target bacterial inocula. Also, the ease with which filamentous phages can be genetically manipulated to express a desired peptide to track and control pathogens and contaminants makes them useful in biosensing. Based on ecology and biology of filamentous phages, we developed a hypothesis on the application of phages in environment to derive benefits at different levels of biological organization ranging from individual bacteria to ecosystem for ecorestoration. We examined the potential applications of filamentous phages in improving bacterial inocula to restore vegetation and to monitor changes in habitat during ecorestoration and, based on our results, recommend a reorientation of the existing framework of using microbial inocula for such restoration and monitoring. Because bacterial inocula and biomonitoring tools based on filamentous phages are likely to prove useful in developing cost‐effective methods of restoring vegetation, we propose that filamentous phages be incorporated into nature‐based restoration efforts and that the tripartite relationship between phages, bacteria, and plants be explored further. Possible impacts of filamentous phages on native microflora are discussed and future areas of research are suggested to preclude any potential risks associated with such an approach.
Keywords: bioremediation, biosensors, ecological theory, filamentous phages, microbial ecology and fitness, restoration ecology
1. INTRODUCTION
Theories in soil biology guide the efforts to restore vegetation in degraded habitats. Natural attenuation—banning any activity that results in environmental degradation—is useful only in those ecosystems that are in the early stages of degradation. Currently, a vast majority of degraded ecosystems show altered abiotic and biotic components and lowered resilience. Consequently, assisted restoration practices, such as planting native species, replacing or treating contaminated soil, and managing water resources, represent the only land restoration options for most of the degraded ecosystems. The Society of Ecological Restoration defines ecorestoration as “the process of assisting the recovery of an ecosystem that has been degraded, damaged or destroyed” (SER, 2004). Restoration practices developed on the principles of plant–microbe mutualism and microbial cooperation, synergism, and antagonism have facilitated ecorestoration (Heneghan et al., 2008; Perring et al., 2015; Young, Petersen, & Clary, 2005).
Over 65% of the earth's surface has been degraded or contaminated, which has resulted in the loss of its potential to benefit human society—which is why ecorestoration is held to be a global need by the United Nations. Restoration practices have site‐specific goals, such as to repair environmental damage after deforestation or overharvesting, to stabilize soil after mining, to restore the productivity of saline soils after heavy irrigation, and to remediate soils contaminated as a result of industrial activity. However, major goals of all land restoration continue to be the restoration of biodiversity, revival of ecosystem services for socio‐economic security, and improved resilience of ecosystems to future environmental change. Restoration may be voluntary, undertaken to improve the quality of life, or mandatory, a legislative directive to ensure sustainable development.
Degraded lands need specialized restoration efforts rather than conventional soil amelioration methods, because such lands are often exposed to multiple sources of stress such as high levels of contaminants, toxins, and pathogens (Perring et al., 2015). Traditionally, soil scientists use agronomic practices and chemical amendments to improve soil fertility (Filiberto & Gaunt, 2013), although different sources of stress need source‐specific efforts to improve soil and plant health. The traditional methods commonly used for treating farmlands or small patches of degraded lands are not only too costly for restoring vast stretches of degraded ecosystems but also of limited efficacy in controlling pathogens and biological toxins and coping with changes in the environment (Figure 1). In contrast, microbial inocula (free‐living, associative, and endosymbiotic) repair normal biological processes affected by degradation, ameliorate contaminated soils, and control phytopathogens in degraded habitats. Microbial inocula are thus an ecologically sound option to speed up revegetation and revive ecosystem services under diverse environmental regimes (Tables 1, 2, 3). Some bacterial genera, such as Bacillus, Bradyrhizobium, Enterobacter, and Pseudomonas, have been widely used as inocula and even commercial formulations have been developed for use with economically and ecologically important plants (Table 1). Microbes may possess multiple traits that may be deployed in combating both abiotic and biotic sources of stress; however, restoration ecologists select bacteria with specific traits to tackle the most serious environmental challenges (Rau et al., 2009; Sharma, Mishra, Rau, & Sharma, 2015). Alternatively, consortia of microbes may also be developed to deal with multiple challenges, but their efficacy is reduced because members of such consortia differ in their environmental and nutritional requirements, and the use of consortia continues to face unpredictable challenges.
Figure 1.
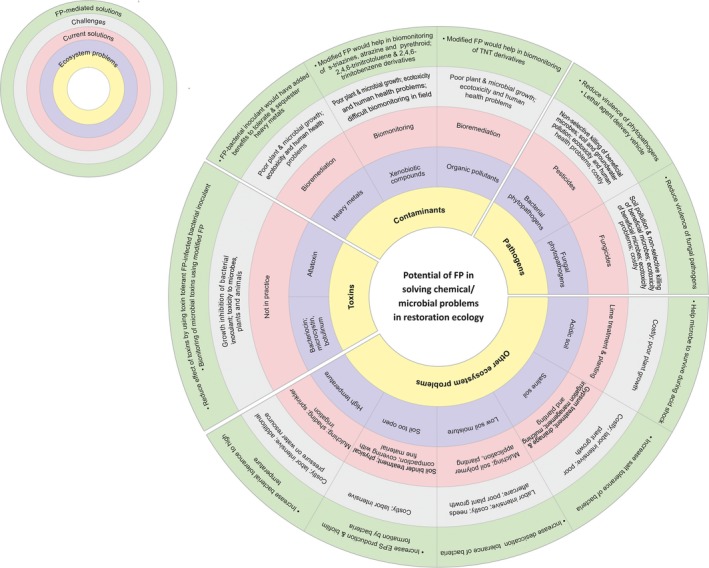
Ecosystem and other environmental challenges for ecological restoration of degraded lands, where current techniques face challenges but filamentous phage has potential to provide solutions. FP, filamentous phage
Table 1.
Potential bacterial targets to explore filamentous phage to improve the benefits of commercial inoculants being used to promote plant growth under different environmental stresses
| Target function | Target organism(s)/activity | Bacterial composition | Trade name of inoculants | Filamentous phage |
|---|---|---|---|---|
| Biofungicide | Rot diseases caused by Fusarium and Rhizoctonia | Bacillus subtilis | Kodiak HB | Needs investigation |
| Seed‐borne disease caused by fungus Ascochyta spp. | Pseudomonas chlororaphis | Cedress; Cedomon | Known for Pseudomonas | |
| Rots, blights, wilt, leaf spot, and mildew | Bacillus subtilis | Biotilis | Needs investigation | |
| Leaf spot caused by different genera of fungus | Pseudomonas fluorescens | Bactvipe | Known for Pseudomonas | |
| Seed‐borne diseases: Common wheat bunt (Tilletia caries), wheat leaf spot (Septoria nodorum) | Pseudomonas chlororaphis | Cerall | Known for Pseudomonas | |
| Bioinsecticide | Lepidoptera larvae and beetles | Bacillus thuringiensisvar. kurstaki | Bioscrop BT16 | Needs investigation |
| Insecticide/miticide: Infection by aphids, psyllids, whiteflies, lygus and mealybugs, thrips and phytophagous mites | Chromobacterium subtsugae strainPRAA4‐1 | Grandevo | Needs investigation | |
| Insect pests of lepidoptera, diptera, coleoptera, hymenoptera | B. thuringiensis var. kurstaki | Lipel | Needs investigation | |
| Bionematicides | Meloidogyne spp, Hetrodera spp, Helicotylenchus spp, Hoplolaimus spp. causing root‐knot, cyst, lesion | Bacillus firmus | Bionemagon | Needs investigation |
| Multifunctional PGPR |
|
Pseudomonas fluorescensA506 | BlightBan A506 | Known for Pseudomonas |
|
P. fluorescensIIHR PF‐2 | Sheathguard | Known for Pseudomonas | |
|
Pseudomonas chlororaphissubsp. aurantiacaSR1 | Liquid PSA | Known for Pseudomonas | |
|
Azotobacter Chrococcum | Bio Azo | Needs investigation | |
|
Azotobacter chroococcum, Pseudomonas fluorescens | Bio Gold | Known for Pseudomonas | |
|
Bacillus, Firmus, Clothianidin | Poncho/Votivo | Needs investigation | |
|
Bacillus species | Si Sol B | Needs investigation | |
| Nutrient Solubilizer/Mobilizer | N2‐fixation | Bradyrhizobium japonicum | Optimize liquid Soybean; Rhizo‐Flo | Needs investigation |
| B. japonicum(USDA 138 for USA, 532C for Canada) | Nodulest 10 | |||
| Acetobacter diazotrophicus | Bio Aceto | |||
| Bradyrhizobium japonicum, Penicillium bilaii | TagTeam LCO | |||
| Delftia acidovorans, Bradyrhizobium japonicum | Bioboost | |||
| Glomus intraradices, Rhizobium leguminosarumbv. viciae | AGTIV | |||
| Bacillus subtilis | HiStick N/T | |||
| Bradyrhizobium japonicum, Trichoderma sp. | Graph‐Ex SA | |||
| Bradyrhizobium japonicum | Dyna‐Start; Vault SP | |||
| Provide inorganic and organic nutrients | Pseudomonas azotoformans | Amase | ||
| Improved bioavailability of phosphate and uptake of N and K | Bacillus amyloliquefaciens, Trichoderma virens | QuickRoots | ||
| Provides N and P | Acidovorax facilis, Bacillus subtilis, B. licheniformis, B. megaterium, B. oleronius, B. marinus, & Rhodococcus rhodochrous | Accomplish LM | ||
| Iron mobilization | Acidithiobacillus ferrooxidan | Fe Sol B | ||
| K Mobilization through production of organic acids and enzymes | Frateuria aurantia | K Sol B | ||
|
Azotobacter vinelandii‐B1795, Bacillus megateriumB1091, Clostridium pasteurianum, Azospirillumsp., Bacillus subtilis, Rhodobactersp., Lactobacillussp., Trichoderma reesei, Saccharomyces cerevisiae, Streptomycessp. | Microbion UNC | Known for Clostridium; needs investigation for other | |
| Rhizobium leguminosarumbiovar viceae | Nodulator XL | Needs investigation | ||
| Azospirillum brasilense; Rhizobium | Nitrofix and Bioenraiz | |||
| Bradyrhizobiumsp. | Vault SP | |||
| Consortium of Rhizobium | Rhizo‐Flo | |||
| High load of Rhizobium | Primo | |||
| Acetobacter diazotrophicus(MTCC 1226), Azotobacter chroococcum (MTCC 3853), A. vinelandii(NCIM 2821), Azospirillum lipoferum(NCIM 2908), Rhizobium japonicum(NCIM 2743) | Agrilife Nitrofix | |||
| Azospirillum brasilense, Azotobacter vinelandii, Bacillus megaterium, B. polymyxa, Pseudomonas fluorescens, Streptomyces albus | Bactofil A 10 | Known for Pseudomonas; for other needs investigation | ||
| Phosphate solubilization | Bacillus subtilis | Bio Phospho | Needs investigation | |
| Bacillus megaterium | Bio Phos | |||
| Pseudomonas striata(NCIM 2847), Bacillus polymyxa(NCIM 2188), B. megaterium(NCIM 2087) | P Sol B | Known for Pseudomonas | ||
| Zinc mobilization | T. thiooxidansNCIM‐5065 | Zn Sol B | Needs investigation | |
| Potash solubilization | Frateuria aurantia | Bio Potash | Needs investigation | |
| Soil amendment | Reclaim alkaline soil (oxidizes sulfur and secretes organic acids) | Thiobacillus thiooxidans(NCIM 5069) | S Sol B | Needs investigation |
Table 2.
Details of bacterial inoculants used for ecological restoration, as potential targets for studying filamentous phage‐mediated benefits
| Bacterial genera | Species | Plant growth‐promoting/bioremedifying traits | Benefits to soil, human, and plants | Filamentous phage (known/needs investigation) | Reference |
|---|---|---|---|---|---|
| Agrobacterium | radiobacter | Provide P and N | Vegetation restoration, Sustainable agriculture | Needs investigation | Belimov, Kojemiakov, and Chuvarliyeva (1995), Belimov Kunakova, et al. (1995), Bashan and Holguin (1997), Singh and Kapoor (1999) |
| tumefaciens | Degrade pesticides: atrazine, simazine, s‐triazine | Reduce human exposure to xenobiotics and carcinogens; sustainable agriculture | Prashanthi, Sundaram, Jeyaseelan, and Kaliannan (2017) | ||
| Azospirillum | amazonense, brasilense, doebereinerae | Fix nitrogen, stabilize soil, reduce nutrient load (NH4, P) from industrial water | Improve plant growth; reduce toxicant load in wastewater; sustainable agriculture; commercial biofertilizer, | Needs investigation | de‐Bashan, Moreno, Hernandez, and Bashan (2002), Eckert et al. (2001), Volpon, De‐Polli, and Döbereiner (1981), Pindi and Satyanarayana (2012) |
| lipoferum | Fix nitrogen, provide vitamins, nicotinic acid, IAA, gibberellins | Restoration of arid ecosystems; growth of grasses; sustainable agriculture; commercial biofertilizer | Bashan and Holguin, (1997); Carrillo‐Garcia, Bashan, Diaz Rivera, and Bethlenfalvay (2000); Pindi and Satyanarayana (2012) | ||
| Azotobacter | vinelandii | Fix nitrogen; increase growth of mangrove species | Restoration of mangrove forest; sustainable agriculture | Needs investigation | Bürgmann, Widmer, Sigler, and Zeyer (2003); Kathiresan and Selvam (2006) |
| chroococcum | Microbe‐assisted phytoremediation of heavy metals (Pb, Zn) | Restoration of mine | Wu, Cheung, Luo, and Wong (2006) | ||
| Acetobacter | diazotrophicus | Fix nitrogen | Vegetation restoration, sustainable agriculture | Needs investigation | Pindi and Satyanarayana (2012) |
| Acinetobacter | baumanniiPUCM1029; calcoaceticus | Fix nitrogen; solubilize phosphate; produce IAA | Environmental restoration; sustainable agriculture | CRAϕ | Rokhbakhsh‐Zamin et al. (2011) |
| radioresistens | Degrades different pesticides | Environmental restoration; sustainable agriculture | Acinetobacter baylyi: CRAϕ | Prashanthi et al. (2017) | |
| calcoaceticus, lwoffii, venetianus, sp. RTE1.4, sp. HC8‐3S, sp. A3 | Degrade organic contaminants (crude oil, halogens, phthalate esters, phenols) in soil and water; reduce nutrient load from wastewater | Environmental restoration; wastewater treatment; sustainable industry | CRAϕ | Fondi et al. (2013); Vamsee‐Krishna, Mohan, and Phale (2006) | |
| Bradyrhizobium | japonicum | Nitrogen provider | Vegetation restoration, sustainable agriculture | Needs investigation | Rodriguez‐Navarro, Oliver, Contreras, and Ruiz‐sainz (2011) |
| Bacillus | megaterium | Fix nitrogen; promote mangrove plant growth; phytoextraction of Pb and Zn | Mangrove and mine restoration; sustainable agriculture | Needs investigation | Kathiresan and Selvam (2006), Pindi and Satyanarayana (2012), Wu et al. (2006) |
| licheniformis | Fix nitrogen; solubilize phosphate | Mangrove restoration | Rojas, Holguin, Glick, and Bashan (2001) | ||
| polymyxa | Solubilize phosphate | Vegetation restoration, sustainable agriculture | Pindi and Satyanarayana (2012) | ||
| mucilaginosus | Phytoextraction of heavy metals (Pb and Zn in a mine tailing) | Mine restoration | Wu et al. (2006) | ||
| Sp. | Degrade pesticides(trifluralin, endosulfan, Aldrin, lindane) | Environmental restoration, reduction of human exposure to carcinogen and genotoxicants, sustainable agriculture | Prashanthi et al. (2017) | ||
| Clostridium | glycolicum, collagenovorans | Bioremediation by volatilization of As (V) | Environmental restoration; reduce human exposure to carcinogens and genotoxicants | CAK1ϕ | Michalke, Wickenheiser, Mehring, Hirner, and Hensel (2000), Meyer, Schmidt, Michalke, and Hensel (2007) |
| Erwinia | amylovoraHSA6 | Mineralize aniline | Environmental restoration; sustainable industry, reduce human exposure to mutagenic and carcinogenic xenobiotics | Known from other members of family (Escherichia: M13ϕ, X‐2ϕ, Xϕ, f1ϕ, Ikeϕ, PR6FSϕ, C‐2ϕ, SFϕ, tf‐1ϕ, fdϕ, I2‐2ϕ, If1ϕ, AE2ϕ, Ec9ϕ, HRϕ, ZJ/2ϕ, CUS1ϕ; Yersinia:Ypfϕ; CUS2ϕ; Shigella:SfXϕ) | Li, Jin, and Yu (2010) |
| carotovora | Degrades different pesticides (pyrethroidallethrin, β‐cyfluthrin, bifenthrin, cypermethrin, flumethrin and permethrin) | Prashanthi et al. (2017) | |||
| Enterobacter | cloacae | Remediate heavy metals (Cr VI, Pb, Cd and Ni II) and radioactive elements | Environmental restoration, sustainable industry, reduce human exposure to toxicants | Prashanthi et al. (2017), Singh, Walker, Morgan, and Wright (2004), George, Gupta, Gopal, Thomas, and Thomas (2013), Gupta, Saxena, Gopal, and Tilak (2003), Gontia‐Mishra, Sapre, Sharma, and Tiwari (2016) | |
| ludwigii | Assist wheat to tolerate drought | Sustainable agriculture | |||
| Sp.B‐14 | Degrades organopesticides (chlorpyrifos, fonofos, terbufos) | Environmental restoration, sustainable agriculture, reduce human exposure to carcinogens | |||
| Sp. RNF 267, EG‐ER‐1, KG‐ER‐1 | Promote plant growth (coconut palms and maize), fix nitrogen | Sustainable agriculture, increase crop yield | |||
| Escherichia | coli | Degrades pesticides (atrazine, simazine, s‐triazine), promote plant growth, biocontrol of pathogen by producing bacteriocin (colicins); remediation of heavy metal (Methylmercury); Siderophore production | Reduce exposure of xenotoxic toxicant, carcinogens, Bioremediation, Sustainable agriculture | Prashanthi et al. (2017), Beneduzi, Ambrosini, and Passaglia (2012), Kane et al. (2016), Searle, Méric, Porcelli, Sheppard, and Lucchini (2015) | |
| Flavobacterium | sp. | Degrade pesticides (cadusafos, diazinon, dichlorovos, ethoprophos, fenamiphos, fenitrothion, isazofos, isofenphos, isoxathion, malathion, methylparathion, monocrotophos, paraoxon, parathion, phosphomidon and quinalphos); Solubilize phosphate | Environmental restoration, sustainable agriculture, reduce human exposure to carcinogens | Needs investigation | Prashanthi et al. (2017), Pindi and Satyanarayana (2012) |
| Frateuria | aurentia | Solubilize potash | Vegetation restoration, sustainable agriculture | Needs investigation | Pindi and Satyanarayana (2012) |
| Herbaspirillum | seropedicae, | Fix nitrogen | Vegetation restoration, sustainable agriculture | Needs investigation | Pindi and Satyanarayana (2012) |
| rubisubalbicans | |||||
| Sp. | Degrade pesticides (trifluralin) | Environmental restoration, sustainable agriculture, reduce human exposure to carcinogens | Prashanthi et al. (2017) | ||
| Micrococcus | luteus | Degrade pesticides (aldrin and lindane) | Environmental restoration, sustainable agriculture, reduce human exposure to carcinogens | Needs investigation | Prashanthi et al. (2017) |
| yunnanensisSMJ12 | Fix nitrogen, solubilize phosphate, produce IAA, siderophores, and 1‐aminocyclopropane‐1‐carboxylate (ACC) deaminase, | Environmental restoration, sustainable agriculture | Mesa et al. (2015) | ||
| Methylobacterium | sp. CBMB20 and sp. CBMB110 | Produce cytokinin and promote sugarcane, tomato, and red pepper crop yield | Sustainable agriculture | Needs investigation | Madhaiyan et al. (2005), Ryu et al. (2006) |
| Neisseria | flavescens | Bioremediation of atmospheric hydrocarbons; accelerate phytoremediation of oil contaminants | Environmental restoration, sustainable industry, reduce human exposure to xenotoxicants | Known from genus (N. gonorrhoeae: NgoΦ6, NgoΦ7, NgoΦ8, NgoΦ9; N. meningitides: MDAΦ, NFϕ; MDAϕ; Nf1‐Aϕ; Nf3‐Aϕ; Nf1‐B1ϕ; B2ϕ; Nf1‐C1ϕ; C2ϕ; C3ϕ; C4ϕ; Nf4‐G2ϕ; G3ϕ; G5ϕ) | Ali et al. (2015) |
| Phyllobacterium | Sp. | Solubilize phosphate; fix nitrogen | Enhance mangrove growth | Needs investigation | Rojas et al. (2001) |
| Propionibacteria | acnes | Bioremediation of atmospheric hydrocarbons and accelerate phytoremediation of oil contaminants | Environmental restoration, sustainable industry | B5ϕ | Ali et al. (2015) |
| Sp. | Provide nitrogen | Vegetation restoration, sustainable agriculture | Sellstedt and Richau (2013) | ||
| Pseudomonas | aeruginosaUCP1567 | Degradation and decolorization of Black B azo dye | Environmental restoration; reduce exposure of xenotoxic toxicants and carcinogens; bioremediation; sustainable agriculture | Known from genus (P. aeruginosa Pf1ϕ; Pf2ϕ; Pf3ϕ; Pf4ϕ; Pf5ϕ; Pf7ϕ; Pf‐LESB58ϕ) | Vilar Jr, Cavalcanti, da Silva, Andrade, and Campos‐Takaki (2015) |
| aeruginosa | Degrade pesticides (HCH, endosulfan) | Prashanthi et al. (2017) | |||
| cepacia | Degrade pesticides (endosulfan) | ||||
| diminuta | Degrade pesticides (chlorpyrifos, fonofos, terbufos) | ||||
| fluorescens | Degrade pesticides (oxadiazon, chlorsulfuron, metsulfuronmethyl); enhance metal uptake in plants | Prashanthi et al (2017), Berg (2009), Vessey, (2003), Chanway, (1997) | |||
| fluorescens | Biopesticide for fungus | Sustainable agriculture of cotton | Wang, Wang, and Zhou (2004) | ||
| maltophilia | Capable of degrading pesticides: Dicamba | Environmental restoration, sustainable agriculture | Prashanthi et al. (2017) | ||
| pseudoalcaligenes | Degrade pesticides (aldrin and lindane) | ||||
| putida | Degrade pesticides (Cadusafos, diazinon, dichlorovos, ethoprophos, fenamiphos, fenitrothion, gramoxone, HCH, isazofos, isofenphos, isoxathion, malathion, matancha, methylparathion, monocrotophos, paraoxon, phosphomidon, parathion, quinalphos, vinclozolin) | ||||
| spinosa | Degrade pesticides (endosulfan) | Prashanthi et al. (2017) | |||
| stutzeri | Degrade pesticides (pyrethroidallethrin, β‐cyfluthrin, bifenthrin, cypermethrin, flumethrin and permethrin) | ||||
| striata, rathonis | Solubilize phosphate | Revegetation,sustainable agriculture | Pindi and Satyanarayana (2012) | ||
| vesicularis, diminuta | Enhance growth of algae (Chlorella) | Mouget, Dakhama, Lavoie, and Noüe (1995) | |||
| Sp. | Degrade pesticides (2,4‐D, atrazine, chlorotoluron, diuron, isoproturon, linuron, monolinuron, propoxur, simazine, s‐triazine, trifluralin); Pentachlorophenol; produce cytokinin | Environmental restoration; sustainable agriculture and industry; disease resistance in plants | Prashanthi et al. (2017) | ||
| Pseudoalteromonas | haloplanktis | Bioremediation of mercury in aquatic environment | Environmental restoration, ecotoxicity prevention, sustainable industry | Known from other members of family (Pseudomonas: Pf1ϕ; Pf2ϕ; Pf3ϕ; Pf4ϕ; Pf5ϕ; Pf7ϕ; Pf‐LESB58ϕ) | Lohara et al. (2001) |
| sp. SCSE709–6 | Bioremediation of Cd(II) under high salinity, pH, and temperature | Zhou, Zhang, Ma, Zhou, and Zhang (2013) | |||
| Rhizobium | leguminosarum | Provide nitrogen | Vegetation restoration, sustainable agriculture | Needs investigation | Young et al. (2006); NFTA (1986) |
| sp. | Siderophore (carboxylate) production | Mosa, Saadoun, Kumar, Helmy, and Dhankher (2016) | |||
| sp. | IAA producer | Datta and Basu (2000), Bhattacharyya and Pati (2000) | |||
| sp. | Remediation of heavy metal contaminated soils | Reduce exposure of xenotoxicants, carcinogens, bioremediation | Wei and Ma (2010) | ||
| Remediation of As contaminated sites | Reichman (2007), Carrasco et al. (2005) | ||||
| galegae | Remediation of benzene, toluene, and/or xylene (BTX) from soil | Good growth, nodulation, nitrogen fixation, and a strong rhizosphere occurred in soils contaminated with oil or spiked with m‐toluate, a model compound representing BTX | Suominen, Jussila, Mäkeläinen, Romantschuk, and Lindström (2000) | ||
| Ralstonia | basilensis | Capable of degrading pesticides: Atrazine, simazine, s‐triazine | Environmental restoration, sustainable agriculture | Known from genus (R. solanacearum: PE226ϕ; RSM1ϕ; RSS1ϕ; RSM3ϕ; RSS0ϕ; p12Jϕ) | Prashanthi et al. (2017) |
| solanacearum | Bioremediation of atmospheric hydrocarbons; accelerate phytoremediation of oil contaminants | Ali et al. (2015) | |||
| pickettii | Solubilize phosphate | Kailasan and Vamanrao (2015) | |||
| Sinorhizobium | fredii | Fix nitrogen | Sustainable agriculture | Needs investigation | Rodriguez‐Navarro et al. (2011) |
| meliloti | Remediation of polycyclic aromatic hydrocarbons (PAH) | a promising bioremediation strategy for aged PAH‐contaminated soils | Teng et al. (2011) | ||
| Shewanella | oneidensis | Bioremediation of heavy metals and radionuclide Cr(VI), Fe(III), Mn(IV), U(VI), and V(V). | Environmental restoration; sustainable agriculture; reduce human exposure to genotoxicants | SW1ϕ | Fredrickson et al. (2008) |
| Sp. | Provide indole; solubilize phosphate | Environmental restoration; sustainable agriculture | Fredrickson et al. (2008) | ||
| Shigella | flexneri | Bioremediation of hydrocarbons and oil from soil | Environmental restoration, sustainable industry | SfXϕ | Duniya, Maikaje, Umar, Ponchang, and Daniel (2016) |
| Stenotrophomonas | acidaminiphila | Degrade pesticides (Pyrethroidallethrin, β‐cyfluthrin, bifenthrin, cypermethrin, flumethrin and permethrin) | Environmental restoration; sustainable agriculture | Known from genus (S. maltophilia:SMA9ϕ; SHP2ϕ) | Prashanthi et al. (2017) |
| maltophilia | Tolerate salt; provide resistance in wheat against biotic and abiotic stress | Singh and Jha (2017) | |||
| maltophilia | Bioremediation of atmospheric hydrocarbons; accelerate phytoremediation of oil contaminants | Ali et al. (2015) | |||
| Salinicola | peritrichatus SMJ30 | Solubilize phosphate; produce IAA, siderophores and 1‐aminocyclopropane‐1‐carboxylate (ACC) deaminase; fix nitrogen | Environmental restoration; sustainable agriculture | Needs investigation | Mesa et al. (2015) |
| Thermus | scotoductus | Bioremediation of toxic metals and radionuclides (Cr, Fe, Co, Tc, U, etc.) in the hot spring wastewater or heated nuclear waste streams. | Environmental restoration, sustainable industry, hazardous waste management, wastewater treatment | Known from genus (T. Thermophiles: PH75ϕ) | Opperman and van Heerden (2008), Slobodkin (2005) |
| thermophilus | Root stabilizer | Sustainable agriculture | Singh, Sarma, and Keswani (2017), Stetter (1999) | ||
| HR13 | Bioremediation of from hot waste water and anaerobic condition | Environmental restoration, sustainable industry | Gihring and Banfield (2001) | ||
| Sp. | Bioremediation of minerals selenite and tellurite | Slobodkin, Sokolova, Lysenko, and Wiegel (2006), Sokolova et al. (2004), Chiong, Barra, González, and Vásquez (1988), Chiong, González, Barra, and Vásquez (1988) | |||
| Vibrio | fischeri | Degrade pesticides (diuron, chlorotoluron, isoproturon, monolinuron and linuron) | Environmental restoration, sustainable industry | Known from genus (V. cholera:493ϕ; CTXϕ; fs1ϕ; fs2ϕ; v6ϕ; Vf12ϕ; Vf33ϕ; VSKϕ; CTXϕ; pre‐CTXϕ; KSF‐1ϕ; VEJϕ; ND1‐fs1ϕ; VCYϕ; VGJϕ; VSKKϕ; VfO4K68ϕ; VfO3K6 (f237)ϕ; VfO3K6ϕ; CTX‐nctϕ) | Prashanthi et al. (2017) |
| sagamiensisSMJ18 | Fix nitrogen, solubilize phosphates, produce IAA, siderophores, and 1‐aminocyclopropane‐1‐carboxylate (ACC) deaminase. | Vegetation restoration, sustainable agriculture | Mesa et al. (2015) | ||
| Xanthomonas | axonopodis | IAA producer | Environmental restoration, sustainable industry | XacF1ϕ | Costacurta, Mazzafera, and Rosato (1998); Ahmad et al. (2014) |
| Yersinia | frederiksenii | Degrade pesticides (pyrethroidallethrin, β‐cyfluthrin, bifenthrin, cypermethrin, flumethrin and permethrin) | Environmental restoration, sustainable industry | Known from genus (Y.pestis: Ypfϕ; CUS2ϕ) | Prashanthi et al. (2017) |
Table 3.
