Abstract
Knee osteoarthritis (OA) is a leading cause of pain and disability. Although conventional treatments show modest benefits, pilot and phase I/II trials with bone marrow (BM) and adipose‐derived (AD) mesenchymal stromal cells (MSCs) point to the feasibility, safety, and occurrence of clinical and structural improvement in focal or diffuse disease. This study aimed to assess the safety and efficacy of the intra‐articular injection of single or repeated umbilical cord‐derived (UC) MSCs in knee OA. UC‐MSCs were cultured in an International Organization for Standardization 9001:2015 certified Good Manufacturing Practice‐type Laboratory. Patients with symptomatic knee OA were randomized to receive hyaluronic acid at baseline and 6 months (HA, n = 8), single‐dose (20 × 106) UC‐MSC at baseline (MSC‐1, n = 9), or repeated UC‐MSC doses at baseline and 6 months (20 × 106 × 2; MSC‐2, n = 9). Clinical scores and magnetic resonance images (MRIs) were assessed throughout the 12 months follow‐up. No severe adverse events were reported. Only MSC‐treated patients experienced significant pain and function improvements from baseline (p = .001). At 12 months, Western Ontario and Mc Master Universities Arthritis Index (WOMAC‐A; pain subscale) reached significantly lower levels of pain in the MSC‐2‐treated group (1.1 ± 1.3) as compared with the HA group (4.3 ± 3.5; p = .04). Pain Visual Analog scale was significantly lower in the MSC‐2 group versus the HA group (2.4 ± 2.1 vs. 22.1 ± 9.8, p = .03) at 12 months. For total WOMAC, MSC‐2 had lower scores than HA at 12 months (4.2 ± 3.9 vs. 15.2 ± 11, p = .05). No differences in MRI scores were detected. In a phase I/II trial (NCT02580695), repeated UC‐MSC treatment is safe and superior to active comparator in knee OA at 1‐year follow‐up. stem cells translational medicine 2019;8:215&224
Keywords: Osteoarthritis, Knee, Mesenchymal stromal cells, Pain, Disability
Significance Statement.
Osteoarthritis is the main disabling musculoskeletal disorder in adults, for which presently available treatments are only of marginal benefit. This trial provides evidence of safety and efficacy of a highly accessible allogeneic cell source that had not been tested in knee osteoarthritis, in spite of its well‐known biological advantages. Even if these results should be confirmed in larger trials, they point the way to a simple, scalable cell‐based therapy open to repeated applications with no need for invasive surgical procedures.
Introduction
Osteoarthritis (OA) is the most common joint disease, leading to chronic pain, poor quality of life, and increased mortality 1, 2, 3. This imposes a major social burden due to elevated health care costs and premature workforce retirement 4, 5. However, despite decades of research, no true disease‐modifying OA drugs are described, and clinical effects of pharmacological interventions remain of short duration. In consequence, current aims have been directed toward the development of newer cell‐based therapies. Initial attempts aiming at joint repair with autologous chondrocytes 6, 7, thus requiring surgical harvest and implantation, have been in part replaced by the use of mesenchymal stromal cells (MSCs), because they have both the capacity for self‐renewal and expansion, as well as inflammation‐dependent homing mechanisms associated with tissue restoring properties 8, 9, 10.
In animal models of induced OA, from mouse to horse, MSC transplantation has been able to prevent, halt, or even reverse cartilage degradation 11, 12, 13, 14, 15. Most human data from clinical series emerge from individuals with underlying OA‐predisposing conditions that require surgical intervention, such as meniscal tears or focal chondral defects (FCDs). In such cases, improvements in pain, histologic, and magnetic resonance imaging (MRI) morphologic scores have been noted 16, 17, 18.
However, MSC therapy is also being assessed now in patients with diffuse knee OA. Indeed, three pilot studies of autologous cell therapy with bone marrow (BM)‐MSCs or adipose‐derived (AD)‐MSCs have been reported, suggesting early evidence of clinical efficacy at 6 months follow‐up 19, 20, 21. In addition, a single phase I trial has tested allogeneic BM‐MSCs, also reporting improvements in joint pain and function 22. Since we and others have shown that umbilical cord‐derived‐MSCs (UC‐MSCs) exhibit higher clonogenic, proliferative, and migration potential than BM‐MSCs, as well as improved secretion of relevant chondrogenic factors 23, 24, interest in UC‐MSCs as a more potent cell source suitable for allogeneic MSC‐based therapy has expanded 25. More than 3,000 patients have received allogeneic MSC treatment for different conditions, and no event linked to allo reactivity has been reported 26.