Details of top ten phytopathogenic bacteria and their associated filamentous phages
| Bacterial genus | Species | Pathogenic traits | Filamentous phage | Reference |
|---|---|---|---|---|
| Agrobacterium | tumefaciens | Crown gall disease in over 140 species of eudicots | Needs investigation | |
| Dickeya | dadantii solani | Soft rot disease | Known from other members of family Enterobacteriaceae (Escherichia: M13ϕ, X‐2ϕ, Xϕ, f1ϕ, Ikeϕ, PR6FSϕ, C‐2ϕ, SFϕ, tf‐1ϕ, fdϕ, I2–2ϕ, If1ϕ, AE2ϕ, Ec9ϕ, HRϕ, ZJ/2ϕ, CUS1ϕ ; Yersinia:Ypfϕ; CUS2ϕ;Shigella:SfXϕ) | Vrancken, Holtappels, Schoofs, Deckers, and Valcke (2013), Starr and Chatterjee (1972) |
| Erwinia | amylovora | Fire blight, bacterial wilt; necrosis of tissue | ||
| tracheiphila | ||||
| Pectobacterium | carotovorum atrosepticum | Soft rot and blackleg disease | ||
| Pseudomonas | aeruginosaPAO1 and PA14 | Root infection and plant mortality; | Pf1ϕ; Pf2ϕ; Pf3ϕ; Pf4ϕ; Pf5ϕ; Pf7ϕ; Pf‐LESB58ϕ | Walker et al. (2004) |
| putidaKT2440 | Phage reduces bacterial fitness in rhizosphere | Pspu28 | Quesada, Soriano, and Espinosa‐Urgel (2012) | |
| syringae | Phytopathogen | Known from P. aeruginoasa | Marcelletti and Scortichini (2014) | |
| Ralstonia | solanacearum | Pathogen: bacterial wilt | PE226ϕ; RSM1ϕ; RSS1ϕ; RSM3ϕ; RSS0ϕ; p12Jϕ | Peeters, Guidot, Vailleau, and Valls (2013) |
| Xanthomonas | campestris pv. citri | Citrus canker, bacterial leaf spot | Cf16ϕ; Cf1cϕ; Cf1tϕ; Cf1tvϕ; Lfϕ; Xfϕ; Xfoϕ; Xfvϕ; Cfϕ; Lfϕ; Xoϕ; Xvϕ; Xf2ϕ | Rodriguez et al. (2012), Potnis et al. (2015), Thieme et al. (2005) |
| campestris pv. vesicatoria | Foliage and fruit spot disease | ϕXv | Lin et al., (1994) | |
| oryzaepv. oryzae | Bacterial leaf blight in rice | ϕXo | ||
| Xylella | Fastidiosa | Phytopathogen: Olive quick decline syndrome, bacterial leaf scorch, oleander leaf scorch, coffee leaf scorch, alfalfa dwarf, phony peach disease, Pierce's disease of grapes and citrus variegated chlorosis | Xff1ϕ | Hopkins and Purcell (2002) |
| Yersinia | pseudotuberculosisand enterocolitica | Infect plants: Affect quality of pasture land and crop plants; cause human disease | Known only from Y. pestis: Ypfϕ; CUS2ϕ | Fukushima, Shimizu, and Inatsu (2011) |
| Fetherston, Lillard, and Perry (1995) |
As a result of advances in molecular and genomic studies, bacterial viruses (bacteriophages or simply phages) have emerged as one of the key elements governing the structure and functioning of microbial communities (Kauffman et al., 2018). Phages play an important role in developing a resilient microbial community (Koskella & Brockhurst, 2014; Silveira & Rohwer, 2016) and in driving adaptation, competition, and antagonism in bacteria and thereby influence the evolution of bacteria and the assembly of microbial community (Rodriguez‐Valera et al., 2009; Mai‐Prochnow et al., 2015; Shapiro, Williams, & Turner, 2016) (Figure 2). However, applying these theories of microbial ecology to ecorestoration has not received due attention.
Figure 2.
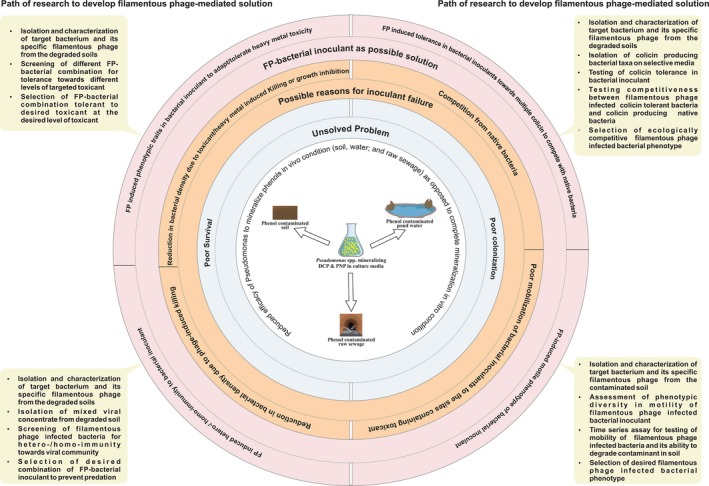
An unsolved problem related to exploiting bioremediation potential of Pseudomonas in phenol contaminated field environment highlighting the potential of filamentous phage to provide solutions and path of research to arrive at them (based on Goldstein, Mallory, & Alexander, 1985; Mrozik, Miga, & Piotrowska‐Seget, 2011). FP, filamentous phage.
Among phages, the use of filamentous phages—rod‐shaped single‐stranded circular DNA viruses characterized by a long helical nonenveloped protein coat (King, Lefkowitz., Adams, & Carstens, 2011)—has evolved from answering fundamental questions in biology to developing biotechnological tools (Rakonjac, 2012; Rakonjac, Bennett, Spagnuolo, Gagic, & Russel, 2011). Filamentous phages are of interest to microbial ecologists and biotechnologists because the phages have unique morphological, biological, and genomic features. The life cycle of these phages is marked by chronic infections: The phages multiply continuously within their bacterial host, which releases them into the immediate environment without undergoing cell lysis (Calendar & Inman, 2005; Maniloff, Cadden, & Putzrath, 1981; Rakonjac et al., 2011).Owing to their small and simple genome, filamentous phage can be easily manipulated to display on their surface a variety of peptides or polypeptides, which makes them useful in developing versatile biosensors (Harper & Kutter, 2008; Rakonjac et al., 2011; Table 4). These phages, therefore, have been studied extensively for their morphology and biology (Das, 2014; Krupovic & Forterre, 2015; Marvin, 1998; Opella, Zeri, & Park, 2008; Rakonjac, 2012), for their influence on the physiology of their bacterial hosts (Mai‐Prochnow et al., 2015), for their traditional applications in displaying specified peptides or proteins (Benhar, 2001; Kehoe & Kay, 2005; Willats, 2002), and for their nontraditional applications to develop tools for diagnostic purposes and in nanobiotechnology and synthetic biology for exploring the secretomes of microbes (Rakonjac et al., 2011; Henry, Arbabi‐Ghahroudi, & Scott, 2015; Gagic, Ciric, Wen, Ng, & Rakonjac, 2016; Szekely & Breitbart, 2016; Mai‐Prochnow et al., 2015).
Table 4.
Comparison of structural, biological, genomic, and functional properties of filamentous phages to that of tailed phages
| Property | Character | Filamentous phage | Tailed phages |
|---|---|---|---|
| Classification |
Order Family |
Not assigned Inoviridae, Plectrovirus |
Caudovirales
Myoviridae, Podoviridae, Siphoviridae |
| Morphology | Shape | Long cylindrical | Diverse shape (vary in tail length and type) |
| Segmentation | Nonsegmented body | Generally segmented body (head, collar, tail, etc.) | |
| Genome | Genome size respect to body length | Smaller genome size as compared to body length (almost double; 3–9.5 kb) | High genome size |
| Host | Host | Infect F+ bacteria only | No preference for F+ or F − |
| Receptor | Receptor | Pilus of the male bacteria | Receptor present on the cell surface |
| No. of receptor | Limited numbers (2–4 per cell) | Many (up to few hundreds per host cell) | |
| Adsorption rate | Adsorption rate constant = 3 × 10−11 cm3/min | Adsorption rate constant = 2.4 × 10−9 cm3/min | |
| Infection | Nature | Chronic | Lytic, Temperate |
| Life cycle | Type | 3 types (episomal, constitutively replicating lysogen and inducible lysogen) | 2 types (lytic and lysogenic) |
| Progeny release | Lysis | Does not kill its host | Kills the host |
| Progeny release | Progeny released throughout the life span of the host | Progeny released once in host's life | |
| Detection | Plaque formation | Absent or turbid (in some cases) | Clear, turbid or centered |
| Observation on plate assay | Difficult | Easy | |
| Electron#x2010;microscopic assay | Generally neglected as debris or pili of bacteria because of its shape and size | Quite easy to distinguish due to characteristics shape and size |
The growing knowledge of the ecology of filamentous phages from diverse bacterial genera and environmental settings makes them an important biological resource for environmental restoration (Fauquet, Mayo, Maniloff, Desselberger, & Ball, 2005; Rakonjac, 2012; Henry et al., 2015; Szekely & Breitbart, 2016; Mai‐Prochnow et al., 2015). More than sixty filamentous phages have been reported from terrestrial and aquatic ecosystems in temperate and tropical regions (Fauquet, Mayo, Maniloff, Desselberger, & Ball, 2005; Rakonjac, Bennett, Spagnuolo, Gagic, & Russel, 2011). Metagenomic analyses show a high frequency of filamentous phages in such contaminated environments as industrial wastewater and sewage disposal sites, which need ecorestoration (Alhamlan, Ederer, Brown, Coats, & Crawford, 2013; Cantalupo et al., 2011). A recent and exhaustive metavirome study also showed the prevalence of filamentous phages in many diverse environments including soils and sediments, saline water and freshwater, and air and food (Szekely & Breitbart, 2016). Bacteria associated with higher animals, insects, corals, and even people (as gut bacteria) also harbor filamentous phages (Weynberg, Voolstra, Neave, Buerger, & van Oppen, 2015), and they have been reported from bacterial genera associated with ecologically important plant families (Brassicaceae, Poaceae, Rutaceae, and Solanaceae) widely employed in environmental restoration (Berg, Marten, & Ballin, 1996; Tseng, Lo, Lin, Pan, & Chang, 1990). Despite emerging evidence on the prevalence of filamentous phages, current knowledge of the ecology of filamentous phages in soil and plants and of their potential application in restoration continues to be limited because of the challenges in purifying and identifying the phages and in assaying their activities.
Infection by filamentous phages affects the fitness of their bacterial hosts, a feature that can be exploited for securing desired ecological benefits. Mostly, a phage infection enhances the host's ability to combat abiotic and biotic stress, to invade a new habitat, and to partake in the development of microbial communities (Askora & Yamada, 2015; Bille et al., 2005; Derbise & Carniel, 2014; Jian, Xiao, & Wang, 2013; Mai‐Prochnow et al., 2015; Rice et al., 2009; Shapiro et al., 2016; Waldor & Mekalanos, 1996; Webb, Lau, & Kjelleberg, 2004; Yu et al., 2015). However, at times a phage infection lowers the fitness of the host bacterium, which is also beneficial if the bacterial host happens to be a plant pathogen (Ahmad, Askora, Kawasaki, Fujie, & Yamada, 2014; Yamada, 2013). Filamentous phages are useful for manipulating bacteria for environmental applications because the phages are stably produced in their bacterial hosts and are easy to manipulate using genetic and chemical methods—however, they remain underexploited in current practice. A chronic infection by a filamentous phage induces long‐term changes in the host physiology, which is desirable for developing microbial inocula.
Besides a relatively persistent relationship with the bacterial host, filamentous phages also show high host specificity up to the level of a strain, which qualifies them as a stable biomarker of their host (Henry et al., 2015; Lin et al., 1999). For example, ϕLf filamentous phage infects only Xanthomonas campestris pv campestris,whereas ϕXv filamentous phage infects only X. campestrispv. vesicatoria: Cross‐inoculation of the filamentous phages and their bacterial hosts did not result in successful infection. Gene III (gIII) of the phage encodes a virion‐associated protein (pIII), which shows structural features essential for a phage to be adsorbed on the surface of its host. A hybrid phage of ϕXv with pIII derived from ϕLf could infect X. campestrispv. campestris successfully but not X. campestrispv. vesicatoria, showing that the host specificity is governed by gIII. As filamentous phages possess unique forms of pIII, such high host specificity makes them useful in tracking and infecting bacterial inocula for environmental applications.
Based on the role of filamentous phages in soil and the ease with which they can be put to biotechnological use, we want to highlight their potential for environmental applications. Specifically, we examined the evidence on (a) the influence of filamentous phages on the ecological and evolutionary potential of their bacterial hosts and (b) the use of filamentous phages in developing biosensing tools for environmental monitoring of microbes and contaminants. We neither provide an in‐depth comparison of different bacterial technologies for environmental restoration nor suggest that the use of filamentous phages along with bacterial inocula can solve every environmental problem. Instead, we highlight the opportunities that filamentous phage present to a practitioner of environmental restoration, especially to design appropriate bacterial inocula and to develop efficient biomonitoring tools. We examine the role of filamentous phages in community ecology and assembly, particularly microbial adaptation, synergism, competition, and antagonism. Finally, we identify priority research areas to realize the potential environmental benefits of filamentous phages and to prevent possible risks in their environmental applications.
2. POTENTIAL AREAS FOR THE APPLICATION OF FILAMENTOUS PHAGES IN ENVIRONMENTAL RESTORATION FOR SUSTAINABLE DEVELOPMENT
Environmental restoration is acceptable to ecological economists as a tool for ensuring human well‐being and developing a sustainable society, which is characterized by improved soil health, reduced negative impacts of industrial activity, and lower poverty (Lei, Pan, & Lin, 2016; Martin, 2017; Millennium Ecosystem Assessment, 2005; Sachs & Reid, 2006; Tallis, Kareiva, Marvier, & Chang, 2008). In fact, the policy to encourage environmental restoration proved promising in helping people to escape the poverty trap in China (Cao, Zhong, Yue, Zeng, & Zeng, 2009). Poverty traps represent a vicious circle formed due to a complex interaction between the poverty and environmental degradation, in which “poverty leads to environmental degradation, and environmental degradation then deepens poverty” (Tallis et al., 2008). Poverty forces the native people to engage in unsustainable exploitation of natural resources, which degrades the environment and reduces the resource base for the poor people. Environmental degradation makes the land unproductive, therefore, reduces the income of native people. In this context, ecological restoration programs, which take into account the livelihood of the native people, also restore ecosystem goods and services besides economic and social development. In Changting County of China, the ecological restoration resulted in reduced soil erosion (68.3%), increased vegetation cover (75%), and species number (6 times) accompanied with increased employment (12.4%) and net income (11.2%) of native people (Cao et al., 2009). Policymakers, ecologists, economists, and social scientists were unanimous in emphasizing that ecosystem restoration was vital to achieving at least 7 of the 17 Sustainable Development Goals (SDGs) outlined by the United Nations as part of Transforming our World: The 2030 Agenda for Sustainable Development (United Nations, 2015). Those seven goals are as follows. Goal 1: End poverty in all its forms everywhere; Goal 2: End hunger, achieve food security and improved nutrition and promote sustainable agriculture; Goal 3: Ensure healthy lives and promote well‐being for all at all ages; Goal 6: Ensure availability and sustainable management of water and sanitation for all; Goal 8: Promote sustained, inclusive and sustainable economic growth, full and productive employment and decent work for all; Goal 13: Take urgent action to combat climate change and its impacts; and Goal 15, which specifically mentions ecorestoration: “Protect, restore and promote sustainable use of terrestrial ecosystems, sustainably manage forests, combat desertification, and halt and reverse land degradation and halt biodiversity loss.”
Soil restoration may involve ex situ or in situ methods to treat and restore degraded lands (Azubuike, Chikere, & Okpokwasili, 2016). In ex situ treatment methods, soil is removed from the affected site and treated either in a bioreactor or on the ground to trigger microbial degradation by manipulating environmental factors such as oxygen, moisture, and nutrients. We may manipulate environmental factors in a bioreactor using an automated controlling system, whereas in the treatment on the ground, we may add organic matter (compost) or fertilizers to the soil, with tillage (land farming) or without tillage (soil biopiles). In situ methods, on the other hand, involve little or no disturbance to soil structure and rely on either natural attenuation by natural physico‐chemical and biological processes or on assisted restoration through enhanced microbial activity. Microbial activity can be enhanced by injecting nutrients, water, chemicals, and even air, using underground pipes, to the contaminated site, that is to the saturated soil zone (biosparging) or to the unsaturated zone (bioventing; Azubuike et al., 2016). Bioventing may be combined with vacuum‐enhanced pumping for treating the contaminants in saturated and unsaturated zones (bioslurping). We may also either introduce specific bacteria to enrich the target bacteria (bioaugmentation) or add specific nutrients to stimulate the activity of targeted bacteria (biostimulation; Malhotra, Mishra, Karmakar, & Sharma, 2017).
In situ and ex situ soil treatments involve costly and labor‐intensive physicochemical methods, and copious use of water puts additional pressure on existing water resources. Also, the use of chemicals to control pathogens adds to the cost, pollutes soil and water, and harms even useful microbes. To avoid these adverse effects, restoration ecologists prefer assisted phytoremediation, which uses plants and their associated microbes to remove or manage the toxicants through such biological processes as rhizofiltration, phytostabilization or biotransformation, biosorption, bioaccumulation or phytoextraction, biodegradation, and biovolatilization (Pilon‐Smits, 2005). Restoration ecologists also select microbial inocula based on their potential to promote plant growth. Microbes promote plant growth by improving soil properties, solubilizing minerals, mobilizing nutrient supply to plants, producing plant growth regulators, and controlling phytopathogens (Rau et al., 2009; Sharma, Mishra, Mohmmed, & Babu, 2011; Sharma, Mohmmed, Mishra, & Babu, 2005; Wubs, Putten, Bosch, & Bezemer, 2016). Thus, the link between below‐ground biodiversity (microbes) and above‐ground biodiversity (plants) is fundamental to ecorestoration. Because contaminated lands harbor fewer forms of life and in smaller numbers, for assisted restoration of vegetation we need to rely on bacteria that not only promote plant growth but are also ecologically competitive (Wubs et al., 2016).
To improve the efficacy of assisted restoration of vegetation, we need to make the outcomes of restoration more predictable and to develop ultrasensitive tools to assess the functionality of sites under restoration (Halme et al., 2013). The tremendous in vitro potential of selected microbes, plants, and a combination of plants and microbes has not been fully realized in the field because these organisms are sensitive to biological toxins, pathogens, contaminants, and such ecosystem challenges as acid or saline soil, inadequate moisture, extreme temperatures, and open soil (Figure 1). Microbial inocula chosen on the basis of their activity in vitro may fail to show similar activity in the field (Figure 2). These challenges may be due to the failure of microbial inocula (poor survival and colonization) or the lack of real‐time biomonitoring tools for tracking the inocula, pathogens, and contaminants—and both can be countered by using filamentous phages to modify the ecophysiology of their bacterial hosts suitably and as ultrasensitive biosensors for real‐time biomonitoring. Filamentous phages carry genes or influence the expression of bacterial genes that help the bacterial hosts to adapt to abiotic and biotic stresses (Shapiro et al., 2016) by developing tolerance to microbial toxins and such abiotic sources of stress in the environment as salinity, desiccation, high temperatures, and high levels of contaminants (Secor et al., 2015; Shapiro et al., 2016; Yu et al., 2015). Filamentous phages may also make their bacterial hosts less virulent or lower our dependency on pesticides to control pathogens, thereby contributing to sustainable restoration (Ahmad et al., 2014; Askora, Kawasaki, Fujie, & Yamada, 2012). Modified filamentous phages may also produce specific peptides or proteins that are useful in monitoring targeted toxins, toxicants, and pesticides in the environment and in holding soil particles together to reduce soil erosion (Curtis, Dunbar, & Macgillivray, 2013; Curtis, Hewitt, & Macgillivray, 2009; Curtis, Macgillivray, & Dunbar, 2011; Goldman et al., 2003; Goldman, Pazirandeh, Charles, Balighian, & Anderson, 2002). In fact, filamentous phages can bridge the gap between the efficacy of microbial activity in vitro and in the field. For example, Pseudomonas can mineralize phenols in vitro but not in vivo in the presence of contaminated soil, water, and raw sewage (Goldstein et al., 1985; Mrozik et al., 2011; Figure 2). This failure of Pseudomonasto mineralize phenols completely has been attributed to several factors, such as high levels of predation of Pseudomonasby phages, stiff competition from native bacteria, greater sensitivity to bacterial toxins, and inadequate mobility of Pseudomonas. These factors reduce the viability and survivability of inocula. Filamentous phages have the potential to meet such challenges to the expression of microbial activity in vivo. For example, bacteria infected with filamentous phages proved immune to predation by other phages and to bacterial toxins, were mobile enough to reach the toxicants, and capable enough to degrade them (Addy, Askora, Kawasaki, Fujie, & Yamada, 2012b; Chouikha, Charrier, Filali, Derbise, & Carniel, 2010; Kimsey & Waldor, 1998; Sun & Webster, 1986; Yang et al., 2010). The path of research to develop remediation methods based on filamentous phages that improve microbial action is shown in Figure 2.
Filamentous phages can drive the ecology and evolution of microbial communities (Figure 3). Greater understanding of the role of filamentous phages in promoting host fitness and host diversity and, in turn, their impact on the dynamics of host populations, community composition, and nutrient cycling will help in developing and applying sustainable microbial technologies (Figure 3). Filamentous phages mediate lateral transfer of genes between bacterial strains and drive the evolution of bacterial hosts (Faruque et al., 2005) and also induce phenotypic changes in their bacterial hosts (Table 5), changes that affect the growth of the associated plants directly or indirectly. Based on a meta‐analysis of such studies, we identified potential areas of research to exploit the tripartite relationship between phages, bacteria, and plants (Figure 4). Greater environmental fitness of phage‐infected bacterial hosts strengthens our hypothesis of using filamentous phages that infect plant growth‐promoting rhizobacteria (PGPR) to develop the next generation of bacterial inocula. These inocula consisting of PGPR assist plants in colonizing degraded environments either directly, by promoting root growth, enriching the soil with nutrients, and increasing chelator‐mediated uptake of nutrients, or indirectly, by controlling pathogens and reducing the level of contaminants (Kaur, Pandove, & Gangwar, 2018). However, filamentous phages should also enable PGPR to outcompete native bacteria in colonizing the soil and the rhizosphere. To achieve this goal, we recommend co‐inoculation with filamentous phages and bacteria for ecorestoration (Figures 3 and 4). To this end, we first need to identify the filamentous phages that can bring about the desired changes in the biology and ecology of target bacteria to improve their efficacy as inoculants. Secondly, we need to identify filamentous phages—or modify them—to develop biosensors to track the inocula and the contaminants through time and space. Research in these areas will contribute to making microbial technologies both sustainable and effective.
Figure 3.
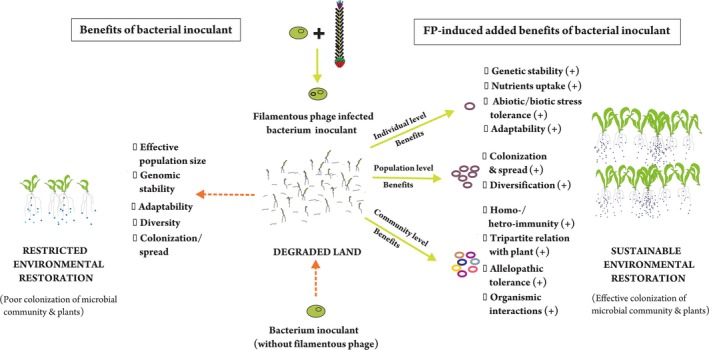
Potential significance of filamentous phages to enhance the ecological and evolutionary potential of the bacterial community to ensure vegetation development at degraded lands
Table 5.
Experimental evidences showing influence of filamentous phage on competitiveness of host bacteria
| Influence on host bacterial competitiveness | Filamentous phage – host bacterium | Changes in host phenotype | Reference |
|---|---|---|---|
| Phages in theory of bacterial adaptation: As an agent to improve adaptation of bacterial host toward abiotic stresses | |||
| Increase in tolerance to high temperature (35°C) | Xf2 – X. campestrispv. Oryzae N5850 | • Altered growth pattern (a slow growth in first 60 hr followed by fast growth) | Kamiunten and Wakimoto (1981) |
| Development of adaptive phenotype due to reduction in rate of cell division and growth rate | M13 – E. coli S‐26 | • Reduction in growth rate due to increase in mean generation time (25%) and duration of lag phase | Brown and Dowell (1968) |
| φRSM (φRSM3, φRSM4) – R. solanacearum (MAFF730139, MAFF106611, UW551 ) | • Reduction in growth rate by ~60% | Askora, Kawasaki, Usami, Fujie, and Yamada (2009) | |
| φRSS1, φRSM1 – R. solanacearum C319; Ps29 | Yamada et al. (2007) | ||
| φM13 – E. coli W6 | Wan and Goddard (2012) | ||
| φM13‐km – E. coli TOP10F | • Reduction in growth rate | Lin et al. (2011) | |
| M13 – E. coli HfrC | Roy & Mitra (1970) | ||
| f327 – Pseudoalteromonas sp. BSi20327 | Yu et al. (2015) | ||
| M13 – Escherichia coli 112‐12 | Salivar, Tzagoloff, and Pratt (1964) | ||
| Cf1c – X. campestris pv. Citri | Kuo, Tan, Su, and Yang (1991) | ||
| Tolerant to radiation | φRSS1, φRSM1 – R. solanacearum C319; Ps29 | • Increase dark coloration and pigmentation | Yamada et al. (2007) |
| φRSM (φRSM3, φRSM4) – R. solanacearum (MAFF730139, MAFF106611 UW551 ) | Askora et al. (2009) | ||
| Regulation of host bacterial community under seasonal fluctuation in extreme arctic environment | f327 – Pseudoalteromonas sp. BSi20327 | • Reduction in cell density and tolerance to NaCl and H2O2 coupled with increase in motility and chemotaxis (escape from nutrient‐deficient, highly saline environments during arctic winter; and avoid over blooming under H2O2, nutrient and radiation abundance of arctic summer) | Yu et al. (2015) |
| Development of freeze‐fracture resistance | fd – E. coli HB11 | • Increased total lipid content (25%) of outer membrane without affecting relative concentration of phospholipids | Bayer and Bayer (1986) |
| Provide adaptive fitness to ensure survival in limited‐energy deep sea environment | SW1 – Shewanella piezotolerans WP3 | • Reduction in swarming motility due to decreased production of lateral flagella with concomitant increase in number of filamentous phages | Jian, Xiao, and Wang (2013) |
| Increase in adaptation to tolerate and sequester high levels of copper and other heavy metals | Unknown – Ralstonia pickettii strains (12D and 12J) | • Horizontal transfer of metal resistance and transporter genes, and zot‐like toxin | Yang et al. (2010) |
| Increase in tolerance to alkaline pH and salt stress; maintenance of redox and energy state | f1 – E. coli | • Induction of phage shock protein response (secretion of pIV secretin); maintenance of PMF‐ and ATP‐dependent protein secretion | Joly et al. (2010) |
| Tolerant to desiccation | Pf – P. aeruginosa | • Increased cellular viscosity, aggregation, and adhesion; promotion of liquid crystalline organization of biofilm matrix | Secor et al. (2015) |
| Generation of high cellular energy during early growth phase; reduction in survival during acid shock | M13 – E. coli | • Upregulation of phosphotransferase; downregulation of acid stress and stationary phase transition genes; impairment of oxidative and acid‐resistance systems | Karlsson, Malmborg‐Hager, Albrekt, and Borrebaeck (2005) |
| Phages in theory of microbial competition: As an agent to protect the bacterial inoculant from allelopathy effect | |||
| Development of tolerance to multiple colicins (E1, E2, and E3) | f1 – E. coli K38 | • Increase in deoxycholate sensitivity, leakage of b‐lactamase, and number of defective F‐pili | Boeke, Model, and Zinder (1982) |
| f1 – E. coli GM1, JM1 | • Modifications in tolA and tolB colicin transporter | Sun and Webster (1986) | |
| Provide heteroimmunity | CTXφ – V. cholerae | • Divergence of phage repressors and their cognate operators (rstR‐ig‐2) | Kimsey and Waldor (1998) |
| Provide homoimmunity | YpfΦ – Y. pestis biovar Orientalis (CO92), Antiqua (IP550‐HC1), Medievalis (IP1865–12)) | • Stable integration of YpfΦ genome as multiple tandem repeats into host chromosome providing homoimmunity to phages | Chouikha et al. (2010) |
| Phage in theories of microbial colonization and antagonism: As an agent to ensure better colonization of host bacteria and control bacterial pathogens | |||
| Increase in colonization potential to new surfaces by increase virulence, transmissibility, infect wide host range, toxin production, biofilm and aggregation | Nf or MDA – Neisseria meningitidis Z2491 |
|
Bille et al. (2005), Joseph et al. (2011) |
| Pf4 – P. aeruginosa PAO1 |
|
Rice et al. (2009) | |
| CTXφ – Vibrio cholerae O395 |
|
Waldor and Mekalanos (1996) | |
| Xf2 – X. campestris pv. Oryzae N5850 |
|
Kamiunten and Wakimoto (1981) | |
| Ypfφ – Yersinia pestis biovar Orientalis |
|
Derbise et al. (2007) | |
| φRSS1 – R. solanacearum MAFF 106603 and MAFF 106611 |
|
Addy, Askora, Kawasaki, Fujie, and Yamada (2012a) | |
| VPIφ – V. cholera strains (N16961 and 395) |
|
Li, Kotetishvili, Chen, and Sozhamannan (2003) | |
| fs2 – V. cholera O1 |
|
Nguyen et al. (2008) | |
| Cf1c – X. campestris pv. Citri |
|
Kuo et al. (1991) | |
| Pf4 – P. aeruginosa PAO1 |
|
Webb et al. (2004) | |
| f1, c2 – Enterobacteria sp. |
|
Kuo, Yang, Chen, and Kuo (2000) | |
| Pf – P. aeruginosa |
|
Secor et al. (2015) | |
| φRSS1, φRSM1 (Ff‐type) – R. solanacearum C319; Ps29 |
|
Yamada et al. (2007) | |
| Pf1 – Pseudomonas aeruginosa PAO1 |
|
Whiteley et al. (2001) | |
| PE226 – R. solanacearum SL341 |
|
Murugaiyan et al. (2010) | |
| YpfΦ – Y. pestis biovar Orientalis (CO92), Antiqua (IP550‐HC1), Medievalis (IP1865‐12) |
|
Chouikha et al. (2010) | |
| Enhance host cell aggregation for colonization but reduced virulence | XacF1 – Xanthomonas axonopodis pv. citri |
|
Ahmad et al. (2014) |
| φRSM1 and φRSM3 – R. solanacearumMAFF 106603 and MAFF 106611 |
|
Addy et al. (2012b) | |
| φRSM (φRSM3, φRSM4) – R. solanacearum (MAFF730139, MAFF106611 UW551 ) |
|
Askora et al. (2009) | |
| Phages in theory of community assembly and evolution: As an agent to influence evolutionary potential of bacterial inoculant and trigger microbial community development | |||
| Reduction in conjugative ability and plasmid transfer function | f1 – E. coli K38 | • Number of defective F‐pili | Boeke et al. (1982) |
| Ike – E. coli K12 RM98 | • Alteration in cell membrane proteins | Iyer , Darby, and Holland, (1976) | |
| Diversification in Isogenic population | M13 – E. coli | • Induction of high variability in individual viral production than other phenotypic traits in isogenic bacterial population | De Paepe, De Monte, Robert, Lindner, and Taddei et al. (2010) |
| Maintenance of conjugation rate and spread of antibiotic resistance within population | φM13 – E. coli W6 | • Reduction in conjugation efficiency by ~10% | Wan and Goddard (2012) |
| φM13‐km – E. coli TOP10F | • Reduction in average number of pili; decrease in conjugation rate with increase in pfu/ml | Lin et al. (2011) | |
| Evolution and Development of superinfective forms and virulent pathogenic variants due to high frequency of mutations | Cf1c – X. campestris pv. Citri | • Variation in gene structure and sequence | Kuo et al. (1991) |
| f1, c2 – Enterobacteria sp. | • Loss of cell viability and reduction in rates of RNA and protein synthesis | Kuo et al. (2000) | |
Figure 4.
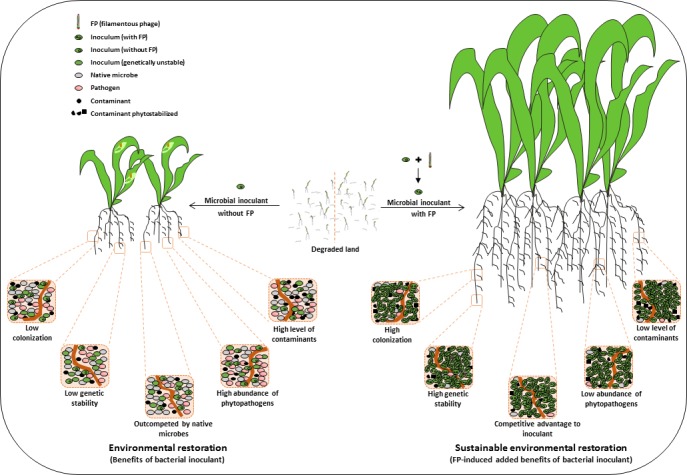
Potential of filamentous phages to assist in the tripartite relation of phage–bacteria–plant to positively influence the upstream effects on plant health, growth, and colonization for ecosystem restoration
3. PLANT‐ASSOCIATED BACTERIA IN ECORESTORATION: CURRENT STATUS AND OPPORTUNITIES FOR FILAMENTOUS PHAGES
3.1. Bacteria as restoration inocula: Potential targets for modulation by filamentous phages
The bacteria used in commercial formulations as inocula for such environmental applications as improving soil health and promoting plant growth serve as potential targets for research involving filamentous phages. Table 1 lists such bacterial taxa and their specific beneficial traits (solubilizing and mobilizing nutrients, ameliorating soils, and controlling phytopathogens). At least 47 such commercial formulations are available in the global market. The single‐function formulations use a single bacterial strain or a consortium of bacterial species for improving soil health and promoting plant growth (Table 1), whereas multifunctional formulations rely either on a consortium of bacteria with different activities or a single bacterial strain with multiple desired traits. The most common bacterial genera in these formulations include Acetobacter, Acidithiobacillus, Acidovorax, Azospirillum, Azotobacter, Bacillus, Bradyrhizobium, Chromobacterium, Delftia, Frateuria, Lactobacillus, Pseudomonas, Rhizobium, Rhodobacter, and Thiobacillus. To make these formulations more effective, we suggest that these genera be used as potential targets for exploring the benefits of filamentous phages, although filamentous phages for Pseudomonas have already been reported.
Some genera also show potential to remediate soil and water contaminated with inorganic and organic toxicants (Table 2) and therefore form another set of target bacteria for phage research. For example, Azotobacter, Bacillus, Clostridium, Enterobacter, E. coli, Pseudoalteromonas, Rhizobium, Shewanella, and Thermus help in dealing with different heavy metals (Table 2). Agrobacterium, Acinetobacter, Bacillus, Erwinia, Enterobacter, E. coli, Flavobacterium, Herbaspirillum, Micrococcus, Pseudomonas, Ralsotonia, Stenotrophomonas, Vibrio, and Yersinia degrade different classes of pesticides (Umadevi, Ayyasamy, & Rajakumar, 2017). However, Acinetobacter, Erwinia, Neisseria, Propionibacterium, Pseudomonas, Ralstonia, Rhizobium, Shigella, and Stenotrophomonas degrade diverse organic contaminants including aromatic amines, azo dyes, phenols, benzenes, toluenes, xylenes, oils, polyaromatic hydrocarbons, halogens, and phthalate esters (Table 2).
Besides their potential in environmental remediation, some bacterial genera also promote plant growth through such means as mineral solubilization, nitrogen fixation, phytohormone production, and antagonism toward pathogens (Table 2). These bacteria can enhance plant growth because they can solubilize insoluble phosphates. Such phosphate‐solubilizing bacteria (PSBs) include Acinetobacter, Agrobacterium, Bacillus, Flavobacterium, Lysinibacillus, Microbacterium, Micrococcus, Paenibacillus, Pseudomonas, Ralstonia, Salinicola, Serratia, Shewanella, and Vibrio, and some of them may also fix nitrogen, namely Agrobacterium, Azospirillum, Azotobacter, Acinetobacter, Bacillus, Bradyrhizobium, Rhizobium, Sinorhizbium, Ensifer, Mesorhizobium, Herbaspirilum, Micrococcus, Phylobacterium, Salinicola, and Vibrio.Some bacteria produce indole acetic acid (IAA), a plant growth regulator essential for plants to colonize degraded sites. These IAA‐producing bacteria include Azospirillum, Acinetobacter, Microbacterium, Micrococcus, Rhizobium, Salinicola, Vibrio, Xanthomonas, and Vibrio. Some bacteria are antagonistic to phytopathogens; these include Bacillus, Micrococcus, E. coli, Paenibacillurs, Rhizobium, Pseudomonas, Salinicola, and Vibrio.Therefore, research on filamentous phages to infect these bacterial genera deserves higher priority.
3.2. Bacteria with known filamentous phages potentially useful in restoration
Filamentous phages have been reported from many bacterial genera potentially useful in ecorestoration. These genera include Acinetobacter, Clostridium, Enterobacteria, Neisseria, Propionibacterium, Pseudoalteromonas, Pseudomonas, Ralstonia, Shewanella, Shigella, Stenotrophomonas, Thermus, Vibrio, Xanthomonas, Xylella, and Yersinia (Addy et al., 2012b; Ahmad et al., 2014; Derbise et al., 2007; Jian et al., 2013; Kuo et al., 2000; Waldor & Mekalanos, 1996; Whiteley et al., 2001; Yu et al., 2015; Table 2). These genera include species that cause diseases in plants and animals, show bioremediation activity, and promote plant growth. The filamentous phage‐mediated ecological fitness of host bacteria has been investigated in selected species for pathogenicity, survival, colonization, multiplication, and distribution in a given ecological niche (Table 5). However, other species that may be potentially useful in bioremediation and restoration of vegetation are yet to be fully exploited.
Most bacterial genera also include species, which have association with plants. For example, Acinetobacter, Enterobacter, Pseudoalteromonas, Pseudomonas, Ralstonia, Shewanella, Stenotrophomonas, Vibrio, Xanthomonas, and Xylella represent predominant endophytes, rhizobacteria, or both, with beneficial effects on plant growth (Bhattacharyya & Jha, 2012; Borriss, 2011; Chandra & Singh, 2016; Kobayashi & Palumbo, 2000; Tilak et al., 2005). Neisseria, Propionibacterium, Ralstonia, and Stenotrophomonas form hydrocarbon‐degrading bacterial communities that inhabit the phyllosphere of plant species that have been widely used for phytoremediation of air and soil contaminated with hydrocarbons (Al‐Awadhi et al., 2013; Al‐Mailem et al., 2010). Pseudoalteromonas shioyasakiensis and Vibrio sagamiensis SMJ18 are important members of endophytic bacterial populations that inhabit Spartina maritima, a species of cordgrass that accumulates heavy metals and is found in most of the polluted estuaries worldwide (Mesa et al., 2015). Of these bacteria, Pseudomonas spp. have been widely exploited for bioremediation. Pseudomonasalso produces diverse molecules to promote plant growth: for example, P. fluorescens produces siderophores to promote the growth of plants; P. chlororaphis produces phenazine, an antibiotic to control fungal pathogens; and P. aurantiaca secretes di‐2,4‐diacetylfluoroglucylmethane, an antibiotic to control Gram‐positive bacterial pathogens.
In fact, species of these bacterial genera have shown their potential in bioremediation and they have been reported from contaminated sites that need to be restored. For example, Acinetobacter spp. (A. calcoaceticus MM5, A. lwoffii ISP4, A. venetianus, Acinetobacter sp. RTE1.4, Acinetobacter sp. HC8‐3S, and Acinetobacter sp. A3) degrade such aromatic contaminants as crude oil, halogens, phthalate esters, and phenols in soil and water (Fondi et al., 2013; Vamsee‐Krishna et al., 2006). Acinetobacter spp. have also been employed for treating industrial wastewater (Liu et al., 2016), and a protocol has also been developed for their mass multiplication. Strains of Acinetobacter, Neisseria, Xanthomonas, and Pseudomonas dominate in a petroleum‐degrading consortium purified from contaminated soils in China (Xu et al., 2011). Thermus spp. (T. scotoductus, T. thermophilus DSM 579, and T. aquaticus DSM 625) favor terrestrial hot springs but T. thermophilus HB8 has also been reported in organic waste, sewage sludge compost, thermogenic compost, cattle manure, and garden waste (Fujio & Kume, 1991; Marteinsson, Birrien, Raguenes, Costa, & Prieur, 1999). These bacterial genera and species have a high potential for processing and bioremediation of wastes even at temperatures as high as 65–84°C.
Some bacterial genera infected with known filamentous phages also show features useful for ecorestoration (bioremediation, promotion of plant growth, and development of vegetation). For example, Clostridiumis one of the common bacterial genera reported from the rhizosphere (Dinesh et al., 2015): C. glycolicum and C. collagenovorans volatilize As (V) (Meyer, et al., 2007; Michalke, et al., 2000). Singh et al. (2004) reported the potential of Enterobacter cloacae B2‐DHA to bioremediate heavy metals (Cr VI, Pb, Cd, and Ni II) and radioactive elements and that of Enterobacter B‐14 to biodegrade organophosphate pesticides in contaminated soils. A bacterial consortium comprising Pseudomonas, Acinetobacter, and Neisseria mineralizes DDT (Carrillo‐Pérez, Ruiz‐Manríquez, & Yeomans‐Reina, 2004) and a consortium comprising Acinetobacter faecalis, Neisseria elongate, and Staphylococcus sp. efficiently degrades crude petroleum oil (Mukred, Hamid, Hamzah, & Yusoff, 2008). Pseudoalteromonas has proved useful in bioremediation of substrates contaminated with inorganic and organic pollutants. For example, Pseudoalteromonas sp. SCSE709‐6 from the deep sea showed a great capacity (96%) to remove Cd(II) at varying temperatures and varying levels of pH and salinity (Zhou, et al., 2013). PseudoalteromonasTG12 solubilizes Fe, accumulates different metals (Gutiérrez, Shimmield, Haidon, Black, & Green, 2008), and degrades alkanes and cycloalkanes (Dubinsky et al., 2013). Pseudoalteromonas and Vibrio purified from sediments in San Diego Bay degraded hydrocarbons and a toxic organic pollutant (phenanthrene or chrysene; Coelho, Rivonkar, Bhavesh, Jothi, & Sangodkar, 2003; Melcher, Apitz, & Hemmingsen, 2002), and P. haloplanktis from Minamata Bay, Japan, was investigated for its resistance to mercury (Lohara et al., 2001) and as a model organism for genetic manipulation in bioremediation studies (Kivelä, Madonna, Krupovìč, Tutino, & Bamford, 2008). Pseudomonas spp. have received attention for their bioremediation potential to deal with diverse organic contaminants. For example, P. alcaligenes, P. mendocina, and P. putida degrade polycyclic aromatic hydrocarbons (e.g., toluene); P. veronii degrades different simple aromatic compounds; P. resinovorans degrades aromatic heterocyclic compounds (e.g., carbazole and quinoline); P. stutzeri KC degrades haloalkanes (e.g., carbon tetrachloride); and P. pseudoalcaligenes uses cyanide as a nitrogen source. In populations of Ralstonia pickettii12D and 12J, infection by filamentous phages makes their bacterial hosts more adaptable to heavy metals by increasing horizontal gene transfer in the bacterial populations (Yang et al., 2010). The metabolic versatility of Shewanella also makes it a member important in cycling metals and organic matter (Fredrickson et al., 2008). For example, S. oneidensis has the potential to remediate substrates contaminated with Cr(VI), Fe(III), Mn(IV), U(VI), and V(V). The role of Shewanella in nutrient cycling becomes important because Mn is the second most abundant metal in the earth's crust, an essential trace element for all living organisms, and also influences the cycling of other elements. Thermus scotoductus is widely used for immobilizing toxic metals and radionuclides (Cr, Co, Fe, Tc, U, etc.) from wastewater from hot springs or heated streams of nuclear waste (Brim, Venkateshwaran, Kostandarithes, Fredrickson, & Daly, 2003; Kashefi & Lovley, 2000; Kieft et al., 1999; Opperman & van Heerden, 2008; Slobodkin, 2005). Thermus oshimai can remove heavy metals (Poli et al., 2009), and Thermus sp. removes selenite and tellurite (Chiong et al. 1988; Slobodkin et al. 2006; Sokolova et al., 2004).
Enterobacter, Pseudoaltermonas, and Vibriospecies are not only significant for bioremediation but also for promoting plant growth. Enterobacter sp. RNF 267 promotes the growth of coconut palms (Cocos nucifera) and maize (George, 2013), and inoculation of green gram (Vigna radiata) with Enterobacter EG‐ER‐1 and KG‐ER‐1 together with Bradyrhizobium sp. increased nodulation (Gupta et al. 2003). P. shioyasakiensis and V. sagamiensis SMJ18 tolerate not only salt and heavy metals (As, Cu, and Zn) but also show multiple traits that promote plant growth: They can fix nitrogen; solubilize phosphates; and produce IAA‐, siderophores, and ACC (1‐aminocyclopropane‐1‐carboxylate deaminase). Spartina maritima inoculated with V. sagamiensis SMJ18 shows more efficient photosynthesis, greater intrinsic water‐use efficiency, and lower metal uptake—which is why the combination of V. sagamiensis and S. maritima has been recommended for ecorestoration of polluted estuaries.
As most of the above‐mentioned bacterial genera can be commercialized and filamentous phages of these genera are known (Table 2), co‐inoculation with bacteria and filamentous phages needs to be tested for environmental use (Figures 3 and 4).
3.3. Phytopathogenic bacteria particularly useful in ecorestoration as targets of research on filamentous phages
Filamentous phages of bacterial phytopathogens also provide an opportunity to improve assisted phytoremediation as part of ecorestoration (Table 3; Figure 4). Filamentous phages have been characterized for six of the world's ten most serious bacterial phytopathogens. These six pathogens belong to four genera, namely Pseudomonas, Ralstonia, Xanthomonas, and Xylella (Mansfield et al., 2012). For three more phytopathogenic bacteria, namely Dickeya, Erwinia, and Pectobacterium, filamentous phages have been reported from related bacterial genera. Filamentous phages of other phytopathogenic genera, namely Acinetobacter, Clostridium, and Pseudoalteromonas, are also relevant because these genera also have species that are pathogenic to plants used for remediation. However, filamentous phages of Propionibacterium and Yersinia, opportunistic pathogens of the human body, are also important because these pathogens use plants as temporary hosts and therefore need to be controlled. Filamentous phages determine the pathogenicity of some bacteria, which is why the biochemical and molecular mechanisms of phages–bacteria interactions can be the key to developing biocontrol methods for phytopathogens. Filamentous phages of avirulent bacterial strains would also be useful as competitors to the filamentous phages of virulent bacterial strains.
4. FILAMENTOUS PHAGES TO BOOST ENVIRONMENTAL COMPETITIVENESS OF BACTERIAL INOCULA
In many bacteria, filamentous phages influence the expression of phenotypic traits and trigger the appropriate ecophysiological mechanisms that help the bacteria to adapt better to sources of stress in the environment. Sometimes, a phage infection triggers a high level of cellular organization to prevent the host cells from being exposed to the sources of stress in the outside environment (Table 5). Experimental evidence shows that filamentous phages drive the ecological success of their host bacteria in a given niche. Such mechanisms have been investigated in Acinetobacter, Clostridium, Enterobacteria, Neisseria, Propionibacterium, Pseudoalteromonas, Pseudomonas, Ralstonia, Shewanella, Shigella, Stenotrophomonas, Thermus, Vibrio, Xanthomonas, Xylella, and Yersinia (Addy et al., 2012b; Ahmad et al., 2014; Derbise et al., 2007; Jian et al., 2013; Kuo et al., 2000; Waldor & Mekalanos, 1996; Whiteley et al., 2001; Yu et al., 2015). As discussed earlier, these genera are also important in bioremediation and in promoting plant growth. Filamentous phages therefore have the potential to improve the ecological and evolutionary potential of bacterial inocula so that the bacteria survive environmental stress, evolve in the changing environment, and contribute to the growth of plants (Figure 3).
4.1. Phages and microbial adaptation
Filamentous phages influence the growth of their bacterial hosts to increase the adaptive potential of the hosts (Table 5). For example, E. coli(112‐12, S‐26, W6), Pseudoalteromonas sp. (f327), Ralstonia solanacearum (C319, Ps29), and Xanthomonas campestris(pv. N5850) infected with filamentous phages show slower growth and greater adaptability to stress (Brown & Dowell, 1968; Kamiunten & Wakimoto, 1981; Salivar, et al., 1964; Wan & Goddard, 2012; Yamada et al., 2007; Yu et al., 2015). The bacterial host survives either because its growth is put on hold until favorable conditions return or because it gets adequate time to activate appropriate mechanisms to combat stress from abiotic sources. Therefore, E. coli infected with a filamentous phage shows reduced growth but greater resilience to changes in the environment; the noninfected and fast‐growing bacterial hosts, on the other hand, show a high metabolic rate and use up the energy for growth, thereby becoming more susceptible to stress (Tamman, Ainelo, Ainsaar, & Hõrak, 2014; Tuomanen, Cozens, Tosch, Zak, & Tomasz, 1986; Yu et al., 2015). Such phage‐mediated phenotypic and ecophysiological changes in bacterial hosts are immensely useful in ecorestoration. These changes in bacterial inocula will help the bacterial hosts to adapt to stress from abiotic sources, to maintain effective bacterial populations, and to perform their desired ecological functions (Arora, Tiwari, & Singh, 2014; Gopalakrishnan et al., 2015; Lahav, 1962; Malusá, Sas‐Paszt, & Ciesielska, 2012).
Other evidence shows that filamentous phages enhance the adaptive potential of bacterial hosts by influencing specific phenotypic traits or biological processes (Table 5). In E. coli HB11, infection from fd phage increases the total lipid content, which helps the host to resist freeze‐fracture stress (Bayer & Bayer, 1986). Cells of Shewanella piezotolerans WP3 infected by the SW1 phage show fewer lateral flagella and poor swarming motility (Jian et al., 2013), which enables the bacteria to survive the limited‐energy environment. In Ralstonia pickettii(12D, 12J), filamentous phages mediate horizontal gene transfer and enable the bacterial host to adapt to a high level of Cu and other heavy metals in lake sediment (Yang et al., 2010). M13‐km phage infection of E. coli TOP10F decreases conjugation and prevents the spread of antibiotic resistance genes in bacterial populations (Lin et al., 2011); M13 infection of E. coliK12 leads to loss of lipopolysaccharide, which makes the strain more susceptible to actinomycin D (Roy & Mitra, 1970b); and f1‐infection of E. coli enables the bacterial cell envelope—by means of phage shock proteins in the bacterial host—to tolerate stress in many forms (high pH, high concentration of salts, etc.; Joly et al., 2010).
Besides these mechanisms, filamentous phages also trigger highly structured organization of bacterial populations or communities (biofilm, for example), which protects their members from several sources of environmental stress (Table 5): Suspensions of Pseudomonas aeruginosa cells infected with Pf filamentous phage become more viscous, which helps the host cells to aggregate and adhere together to form a biofilm, which enables the assembly to survive desiccation and offers protection from aminoglycoside antibiotics and toxic chemicals (Secor et al., 2015; Webb et al., 2004). A biofilm consisting of multiple species is a cross‐species communication network that enables the constituent species to use nutrients including C more effectively when they are in short supply (Flemming et al., 2016). Based on the evidence discussed here, we suggest that (a) filamentous phages of target bacteria be isolated and analyzed to develop ecologically competitive bacterial inocula and (b) bacteria used in commercial inocula and potential PGPR be used as hosts to isolate suitable filamentous phages and the adaptive potential of such phages–bacteria co‐inoculation be tested.
4.2. Phages to make beneficial bacteria more competitive
Bacteria that form the inocula used for remediation may tolerate stress from abiotic sources but they also need to overcome biotic adversaries, especially the well‐adapted native microbial species (Amarger, 1981; Barnet, 1980; Bashan, 1998; Díaz‐Ramírez, Escalante‐Espinosa, Schroeder, Fócil‐Monterrubio, & Ramírez‐Saad, 2013; Gopalakrishnan et al., 2015; Vincent, 1954). Some researchers believe that stressful habitats favor toxin producers, which invade such habitats and outcompete the toxin‐susceptible strains (Hibbing, Fuqua, Parsek, & Peterson, 2010; Majeed, Gillor, Kerr, & Riley, 2011; Riley & Gordon, 1999). In fact, the markedly lower biodegradation efficacy of bacterial inocula in vivo than that in in vitro has been attributed to their sensitivity to toxins produced by native bacteria (Goldstein et al., 1985; Mrozik et al., 2011).
Infection of E. coli (K38, GM1, JM1) and V. cholerae by filamentous phages not only increases the ability of the hosts to tolerate toxins but also makes the hosts resistant to infection by other (homo‐ or heterologous) phages. In E. coli, infection by f1 filamentous phage leads to irreversible changes in the membrane protein that serves as a common receptor for colicin and phages (Table 5; Boeke et al., 1982; Sun & Webster, 1986; Zinder, 1973). Therefore, we propose the use of filamentous phages to develop ecologically competitive bacterial inoculants that can tolerate toxins and resist other phages in the soil. In E. coliK38, infection by filamentous phage triggers a phenotypic change in the membrane, which makes it more sensitive to deoxycholate, promotes leakage of β‐lactamase, and increases the number of defective pilli on the host cell. Due to these changes, E. coliK38 tolerates colicins but shows a reduced frequency of conjugation (Boeke et al., 1982). Cells of Vibrio cholerae infected with CTXф develop heteroimmunity against lambdoid phages and show a divergence in phage repressors and their cognate operators (rstR‐og‐2; Kimsey & Waldor, 1998). These changes confer a competitive advantage on the infected cells in countering attack by other bacterial species (Davies & Davies, 2010; Feldgarden & Riley, 1998). Thus, filamentous phages have the potential to protect their hosts from biotic sources of stress as well, both chemical and viral, which are common features of the ecosystem in which the inoculants find themselves.
4.3. Phages to improve colonizing abilities of bacteria
Bacterial inocula used in remediation should not only survive, by competing successfully with other microbes, but also thrive, colonizing the contaminated sites to provide the desired ecological benefit. Bacteria can become virulent after infection by filamentous phages: Such virulent bacteria can then invade and colonize a particular niche (a habitat or an organism; Table 5). The most noteworthy examples of such bacterium–phage association include Neisseria meningitidisand Nf/MDA (Bille et al., 2005), Pseudomonas aeruginosa and фPf4 (Rice et al., 2009), Ralstonia solanacearum and φRSS1 (Addy et al., 2012b), Vibrio cholerae and CTXø (Waldor & Mekalanos, 1996), Xanthomonas campestris and Xf2 (Kamiunten & Wakimoto, 1981), and Yersinia pestis and YpfΦ (Derbise & Carniel, 2014).
In these bacterial hosts, filamentous phages either carry the virulence gene(s) (Addy et al., 2012b; Derbise & Carniel, 2014; Waldor & Mekalanos, 1996) or regulate the expression of virulence factors (Addy, Askora, Kawasaki, Fujie, & Yamada, 2012a, b; Ahmad et al., 2014). For example, N. meningitidisand Y. pestis are transformed into virulent strains capable of causing epidemics after receiving the toxin gene from Nf or MDA and from YpfΦ phage, respectively (Bille et al., 2005; Derbise et al., 2007). The filamentous phage CTXΦ transfers to V. choleraeO395 the gene ctxAB, which encodes the cholera toxin (Waldor & Mekalanos, 1996). Other strains of V. cholerae, namely N16961 and 395, develop into potential pathogens after they receive the gene vibrio pathogenicity island (VPI) from the filamentous phage VPIΦ (Li et al., 2003): That potential is realized if a helper phage, namely fs2, infects V. choleraeO1 thereby converting the host into a virulent strain. The phage‐mediated transfer of rstCgene into V. choleraeO1 increases the production of the cholera toxin and triggers the multiplication of the resident CTXΦ phage, thus making the host highly virulent (Nguyen et al., 2008).
Filamentous phages can also convert pathogens into their superinfective forms by changing the phenotypic traits associated with virulence. For example, Xf2 infection converts Xanthomonas campestris pv oryzaeN5850 into a highly virulent phytopathogen by increasing the production of extracellular polysaccharide (Kamiunten & Wakimoto, 1981). Virulent pathogenic variants of X. campestrispv citrievolve after infection by CF1c phage (Kuo et al., 1991), and infection by the phage PE226 transfers into Ralstonia solanacearumSL341 the genes responsible for producing toxins and thus widens the host range of the pathogen (Askora, Kawasaki, Usami, Fujie, & Yamada, 2009). Similarly, Pseudomonas aeruginosaPAO1 evolves into a superinfective phenotype after infection with the phage Pf4 (Rice et al., 2009; Webb et al., 2004). Other plant‐associated bacteria, such as E. coli(Bayer & Bayer, 1986), Enterobacteria(Kuo et al., 2000), P. aeruginosa(Secor et al., 2015), and R. solanacearum(MAFF106603 and MAFF106611; C319 and Ps29), also evolve into infective phenotypes if they are infected by their specific filamentous phages (Addy et al., 2012b; Yamada et al., 2007; Table 5). Although E. coli and Enterobacter are part of the microbiome of the human gut, recent research has shown that these bacteria are also native soil bacteria, which promote plant growth and remediate the environment. For example, E. coli has been reported from soils from seven geo‐climatic zones of India, and inoculating Zea mays with E. coli enhances nutrient uptake and plant growth (Nautiyal & Shono, 2010). In fact, plants exert niche‐specific selection pressure on the organisms associated with them; for example, strains of E. coli associated with plants are a distinct phenotype and make an independent phylogroup different from that purified from mammalian hosts (Méric, Kemsley, Falush, Saggers, & Lucchini, 2013). E. coli strain USML2 has been shown to be a growth‐promoting endophyte in leaves of the oil palm (Elaeis guineensis; Tharek, Sim, Khairuddin, Ghazali, & Najimudin, 2017). Different species of Enterobacter have been reported as rhizobacteria associated with different plant species (E. sakazakii and E. agglomerans with soybean; E. cloacae with citrus, maize, and soybean; and E. asburiae with sweet potato). These species possess multiple growth‐promoting traits and enhance plant growth (Ramesh, Sharma, Sharma, Yadav, & Joshi, 2014). Recent studies suggest that E. coliand Enterobacter also occur as endophytes in different plants and enhance nutrient uptake and growth of their hosts plants and are useful for environmental clean‐up (Santoyoa, Moreno‐Hagelsieb, Orozco‐Mosqueda, & C., & Glick, B.R., 2016). Based on these examples, we propose the use of filamentous phages to enable bacterial inocula to colonize contaminated habitats, control phytopathogens, and promote plant growth for ecorestoration.
Filamentous phage‐infected P. aeruginosashows high potential to colonize different habitats because of its ability to form biofilms, which have a liquid crystalline organizational structure (Rice et al., 2009; Secor et al., 2015). The biofilm is not an inert structure but a cooperative and interactive network that develops into an ecologically cohesive microbial community, which can rapidly colonize the target site (Hengzhuang, Wu, Ciofu, Song, & Høiby, 2011, 2012; Høiby et al., 2011; Høiby, Bjarnsholt, Givskov, Molin, & Ciofu, 2010). In fact, Pf1‐infected P. aeruginosaPAO1 strains outcompete the noninfected strains in forming a biofilm and also form a cohesive group for exchanging genes—exchanges from which the noninfected strains are excluded (Whiteley et al., 2001). Such gene exchanges within a biofilm help the bacterial hosts to develop into superinfective phenotypes that can adapt to and colonize new surfaces effectively (Rice et al., 2009; Webb et al., 2004). Lytic phages specific to native bacteria can also create a niche for the bacterial inocula (Kuykendall & Hashem, 1998; van Elsas & van Overbeek, 1993). Therefore, a consortium of lytic phages, filamentous phages, and the target bacterial inocula should be tested for improved colonization by the bacterial inocula of the contaminated site (Figures 3, 4).
Further, the genome of a filamentous phage itself can be manipulated to increase the ability of bacterial hosts that make up the inoculum to colonize diverse environments. Immediate opportunities for such manipulation are available in some plant‐associated bacterial genera such as Pseudomonas, Ralstonia, and Xanthomonas for which filamentous phages have already been reported (Table 5). At the same time, the little‐explored plant growth‐promoting bacteria such as Acinetobacter, Clostridium, E. coli, Enterobacter, Propionibacteria, Shewanella, and Vibrio(Table 2) need immediate attention. Concerted efforts are required to extend the similarities in the relationships between filamentous phages and bacteria as revealed in laboratory tests to the bacteria used in commercial formulations and for ecorestoration (Table 1).
4.4. Phages to promote community assembly and evolution
A bacterial strain with high genetic stability confers desirable benefits in terms of plant growth and soil health after inoculation; however, successive generations of the strain should also have the ability to diversify and adapt to the changing environment (Sharma, Mishra, Mohmmed, et al., 2011). Filamentous phage may promote genetic stability as well as genetic diversity in a bacterial population depending upon the relative proportions of the filamentous phages and their bacterial hosts.
Environmental stress promotes genetic instability in bacteria; as a result, desirable bacterial phenotypes progressively disappear from the population (Mohmmed, Sharma, Ali, & Babu, 2001; Rau et al., 2009; Sharma et al., 2005; Sharma, Mishra, Mohmmed, et al., 2011; Sharma, Mishra, Rau, & Sharma, 2011). Plasmids, which are extrachromosomal genetic elements, carry environmentally relevant gene(s) in bacteria and help them to adapt to specific niches or confront environmental challenges. Often, the desired bacterial phenotype is lost after inoculation because the plasmid that encodes ecologically competitive gene(s) is lost (Bergstrom, Lipsitch, & Levin, 2000; Hall et al., 2015; Hall, Wood, Harrison, & Brockhurst, 2016; Harrison & Brockhurst, 2012; Mohmmed et al., 2001). At a high phage‐to‐bacterium ratio, infection by the filamentous phage inhibits conjugation in E. colipopulation (K38 and TOP10F) and ensures genetic stability (Boeke et al., 1982; Lin et al., 2011; Table 5). However, the filamentous phage and the F − recipient bacterial cells compete for a common receptor (pilus) of F+ donor bacterial cells. Therefore, the ratio of phage to F − recipient bacterial cells determines which of the two events will be more frequent: infection of F+ donor bacterial cells by the filamentous phage or conjugation between F+ and F − bacterial cells (Novotny, Knight, & Brinton, 1968; Ou, 1973; Wan & Goddard, 2012). The role of filamentous phages in preventing conjugation (Lin et al., 2011; Wan & Goddard, 2012) shows their potential in ecorestoration to maintain genetic stability of bacterial inocula. In fact, the role of protein g3p of a filamentous phage in hindering conjugation process has already been demonstrated. Although a filamentous phage infects and persists within its bacterial host, regular reacquisition by the host may be required to maintain such infected bacteria in a population in sufficient numbers. Also, prior knowledge of the right ratio of filamentous phages to bacterial cells that ensures genetic stability in target bacteria is a prerequisite to using filamentous phages as co‐inoculants.
Independent studies on E. colistrains have confirmed the potential of M13 filamentous phage to trigger genetic heterogeneity in an isogenic population of E. coli(De Paepe et al., 2010) or to maintain genetic homogeneity in E. coli W6 population (Wan & Goddard, 2012; Table 5). Using quantitative analysis, Lin et al. (2011) showed that as the M13 filamentous phage‐to‐E. coli ratio increases, the conjugation frequency decreases: A lower ratio favors conjugation and gene exchange, whereas a high ratio lowers the frequency of conjugation. We suggest that strains of bacterial inoculants be examined for the filamentous phages associated with them and the optimum ratio of filamentous phages to their bacterial hosts be determined for triggering gene exchange and genome diversification in the host population.
However, it is important to ask a fundamental question: Are the filamentous phages that trigger genetic stability or promote genetic diversity in bacterial populations different for different bacterial species or is the difference due to the relative proportions of phages and bacteria? To answer this question, we must isolate filamentous phages from different bacterial genera or species from the natural environment and then analyze the impact of their relationships on the ecology of the bacterial hosts using well‐designed laboratory studies. We may use bacterial species with known filamentous phages for identifying the optimal ratio of phages to bacteria for genetic stability and that for genetic diversity in bacterial populations. Such studies will guide in situ genetic engineering of bacterial inocula. Figure 6 outlines a suggested path of research for developing ecologically competitive phage–bacterium inocula based on the ecological impact of the phages‐to‐bacteria ratio.
Figure 6.
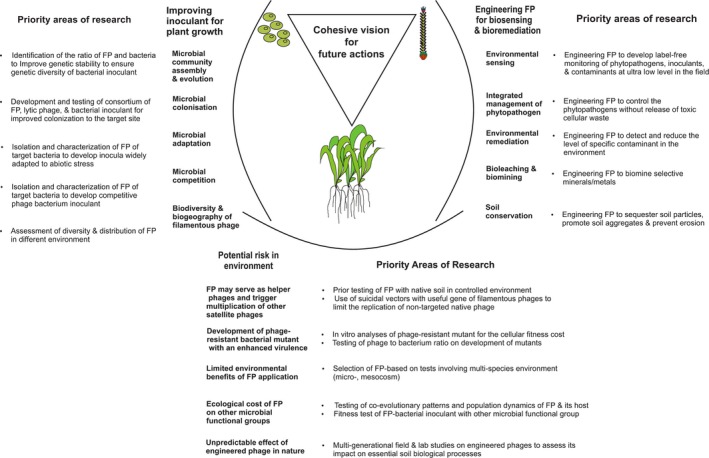
Outline of multi‐branched course of research proposed with a cohesive vision for future actions for environmental application of filamentous phage (i) for improving inoculants for plant growth, (ii) for engineering filamentous phage for biosensing and bioremediation, and (iii) for preventing potential risks of filamentous phage application in the environment. FP, filamentous phage
5. ROLE OF FILAMENTOUS PHAGES IN THE MONITORING BACTERIAL INOCULA AND IN DETECTING AND CONTROLLING PLANT PATHOGENS AND CHEMICAL CONTAMINANTS
5.1. Phages as sensors
The success of restoration efforts depends on the tools available for detecting and controlling pathogens and contaminants. To evaluate and guide restoration, restoration ecologists monitor inocula, pathogens, and contaminants in time and space (Felici et al., 2008; Harvey, 1993; Lynch et al., 2004; van Elsas, Duarte, Rosado, & Smalla, 1998). However, to monitor multiple contaminants and pathogens in degraded environments, we need different physicochemical and biological methods. Because we employ a consortium of bacterial strains and species to tackle multiple contaminants and pathogens, monitoring a consortium (multiple targets) for survival, colonization, and performance also warrants the use of an array of biological methods.
Compared to the conventional monitoring tools, those based on filamentous phages can be more easily tailored for diverse targets and even for detecting a target when it is present in ultralow levels and that too in real time (Figure 5). Tracking of organisms (bacteria, viruses, etc.) and biological materials (spores, toxins, proteins, and DNA) relies on different methods, which may be (a) microbiological (culture, colony counting, chemical and biological plate assays), (b) biochemical and immunological (enzyme‐ or antibody‐based assays), and (c) biomolecular (marker sequences, gene expression, polymerase chain reaction, etc.). Similarly, the tracking of toxicants involves chemical and analytical methods (titrimetric, spectrophotometric, fluorometric, chemiluminescence, etc.). Despite immense advancements, the use of biochemical and molecular methods for environmental studies continues to face such challenges as high costs, the need to process samples, longer time required for assays, and low sensitivity of methods aimed at detecting biological targets in samples from the environment (Felici et al., 2008; Liu, Li, Khan, & Zhu, 2012). In contrast, filamentous phage‐based sensors are economical, can be used in real time without having to process the samples, and detect targets even when they are present at ultralow concentrations in the environment. Because of these benefits, such biosensors may become an important item in the toolkit of the restoration ecologist.
Figure 5.
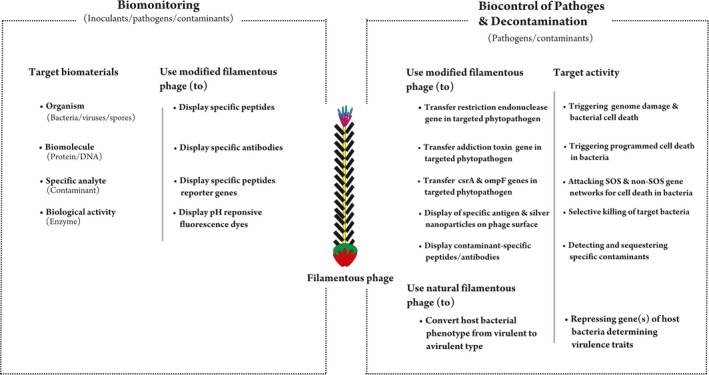
Potential significance of filamentous phages to develop efficient biomonitoring system for tracking and management of targeted inoculated strain, pathogens, and contaminants
Filamentous phages offer flexibility in modifying surface proteins to develop target‐specific receptors or probes and ease in combining different transducers or sensor surfaces such as nano‐ or micromechanical, electrochemical, and optical sensing platforms (Bernard & Francis, 2014; Sagona, Grigonyte, MacDonald, & Jaramillo, 2016; Templier, Roux, Roupioz, & Livache, 2016; Tables 6 and 7). Depending upon the sensor platform, biosensors differ in the principles of transduction of the signal and detection of the analyte. The ease and flexibility in tailoring the surface of the filamentous phage using genetic and chemical methods offer a better opportunity to restoration ecologists to develop ultrasensitive, specific, and cost‐effective biosensors for targets that may differ in size and their chemical nature. Such biosensors detect specific targets at ultralow levels in complex environmental samples without any prior treatment. Besides these properties, filamentous phage‐based sensors are stable and robust even under harsh environments and can be reused by regenerating the receptor surface, which makes them ideal for environmental use (Rakonjac et al., 2011; Singh, Poshtiban, & Evoy, 2013). In restoration ecology, such biosensors may serve as bioindicators (specific to bacteria, viruses) or as biomarkers (specific to pathogen‐specific biomolecules such as DNA and protein or to a specific biological activity). Because they are robust, such biosensors may track different biomaterials in complex environmental samples both in vivo (on plant surfaces and inside tissues) and in vitro (Table 5).
Table 6.
Contributions of modified filamentous phages in detection and control of pathogens in environment
| Filamentous phage | Modification in filamentous phage | Biosensor type and working principle | Target biomaterial | Sensitivity/specificity | References |
|---|---|---|---|---|---|
| Phages in theory of environmental sensing: As an agent to develop sensitive biosensor to track pathogens | |||||
| E2 from fd and 7b1 from M13 phage libraries | Expression of Salmonella typhimurium‐specific peptide (VTPPTQHQ) on pVIII | Quartz crystal microbalance (QCM):Modified phage immobilized on QCM platform detects the pathogen based on Piezoelectric effect. | Salmonella typhimurium | Detects 102 cells/ml with a response time of <180 s | Olsen et al. (2007) |
| JRB7, clone from f8/8 landscape phage library | Expression of B. antracis‐specific peptide on pVIII | Magnetoelastic resonators (ME):Recombinant phage immobilized onto ME platform coated with gold surface binds to antigen | B. anthracis spores | Detects 103 spores/ml in vitro conditions | Shen, Lakshmanan, Mathison, Petrenko, and Chin (2009) |
| E2 derived from fd landscape phage library | Expression of S. typhimurium‐specific peptide on pVIII | Magnetoelastic resonators (ME):Phage nanoprobe immobilized on ME detects binding of pathogen as decrease in resonance frequency | S. typhimurium | Detects 5×102 cfu/ml of pathogen on the surface of tomato | Li, Johnson, et al. (2010), Li, Li, et al. (2010) |
| M13 | M13 displaying peptide specific to target pathogen | Lithographically patterned nanowire‐electrodeposition (LPNE):Virus functionalized nanowires developed by grafting modified phage on poly 3,4‐ethylenedioxythiophene (PEDOT) would bind specific antigen. | Specific antigen on targeted pathogen | Detects antigen (20 nM–99 nM) in in vitro and in vivo conditions | Arter, Taggart, Mclntire, Penner, and Weiss (2010) |
| M13 (Phagemid M13KO7) | Expression of the reporter gene, alkaline phosphatase on pVIII with the help of helper phage M13KO7 | Amperometric Electrochemical Biosensors:Phage immobilized on the electrode detects the target organism in environmental samples based on the activity of reporter enzyme | E. coliTG1 | Detection of 1 cfu/ml in <3 hr; 10‐fold increase in the sensitivity of the biosensor (due to enzyme activity) as compared to other phage‐based biosensors | Neufeld et al. (2005) |
| M13 | Expression of prostate‐specific membrane antigen (PSMA) specific peptide (CALCEF ‐LG) on pVIII | Electrochemical impedance spectroscopy (EIS): Electrode developed by covalent binding of phage to the gold surface generates electrical signal on binding of antigen | PSMA | 120 nM of the target protein | Yang et al. (2006) |
| M13 | pH‐responsive cyanine dye (HCyC‐646) is covalently attached to pVIII | Near Infrared Fluorescence Ratiometric pH Imaging: A bright pH‐responsive ratiometric imaging platform, developed by ligation of dye to the phage surface, measures the emission signal on pH change | Optically diffused tissue | Pathogenesis associated changes in acid–base homeostasis are analyzed by pH measurements both intracellularly and through optically diffused tissue2008 | Hilderbrand et al. (2008) |
| Modified M13 | Modified pIII binds specifically to target antigens, whereas modified pVIII reacts with signal‐producing gold nanoparticles (Au NPs) | Single bioanalytical platform for antigen detection and identification:DNA‐conjugated phage detects (optically/spectroscopically) and identifies the antigen (DNA microarray) | Protein detection and identification | Real time, reagent free detection of antigen (detection limit 25 fmole) and its identification in a high‐throughput manner | Lee, Domaille, and Cha (2012) |
| R5C2 | Expression of streptavidin‐binding peptide NH2–ANRLP CHPQFPCTSHE on pVIII | Opto‐fluidic ring resonator (OFRR) – Lab‐on‐Chip device:Phage integrated on OFRR platform acts as a receptor for detection of analyte and enables label‐free detection of the analyte in small volume | Protein/DNA/Virus | Real‐time detection of protein/DNA (100 pM; Kdapparent 25 pM) and virus particles (2.3 × 103 pfu/ml) | Zhu et al. (2008) |
| A clone from f8/8 landscape phage library | Expression of EPRLSPHS peptide on pVIII protein | Modified phage displaying unique landscape on its body binds antigen with extremely high efficiency and specificity | Spores of Bacillus anthracis | Up to 70‐fold high specificity to spores of B. anthracis than other Bacillusspp. | Brigati et al. (2004) |
| Phages in theory of antagonism and biocontrol: As an agent to biocontrol of phytopathogens | |||||
| M13 | Phages encode restriction endonuclease BglII gene (M13R) or λS holin gene (M13S105, M13VIIIS105) | Genetically modified (GM) phage as bactericidal agents: Modified phage kills bacterial host without lysis and release of toxin in environment | E. coli strain MC4100F¢ | >99% killing of pathogen within 2 hr | Hagens and Bläsi (2003) |
| Pf3 | Phage export protein gene is replaced with BglII endonuclease gene | Nonreplicating GM phage as bactericidal agents:Modified phage kills host bacteria without lysis and release of endotoxins | Pseudomonas aeruginosa | Phage treatment effectively control P. aeruginosa population in in vivo condition with a minimal lethal dose | Hagens, Habel, von Ahsen, von Gabain, and Blasi (2004) |
| Nonlytic, M13 phagemid | Encode the addiction toxins modules (Gef and ChpBK) | Recombinant Phage as a Lethal‐Agent Delivery Vehicle:Modified phage delivers addiction toxins module to elicit programmed cell death in bacterial host | E. coli ER2738 | 94%–98% reduction in in vivo condition within 5 hr | Westwater et al. (2003) |
| M13mp18 phage | Expression of genes (csrA and ompF) that target multiple antibiotics simultaneously | Adjuvant therapy to treat antibiotic‐resistant bacteria:Modified phages trigger SOS and non‐SOS gene networks that are not directly targeted by antibiotics (e.g., aminoglycosides and β‐lactams) kills antibiotic‐resistant bacteria, biofilm cells, and persister cells | E. coli EMG2 and RFS289 | Modified phage kills antibiotic‐resistant bacteria, biofilm cells, and persister cells | Lu and Collins (2009) |
| M13 | Predominant expression of three glutamic acid (E3) residues on pVIII | Silverized Antimicrobial Phage Fibers: Modified phages immobilized to the fibers electrostatically bind silver ions which on subsequent reduction to metallic silver provide antibacterial properties to the fibers | Staphylococcus epidermidis and E. coli | Biocidal fibers show bactericidal activity up to 300 □m distance within a 2 hr exposure; kills 109 cfu/ml with 20 hr continued exposure | Mao, Belcher, and Van Vliet (2010) |
| fRSM (φRSM1 or φRSM3) | Phages found in natural association with host bacteria R. solanacearum MAFF 106603 and MAFF106611 | Phage therapy reduces virulence of phytopathogen: Phage‐infected host cells are characterized with reduction in: (i) twitching motility; (ii) number of type IV pili (Tfp); (iii) β‐1,4‐endoglucanase (Egl) activity; (iv) extracellular polysaccharide (EPS) production, and (iii) expression of certain genes (egl, pehC, phcA, phcB, pilT, and hrpB). | R. solanacearum MAFF 106603 and MAFF 106611 | Loss of virulence to cause wilting to tomato plant (105 cfu wild type trigger wilting within 3 days and cause plant death within 5–7 days) | Addy et al. (2012a) |
| φXacF1 | Phage found in natural association with host bacteria X. axonopodis pv. citri MAFF673010 and MAFF301080 | Phage therapy reduces virulence of pathogen:φXacF1‐infected X. axonopodis pv. citri strains are characterized with lower levels of extracellular polysaccharide production, reduced twitching motility, slower growth rate, and a dramatic reduction in virulence. | X. axonopodis pv. citri MAFF673010 and MAFF301080 | Extreme reduction in virulence, failed to cause citrus canker in lemon even after 4 weeks of post‐infection | Ahmad et al. (2014) |
Table 7.
Contributions of modified filamentous to detect and detoxify the contaminants in environment
| Filamentous phage | Modification in filamentous phage | Integrating component and working principle | Target biomaterial/potential target | Sensitivity/specificity | References |
|---|---|---|---|---|---|
| Phages in theories of environmental sensing and remediation: As an agent to detect and sequester the contaminants | |||||
| Affinity selected M13 clones | Expression of TNT/TNB‐specific peptide on pIII, minor coat protein; Cy5 dye covalently linked to free amino groups on phage surface | Phage‐based immunofluorescence:Modified phage integrated into continuous flow immuno sensor platform detects TNT in environmental samples; fluorescence intensity is taken as a measure of TNT bound to phages | 2,4,6‐trinitrotoluene (TNT) and 2,4,6‐trinitrobenzene (TNB) derivatives | ELISA‐based detection of 10 ng/ml TNT in environmentally relevant, nonphysiological medium, artificial seawater | Goldman et al. (2002) |
| M13 selected from random heptapeptide library | Expression of KPLLMGS, QPKGPKQ and TPTTYKV peptide on pIII | Mineral Processing:Modified phages with KPLLMGS and QPKGPKQ bound specifically to sphalerite and those with TPTTYKV showed specificity to both sphalerite and chalcopyrite; | Sphalerite (ZnS) and chalcopyrite (CuFeS2) in environmental sample | Mineral detection within 1 h of treatment at room temperature | Curtis et al. (2009) |
| VP12 and VP14 selected from P8/8 library | Expression of DSQKTNPS (VP12) and DGQKASNS (VP14) at pVIII | Mineral aggregation:Phage with charged and polar residues on the surface induce aggregation and removal of physical contaminants (via salt bridges) from oil sand tailings in a wide range of pH and ionic strength. | Sphalerite (ZnS) and chalcopyrite (CuFeS2) | 5 × 1011–5 × 1012phages/ml induced aggregation of fine clay (<45 μm); 1,012 phage/ml settled ~99% of the mineral ore in 10 s; | Curtis et al. (2011), Curtis et al. (2013) |
| ScFv phage display library of M13 | Phage expressed single chain antibodies (fused heavy and light chain variable domains) on pIII | Recombinant antibody labeled phage: Phage with TNT specific fluorescence labeled antibodies detects TNT in environment | TNT and derivatives | Detects TNT in ultralow level (1 ng/ml) in aqueous solution | Goldman et al. (2003) |
| M13 from ScFv phage display library | Phage expressed single chain antibodies on the coat proteins are selected | Noncompetitive Immunoassay for Small molecules:phage expressing fluorescence labeled recombinant antibodies detects morphine in environment by measuring FRET signal | Small analytes like morphine | Detects ultralow levels (5 ng/ml) of morphine within 2 min even in the presence of structurally similar analytes | Pulli, Höyhtyä, Söderlund, and Takkinen (2005) |
| 1G40 | Phage expressed reporter gene ( β‐galactosidase) at pVIII | Surface Plasmon Resonance (SPR) Sensors: Phage immobilized on the gold surface of SPR biosensor chip detects the substrate based on the activity of reporter enzyme. | Substrate for the reporter gene (e.g., β‐galactose for β‐galactosidase) | Detects contaminant even at 10−7M | Nanduri, Bhunia, Tu, Paoli, and Brewster (2007 ) |
| Modified M13mp18 | A short random 20 bp DNA sequence is inserted at SinI site of the phage genome | Bacteriophage biotracing: Modified phage used for tracing the pollution source in environmental condition; analyses is based on plaque assay and identification of unique sequence | Modified bacteriophage | Detects even 1pfu/ml in presence of wild type (M13) and other closely related phages (F1, Fd); simultaneous tracing of a number of pollution sources is feasible | Daniell, Davy, and Smith (2000) |
Micromechanical biosensors have exploited modified filamentous phages to develop sensors for detecting bacterial cells (analyte) at ultralow levels. Quartz crystal microbalances (QCMs), magnetoelastic (ME) resonators, and nano‐cantilevers represent the micromechanical type of biosensors. The analyte mass accumulates on the surface of the micromechanical biosensor and results in a corresponding shift in the vibrational resonance of the transducer. A QCM sensor comprises a piezoelectric plate coated with metallic electrodes on both sides. The modified filamentous phage immobilized on the surface of a QCM detects the wet mass of the target bacteria even in nanograms. Bacterial cells bound to the phage‐displayed probe cause a resonance shift under a magnetic field, which makes detection possible in real time. An ME sensor consists of an amorphous ferromagnetic ribbon, which is an advantage in environmental monitoring due to its small size, low cost, and passive and wireless nature. Detecting a target requires neither prior sample preparation nor enrichment of bacterial cells. Biosensors in the form of QCM with E2 phage modified to express specific peptides detected extremely low numbers of the target pathogen (Salmonella typhimurium: 102 cfu/ml) within 3 min (Huang et al., 2007; Lee, Song, Hwang, & Lee, 2013); an ME‐filamentous phage biosensor also detected bacterial pathogen in ultralow numbers (S. typhimurium: 50 cfu/ml) on the surface of tomato (Li, Johnson, et al., 2010; Li, Li, et al., 2010); and another ME biosensor with modified JRB7 detected spores of B. anthracis(103 spores/ml) in vitro.
Electrochemical biosensors have used filamentous phages to detect chemical changes in a cellular environment: The sensor phages display target‐specific peptides, which detect and report the analyte as a change in current (amperometric sensor), impedance (impedance sensor), and voltage potential (light‐addressable potentiometric sensor). Modified M13 helper phage expresses an electrochemically active reporter, such as alkaline phosphatase, at the surface; the reporter measures the current flow (oxidation‐reduction reaction) and detects the signal in an amperometric sensing system. An amperometric electrochemical biosensor with modified M13 detected E. coli TG1 at concentrations as low as 1 cfu/ml by monitoring the activity of the reporter enzyme (Neufeld, Mittelman, Buchner, & Rishpon, 2005). A filamentous phage‐based imaging system also detects pathogen‐related chemical changes (acid–base homeostasis) in optically diffuse tissue (Hilderbrand, Kelly, Niedre, & Weissleder, 2008); for example, phage M13 was modified to ligate a pH‐responsive cyanine dye (HCyc‐646) to pVIII and thus developed into a ratiometric probe. The engineered phage‐based sensor can also use impedance spectroscopy, an electrochemical technique, for such applications. These methods are highly sensitive and easy: For example, a biosensor with engineered M13 filamentous phage covalently attached to a gold electrode measures electrical impedance over a wide range of frequencies (in kHz) and can detect ~120 nanomolar prostate‐specific membrane antigen at signal‐to‐noise ratios greater than 10. Filamentous phage‐based light‐addressable potentiometric sensors (LAPS) represent another such biosensor, which is composed of a semiconductor‐insulator base activated by directed light pulses. These label‐free biosensors detect target enzyme activity or cellular pH, redox condition, or ion gradients, and LAPS have proved flexible enough to modify covalently with as many as four different phages.
Optical sensors, which rely on either spectrometry‐based or resonance‐based sensing, have the potential to detect target biomolecules at ultralow levels. Spectrometry‐based methods such as UV/Vis spectrometry, bio/chemiluminescence, fluorescence or phosphorescence spectrometry, and infrared spectrometry measure changes in intensity at a particular wavelength whereas resonance‐based methods such as surface plasmon resonance (SPR), fluorescence resonance energy transfer (FRET), and colorimetry measure changes in chemical properties upon a change in the wavelength. Opto‐fluidic ring resonator (OFRR) integrates microfluidics and photonic sensing technologies to develop an ultrasensing platform for detecting the target at ultralow levels (as low as nanoliters or at concentrations of 10 pg/mm2). Low cost, high sensitivity, and good reusability of filamentous phage‐based OFRR biosensors make them a promising platform for detection of biomolecules in the environment (Table 6): An OFRR (a lab‐on‐a‐chip device) comprising immobilized phage R5C2 on silicon microfluidics detected targeted protein/DNA at picomolar levels in real time (Suter et al., 2008; Zhu, White, Suter, & Fan, 2008; Zhu, White, Suter, Zourob, & Fan, 2008).
Filamentous phage‐based field effect transistors (FET) can potentially track target viruses in environmental samples: FET comprise a source, a gate, and drain electrodes and detect the target biomolecule in a fluid system based on the change in current–voltage characteristics of the transistor (electrical transduction). Such an electrical biosensor houses a modified filamentous phage as a probe and a semiconductor device as a transducer. The miniaturization and integration into a small chip make the biosensor useful for high‐throughput analysis, whereas integration of nanowires provides an opportunity to develop next‐generation ultrasensitive biosensors: label‐free polymer‐nanowires‐based FET transistors or chemiresistors as biosensors detected bacterial pathogens even at 1 cfu/ml and phages at 103 pfu/ml in untreated environmental samples (Lee et al., 2013).
Thus, filamentous phages as biosensors are fast, reliable, and easy to use and do not rely on costly probes and are particularly suitable for environmental restoration programs because they are highly specific and highly sensitive, can be mass‐produced cheaply, and can withstand harsh environments.
5.2. Phages for biological control
Quick detection and control of phytopathogens facilitate plant recruitment and restoration of vegetation (Al‐Karaki, 2013; Barea, 2015; Bashan, 1998; Sharma et al., 2005). Filamentous phages (modified and natural) can help in detecting and controlling targeted pathogens in environmental samples containing a mixture of even closely related strains. Filamentous phages control the emergence of bacterial pathogens through their effects on the physiology of their bacterial hosts (Table 7; Figure 5). Recombinant filamentous phages have been developed to target such bacterial phytopathogens as Pseudomonas (P. putida, P. aeruginosa), E. coli(Hagens & Bläsi, 2003), and Ralstonia solanacearum (Yamada, 2013) and filamentous phages have been genetically modified to be antagonistic toward bacterial pathogens through (a) expression of restriction endonucleases, (b) initiation of programmed cell death, (c) development of sensitivity to antimicrobial substances, and (d) development of oxidative burst in host cells (Table 6).
Filamentous phages have been modified as environmentally safe biocontrol agents for target pathogens. Filamentous phages are host‐specific but their nonlytic life cycles initially limited their use in biological control of pathogens (Loc‐Carrillo & Abedon, 2011; Henry et al., 2015; Mai‐Prochnow et al., 2015). However, filamentous phages may serve as a vehicle for delivering toxins into target pathogenic strains—the nonlytic life cycle then becomes an advantage because it prevents the release of toxic cellular waste or endotoxins into the immediate environment (Goodridge, 2010; Lu & Koeris, 2011; Viertel, Ritter, & Horz, 2014) and also prevents undesirable changes in the structure and functioning of the microbial community due to the release of toxic waste from the lysed pathogenic cells. For example, a modified M13 phage delivers “addiction toxin” genes (Gef and ChpBK), which triggers programmed cell death in target bacteria in vivo (Table 6). Such “suicide” systems have shown their potential to control some environmentally significant bacteria, namely Pseudomonas (P. putida, P. aeruginosa) and E. coli(Hagens & Bläsi, 2003). Phage M13 has been modified to encode restriction endonuclease (BglII) for killing the target bacteria with efficiency comparable to that of lytic phages. Therefore, modified filamentous phages not only control the target bacteria but also minimize the risk of the toxins being released into soil. Considering these benefits, we believe that filamentous phage‐based biocontrol of pathogens will also add to the efficacy of bacterial inocula in restoration of vegetation.
The use of filamentous phages to control pathogens that are resistant to multiple antibiotics is yet to win the attention of restoration ecologists that it deserves. For example, M13mp18 modified phage targets SOS and non‐SOS networks of bacterial hosts and controls antibiotic‐resistant and persister strains of E. coli(EMG2, RFS289; Lu & Collins, 2009). Also, silverized antimicrobial phage fibers developed with E3 modified M13 kill bacterial contaminants in water during filtration through phage‐coated fibers (Mao et al., 2010; Table 6). Fusion phages (fd‐pIIICTX) can control diverse pathogens as effectively as phage cocktails or mixtures can (Pires, Cleto, Sillankorva, Azeredo, & Lu, 2016). In some cases, natural filamentous phages can also reduce the pathogenicity of their bacterial host. Such filamentous phages offer an opportunity for environmentally safe biocontrol of pathogens. For example, Addy et al. (2012a) reported a filamentous phage (φRSM) of the wilt‐causing bacterial pathogen Ralstonia solanacearum: φRSM infection made the pathogen less virulent and thus prevented damage to its host plants (Yamada, 2013). Ecologists have also been interested in φRSM because R. solanacearumis a serious threat to phytorestoration programs (Zhang et al., 2017), for example to programs to grow tobacco for ecorestoration of nutrient‐deficient and contaminant‐rich soils. In fact, degraded soils show greater abundance of not only R. solanacearumbut also of pathogenic members of Pseudomonas, Erwinia, and Xanthomonas. Controlling R. solanacearum is a challenge because it survives as a latent infection in indigenous weeds for many years (Hayward, 1991; Wenneker et al., 1999). However, it would also be useful to isolate and engineer filamentous phages of other pathogenic bacteria and test the potential of these phages to control the pathogens of other wild plants. Such use of filamentous phages to lower pathogenicity, virulence, and spread of dreadful phytopathogens may mark a new milestone in restoration programs.
The increasing numbers of bacterial genera discovered to have an association with filamentous phages and the ease with which the phage genome can be modified to produce antimicrobial peptides represent an untapped but most promising opportunity for biocontrol of pathogens. Using suitably modified filamentous phages along with other biocontrol agents can revolutionize the integrated management of phytopathogens. However, these phages are at times unstable in their host populations, even when they do not result in the evolution of host resistance (Lerner & Model, 1981). This characteristic, and the loss of filamentous phages during molecular applications (Mai‐Prochnow et al., 20155), is obstacles to their use as inhibitors of pathogenic bacteria.
However, other species or strains of Ralstoniaalso develop symbiotic endophytic associations with plant species that show high potential for phytoremediation. For example, poplars (Populus spp.) and willows (Salix spp.), which are used for phytoremediation, also carry Ralstoniaas an endophyte besides Acinetobacter, Pseudomonas, and Xanthomonas. Ralstoniais also an endophyte of Anthurium, Brassica juncia, Geranium, Gerbera, Heliconia, Mimosa, Salvia, sunflower, tobacco, Verbena, and Zinnia, which are also used for phytoremediation of sites contaminated with inorganic and organic pollutants (Ikeura et al., 2016; Kabra, Khandare, Kurade, & Govindwar, 2011; Liu, Xin, & Zhou, 2017; Madera‐Parra, Peña‐Salamanca, Pena, Rousseau, & Lens, 2015; Mahdieh, Yazdani, & Mahdieh, 2013). For example, R. eutropha was effective in combination with sunflower (Helianthus) for phytoremediation of sites contaminated with Cd and Zn (Marques, Moreira, Franco, Rangel, & Castro, 2013), with maize for phytoremediation of sites contaminated with Cd (Moreira, Marques, Franco, Rangel, & Castro, 2014), and with Juncus acutus for biotransformation of Cr(VI) (Dimitroula et al., 2015). Filamentous phages that affect members of Ralstoniaare useful in controlling phytopathogenic strains of the bacteria and in improving the efficacy of endophytic bacterial strains used in phytoremediation.
5.3. Phages for remediation of contaminated sites
Restoration ecologists use microbial technologies for remediation of contaminated sites and for restoring ecosystem goods and services (UNCCD, 2013), and filamentous phages have been engineered to detect contaminants (physical, organic, and inorganic) and to remove or to reduce them in the environment. To assess the success of such efforts, it is necessary to examine the environment for the presence of such contaminants (Brown, 1997; Henry et al., 2015; Viertel et al., 2014; Table 3).
Phages display specific peptides on phage coat proteins (pVIII and pIII), which have been used for detecting, binding to, or decomposing the contaminants (Henry et al., 2015; Nambudripad, Stark, & Makowski, 1991; Petrenko & Makowski, 1993; Thiriot, Nevzorova, & Opella, 2005). Display technology can screen billions of potential toxicants and help in developing fusion phages that retain not only their infectivity and immunogenicity but also their degradation ability (Lerner, Benkovic, & Schultz, 1991). Such properties of modified phages make them potential co‐inoculants with the host bacteria to extract specific pollutants from the environment. For remediation of contaminated soils, phage inoculation thus represents a cost‐effective method of setting up a factory in situ to produce large quantities of contaminant‐specific biomolecules for remediation of the soil environment. This environment‐friendly approach may not be a permanent solution for bioremediation but it is worthwhile to test its potential to reduce the concentration of contaminants in the environment.
Modified filamentous phages also offer another method of separating and processing minerals (bioleaching or biomining) for restoring abandoned mines. For example, modified phages show specificity and selectivity in binding to environmental sources of sphalerite (ZnS, a major ore of zinc) and chalcopyrite (CuFeS2,a major ore of copper; Table 7). These phages separate sphalerite efficiently despite the presence of such natural contaminants as silica (a waste mineral) and pyrite (Curtis et al., 2009). The modified phages can potentially mine minerals and metals selectively from natural ore and remediate abandoned mines by acting on waste and industrial scrap and thus contribute to economic and ecological security.
Filamentous phages VP12 and VP14 have been modified to display a specific peptide (DSQKTNPS on pVIII) that sequesters physical pollutants (fine silt and clay particles; Curtis et al., 2011; Curtis et al., 2013). In fact, the mechanism by which the peptide binds to chalcopyrite has also been elucidated: The two phages sequester on their surface particles smaller than 45 µm in diameter under a wide range of pH (3–11) and cation concentrations. The potential of such phages to aggregate soil particles and thereby prevent erosion can be tapped as a novel strategy in soil management.
Phages have also been modified to display peptides (12‐mer) specific to organic contaminants, namely 2,4,6‐trinitrotoluene (TNT) and 2,4,6‐trinitrobenzene (TNB; Table 7). Such modified phages can detect the target organic contaminants at ultralow levels (10 ng/ml) even in heterogeneous environmental samples (Goldman et al., 2002). Modified M13 phages displaying antibodies detected TNT and its derivatives even at concentrations of 1 ng/ml (Goldman et al., 2003). The modified phages displaying specific antibodies are highly selective and sensitive and can detect even minute quantities of such contaminants as morphine at ultralow levels (5 ng/ml within 2 min) in environmental samples (Pulli et al., 2005). Therefore, co‐inoculation of modified phages with plant growth‐promoting bacteria will serve as a supplementary biotechnology to sequester and detoxify targeted contaminants and to speed up revegetation of degraded lands.
6. PRIORITY AREAS OF RESEARCH TO MINIMIZE THE POTENTIAL RISK TO THE ENVIRONMENT FROM THE APPLICATION OF FILAMENTOUS PHAGES
So far, filamentous phages have been presented in a positive light. However, as with invasive species and transgenic organisms, the potential risks from introducing filamentous phages that are foreign to degraded soils must not be overlooked. Even if a phage is native to the soil, phage inoculation may disturb the phage‐to‐bacteria ratio, which is crucial to many biological processes in soil (Meaden & Koskella, 2013; Reyes et al., 2010). Because bacterial inoculation aims to restore adversely affected soil processes in degraded lands, the risk from using modified or natural filamentous phages is minimal (Sharma et al., 2015; Sharma, Mishra, Mohmmed, & Babu, 2008; Sharma, Mishra, Rau, et al., 2011). Based on a cost‐benefit analysis, we maintain that the benefits of filamentous phages outweigh the risks from deploying them in damaged ecosystems (Figure 6). Also, the impact of inoculation with filamentous phages is hard to predict unless we have prior knowledge of their host range and the proportion of filamentous phage‐infected bacteria in bacterial populations. Based on the low density and diversity of bacterial communities in degraded environments, we believe that the immediate benefits of phages outweigh the possible risks (Figures 5and 6).
Expansion of the host range of nonnative or modified filamentous phages and the activation of unknown silent phages in bacterial populations in inoculated soils are other potential risks from phage application. Filamentous phages are highly host‐specific and rarely extend their host range to include other strains, species, or genera (Hyman & Abedon, 2010; Piekarowicz et al., 2014). To preclude the possible risk of filamentous phages serving as helper phages for the multiplication of satellite phages (Rakonjac et al., 2011), it is essential to conduct prior experiments with native soils (Figure 6). Alternatively, to limit the replication of nontargeted phages in soil, we may load the useful genes from filamentous phages into a suicide vector (Addy, Askora, Kawasaki, Fujie, & Yamada, 2014; Huber & Waldor, 2002; Martínez & Campos‐Gómez, 2016; Pant et al., 2015). To ascertain the possible impact of introduced phages on the functioning of microbial communities, we need to generate relevant knowledge using ecologically relevant laboratory‐ and field‐based studies in a multi‐species environment (microcosm or mesocosm).
Evolution of phage‐resistant but virulent bacterial mutants is another common concern (Hosseinidoust, Ven, & Tufenkji, 2013). However, in the environment, selection may work against the multiplication of such mutants. Bacteria with mutated pili develop resistance to filamentous phages but show reduced fitness. It may be noted that the pilus helps a bacterium to adhere to suitable objects, move, colonize, and spread into the environment. At the same time, the phages‐to‐bacteria ratio affects the frequency of conjugation in bacterial populations. Therefore, we propose that the population dynamics of filamentous phages and their bacterial hosts in a given environment be examined before deploying filamentous phages for remediation and the impact of population dynamics on the frequency of conjugation and on the functioning of host bacteria be estimated to develop strategies for the use of filamentous phages.
Finally, we need to consider the costs and benefits of the environmental use of filamentous phages based on in vitro studies, which are characterized by a stable environment with few limiting factors. It is possible that the benefits will be lower in the fluctuating natural environments with many limiting factors. However, even marginal improvements in the efficacy of bacterial inoculants due to filamentous phages are worthwhile and particularly important in restoring degraded lands. The costs and benefits are context dependent. The effect of a phage on the bacterial cell is likely to have bottom‐up consequences on bacterial populations, on microbial communities, and on other associated organisms (plants and animals). Such effects of phage inoculation on interactions between organisms will have ecological costs in terms of its impacts on other functional groups of microbes such as associative cooperators, competitors, and predators. Therefore, we suggest that the ecological cost of the application of filamentous phages be estimated and used as the foundation for developing suitable methods for co‐inoculation with bacteria and filamentous phages (Figure 6).
7. CONCLUSIONS
Theories related to the ecology of soil microbes have guided microbe‐assisted environmental restoration programs. Applying such theories to phage–bacterium interactions will improve microbial inoculation technologies for revegetation of degraded lands. Growing knowledge of the ecology of filamentous phages of different bacterial genera in diverse environmental settings shows the potential of phages as an emerging bioresource suitable for environmental applications. Co‐inoculation of bacteria with filamentous phages will increase the ecological and evolutionary potential of microbial communities in degraded lands because filamentous phages will help bacterial inocula to colonize degraded sites subjected to multiple abiotic and biotic sources of stress. Further, the ease with which the genome of filamentous phages can be manipulated to express a range of peptides and proteins makes such phages a real‐time sensing tool for environmental restoration. Sensors based on filamentous phages can detect inocula, pathogens, and contaminants in environmental samples at ultralow levels and will not only contribute to more informed decisions related to restoration but also save time and resources. We recommend that restoration ecologists exploit filamentous phages to enhance the ecophysiological capabilities of host bacteria and to find new filamentous phages and understand their ecological relevance. Researchers in environmental remediation will benefit from studying the ecology of filamentous phages to shape bacterial populations for a given purpose; in turn, the exercise of shaping bacterial populations will help the researchers to understand the ecology of phage better. The priority research areas identified in this review will help to realize the potential of phage–bacterium co‐inoculation in environmental restoration and, at the same time, minimize the possible risks from deploying phages. Such use of filamentous phages can usher a tectonic shift in the science and practice of ecorestoration.
CONFLICT OF INTEREST
Authors declare no conflict of interest.
AUTHOR'S CONTRIBUTION
Conceived the hypothesis: RSS, VM, SK. Analyzed the literature/data: SK, VM, RSS, PK. Wrote the paper: RSS, SK, VM, PK.
ACKNOWLEDGMENTS
We acknowledge the anonymous reviewers and editors for their critical comments and constructive suggestions that helped us to improve the manuscript. We thank Yateendra Joshi and R. Geeta for copy‐editing the MS. We acknowledge Inderjit Singh for his critical comments and constructive suggestions. We thank Aakansha Tyagi in extending support while copy‐editing the MS. We also thank the University of Delhi for the Faculty Research Grant and the Department of Science & Technology, Government of India, for the DU‐DST PURSE Grant (Phase II) to RSS and VM. SK and PK wish to thank the University Grants Commission for the Junior Research Fellowship.
Sharma RS, Karmakar S, Kumar P, Mishra V. Application of filamentous phages in environment: A tectonic shift in the science and practice of ecorestoration. Ecol Evol. 2019;9:2263–2304. 10.1002/ece3.4743
Contributor Information
Radhey Shyam Sharma, Email: radheyss26@gmail.com, Email: rads26@hotmail.com.
Vandana Mishra, Email: mistletoe_h@hotmail.com.
DATA ACCESSIBILITY
All the data used for developing the proposals and recommendations presented in this paper were sourced from published studies, and appropriate references are provided.
REFERENCES
- Addy, H. S. , Askora, A. , Kawasaki, T. , Fujie, M. , & Yamada, T. (2012a). Loss of virulence of the phytopathogen Ralstonia solanacearum through infection by φRSM filamentous phages. Phytopathology, 10, 469–477. [DOI] [PubMed] [Google Scholar]
- Addy, H. S. , Askora, A. , Kawasaki, T. , Fujie, M. , & Yamada, T. (2012b). The filamentous phage ϕRSS1 enhances virulence of phytopathogenic Ralstonia solanacearum on tomato. Phytopathology, 102, 244–251. [DOI] [PubMed] [Google Scholar]
- Addy, H. S. , Askora, A. , Kawasaki, T. , Fujie, M. , & Yamada, T. (2014). Disruption of gspD and its effects on endoglucanase and filamentous phage secretion in Ralstonia solanacearum . Procedia Environmental Sciences, 20, 753–759. 10.1016/j.proenv.2014.03.090 [DOI] [Google Scholar]
- Ahmad, A. A. , Askora, A. , Kawasaki, T. , Fujie, M. , & Yamada, T. (2014). The filamentous phage XacF1 causes loss of virulence in Xanthomonas axonopodis pv. citri, the causative agent of citrus canker disease. Frontiers in Microbiology, 5, 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Awadhi, H. , Dashti, N. , Khanafer, M. , Al‐Mailem, D. , Ali, N. , & Radwan, S. (2013). Bias problems in culture‐independent analysis of environmental bacterial communities: A representative study on hydrocarbonoclastic bacteria. SpringerPlus, 2, 369 10.1186/2193-1801-2-369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhamlan, F. S. , Ederer, M. M. , Brown, C. J. , Coats, E. R. , & Crawford, R. L. (2013). Metagenomics‐based analysis of viral communities in dairy lagoon wastewater. Journal of Microbiological Methods, 92, 183–188. 10.1016/j.mimet.2012.11.016 [DOI] [PubMed] [Google Scholar]
- Ali, N. , Al‐Awadhi, H. , Dashti, N. , Khanafer, M. , El‐Nemr, I. , Sorkhoh, N. , & Radwan, S. S. (2015). Bioremediation of atmospheric hydrocarbons via bacteria naturally associated with leaves of higher plants. International Journal of Phytoremediation, 17, 1160–1170. 10.1080/15226514.2015.1045125 [DOI] [PubMed] [Google Scholar]
- Al‐Karaki, G. N. (2013). The role of mycorrhiza in the reclamation of degraded lands in arid environments In Shahid S. A., Taha F. K., & Abdelfattah M. A. (Eds.), Developments in soil classification, land use planning and policy implications: Innovative thinking of soil inventory for land use planning and management of land resources (pp. 823–836). Dordrecht, The Netherlands: Springer. [Google Scholar]
- Al‐Mailem, D. M. , Sorkhoh, N. A. , Marafie, M. , Al‐Awadhi, H. , Eliyas, M. , & Radwan, S. S. (2010). Oil phytoremediation potential of hypersaline coasts of the Arabian Gulf using rhizosphere technology. Bioresource Technology, 101, 5786–5792. 10.1016/j.biortech.2010.02.082 [DOI] [PubMed] [Google Scholar]
- Amarger, N. (1981). Selection of Rhizobium strains on their competitive ability for nodulation. Soil Biology and Biochemistry, 13, 481–486. 10.1016/0038-0717(81)90038-9 [DOI] [Google Scholar]
- Arora, N. K. , Tiwari, S. , & Singh, R. (2014). Comparative study of different carriers inoculated with nodule forming and free living plant growth promoting bacteria suitable for sustainable agriculture. Journal of Plant Pathology & Microbiology, 5, 229–231. [Google Scholar]
- Arter, J. A. , Taggart, D. K. , Mclntire, T. M. , Penner, R. M. , & Weiss, G. A. (2010). Virus‐PEDOT nanowires for biosensing. Nano Letters, 10, 4858–4862. 10.1021/nl1025826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askora, A. , Kawasaki, T. , Fujie, M. , & Yamada, T. (2012). Loss of virulence of the phytopathogen Ralstonia solanacearum through infection by φRSM filamentous phages. Phytopathology, 10, 469–477. [DOI] [PubMed] [Google Scholar]
- Askora, A. , Kawasaki, T. , Usami, S. , Fujie, M. , & Yamada, T. (2009). Host recognition and integration of filamentous phage ϕRSM in the phytopathogen, Ralstonia solanacearum . Virology, 384, 69–76. 10.1016/j.virol.2008.11.007 [DOI] [PubMed] [Google Scholar]
- Askora, A. , & Yamada, T. (2015). Two different evolutionary lines of filamentous phages in Ralstonia solanacearum: Their effects on bacterial virulence. Frontiers in Genetics, 6, 217–223. 10.3389/fgene.2015.00217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azubuike, C. C. , Chikere, C. B. , & Okpokwasili, G. C. (2016). Bioremediation techniques–classification based on site of application: Principles, advantages, limitations and prospects. World Journal of Microbiology and Biotechnology, 32, 180–197. 10.1007/s11274-016-2137-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barea, J. M. (2015). Future challenges and perspectives for applying microbial biotechnology in sustainable agriculture based on a better understanding of plant‐microbe interactions. Journal of Soil Science and Plant Nutrition, 15, 261–282. [Google Scholar]
- Barnet, Y. M. (1980). The effect of rhizobiophages on populations of Rhizobium trifolii in the root zone of clover plants. Canadian Journal of Microbiology, 26, 572–576. [DOI] [PubMed] [Google Scholar]
- Bashan, Y. (1998). Inoculants of plant growth‐promoting bacteria for use in agriculture. Biotechnology Advances, 16, 729–770. 10.1016/S0734-9750(98)00003-2 [DOI] [Google Scholar]
- Bashan, Y. , & Holguin, G. (1997). Azospirillum‐plant relationships: Environmental and physiological advances (1990–1996). Canadian Journal of Microbiology, 43, 103–121. [DOI] [PubMed] [Google Scholar]
- Bayer, M. E. , & Bayer, M. H. (1986). Effects of bacteriophage fd infection on Escherichia coli HB11 envelope: A morphological and biochemical study. Journal of Virology, 57, 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belimov, A. A. , Kojemiakov, A. P. , & Chuvarliyeva, C. V. (1995). Interaction between barley and mixed cultures of nitrogen fixing and phosphate‐solubilising bacteria. Plant and Soil, 173, 29–37. [Google Scholar]
- Belimov, A. A. , Kunakova, A. M. , Vasilyeva, N. D. , Gruzdeva, E. V. , Vorobiev, N. I. , Kojemiakov, A. P. , … Sokova, S. A. (1995). Relationship between survival rates of associative nitrogen‐fixers on roots and yield response of plants to inoculation. FEMS Microbiology Ecology, 17, 187–196. [Google Scholar]
- Beneduzi, A. , Ambrosini, A. , & Passaglia, L. M. P. (2012). Plant growth‐promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genetics and Molecular Biology, 35, 1044–1051. 10.1590/S1415-47572012000600020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhar, I. (2001). Biotechnological applications of phage and cell display. BiotechnologyAdvances, 19, 1–33. 10.1016/S0734-9750(00)00054-9 [DOI] [PubMed] [Google Scholar]
- Berg, G. (2009). Plant‐microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Applied and Environmental Microbiology, 84, 11–18. 10.1007/s00253-009-2092-7 [DOI] [PubMed] [Google Scholar]
- Berg, G. , Marten, P. , & Ballin, G. (1996). Stenotrophomonas maltophilia in the rhizosphere of oilseed rape—occurrence, characterization and interaction with phytopathogenic fungi. Microbiological Research, 151, 19–27. 10.1016/S0944-5013(96)80051-6 [DOI] [Google Scholar]
- Bergstrom, C. T. , Lipsitch, M., & Levin, B. R. (2000). Natural selection, infectious transfer and the existence conditions for bacterial plasmids. Genetics, 155, 1505–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard, J. M. , & Francis, M. B. (2014). Chemical strategies for the covalent modification of filamentous phage. Frontiers in Microbiology, 5, 734 10.3389/fmicb.2014.00734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya, P. , & Jha, D. (2012). Plant growth‐promoting rhizobacteria (PGPR): Emergence in agriculture. World Journal of Microbiology and Biotechnology, 28, 1327–1350. 10.1007/s11274-011-0979-9 [DOI] [PubMed] [Google Scholar]
- Bhattacharyya, R. N. , & Pati, B. R. (2000). Growth behaviour and indole acetic acid (IAA) production by a Rhizobium isolated from root nodules of Alysicarpus vaginalis DC. Acta Microbiologica Et Immunologica Hungarica, 47, 41–51. [PubMed] [Google Scholar]
- Bille, E. , Zahar, J.‐R. , Perrin, A. , Morelle, S. , Kriz, P. , Jolley, K. A. , … Tinsley, C. R. (2005). A chromosomally integrated bacteriophage in invasive meningococci. The Journal of Experimental Medicine, 201, 1905–1913. 10.1084/jem.20050112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke, J. D. , Model, P. , & Zinder, N. D. (1982). Effects of bacteriophage f1 gene III protein on the host cell membrane. Molecular Genetics and Genomics, 186, 185–192. 10.1007/BF00331849 [DOI] [PubMed] [Google Scholar]
- Borriss, R. (2011). Use of plant‐associated Bacillusstrains as biofertilizers and biocontrol agents in agriculture In Maheshwari D. K. (Ed.), Bacteria in agrobiology: Plant growth responses (pp. 41–76). Berlin, Germany: Springer. [Google Scholar]
- Brigati, J. , Williams, D. D. , Sorokulova, I. B. , Nanduri, V. , Chen, I. H. , Turnbough, C. L. Jr , & Petrenko, V. A. (2004). Diagnostic probes for Bacillus anthracis spores selected from a landscape phage library. Clinical Chemistry, 50, 1899–1906. 10.1373/clinchem.2004.038018 [DOI] [PubMed] [Google Scholar]
- Brim, H. , Venkateshwaran, A. , Kostandarithes, H. M. , Fredrickson, J. K. , & Daly, M. J. (2003). Engineering Deinococcus geothermalis for bioremediation of high‐temperature radioactive waste environments. Applied and Environmental Microbiology, 69, 4575–4582. 10.1128/AEM.69.8.4575-4582.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, L. R. , & Dowell, C. E. (1968). Replication of coliphage M‐13. I. Effects on host cell after synchronized infection. Journal of Virology, 2, 1290–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, S. (1997). Metal‐recognition by repeating polypeptides. Nature Biotechnology, 15, 269–272. 10.1038/nbt0397-269 [DOI] [PubMed] [Google Scholar]
- Bürgmann, H. , Widmer, F. , Sigler, W. V. , & Zeyer, J. (2003). mRNA extraction and reverse transcription‐PCR protocol for detection of nifH gene expression by Azotobacter vinelandii in soil. Applied and Environmental Microbiology, 69, 1928–1935. 10.1128/AEM.69.4.1928-1935.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calendar, R. L. , & Inman, R. (2005). Phage biology In Waldor M. K., Friedman D. I., & Adhya S. L. (Eds.), Phages: Their role in bacterial pathogenesis and biotechnology (pp. 18–36). Washington, DC: ASM Press. [Google Scholar]
- Cantalupo, P. G. , Calgua, B. , Zhao, G. , Hundesa, A. , Wier, A. D. , Katz, J. P. , …, & Pipas, J. M. . (2011). Raw sewage harbors diverse viral populations. Mbio, 2, e00180–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, S. , Zhong, B. , Yue, H. , Zeng, H. , & Zeng, J. (2009). Development and testing of a sustainable environmental restoration policy on eradicating the poverty trap in China's Changting County. Proceedings of National Academy of Sciences USA, 106, 10712–10716. 10.1073/pnas.0900197106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco, J. A. , Armario, P. , Pajuelo, E. , Burgos, A. , Caviedes, M. A. , Lopez, R. , … Palomares, A. J. (2005). Isolation and characterisation of symbiotically effective Rhizobium resistant to arsenic and heavy metals after the toxic spill at the Aznalcollar pyrite mine. Soil Biology and Biochemistry, 37, 1131–1140. [Google Scholar]
- Carrillo‐Garcia, A. , Bashan, Y. , Diaz Rivera, E. , & Bethlenfalvay, G. J. (2000). Effects of resource island soils, competition, and inoculation with Azospirillumon survival and growth of Pachycereus pringlei, the giant cactus of the Sonoran Desert. Ecological Research, 8, 65–73. 10.1046/j.1526-100x.2000.80009.x [DOI] [Google Scholar]
- Carrillo‐Pérez, E. , Ruiz‐Manríquez, A. , & Yeomans‐Reina, H. (2004). Aislamiento, identifi cación y evaluación de un cultivo mixto de microorganismos con capacidad para degradar DDT. Revista Internacional De Contaminación Ambiental, 20, 69–75. [Google Scholar]
- Chandra, P. , & Singh, E. (2016). Applications and mechanisms of plant growth‐stimulating rhizobacteria In Choudhary D., Varma A., & Tuteja N. (Eds.), Plant‐microbe interaction: An approach to sustainable agriculture (pp. 37–62). Singapore, Singapore: Springer. [Google Scholar]
- Chanway, C. P. (1997). Inoculation of tree roots with plant growth promoting soil bacteria: An emerging technology for reforestation. Journal of Forest Science, 43, 99–112. [Google Scholar]
- Chiong, M. , Barra, R. , González, E. , & Vásquez, C. (1988). Resistance of Thermus spp. to potassium tellurite. Applied and Environmental Microbiology, 54, 610–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiong, M. , González, E. , Barra, R. , & Vásquez, C. (1988). Purification and biochemical characterization of tellurite‐reducing activities from Thermus thermophilus HB8. Journal of Bacteriology, 170, 3269–3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouikha, I. , Charrier, L. , Filali, S. , Derbise, A. , & Carniel, E. (2010). Insights into the infective properties of YpfPhi, the Yersinia pestis filamentous phage. Virology, 407, 43–52. [DOI] [PubMed] [Google Scholar]
- Coelho, J. , Rivonkar, C. U. , Bhavesh, N. S. , Jothi, M. , & Sangodkar, U. M. X. (2003). Biosurfactants production by the quinoline degrading marine bacterium Pseudomonas sp. strain GU 104, and its effect on the metabolism of green mussel Perna viridis L. Indian Journal of Geo‐Marine Sciences, 32, 202–207. [Google Scholar]
- Costacurta, A. , Mazzafera, P. , & Rosato, Y. B. (1998). Indole‐3‐acetic acid biosynthesis by Xanthomonas axonopodis pv. citri is increased in the presence of plant leaf extracts. FEMS Microbiology Letters, 159, 215–220. [Google Scholar]
- Curtis, S. B. , Dunbar, W. S. , & Macgillivray, R. T. (2013). Bacteriophage‐induced aggregation of oils and stailings. Biotechnology and Bioengineering, 110, 803–811. 10.1002/bit.24745 [DOI] [PubMed] [Google Scholar]
- Curtis, S. B. , Hewitt, J. , & Macgillivray, R. T. (2009). Biomining with bacteriophage: Selectivity of displayed peptides for naturally occurring sphalerite and chalcopyrite. Biotechnology and Bioengineering, 102, 644–650. 10.1002/bit.22073 [DOI] [PubMed] [Google Scholar]
- Curtis, S. B. , Macgillivray, R. T. , & Dunbar, W. S. (2011). Effects of bacteriophage on the surface properties of chalcopyrite (CuFeS(2).), and phage‐induced flocculation of chalcopyrite, glacialtill, and oils and stailings. Biotechnology and Bioengineering, 108, 1579–1590. 10.1002/bit.23097 [DOI] [PubMed] [Google Scholar]
- Daniell, T. J. , Davy, M. L. , & Smith, R. J. (2000). Development of a genetically modified bacteriophage for use in tracing sources of pollution. Journal of Applied Microbiology, 88, 860–869. 10.1046/j.1365-2672.2000.01028.x [DOI] [PubMed] [Google Scholar]
- Das, B. (2014). Mechanistic insights into filamentous phage integration in Vibrio cholerae . Frontiers in Microbiology, 650, 1–9. 10.3389/fmicb.2014.00650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta, C. , & Basu, P. S. (2000). Indole acetic acid production by a Rhizobium species from root nodules of a leguminous shrub, Cajanus cajan . Microbiological Research, 155, 123–127. 10.1016/S0944-5013(00)80047-6 [DOI] [PubMed] [Google Scholar]
- Davies, J. , & Davies, D. (2010). Origins and evolution of antibiotic resistance. Microbiology and Molecular Biology Reviews, 74, 417–433. 10.1128/MMBR.00016-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Paepe, M. , De Monte, S. , Robert, L. , Lindner, A. B. , & Taddei, F. (2010). Emergence of variability in isogenic Escherichia coli populations infected by a filamentous virus. PLoS ONE, 5, e11823 10.1371/journal.pone.0011823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de‐Bashan, L. E. , Moreno, M. , Hernandez, J.‐P. , & Bashan, Y. (2002). Removal of ammonium and phosphorous ions from synthetic wastewater by the microalgae Chlorella vulgariscoimmobilized in alginate beads with the microalgae growth‐promoting bacterium Azospirillum brasilense . Water Research, 36, 2941–2948. [DOI] [PubMed] [Google Scholar]
- Derbise, A. , & Carniel, E. (2014). YpfΦ: A filamentous phage acquired by Yersinia pestis . Frontiers in Microbiology, 5, 701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbise, A. , Chenal‐Francisque, V. , Pouillot, F. , Fayolle, C. , Prévost, M. C. , Médigue, C. , … Carniel, E. (2007). A horizontally acquired filamentous phage contributes to the pathogenicity of the plague bacillus. Molecular Microbiology, 63, 1145–1157. 10.1111/j.1365-2958.2006.05570.x [DOI] [PubMed] [Google Scholar]
- Díaz‐Ramírez, I. , Escalante‐Espinosa, E. , Schroeder, R. A. , Fócil‐Monterrubio, R. , & Ramírez‐Saad, H. (2013). Hydrocarbon biodegradation potential of native and exogenous microbial inocula in Mexican tropical soils In Chamy R., & Rosenkranz F. (Eds.), Biodegradation of hazardous and special products (pp. 155–178). Rijeka, Croatia: Tech. [Google Scholar]
- Dimitroula, H. , Syranidou, E. , Manousaki, E. , Nikolaidis, N. P. , Karatzas, G. P. , & Kalogerakis, N. (2015). Mitigation measures for chromium‐VI contaminated groundwater ‐ The role of endophytic bacteria in rhizofiltration. Journal of Hazardous Materials, 281, 114–120. 10.1016/j.jhazmat.2014.08.005 [DOI] [PubMed] [Google Scholar]
- Dinesh, R. , Anandaraj, M. , Kumar, A. , Bini, Y. K. , Subila, K. P. , & Aravind, R. (2015). Isolation, characterization, and evaluation of multi‐trait plant growth promoting rhizobacteria for their growth promoting and disease suppressing effects on ginger. Microbiological Research, 173, 34–43. 10.1016/j.micres.2015.01.014 [DOI] [PubMed] [Google Scholar]
- Dubinsky, E. A. , Conrad, M. E. , Chakraborty, R. , Bill, M. , Borglin, S. E. , Hollibaugh, J. T. , … Tom, L. M. (2013). Succession of hydrocarbon‐degrading bacteria in the aftermath of the Deepwater Horizon oil spill in the Gulf of Mexico. Environmental Science & Technology, 47, 10860–10867. [DOI] [PubMed] [Google Scholar]
- Duniya, D. A. , Maikaje, D. B. , Umar, Y. A. , Ponchang, A. W. , & Daniel, A. (2016). Molecular characterization and determination of bioremediation potentials of some bacteria isolated from spent oil contaminated soil mechanic workshops in Kaduna Metropolis. World Applied Sciences Journal, 34, 750–759. [Google Scholar]
- Eckert, B. , Weber, O. B. , Kirchhof, G. , Halbritter, A. , Stoffels, M. , & Hartmann, A. (2001). Azospirillum doebereinerae sp. nov., a nitrogen‐fixing bacterium associated with the C4‐grass Miscanthus. International Journal of Systematic and Evolutionary Microbiology, 51, 17–26. [DOI] [PubMed] [Google Scholar]
- Faruque, S. M. , Islam, M. J. , Ahmad, Q. S. , Faruque, A. S. G. , Sack, D. A. , Nair, G. B. , & Mekalanos, J. J. (2005). Self‐limiting nature of seasonal cholera epidemics: Role of host‐mediated amplification of phage. Proceedings of the National Academy of Sciences, 102, 6119–6124. 10.1073/pnas.0502069102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauquet, C. M. , Mayo, M. A. , Maniloff, J. , Desselberger, U. , & Ball, L. A. (2005). Virus Taxonomy : VIIIth Report of the International Committee on Taxonomy of Viruses. London, UK: Elsevier/Academic Press. [Google Scholar]
- Feldgarden, M. , & Riley, M. A. (1998). High levels of colicin resistance in Escherichia coli . Evolution, 52, 1270–1276. [DOI] [PubMed] [Google Scholar]
- Felici, C. , Vettori, L. , Giraldi, E. , Forino, L. M. C. , Toffanin, A. , Tagliasacchi, A. M. , & Nuti, M. (2008). Single and co‐inoculation of Bacillus subtilis and Azospirillum brasilense on Lycopersicon esculentum: Effects on plant growth and rhizosphere microbial community. Applied Soil Ecology, 40, 260–270. 10.1016/j.apsoil.2008.05.002 [DOI] [Google Scholar]
- Fetherston, J. D. , Lillard, J. W. , & Perry, R. D. (1995). Analysis of the pesticin receptor from Yersinia pestis: Role in iron-deficient growth and possible regulation by its siderophore. Journal of Bacteriology, 177(7), 1824–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiberto, D. M. , & Gaunt, J. L. (2013). Practicality of biochar additions to enhance soil and crop productivity. Agriculture, 3(4), 715–725. 10.3390/agriculture3040715 [DOI] [Google Scholar]
- Flemming, H.‐C. , Wingender, J. , Kjelleberg, S. , Steinberg, P. D. , Rice, S. A. , & Szewzyk, U. (2016). Biofilms: An emergent form of bacterial life. Nature Reviews Microbiology, 14, 563–575. 10.1038/nrmicro.2016.94 [DOI] [PubMed] [Google Scholar]
- Fondi, M. , Rizzi, E. , Emiliani, G. , Orlandini, V. , Berna, L. , Papaleo, M. C. , … Fani, R. (2013). The genome sequence of the hydrocarbon‐degrading Acinetobacter venetianus VE‐C3. Research in Microbiology, 164, 439–449. 10.1016/j.resmic.2013.03.003 [DOI] [PubMed] [Google Scholar]
- Fredrickson, J. K. , Romine, M. F. , Beliaev, A. S. , Auchtung, J. M. , Driscoll, M. E. , Gardner, T. S. , … Tiedje, J. M. (2008). Towards environmental systems biology of Shewanella . Nature Reviews Microbiology, 6, 592–603. 10.1038/nrmicro1947 [DOI] [PubMed] [Google Scholar]
- Fujio, Y. , & Kume, S. (1991). Isolation and identification of thermophilic bacteria from sewage sludge compost. Journal of Fermentation and Bioengineering, 72, 334–337. 10.1016/0922-338X(91)90082-R [DOI] [Google Scholar]
- Fukushima, H. , Shimizu, S. , & Inatsu, Y. (2011). Yersinia enterocolitica and Yersinia pseudotuberculosis detection in foods. Journal of Pathogens, 2011(735308), 1–9. 10.4061/2011/735308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagic, D. , Ciric, M. , Wen, W. X. , Ng, F. , & Rakonjac, J. (2016). Exploring the secretomes of microbes and microbial communities using filamentous phage display. Frontiers in Microbiology, 7, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George, P. , Gupta, A. , Gopal, M. , Thomas, L. , & Thomas, G. V. (2013). Multifarious beneficial traits and plant growth promoting potential of Serratia marcescens KiSII and Enterobacter sp. RNF 267 isolated from the rhizosphere of coconut palms (Cocos nucifera L.). World Journal of Microbiology and Biotechnology, 29, 109–117. [DOI] [PubMed] [Google Scholar]
- Gihring, T. M. , & Banfield, J. F. (2001). Arsenite oxidation and arsenate respiration by a new Thermus isolate. FEMS Microbiology Letters, 204, 335–340. 10.1111/j.1574-6968.2001.tb10907.x [DOI] [PubMed] [Google Scholar]
- Goldman, E. R. , Hayhurst, A. , Lingerfelt, B. M. , Iverson, B. L. , Georgiou, G. , & Anderson, G. P. (2003). 2,4,6‐Trinitrotoluene detection using recombinant antibodies. Journal of Environmental Monitoring, 5, 380–383. 10.1039/b302012f [DOI] [PubMed] [Google Scholar]
- Goldman, E. R. , Pazirandeh, M. P. , Charles, P. T. , Balighian, E. D. , & Anderson, G. P. (2002). Selection of phage displayed peptides for the detection of 2,4,6‐trinitrotoluene in seawater. Analytica Chimica Acta, 457, 13–19. 10.1016/S0003-2670(01)01246-6 [DOI] [Google Scholar]
- Goldstein, R. M. , Mallory, L. M. , & Alexander, M. (1985). Reasons for possible failure of inoculation to enhance biodegradation. Applied and Environmental Microbiology, 50, 977–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gontia‐Mishra, I. , Sapre, S. , Sharma, A. , & Tiwari, S. (2016). Amelioration of drought tolerance in wheat by the interaction of plant growth‐promoting rhizobacteria. Plant Biology, 18, 992–1000. 10.1111/plb.12505 [DOI] [PubMed] [Google Scholar]
- Goodridge, L. D. (2010). Designing phage therapeutics. Current Pharmaceutical Biotechnology, 11, 15–27. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan, S. , Sathya, A. , Vijayabharathi, R. , Varshney, R. K. , Gowda, C. L. L. , & Krishnamurthy, L. (2015). Plant growth promoting rhizobia: Challenges and opportunities. 3 Biotech, 5(4), 355–377. 10.1007/s13205-014-0241-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, A. , Saxena, A. K. , Gopal, M. , & Tilak, K. V. B. R. (2003). Effects of co‐inoculation of plant growth promoting rhizobacteria and Bradyrhizobium sp. (Vigna) on growth and yield of green gram [Vigna radiata (L.) Wilczek]. Tropical Agriculture‐London Then Trinidad, 80, 28–35. [Google Scholar]
- Gutiérrez, T. , Shimmield, T. , Haidon, C. , Black, K. , & Green, H. D. (2008). Emulsifying and metal ion binding activity of a glycoprotein exopolymer produced by Pseudoalteromonas sp. strain TG12. Applied and Environmental Microbiology, 74, 4867–4876. 10.1128/AEM.00316-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagens, S. , & Bläsi, U. (2003). Genetically modified filamentous phage as bactericidal agents: A pilot study. Letters in Applied Microbiology, 37, 318–323. 10.1046/j.1472-765X.2003.01400.x [DOI] [PubMed] [Google Scholar]
- Hagens, S. , Habel, A. , von Ahsen, U. , von Gabain, A. , & Blasi, U. (2004). Therapy of experimental Pseudomonas infections with a nonreplicating genetically modified phage. Antimicrobial Agents and Chemotherapy, 48(10), 3817–3822. 10.1128/AAC.48.10.3817-3822.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, J. P. , Harrison, E. , Lilley, A. K. , Paterson, S. , Spiers, A. J. , & Brockhurst, M. A. (2015). Environmentally co‐occurring mercury resistance plasmids are genetically and phenotypically diverse and confer variable context‐dependent fitness effects. Environmental Microbiology, 17, 5008–5022. 10.1111/1462-2920.12901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, J. P. , Wood, A. J. , Harrison, E. , & Brockhurst, M. A. (2016). Source–sink plasmid transfer dynamics maintain gene mobility in soil bacterial communities. Proceedings of the National Academy of Sciences, 113, 8260–8265. 10.1073/pnas.1600974113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halme, P. , Allen, K. A. , Auniņš, A. , Bradshaw, R. H. , Brūmelis, G. , Čada, V. , … Ikauniece, S. (2013). Challenges of ecological restoration: Lessons from forests in northern Europe. Biological Conservation, 167, 248–256. 10.1016/j.biocon.2013.08.029 [DOI] [Google Scholar]
- Harper, D. R. , & Kutter, E. (2008). Bacteriophage: Therapeutic uses In Chichester U. K. (Ed.), The Encyclopedia of Life Sciences (pp. 1–7). John Wiley & Sons. [Google Scholar]
- Harrison, E. , & Brockhurst, M. A. (2012). Plasmid‐mediated horizontal gene transfer is a coevolutionary process. Trends in Microbiology, 20, 262–267. 10.1016/j.tim.2012.04.003 [DOI] [PubMed] [Google Scholar]
- Harvey, R. W. (1993). Fate and transport of bacteria injected into aquifers. Current Opinion in Biotechnology, 4, 312–317. 10.1016/0958-1669(93)90101-2 [DOI] [Google Scholar]
- Hayward, A. C. (1991). Biology and Epidemiology of Bacterial Wilt Caused by Pseudomonas Solanacearum. Annual Review of Phytopathology, 29, 65–87. 10.1146/annurev.py.29.090191.000433 [DOI] [PubMed] [Google Scholar]
- Heneghan, L. , Miller, S. P. , Baer, S. , Callaham, M. A. , Montgomery, J. , Pavao‐Zuckerman, M. , … Richardson, S. (2008). Integrating soil ecological knowledge into restoration management. Restoration Ecology, 16, 608–617. 10.1111/j.1526-100X.2008.00477.x [DOI] [Google Scholar]
- Hengzhuang, W. , Wu, H. , Ciofu, O. , Song, Z. , & Høiby, N. (2011). Pharmacokinetics/pharmacodynamics of colistin and imipenem on mucoid and nonmucoid Pseudomonas aeruginosa biofilms. Antimicrobial Agents and Chemotherapy, 55, 4469–4474. 10.1128/AAC.00126-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengzhuang, W. , Wu, H. , Ciofu, O. , Song, Z. , & Høiby, N. (2012). In vivo pharmacokinetics/pharmacodynamics of colistin and imipenem in Pseudomonas aeruginosa biofilm infection. Antimicrobial Agents and Chemotherapy, 56, 2683–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry, K. A. , Arbabi‐Ghahroudi, M. , & Scott, J. K. (2015). Beyond phage display: Non‐traditional applications of the filamentous bacteriophage as a vaccine carrier, therapeutic biologic, and bioconjugation scaffold. Frontiers in Microbiology, 6, 755 10.3389/fmicb.2015.00755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbing, M. E. , Fuqua, C. , Parsek, M. R. , & Peterson, S. B. (2010). Bacteria competition: Surviving and thriving in the microbial jungle. Nature Reviews Microbiology, 8, 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilderbrand, S. A. , Kelly, K. A. , Niedre, M. , & Weissleder, R. (2008). Near infrared fluorescence‐based bacteriophage particles for ratiometric pH imaging. Bioconjugate Chemistry, 19, 1635–1639. 10.1021/bc800188p [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høiby, N. , Bjarnsholt, T. , Givskov, M. , Molin, S. , & Ciofu, O. (2010). Antibiotic resistance of bacterial biofilms. International Journal of Antimicrobial Agents, 35, 322–332. 10.1016/j.ijantimicag.2009.12.011 [DOI] [PubMed] [Google Scholar]
- Høiby, N. , Ciofu, O. , Johansen, H. K. , Song, Z. J. , Moser, C. , Jensen, P. Ø. , … Bjarnsholt, T. (2011). The clinical impact of bacterial biofilms. International Journal of Oral Science, 3, 55–65. 10.4248/IJOS11026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins, D. L. , & Purcell, A. H. (2002). Xylella fastidiosa: Cause of Pierce�s Disease of Grapevine and Other Emergent Diseases. Plant Disease, 86(10), 1056–1066. 10.1094/PDIS.2002.86.10.1056 [DOI] [PubMed] [Google Scholar]
- Hosseinidoust, Z. , Van De Ven, T. G. M. , & Tufenkji, N. (2013). Evolution of Pseudomonas aeruginosavirulence as a result of phage predation. Applied and Environmental Microbiology, 79, 6110–6116. 10.1128/AEM.01421-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, S. , Yang, H. , Johnson, M. , Chen, I. , Petrenko, V. A. , & Chin, B. A. (2007). Simultaneous Detection of Salmonella typhimurium and Bacillus anthracis spores using phage‐based magnetoelastic biosensors. Nanotech, 2, 515–518. [DOI] [PubMed] [Google Scholar]
- Huber, K. E. , & Waldor, M. K. (2002). Filamentous phage integration requires the host recombinases XerC and XerD. Nature, 417, 656–659. 10.1038/nature00782 [DOI] [PubMed] [Google Scholar]
- Hyman, P. , & Abedon, S. T. (2010). Bacteriophage host range and bacterial resistance. Advances in Applied Microbiology, 70, 217–248. [DOI] [PubMed] [Google Scholar]
- Ikeura, H. , Kawasaki, Y. H. , Kaimi, E. , Nishiwaki, J. , Noborio, K. , & Tamaki, M. (2016). Screening of plants for phytoremediation of oil‐contaminated soil. International Journal of Phytoremediation, 18, 460–466. 10.1080/15226514.2015.1115957 [DOI] [PubMed] [Google Scholar]
- Iyer, R. , Darby, V. , & Holland, I. B. (1976). Changes in membrane proteins of Escherichia coli K12 mediated by bacteriophage IKe‐specific plasmids. Biochimica Et Biophysica Acta, 453, 311–318. 10.1016/0005-2795(76)90126-4 [DOI] [PubMed] [Google Scholar]
- Jian, H. , Xiao, X. , & Wang, F. (2013). Role of filamentous phage SW1in regulating the lateral flagella of Shewanella piezotolerans strain WP3 at low temperatures. Applied and Environmental Microbiology, 79, 7101–7109. 10.1128/AEM.01675-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly, N. , Engl, C. , Jovanovic, G. , Huvet, M. , Toni, T. , Sheng, X. , … Buck, M. (2010). Managing membrane stress: The phage shock protein (Psp). response, from molecular mechanisms to physiology. FEMS Microbiology Reviews, 34, 797–827. 10.1111/j.1574-6976.2010.00240.x [DOI] [PubMed] [Google Scholar]
- Joseph, B. , Schwarz, R. F. , Linke, B. , Blom, J. , Becker, A. , Claus, H. , … Schoen, C. (2011). Virulence evolution of the human pathogen Neisseria meningitidis by recombination in the core and accessory genome. PLoS ONE, 6, e18441 10.1371/journal.pone.0018441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabra, A. N. , Khandare, R. V. , Kurade, M. B. , & Govindwar, S. P. (2011). Phytoremediation of a sulphonated azo dye Green HE4B by Glandularia pulchella (Sweet) Tronc. (Moss Verbena). Environmental Science and Pollution Research, 18, 1360–1373. 10.1007/s11356-011-0491-7 [DOI] [PubMed] [Google Scholar]
- Kailasan, N. S. , & Vamanrao, V. B. (2015). Isolation and characterization of Ralstonia pickettii ‐ A novel phosphate solubilizing bacterium from pomegranate rhizosphere from Western India. International Journal of Life Sciences Biotechnology and Pharma Research, 4, 1–9. [Google Scholar]
- Kamiunten, H. , & Wakimoto, S. (1981). Effect of the infection with filamentous phage Xf2 on the properties of Xanthomonas campestris pv. Oryzae . Annals of the Phytopathological Society of Japan, 47, 627–636. 10.3186/jjphytopath.47.627 [DOI] [Google Scholar]
- Kane, A. L. , Al‐Shayeb, B. , Holec, P. V. , Rajan, S. , Le Mieux, N. E. , Heinsch, S. C. , … Gralnick, J. A. (2016). Toward bioremediation of methylmercury using silica encapsulated Escherichia coli harboring the mer operon. PLoS ONE, 11, e0147036 10.1371/journal.pone.0147036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson, F. , Malmborg‐Hager, A. C. , Albrekt, A. S. , & Borrebaeck, C. A. (2005). Genome‐wide comparison of phage M13‐infected vs. uninfected Escherichia coli . Canadian Journal of Microbiology, 51, 29–35. [DOI] [PubMed] [Google Scholar]
- Kashefi, K. , & Lovley, D. R. (2000). Reduction of Fe(Ill), Mn(IV), and toxic metals at 100°C by Pyrobaculum islandicum . Applied and Environmental Microbiology, 66, 1050–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathiresan, K. , & Selvam, M. M. (2006). Evaluation of beneficial bacteria from mangrove soil. Botanica Marina, 49, 86–88. 10.1515/BOT.2006.011 [DOI] [Google Scholar]
- Kauffman, K. M. , Hussain, F. A. , Yang, J. , Arevalo, P. , Brown, J. M. , Chang, W. K. , … Polz, M. F. (2018). A major lineage of non‐tailed dsDNA viruses as unrecognized killers of marine bacteria. Nature, 554, 118–122. 10.1038/nature25474 [DOI] [PubMed] [Google Scholar]
- Kaur, J. , Pandove, G. , & Gangwar, M. (2018). Mitigating the impact of climate change by use of microbial inoculants. The Pharma Innovation Journal, 7(1), 279–288. [Google Scholar]
- Kehoe, J. W. , & Kay, B. K. (2005). Filamentous phage display in the new millennium. Chemical Reviews, 105, 4056–4072. 10.1021/cr000261r [DOI] [PubMed] [Google Scholar]
- Kieft, T. L. , Fredrickson, J. K. , Onstott, T. C. , Gorby, Y. A. , Kostandarithes, H. M. , Bailey, T. J. , … Gray, M. S. (1999). Dissimilatory reduction of Fe(III) and other electron acceptors by a Thermus isolate. Applied and Environmental Microbiology, 65, 1214–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimsey, H. H. , & Waldor, M. K. (1998). CTXf immunity: Application in the development of cholera vaccines. Proceedings of the National Academy of Sciences USA, 95, 7035–7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A. M., Lefkowitz E., Adams M. J., & Carstens E. B. (2011). Virus taxonomy: Ninth report of the International Committee on Taxonomy of Viruses (pp. 375–383). Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- Kivelä, H. M. , Madonna, S. , Krupovìč, M. , Tutino, M. L. , & Bamford, J. K. (2008). Genetics for Pseudoalteromonas provides tools to manipulate marine bacterial virus PM2. Journal of Bacteriology, 190, 1298–1307. 10.1128/JB.01639-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi, D. , & Palumbo, J. (2000). Bacterial endophytes and their effects on plants and uses in agriculture Microbial endophytes (pp. 199–233). New York, NY: Dekker. [Google Scholar]
- Koskella, B. , & Brockhurst, M. A. (2014). Bacteria–phage coevolution as a driver of ecological and evolutionary processes in microbial communities. FEMS Microbiology Reviews, 38, 916–931. 10.1111/1574-6976.12072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupovic, M. , & Forterre, P. (2015). Single‐stranded DNA viruses employ a variety of mechanisms for integration into host genomes. Annals of the New York Academy of Sciences, 1341, 41–53. [DOI] [PubMed] [Google Scholar]
- Kuo, M. Y. , Yang, M. K. , Chen, W. P. , & Kuo, T. T. (2000). High‐frequency interconversion of turbid and clear plaque strains of bacteriophage f1 and associated host cell death. Canadian Journal of Microbiology, 46, 841–847. 10.1139/w00-068 [DOI] [PubMed] [Google Scholar]
- Kuo, T.‐T. , Tan, M.‐S. , Su, M.‐T. , & Yang, M. K. (1991). Complete nucleotide sequence of filamentous phage Cf1c from Xanthomonas campestris pv. citri . Nucleic Acids Research, 19, 2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuykendall, L. D. , & Hashem, F. M. (1998). Enhancement of biological nitrogen fixation using strain‐specific bacterial viruses for biological control of ineffective Bradyrhizobium or Rhizobium In Boland G., & Bolis L. (Eds.), Plant‐Microbe Interactions and Biological Control (pp. 297–308). New York, NY: Marcel Dekker Inc. [Google Scholar]
- Lahav, N. (1962). Adsorption of sodium bentonite particles on Bacillus subtilis . Plant and Soil, 17, 191–208. 10.1007/BF01376224 [DOI] [Google Scholar]
- Lee, J. H. , Domaille, D. W. , & Cha, J. N. (2012). Amplified Protein Detection and Identification through DNA-Conjugated M13 Bacteriophage. ACS Nano, 6(6), 5621–5626. 10.1021/nn301565e [DOI] [PubMed] [Google Scholar]
- Lee, J. W. , Song, J. , Hwang, M. P. , & Lee, K. H. (2013). Nanoscale bacteriophage biosensors beyond phage display. International Journal of Nanomedicine, 8, 3917–3925. 10.2147/IJN.S51894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei, K. , Pan, H. , & Lin, C. (2016). A landscape approach towards ecological restoration and sustainable development of mining areas. Ecological Engineering, 90, 320–325. 10.1016/j.ecoleng.2016.01.080 [DOI] [Google Scholar]
- Lerner, R. A. , Benkovic, S. J. , & Schultz, P. G. (1991). At the crossroads of chemistry and immunology: Catalytic antibodies. Science, 252, 659–667. 10.1126/science.2024118 [DOI] [PubMed] [Google Scholar]
- Lerner, T. J. , & Model, P. (1981). The “steady state” of coliphage f1: DNA synthesis late in infection. Virology, 115, 282–294. 10.1016/0042-6822(81)90111-2 [DOI] [PubMed] [Google Scholar]
- Li, J. , Jin, Z. , & Yu, B. (2010). Isolation and characterization of aniline degradation slightly halophilic bacterium, Erwinia sp. Strain HSA 6. Microbiological Research, 165, 418–426. [DOI] [PubMed] [Google Scholar]
- Li, M. , Kotetishvili, M. , Chen, Y. , & Sozhamannan, S. (2003). Comparative genomic analyses of the vibrio pathogenicity island and cholera toxin prophage regions in nonepidemic serogroup strains of Vibrio cholerae. Applied Environmental Microbiology, 69, 1728–1738. 10.1128/AEM.69.3.1728-1738.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S. , Li, Y. , Chen, H. , Horikawa, S. , Shen, W. , Simonian, A. , & Chin, B. A. (2010). Direct detection of Salmonella typhimurium on fresh produce using phage‐based magnetoelastic biosensors. Biosensors and Bioelectronics, 26, 1313–1319. [DOI] [PubMed] [Google Scholar]
- Li, S. , Johnson, M. L. , Li, Y. , Chen, H. , Banerjee, I. , Chen, I.‐H. , … Chin, B. A. (2010). Micro‐fabricated wireless biosensors for the detection of S. typhimurium in liquids. Proceedings of SPIE, 10.1117/12.849474. [DOI] [Google Scholar]
- Lin, A. , Jimenez, J. , Derr, J. , Vera, P. , Manapat, M. L. , Esvelt, K. M. , … Chen, I. A. (2011). Inhibition of bacterial conjugation by phage M13 and its protein g3p: Quantitative analysis and model. PLoS ONE, 6, e19991 10.1371/journal.pone.0019991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, N. T. , Liu, T. J. , Lee, T. C. , You, B. Y. , Yang, M. H. , Wen, F. S. , & Tseng, Y. H. (1999). The adsorption protein genes of Xanthomonas campestris filamentous phages determining host specificity. Journal of Bacteriology, 181(8), 2465–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, N.-T. , You, B.-Y. , Huang, C.-Y. , Kuo, C.-W. , Wen, F.-S. , Yang, J.-S. , & Tseng, Y.-H. (1994). Characterization of two novel filamentous phages of Xanthomonas. Journal of General Virology, 75(9), 2543–2547. 10.1099/0022-1317-75-9-2543 [DOI] [PubMed] [Google Scholar]
- Liu, J. , Xin, X. , & Zhou, Q. (2017). Phytoremediation of contaminated soils using ornamental plants. Environmental Reviews, e‐12, [DOI] [Google Scholar]
- Liu, W. , Li, L. , Khan, M. A. , & Zhu, F. (2012). Popular molecular markers in bacteria. Molecular Genetics, Microbiology and Virology, 27, 103–107. 10.3103/S0891416812030056 [DOI] [PubMed] [Google Scholar]
- Liu, W. , Røder, H. L. , Madsen, J. S. , Bjarnsholt, T. , Sørensen, S. J. , & Burmølle, M. (2016). Interspecific bacterial interactions are reflected in multispecies biofilm spatial organization. Frontiers in Microbiology, 7, 1366 10.3389/fmicb.2016.01366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loc‐Carrillo, C. , & Abedon, S. T. (2011). Pros and cons of phage therapy. Bacteriophage, 1, 111–114. 10.4161/bact.1.2.14590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohara, K. , Liyama, R. , Nakamura, K. , Silver, S. , Sakai, M. , Takeshita, M. , & Furukawa, K. (2001). The mer operon of a mercury‐resistant Pseudoalteromonas haloplanktis strain isolated from Minamata Bay, Japan. Applied Microbiology and Biotechnology, 56, 736–741. [DOI] [PubMed] [Google Scholar]
- Lu, T. K. , & Collins, J. J. (2009). Engineered bacteriophage targeting gene networks as adjuvants for antibiotic therapy. Proceedings of the National Academy of Sciences, 106, 4629–4634. 10.1073/pnas.0800442106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, T. K. , & Koeris, M. S. (2011). The next generation of bacteriophage therapy. Current Opinion in Microbiology, 14, 524–531. 10.1016/j.mib.2011.07.028 [DOI] [PubMed] [Google Scholar]
- Lynch, J. M. , Benedetti, A. , Insam, H. , Nuti, M. P. , Smalla, K. , Torsvik, V. , & Nannipieri, P. (2004). Microbial diversity in soil: Ecological theories, the contribution of molecular techniques and the impact of transgenic plants and transgenic microorganisms. Biology and Fertility of Soils, 40, 363–385. 10.1007/s00374-004-0784-9 [DOI] [Google Scholar]
- Madera‐Parra, C. A. , Peña‐Salamanca, E. J. , Pena, M. R. , Rousseau, D. P. L. , & Lens, P. N. L. (2015). Phytoremediation of landfill leachate with Colocasia esculenta, Gynerum sagittatum and Heliconia psittacorum in constructed wetlands. International Journal of Phytoremediation, 17, 16–24. [DOI] [PubMed] [Google Scholar]
- Madhaiyan, M. , Poonguzhali, S. , Lee, H. S. , Hari, K. , Sundaram, S. P. , & Sa, T. M. (2005). Pinkpigmented facultative methylotrophic bacteria accelerate germination, growth and yield of sugarcane clone Co86032 (Saccharum officinarum L.). Biology and Fertility of Soils, 41, 350–358. 10.1007/s00374-005-0838-7 [DOI] [Google Scholar]
- Mahdieh, M. , Yazdani, M. , & Mahdieh, S. (2013). The high potential of Pelargonium roseumplant for phytoremediation of heavy metals. Environmental Monitoring and Assessment, 185, 7877–7881. 10.1007/s10661-013-3141-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai‐Prochnow, A. , Hui, J. G. , Kjelleberg, S. , Rakonjac, J. , McDougald, D. , & Rice, S. A. (2015). Big things in small packages: The genetics of filamentous phage and effects on fitness of their host. FEMS Microbiology Reviews, 39, 465–487. 10.1093/femsre/fuu007 [DOI] [PubMed] [Google Scholar]
- Majeed, H. , Gillor, O. , Kerr, B. , & Riley, M. A. (2011). Competitive interactions in Escherichia coli populations: The role of bacteriocins. The ISME Journal, 5, 7111 10.1038/ismej.2010.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra, S. , Mishra, V. , Karmakar, S. , & Sharma, R. S. (2017). Environmental predictors of indole acetic acid producing rhizobacteria at fly ash dumps: Nature‐based solution for sustainable restoration. Frontiers in Environmental Science, 5, 59 10.3389/fenvs.2017.00059. [DOI] [Google Scholar]
- Malusá, E. , Sas‐Paszt, L. , & Ciesielska, J. (2012). Technologies for beneficial microorganisms inocula used as biofertilizers. Scientific World Journal, 2012, 491206 10.1100/2012/491206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniloff, J. , Cadden, S. P. , & Putzrath, R. M. (1981). Maturation of an enveloped budding phage: Mycoplasma virus L2 In DuBow M. S. (Ed.), Bacteriophage assembly (pp. 503–513). New York, NY: AR Liss Inc. [PubMed] [Google Scholar]
- Mansfield, J. , Genin, S. , Magori, S. , Citovsky, V. , Sriariyanum, M. , Ronald, P. , Toth, I. A. N. (2012). Top 10 plant pathogenic bacteria in molecular plant pathology. Molecular plant pathology, 13(6), 614–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, J. Y. , Belcher, A. M. , & Van Vliet, K. J. (2010). Genetically engineered phage fibers and coatings for antibacterial applications. Advanced Functional Materials, 20, 209–214. 10.1002/adfm.200900782 [DOI] [Google Scholar]
- Marcelletti, S. , & Scortichini, M. (2014). Definition of Plant-Pathogenic Pseudomonas Genomospecies of the Pseudomonas syringae Complex Through Multiple Comparative Approaches. Phytopathology, 104(12), 1274–1282. 10.1094/PHYTO-12-13-0344-R [DOI] [PubMed] [Google Scholar]
- Marques, A. P. , Moreira, H. , Franco, A. R. , Rangel, O. A. , & Castro, P. M. (2013). Inoculating Helianthus annus(sun flower) groconin zinc and cadmium contaminated soil with PGPR effect on phytoremediation strategies. Chemosphere, 92, 74–78. [DOI] [PubMed] [Google Scholar]
- Marteinsson, V. T. , Birrien, J. L. , Raguenes, G. , da Costa, M. S. , & Prieur, D. (1999). Isolation and characterization of Thermus thermophilus Gy1211 from a deep‐sea hydrothermal vent. Extremophiles, 3, 247–251. 10.1007/s007920050123 [DOI] [PubMed] [Google Scholar]
- Martin, D. M. (2017). Ecological restoration should be redefined for the twenty‐first century. Restoration Ecology, 25, 668–673. 10.1111/rec.12554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez, E. , & Campos‐Gómez, J. (2016). Pf filamentous phage requires UvrD for replication in Pseudomonas Aeruginosa . Msphere, 1, e00104–e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin, D. A. (1998). Filamentous phage structure, infection and assembly. Current Opinion in Structural Biology, 8, 150–158. 10.1016/S0959-440X(98)80032-8 [DOI] [PubMed] [Google Scholar]
- Meaden, S. , & Koskella, B. (2013). Exploring the risks of phage application in the environment. Frontiers in Microbiology, 4, 358 10.3389/fmicb.2013.00358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher, R. J. , Apitz, S. E. , & Hemmingsen, B. B. (2002). Impact of irradiation and polycyclic aromatic hydrocarbon spiking on microbial populations in marine sediment for future aging and biodegradability studies. Applied and Environmental Microbiology, 68, 2858–2868. 10.1128/AEM.68.6.2858-2868.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méric, G. , Kemsley, E. K. , Falush, D. , Saggers, E. J. , & Lucchini, S. (2013). Phylogenetic distribution of traits associated with plant colonization in Escherichia coli . Environmental Microbiology, 15, 487–501. [DOI] [PubMed] [Google Scholar]
- Mesa, J. , Mateos‐Naranjo, E. , Caviedes, M. A. , Redondo‐Gómez, S. , Pajuelo, E. , & Rodríguez‐Llorente, I. D. (2015). Endophytic cultivable bacteria of the metal bioaccumulator Spartina maritima improve plant growth but not metal uptake in polluted marshes soils. Frontiers in Microbiology, 6, 1450 10.3389/fmicb.2015.01450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, J. , Schmidt, A. , Michalke, K. , & Hensel, R. (2007). Volatilization of metals and metalloids by the microbial population of an alluvial soil. Systematic and Applied Microbiology, 30, 229–238. [DOI] [PubMed] [Google Scholar]
- Michalke, K. , Wickenheiser, E. B. , Mehring, M. , Hirner, A. V. , & Hensel, R. (2000). Production of volatile derivatives of metal(loid)s by microflora involved in anaerobic digestion of sewage sludge. Applied and Environmental Microbiology, 66, 2791–2796. 10.1128/AEM.66.7.2791-2796.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millennium Ecosystem Assessment (2005). Ecosystems and human well‐being: Biodiversity synthesis. Washington, DC: World Resources Institute. [Google Scholar]
- Mohmmed, A. , Sharma, R. S. , Ali, S. , & Babu, C. R. (2001). Molecular diversity of the plasmid genotypes among Rhizobium gene pools of sesbanias from different habitats of a semi‐arid region (Delhi). FEMS Microbiology Letters, 205, 171–178. 10.1111/j.1574-6968.2001.tb10943.x [DOI] [PubMed] [Google Scholar]
- Moreira, H. , Marques, A. P. , Franco, A. R. , Rangel, A. O. , & Castro, P. M. (2014). Phytomanagement of Cd contaminated soils using maize (Zea mays L.) assisted by plant growth promoting rhizobacteria. Environmental Science and Pollution Research, 21, 9742–9753. [DOI] [PubMed] [Google Scholar]
- Mosa, K. A. , Saadoun, I. , Kumar, K. , Helmy, M. , & Dhankher, O. P. (2016). Potential biotechnological strategies for the cleanup of heavy metals and metalloids. Frontiers in Plant Science, 7, 303 10.3389/fpls.2016.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouget, J.‐L. , Dakhama, A. , Lavoie, M. C. , & de la Noüe, J. (1995). Algal growth enhancement by bacteria: Is consumption of photosynthetic oxygen involved? FEMS Microbiology Ecology, 18, 35–43. 10.1111/j.1574-6941.1995.tb00159.x [DOI] [Google Scholar]
- Mrozik, A. , Miga, S. , & Piotrowska‐Seget, Z. (2011). Enhancement of phenol degradation by soil bioaugmentation with Pseudomonas sp. JS150. Journal of Applied Microbiology, 111, 1357–1370. 10.1111/j.1365-2672.2011.05140.x [DOI] [PubMed] [Google Scholar]
- Mukred, A. M. , Hamid, A. A. , Hamzah, A. , & Yusoff, W. M. W. (2008). Enhancement of biodegradation of crude petroleum‐oil in contaminated water by the addition of nitrogen sources. Pakistan Journal of Biological Sciences, 11, 2122–2127. 10.3923/pjbs.2008.2122.2127 [DOI] [PubMed] [Google Scholar]
- Murugaiyan, S. , Bae, J. Y. , Wu, J. , Lee, S. D. , Um, H. Y. , Choi, H. K. , … Lee, S.‐W. (2010). Characterization of filamentous bacteriophage PE226 infecting Ralstonia solanacearum strains. Journal of Applied Microbiology, 110, 296–303. 10.1111/j.1365-2672.2010.04882.x [DOI] [PubMed] [Google Scholar]
- Nambudripad, R. , Stark, W. , & Makowski, L. (1991). Neutron diffraction studies of the structure of filamentous bacteriophage Pf1. Demonstration that the coat protein consists of a pair of alpha‐helices with an intervening, non‐helical surface loop. Journal of Molecular Biology, 220, 359–379. 10.1016/0022-2836(91)90019-3 [DOI] [PubMed] [Google Scholar]
- Nanduri, V. , Bhunia, A. K. , Tu, S. I. , Paoli, G. C. , & Brewster, J. D. (2007). SPR biosensor for the detection of L‐monocytogenes using phage‐displayed antibody. Biosensors and Bioelectronics, 23, 248–252. 10.1016/j.bios.2007.04.007 [DOI] [PubMed] [Google Scholar]
- Nautiyal, P. C. , & Shono, M. (2010). Analysis of the role of mitochondrial and endoplasmic reticulum localized small heat shock proteins in tomato (Lycopersicon esculentum Mill.) plant. Biologia Plantarum, 54, 715–719. 10.1007/s10535-010-0127-7 [DOI] [Google Scholar]
- Neufeld, T. , Mittelman, A. S. , Buchner, V. , & Rishpon, J. (2005). Electrochemical phagemid assay for the specific detection of bacteria using Escherichia coli TG‐1 and the M13KO7 phagemid in a model system. Analytical Chemistry, 77, 652–657. [DOI] [PubMed] [Google Scholar]
- NFTA (1986). Actinorhizal Trees Useful in Cool to Cold Regions In Roshetko J. (Ed.), NFTA (Nitrogen Fixing Tree Association) highlights: a quick guide to useful nitrogen-fixing trees from around the world. Morrilton, AR: Winrock International NFTA. [Google Scholar]
- Nguyen, D. T. , Nguyen, B. M. , Tran, H. H. , Ngo, T. C. , Le, T. H. , Nguyen, H. T. , … Ehara, M. (2008). Filamentous vibriophage fs2 encoding the rstC gene integrates into the same chromosomal region as the CTX phage. FEMS Microbiology Letters, 284, 225–230. [DOI] [PubMed] [Google Scholar]
- Novotny, C. , Knight, W. S. , & Brinton, C. C. Jr (1968). Inhibition of bacterial conjugation by ribonucleic acid and deoxyribonucleic acid male‐specific bacteriophages. Journal of Bacteriology, 95, 314–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen, E. , Sykora, J. , Sorokulova, I. , Neely, W. , Petrenko, V. , Chen, I.‐H. , … Vodyanoy, V. (2007). Phage fusion proteins as bioselective receptors for piezoelectric sensors. ECS Transactions, 2, 9–25. [Google Scholar]
- Opella, S. J. , Zeri, A. C. , & Park, S. H. (2008). Structure, dynamics, and assembly of filamentous bacteriophages by nuclear magnetic resonance spectroscopy. Annual Review of Physical Chemistry, 59, 635–657. 10.1146/annurev.physchem.58.032806.104640 [DOI] [PubMed] [Google Scholar]
- Opperman, D. J. , & van Heerden, E. (2008). A membrane‐associated protein with Cr(VI)‐reducing activity from Thermus scotoductus SA‐01. FEMS Microbiology Letters, 280, 210–218. 10.1111/j.1574-6968.2007.01063.x [DOI] [PubMed] [Google Scholar]
- Ou, J. T. (1973). Inhibition of formation of Escherichia coli mating pairs by f1 and MS2 bacteriophages as determined with a Coulter counter. Journal of Bacteriology, 114, 1108–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant, A. , Anbumani, D. , Bag, S. , Mehta, O. , Kumar, P. , Saxena, S. , … Das, B. (2015). Effect of LexA on Chromosomal Integration of CTXϕ in Vibrio cholerae . Journal of Bacteriology, 198, 268–275. 10.1128/JB.00674-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters, N. , Guidot, A. , Vailleau, F. , & Valls, M. (2013). Ralstonia solanacearum, a widespread bacterial plant pathogen in the post-genomic era. Molecular Plant Pathology, 14(7), 651–662. 10.1111/mpp.12038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perring, M. P. , Standish, R. J. , Price, J. N. , Craig, M. D. , Erickson, T. E. , Ruthrof, K. X. , … Hobbs, R. J. (2015). Advances in restoration ecology: Rising to the challenges of the coming decades. Ecosphere, 6, 1–25. 10.1890/ES15-00121.1 [DOI] [Google Scholar]
- Petrenko, V. A. , & Makowski, L. (1993). Potential applications of phage display to bioremediation In Hinchee R. E., Semprini L., & Ong S. K. (Eds.), Bioremediation of chlorinated and polycyclic aromatic hydrocarbon compounds (pp. 266–280). Columbus, OH: Taylor and Francis Group. [Google Scholar]
- Piekarowicz, A. , Kłyż, A. , Majchrzak, M. , Szczêsna, E. , Piechucki, M. , Kwiatek, A. , … Stein, D. C. (2014). Neisseria gonorrhoeae filamentous phage NgoΦ6 is capable of infecting a variety of Gram‐negative bacteria. Journal of Virology, 88, 1002–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilon‐Smits, E. (2005). Phytoremediation. Annual Review of Plant Biology, 56, 15–39. 10.1146/annurev.arplant.56.032604.144214 [DOI] [PubMed] [Google Scholar]
- Pindi, P. K. , & Satyanarayana, S. D. V. (2012). Liquid microbial consortium‐ a potential tool for sustainable soil health. Biofertilizers & Biopesticides, 3, 4 10.4172/2155-6202.1000124. [DOI] [Google Scholar]
- Pires, D. P. , Cleto, S. , Sillankorva, S. , Azeredo, J. , & Lu, T. K. (2016). Genetically engineered phages: A review of advances over the last decade. Microbiology and Molecular Biology Reviews, 80, 523–543. 10.1128/MMBR.00069-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli, A. , Salerno, A. , Laezza, G. , di Donato, P. , Dumontet, S. , & Nicolaus, B. (2009). Heavy metal resistance of some thermophiles: Potential use of α‐amylase from Anoxybacillus amylolyticus as a microbial enzymatic bioassay. Research in Microbiology, 160, 99–106. 10.1016/j.resmic.2008.10.012 [DOI] [PubMed] [Google Scholar]
- Potnis, N. , Timilsina, S. , Strayer, A. , Shantharaj, D. , Barak, J. D. , Paret, M. L. , … Jones, J. B. (2015). Bacterial spot of tomato and pepper: Diverse Xanthomonas species with a wide variety of virulence factors posing a worldwide challenge. Molecular Plant Pathology, 16(9). 10.1111/mpp.12244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prashanthi, M. , Sundaram, R. , Jeyaseelan, A. , & Kaliannan, T. (2017). Bioremediation and sustainable technologies for cleaner environment. Environmental Science and Engineering, Switzerland, Springer. 10.1007/978-3-319-48439-6 [DOI] [Google Scholar]
- Pulli, T. , Höyhtyä, M. , Söderlund, H. , & Takkinen, K. (2005). One‐step homogeneous immunoassay for small analytes. Analytical Chemistry, 77, 2637–2642. 10.1021/ac048379l [DOI] [PubMed] [Google Scholar]
- Quesada, J. M. , Soriano, M. I. , & Espinosa-Urgel, M. (2012). Stability of a Pseudomonas putida KT2440 bacteriophage-carried genomic island and its impact on rhizosphere fitness. Applied and Environmental Microbiology, 78(19), 6963–6974. 10.1128/AEM.00901-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakonjac, J. (2012). Filamentous bacteriophages: Biology and applications. eLs, 10.1002/9780470015902.a0000777 [DOI] [PubMed] [Google Scholar]
- Rakonjac, J. , Bennett, N. J. , Spagnuolo, J. , Gagic, D. , & Russel, M. (2011). Filamentous bacteriophage: Biology, phage display and nanotechnology applications. Current Issues in Molecular Biology, 13, 51–76. [PubMed] [Google Scholar]
- Ramesh, A. , Sharma, S. K. , Sharma, M. P. , Yadav, N. , & Joshi, O. P. (2014). Plant growth‐promoting traits in Enterobacter cloacae subsp. dissolvens MDSR9 isolated from soybean rhizosphere and its impact on growth and nutrition of soybean and wheat upon inoculation. Agricultural Research, 3, 53–66. 10.1007/s40003-014-0100-3 [DOI] [Google Scholar]
- Rau, N. , Mishra, V. , Sharma, M. , Das, M. , Ahaluwalia, K. , & Sharma, R. S. (2009). Evaluation of functional diversity in rhizobacterial taxa of a wild grass (Saccharum ravennae) colonizing abandoned fly ash dumps in Delhi urban ecosystem. Soil Biology and Biochemistry, 41, 813–821. 10.1016/j.soilbio.2009.01.022 [DOI] [Google Scholar]
- Reichman, S. M. (2007). The potential use of the legume–rhizobium symbiosis for the remediation of arsenic contaminated sites. Soil Biology and Biochemistry, 39, 2587–2593. 10.1016/j.soilbio.2007.04.030 [DOI] [Google Scholar]
- Reyes, A. , Haynes, M. , Hanson, N. , Angly, F. E. , Heath, A. C. , Rohwer, F. , & Gordon, J. I. (2010). Viruses in the faecal microbiota of monozygotic twins and their mothers. Nature, 466, 334–338. 10.1038/nature09199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice, S. A. , Tan, C. H. , Mikkelsen, P. J. , Kung, V. , Woo, J. , Tay, M. , … Kjelleberg, S. (2009). The biofilm life cycle and virulence of Pseudomonas aeruginosa are dependent on a filamentous prophage. ISME Journal, 3, 271–282. 10.1038/ismej.2008.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley, M. A. , & Gordon, D. M. (1999). The ecological role of bacteriocins in bacterial competition. Trends in Microbiology, 7, 129–133. 10.1016/S0966-842X(99)01459-6 [DOI] [PubMed] [Google Scholar]
- Rodriguez-R, L. M. , Grajales, A. , Arrieta-Ortiz, M. , Salazar, C. , Restrepo, S. , & Bernal, A. (2012). Genomes-based phylogeny of the genus Xanthomonas. BMC Microbiology, 12(1), 43 10.1186/1471-2180-12-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez‐Navarro, D. N. , Oliver, I. M. , Contreras, M. A. , & Ruiz‐sainz, J. E. (2011). Soybean interactions with soil microbes, agronomical and molecular aspects. Agronomy for Sustainable Development, 31, 173–190. 10.1051/agro/2010023 [DOI] [Google Scholar]
- Rodriguez‐Valera, F. , Martin‐Cuadrado, A. B. , Rodriguez‐Brito, B. , Pašić, L. , Thingstad, T. F. , Rohwer, F. , & Mira, A. (2009). Explaining microbial population genomics through phage predation. Nature Reviews Microbiology, 7, 828–836. 10.1038/nrmicro2235 [DOI] [PubMed] [Google Scholar]
- Rojas, A. , Holguin, G. , Glick, B. R. , & Bashan, Y. (2001). Synergism between Phyllobacteriumsp. (N‐fixer) and Bacillus licheniformis(P‐solubilizer), both from a semiarid mangrove rhizosphere. FEMS Microbiology Ecology, 35, 181–187. [DOI] [PubMed] [Google Scholar]
- Rokhbakhsh‐Zamin, F. , Sachdev, D. , Kazemi‐Pour, N. , Engineer, A. , Pardesi, K. R. , Zinjarde, S. , … Chopade, B. A. (2011). Characterization of plant‐growth‐promoting traits of Acinetobacter species isolated from rhizosphere of Pennisetum glaucum . Journal of Microbiology and Biotechnology, 21, 556–566. [PubMed] [Google Scholar]
- Roy, A. , & Mitra, S. (1970. a). Increased fragility of Escherichia coli after infection with bacteriophage M13. Journal of Virology, 6(3), 333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy, A. , & Mitra, S. (1970b). Susceptibility of E. coli K‐12 to actinomycin D after infection with phage‐M13. Nature, 228, 365–366. [DOI] [PubMed] [Google Scholar]
- Ryu, J.-H. , Madhaiyan, M. , Poonguzhali, S. , Yim, W.-J. , Indiragandhi, P. , Kim, K.-A. , … Sa, T. (2006). Plant Growth Substances Produced by Methylobacterium spp. and Their Effect on Tomato (Lycopersicon esculentum L.) and Red Pepper (Capsicum annuum L.) Growth. Journal of Microbiology and Biotechnology, 16(10), 1622–1628. [Google Scholar]
- Sachs, J. D. , & Reid, W. V. (2006). Investments toward sustainable development. Science, 312, 1002. [DOI] [PubMed] [Google Scholar]
- Sagona, A. P. , Grigonyte, A. M. , MacDonald, P. R. , & Jaramillo, A. (2016). Genetically modified bacteriophages. Integrative Biology, 8, 465–474. 10.1039/C5IB00267B [DOI] [PubMed] [Google Scholar]
- Salivar, W. O. , Tzagoloff, H. , & Pratt, D. (1964). Some physical‐chemical and biological properties of the rod‐shaped coliphage M13. Virology, 24, 359–371. 10.1016/0042-6822(64)90173-4 [DOI] [PubMed] [Google Scholar]
- Santoyoa, G. , Moreno‐Hagelsieb, G. , Orozco‐Mosqueda, M. C. , & Glick, B. R. (2016). Plant growth‐promoting bacterial endophytes. Microbiological Research, 183, 92–99. 10.1016/j.micres.2015.11.008 [DOI] [PubMed] [Google Scholar]
- Searle, L. J. , Méric, G. , Porcelli, I. , Sheppard, S. K. , & Lucchini, S. (2015). Variation in siderophore biosynthetic gene distribution and production across environmental and faecal populations of Escherichia coli . PLoS ONE, 10, e0117906 10.1371/journal.pone.0117906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secor, P. R. , Sweere, J. M. , Michaels, L. A. , Malkovskiy, A. V. , Lazzareschi, D. , Katznelson, E. , … Bollyky, P. L. (2015). Filamentous bacteriophage promote biofilm assembly and function. Cell Host and Microbe, 18, 549–559. 10.1016/j.chom.2015.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellstedt, A. , & Richau, K. H. (2013). Aspects of nitrogen‐fixing actinobacteria, in particular free‐living and symbiotic Frankia. FEMS Microbiology Letters, 342, 179–186. [DOI] [PubMed] [Google Scholar]
- SER (Society for Ecological Restoration Science & Policy Working Group) . (2004). The SER Primer on Ecological Restoration. www.ser.org/
- Shapiro, J. W. , Williams, E. S. , & Turner, P. E. (2016). Evolution of parasitism and mutualism between filamentous phage M13 and Escherichia coli . PeerJ, 4, e2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, M. , Mishra, V. , Rau, N. , & Sharma, R. S. (2011). Functionally diverse rhizobacteria of Saccharum munja (a native wild grass). Colonizing abandoned morrum mine in Aravalli hills (Delhi). Plant and Soil, 341, 447–459. [Google Scholar]
- Sharma, M. , Mishra, V. , Rau, N. , & Sharma, R. S. (2015). Increased iron‐stress resilience of maize through inoculation of siderophore producer Arthorbacter globiformis BGDa404 from mine. Journal of Basic Microbiology, 56, 719–735. [DOI] [PubMed] [Google Scholar]
- Sharma, R. S. , Mishra, V. , Mohmmed, A. , & Babu, C. R. (2008). Phage specificity and lipopolysaccharides of stem‐ and root‐nodulating bacteria (Azorhizobium caulinodans, Sinorhizobium spp., and Rhizobium spp.). of Sesbania spp. Archives of Microbiology, 189, 411–418. [DOI] [PubMed] [Google Scholar]
- Sharma, R. S. , Mishra, V. , Mohmmed, A. , & Babu, C. R. (2011). Variations in outer‐ membrane characteristics of two stem‐nodulating bacteria of Sesbani rostrata and its role in tolerance towards diverse stress. Current Microbiology, 63, 81–86. [DOI] [PubMed] [Google Scholar]
- Sharma, R. S. , Mohmmed, A. , Mishra, V. , & Babu, C. R. (2005). Diversity in promiscuous group of rhizobia from three Sesbania spp. colonizing ecologically distinct habitats of the semi‐arid Delhi region. Research in Microbiology, 156, 57–67. [DOI] [PubMed] [Google Scholar]
- Shen, W. , Lakshmanan, R. S. , Mathison, L. C. , Petrenko, V. A. , & Chin, B. A. (2009). Phage coated magnetoelastic micro‐biosensors for real‐time detection of Bacillus anthracis spores. Sensors and Actuators B: Chemical, 137, 501–506. 10.1016/j.snb.2009.01.027 [DOI] [Google Scholar]
- Silveira, C. B. , & Rohwer, F. L. (2016). Piggyback‐the‐Winner in host‐associated Microbial Communities. Npj Biofilms and Microbiomes, 2, 16010 10.1038/npjbiofilms.2016.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, B. K. , Walker, A. , Morgan, J. A. W. , & Wright, D. J. (2004). Biodegradation of chlorpyrifos by Enterobacter strain B‐14 and its use in the bioremediation of contaminated soils. Applied and Environmental Microbiology, 70, 4855–4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, H. B. , Sarma, B. K. , & Keswani, C. (2017). Advances in PGPR research. UK: CAB International. [Google Scholar]
- Singh, R. P. , & Jha, P. N. (2017). The PGPR Stenotrophomonas maltophilia SBP‐9 Augments resistance against biotic and abiotic stress in wheat plants. Frontiers in Microbiology, 8, 1945 10.3389/fmicb.2017.01945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, S. , & Kapoor, K. K. (1999). Inoculation with phosphate‐solubilizing microorganisms and a vesicular‐arbuscular mycorrhizal fungus improves dry matter yield and nutrient uptake by wheat grown in a sandy soil. Biology and Fertility of Soils, 28, 139–144. 10.1007/s003740050475 [DOI] [Google Scholar]
- Singh, A. , Poshtiban, S. , & Evoy, S. (2013). Recent Advances in Bacteriophage Based Biosensors for Food-Borne Pathogen Detection. Sensors, 13(2), 1763–1786. 10.3390/s130201763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobodkin, A. I. (2005). Thermophilic microbial metal reduction. Mikrobiologiia, 74, 501–514. 10.1007/s11021-005-0096-6 [DOI] [PubMed] [Google Scholar]
- Slobodkin, A. I. , Sokolova, T. G. , Lysenko, A. M. , & Wiegel, J. (2006). Reclassification of Thermoterrabacterium ferrireducens as Carboxydothermus ferrireducens comb. nov., and emended description of the genus Carboxydothermus . International Journal of Systematic and Evolutionary Microbiology, 56, 2349–2351. 10.1099/ijs.0.64503-0 [DOI] [PubMed] [Google Scholar]
- Sokolova, T. G. , Gonzales, J. M. , Kostrikina, N. A. , Chernyh, N. A. , Slepova, T. V. , Bonch‐Osmolovskaya, E. A. , & Robb, F. T. (2004). Thermosinus carboxydivorans gen. nov., sp. nov., a new anaerobic, thermophilic, carbon‐monoxide‐oxidizing, hydrogenogenic bacterium from a hot pool of Yellowstone National Park. International Journal of Systematic and Evolutionary Microbiology, 54, 2353–2359. [DOI] [PubMed] [Google Scholar]
- Starr, M. P. , & Chatterjee, A. K. (1972). The Genus Erwinia: Enterobacteria Pathogenic to Plants and Animals. Annual Review of Microbiology, 26, 389–426. 10.1146/annurev.mi.26.100172.002133 [DOI] [PubMed] [Google Scholar]
- Stetter, K. O. (1999). Extremophiles and their adaptation to hot environments. FEBS Letters, 452, 22–25. 10.1016/S0014-5793(99)00663-8 [DOI] [PubMed] [Google Scholar]
- Sun, T.‐P. , & Webster, R. E. (1986). fii, a bacterial locus required for filamentous phage infection and its relation to colicin‐tolerant tolA and tolB. Journal of Bacteriology, 165, 107–115. 10.1128/jb.165.1.107-115.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suominen, L. , Jussila, M. M. , Mäkeläinen, K. , Romantschuk, M. , & Lindström, K. (2000). Evaluation of the Galega‐Rhizobium galegaesystem for the bioremediation of oil‐contaminated soil. Environmental Pollution, 107, 239–244. 10.1016/S0269-7491(99)00143-8 [DOI] [PubMed] [Google Scholar]
- Suter, J. D. , White, I. M. , Zhu, H. , Shi, H. , Caldwell, C. W. , & Fan, X. (2008). Label‐free quantitative DNA detection using the liquid core optical ring resonator. Biosensors and Bioelectronics, 23, 1003–1009. 10.1016/j.bios.2007.10.005 [DOI] [PubMed] [Google Scholar]
- Szekely, A. J. , & Breitbart, M. (2016). Single‐stranded DNA phages: From early molecular biology tools to recent revolutions in environmental microbiology. FEMS Microbiology Letters, 363, fnw027 10.1093/femsle/fnw027 [DOI] [PubMed] [Google Scholar]
- Tallis, H. , Kareiva, P. , Marvier, M. , & Chang, A. (2008). An ecosystem services framework to support both practical conservation and economic development. Proceedings of the National Academy of Sciences USA, 105, 9457–9464. 10.1073/pnas.0705797105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamman, H. , Ainelo, A. , Ainsaar, K. , & Hõrak, R. (2014). A moderate toxin, GraT, modulates growth rate and stress tolerance of Pseudomonas putida . Journal of Bacteriology, 196, 157–169. 10.1128/JB.00851-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templier, V. , Roux, A. , Roupioz, Y. , & Livache, T. (2016). Ligands for label‐free detection of whole bacteria on biosensors: A review. TrAC Trends in Analytical Chemistry, 79, 71–79. 10.1016/j.trac.2015.10.015 [DOI] [Google Scholar]
- Teng, Y. , Shen, Y. , Luo, Y. , Sun, X. , Sun, M. , Fu, D. , … Christie, P. (2011). Influence of Rhizobium meliloti on phytoremediation of polycyclic aromatic hydrocarbons by alfalfa in an aged contaminated soil. Journal of Hazardous Materials, 186, 1271–1276. 10.1016/j.jhazmat.2010.11.126 [DOI] [PubMed] [Google Scholar]
- Tharek, M. , Sim, K. S. , Khairuddin, D. , Ghazali, A. H. , & Najimudin, N. (2017). Whole‐genome sequence of endophytic plant growth‐promoting Escherichia coli USML2. GenomeAnnouncements, 5, e00305–e317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thieme, F. , Koebnik, R. , Bekel, T. , Berger, C. , Boch, J. , Buttner, D. , … Kaiser, O. (2005). Insights into Genome Plasticity and Pathogenicity of the Plant Pathogenic Bacterium Xanthomonas campestris pv. vesicatoria Revealed by the Complete Genome Sequence. Journal of Bacteriology, 187(21), 7254–7266. 10.1128/JB.187.21.7254-7266.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiriot, D. S. , Nevzorova, A. A. , & Opella, S. J. (2005). Structural basis of the temperature transition of Pf1 bacteriophage. Protein Science, 14, 1064–1070. 10.1110/ps.041220305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilak, K. , Ranganayaki, N. , Pal, K. , De, R. , Saxena, A. , Nautiyal, C. S. , … Johri, B. (2005). Diversity of plant growth and soil health supporting bacteria. Current Science, 89(1), 136–150. [Google Scholar]
- Tseng, Y. H. , Lo, M. C. , Lin, K. C. , Pan, C. C. , & Chang, R. Y. (1990). Characterization of filamentous bacteriophage ϕLf from Xanthomonas campestris pv. campestris . Journal of General Virology, 71, 1881–1884. [DOI] [PubMed] [Google Scholar]
- Tuomanen, E. , Cozens, R. , Tosch, W. , Zak, O. , & Tomasz, A. (1986). The rate of killing of Escherichia coli by beta‐lactam antibiotics is strictly proportional to the rate of bacterial growth. Journal of General Microbiology, 132, 1297–1304. [DOI] [PubMed] [Google Scholar]
- Umadevi, S. , Ayyasamy, P. M. , & Rajakumar, S. (2017). Biological perspective and role of bacteria in pesticide degradation (pp. 3–12). Cham, Switzerland: Springer; 10.1007/978-3-319-48439-6_1 [DOI] [Google Scholar]
- UNCCD (2013). A stronger UNCCD for a land-degradation neutral world. Issue Brief, Bonn, Germany. [Google Scholar]
- United Nations General Assembly (2015). Transforming our world: The 2030 Agenda for Sustainable Development (p. A/RES/70/1). http://www.refworld.org/docid/57b6e3e44.html [Google Scholar]
- Vamsee‐Krishna, C. , Mohan, Y. , & Phale, P. S. (2006). Biodegradation of phthalate isomers by Pseudomonas aeruginosa PP4, Pseudomonas sp. PPD and Acinetobacter lwoffii ISP4. Applied Microbiology and Biotechnology, 72, 1263–1269. [DOI] [PubMed] [Google Scholar]
- Van Elsas, J. D. , Duarte, G. F. , Rosado, A. S. , & Smalla, K. (1998). Microbiological and molecular biological methods for monitoring microbial inoculants and their effects in the soil environment. Journal of Microbiological Methods, 32, 133–154. 10.1016/S0167-7012(98)00025-6 [DOI] [Google Scholar]
- Van Elsas, J. D. , & Van Overbeek, L. S. (1993). Bacterial responses to soil stimuli In Kjelleberg S. (Ed.), Starvation in bacteria (pp. 55–79). New York, NY: Plenum Press. [Google Scholar]
- Vessey, J. K. (2003). Plant growth promoting rhizobacteria as biofertilizers. Plant and Soil, 255, 571–586. [Google Scholar]
- Viertel, T. M. , Ritter, K. , & Horz, H. P. (2014). Viruses versus bacteria‐novel approaches to phage therapy as a tool against multidrug‐resistant pathogens. Journal of Antimicrobial Chemotherapy, 69, 2326–2336. 10.1093/jac/dku173 [DOI] [PubMed] [Google Scholar]
- Vilar Jr, J. C. , Cavalcanti, D. L. , da Silva, C. A. A. , Andrade, R. F. S. , & Campos‐Takaki, G. M. (2015). Decolorization of Black B azo dye by Pseudomonas aeruginosa . International Journal of Current Microbiology and Applied Sciences, 4, 720–728. [Google Scholar]
- Vincent, J. M. (1954). The root nodules bacteria as factors in clover establishment in the red basaltic soils of the Lismore district. N.S.W.I. A survey of native strains. Australian Journal of Agricultural Research, 5, 55–60. [Google Scholar]
- Volpon, A. G. T. , De‐Polli, H. , & Döbereiner, J. (1981). Physiology of nitrogen fixation in Azospirillum lipoferum Br 17 (ATCC 29 709). Archives of Microbiology, 128, 371 10.1007/BF00405915 [DOI] [Google Scholar]
- Vrancken, K. , Holtappels, M. , Schoofs, H. , Deckers, T. , & Valcke, R. (2013). Pathogenicity and infection strategies of the fire blight pathogen Erwinia amylovora in Rosaceae: State of the art. Microbiology, 159, 823–832. 10.1099/mic.0.064881-0 [DOI] [PubMed] [Google Scholar]
- Waldor, M. K. , & Mekalanos, J. J. (1996). Lysogenic conversion by a filamentous phage encoding cholera toxin. Science, 272, 1910–1914. 10.1126/science.272.5270.1910 [DOI] [PubMed] [Google Scholar]
- Walker, T. S. , Bais, H. P. , D�ziel, E. , Schweizer, H. P. , Rahme, L. G. , Fall, R. , & Vivanco, J. M. (2004). Pseudomonas aeruginosa-plant root interactions. Pathogenicity, biofilm formation, and root exudation. Plant Physiology, 134(1), 320–331. 10.1104/pp.103.027888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan, Z. , & Goddard, N. L. (2012). Competition between conjugation and M13 phage infection in Escherichia coli in the absence of selection pressure: A kinetic study. Genes, Genomes, Genetics, 2, 1137–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C. , Wang, D. , & Zhou, Q. (2004). Colonization and persistence of a plant growth‐promoting bacterium Pseudomonas fluorescensstrain CS85, on roots of cotton seedlings. Canadian Journal of Microbiology, 50, 475–481. [DOI] [PubMed] [Google Scholar]
- Webb, J. S. , Lau, M. , & Kjelleberg, S. (2004). Bacteriophage and phenotypic variation in Pseudomonas aeruginosa biofilm development. Journal of Bacteriology, 186, 8066–8073. 10.1128/JB.186.23.8066-8073.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, G. , & Ma, Z. (2010). Application of rhizobia‐legume symbiosis for remediation of heavy‐metal contaminated soils. Wei Sheng Wu Xue Bao, 50, 1421–1430. [PubMed] [Google Scholar]
- Wenneker, M. , Verdel, M. S. W. , Groeneveld, R. M. W. , Kempenaar, C. , van Beuningen, A. R. , & Janse, J. D. (1999). Ralstonia (Pseudomonas) solanacearum Race 3 (Biovar 2) in Surface Water and Natural Weed Hosts: First Report on Stinging Nettle (Urtica dioica). European Journal of Plant Pathology, 105(3), 307–315. 10.1023/A:1008795417575 [DOI] [Google Scholar]
- Westwater, C. , Kasman, L. M. , Schofield, D. A. , Werner, P. A. , Dolan, J. W. , Schmidt, M. G. , & Norris, J. S. (2003). Use of genetically engineered phage to deliver antimicrobial agents to bacteria: An alternative therapy for treatment of bacterial infections. Antimicrobial Agents and Chemotherapy, 47, 1301–1307. 10.1128/AAC.47.4.1301-1307.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weynberg, K. D. , Voolstra, C. R. , Neave, M. J. , Buerger, P. , & van Oppen, M. J. H. (2015). From cholera to corals: Viruses as drivers of virulence in a major coral bacterial pathogen. Scientific Reports, 5, 17889 10.1038/srep17889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley, M. , Bangera, M. G. , Bumgarner, R. E. , Parsek, M. R. , Teitzel, G. M. , Lory, S. , & Greenberg, E. P. (2001). Gene expression in Pseudomonas aeruginosa biofilms. Nature, 413, 860–864. 10.1038/35101627 [DOI] [PubMed] [Google Scholar]
- Willats, W. G. (2002). Phage display: Practicalities and prospects. Plant Molecular Biology, 50, 837–854. [DOI] [PubMed] [Google Scholar]
- Wu, S. C. , Cheung, K. C. , Luo, Y. M. , & Wong, H. M. (2006). Effects of inoculation of plant growth‐promoting rhizobacteria on metal uptake by Brassica juncea . Environmental Pollution, 140, 124–135. [DOI] [PubMed] [Google Scholar]
- Wubs, E. J. , van der Putten, W. H. , Bosch, M. , & Bezemer, T. M. (2016). Soil inoculation steers restoration of terrestrial ecosystems. NaturePlants, 2, 16107 10.1038/nplants.2016.107 [DOI] [PubMed] [Google Scholar]
- Xu, H. X. , Wu, H. Y. , Qiu, Y. P. , Shi, X. Q. , He, G. H. , Zhang, J. F. , & Wu, J. C. (2011). Degradation of fluoranthene by a newly isolated strain of Herbaspirillum chlorophenolicum from activated sludge. Biodegradation, 22(2), 335–345. 10.1007/s10532-010-9403-7 [DOI] [PubMed] [Google Scholar]
- Yamada, T. (2013). Filamentous phages of Ralstonia solanacearum: Double‐edged swords for pathogenic bacteria. Frontiers in Microbiology, 4, 325 10.3389/fmicb.2013.00325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, T. , Kawasaki, T. , Nagata, S. , Fujiwara, A. , Usami, S. , & Fujie, M. (2007). New bacteriophages that infect the phytopathogen Ralstonia solanacearum . Microbiology, 153, 2630–2639. 10.1099/mic.0.2006/001453-0 [DOI] [PubMed] [Google Scholar]
- Yang, F. , Pecina, D. A. , Kelly, S. D. , Kim, S. H. , Kemner, K. M. , Long, D. T. , & Marsh, T. L. (2010). Biosequestration via cooperative binding of copper by Ralstonia pickettii . Environmental Technology, 31, 1045–1060. [DOI] [PubMed] [Google Scholar]
- Yang, L. M. C. , Tam, P. Y. , Murray, B. J. , McIntire, T. M. , Overstreet, C. M. , Weiss, G. A. , & Penner, R. M. (2006). Virus electrodes for universal biodetection. Analytical chemistry, 78(10), 3265–3270. [DOI] [PubMed] [Google Scholar]
- Young, J. P. W. , Crossman, L. C. , Johnston, A. W. B. , Thomson, N. R. , Ghazoui, Z. F. , Hull, K. H. , … Parkhill, J. (2006). The genome of Rhizobium leguminosarum has recognizable core and accessory components. Genome Biology, 7, R34 10.1186/gb-2006-7-4-r34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, T. P. , Petersen, D. A. , & Clary, J. J. (2005). The ecology of restoration: Historical links, emerging issues and unexplored realms. Ecology Letters, 8, 662–673. 10.1111/j.1461-0248.2005.00764.x [DOI] [Google Scholar]
- Yu, Z. C. , Chen, X. L. , Shen, Q. T. , Zhao, D. L. , Tang, B. L. , Su, H. N. , … Zhang, Y. Z. (2015). Filamentous phages prevalent in Pseudoalteromonas spp. confer properties advantageous to host survival in Arctic sea ice. ISME Journal, 9, 871–881. 10.1038/ismej.2014.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Wang, R. , Chen, S. , Qi, G. , He, Z. , & Zhao, X. (2017). Microbial taxa and functional genes shift in degraded soil with bacterial wilt. Scientific Reports, 7, 39911 10.1038/srep39911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, W. , Zhang, H. , Ma, Y. , Zhou, J. , & Zhang, Y. (2013). Bio‐removal of cadmium by growing deep‐sea bacterium Pseudoalteromonas sp. SCSE709‐6. Extremophiles, 17, 723–731. 10.1007/s00792-013-0554-4 [DOI] [PubMed] [Google Scholar]
- Zhu, H. , White, I. M. , Suter, J. D. , & Fan, X. (2008). Phage‐based label‐free biomolecule detection in an opto‐fluidic ring resonator. Biosensors and Bioelectronics, 24, 461–466. [DOI] [PubMed] [Google Scholar]
- Zhu, H. , White, I. M. , Suter, J. D. , Zourob, M. , & Fan, X. (2008). Opto‐fluidic micro‐ring resonator for sensitive label‐free viral detection. The Analyst, 132, 356–360. [DOI] [PubMed] [Google Scholar]
- Zinder, N. D. (1973). Resistance to colicins E3 and K induced by infection with bacteriophage fl. Proceedings of the National Academy of Sciences USA, 74, 3160–3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data used for developing the proposals and recommendations presented in this paper were sourced from published studies, and appropriate references are provided.