We therefore decided to test this easily accessible and well‐characterized source of MSCs in a phase I/II controlled, randomized, triple‐blind trial, comparing single or double intra‐articular injection of UC‐MSCs, with an approved viscosupplementation treatment, in individuals with knee OA.
Subjects, Materials, and Methods
Study Design
The study was registered at ClinicalTrials.gov (NCT02580695) and planned as a phase I/II randomized, double‐blind, controlled trial assessing the safety and efficacy of single or repeated doses of intra‐articular UC‐MSCs, compared with repeated doses of intra‐articular hyaluronic acid (HA) in patients with knee OA. It was approved prior to patient recruitment by a Chilean Ministry of Health Ethics Committee (CECSSMO131015) and conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki.
Patients
The target population included individuals of 40–65 years of age, recruited between November 2015 and January 2016 at the University of los Andes Clinical Center in Santiago, Chile, and meeting the following inclusion criteria: symptomatic knee OA (defined by daily pain at the affected joint for at least 3 months before inclusion) with grade 1–3 Kellgren‐Lawrence radiographic changes in the targeted knee, without meniscal rupture. Patients were excluded if they had bilateral symptomatic knee OA, condylar or tibial plateau generalized bone marrow edema on MRI, major axial deviation defined by valgus (>10°) or varus (5°) deformity of the involved leg, use of oral or intra‐articular steroids or hyaluronic acid in the past 6 months, ipsilateral hip or ankle pain, local or systemic infection, any form of secondary arthritis, previous malignancy, or body mass index ≥30. All randomized patients provided written informed consent.
Intervention
Forty individuals were screened, leading to the randomized allocation by electronic data entry system of 29 patients at a 1:1:1 ratio, into the three study groups receiving intra‐articular knee injections of HA at baseline and 6 months (Control group, n = 8); UC‐MSCs at baseline and 6 months (MSC‐2 group, n = 9), or UC‐MSCs only at baseline, followed by placebo at 6 months (MSC‐1 group, n = 9; Fig. 1). MSC injections contained 20 × 106 UC‐MSCs in 3 cc of saline with 5% AB plasma, HA injections contained 3 cc of Durolane, and placebo injections contained 5% AB plasma in 3 cc of saline.
Figure 1.
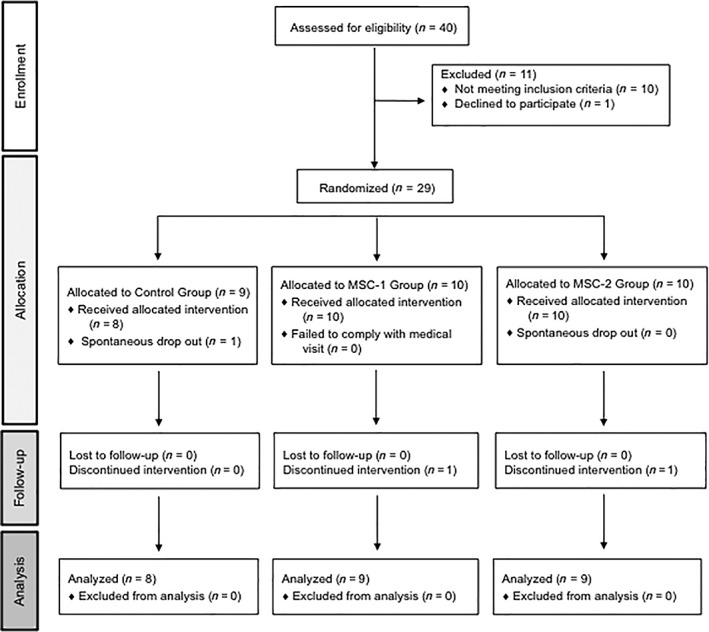
Flow chart. Abbreviation: MSC, mesenchymal stromal cell.
Outcomes
The primary endpoint of the trial was the safety of UC‐MSC treatment, according to the number of treatment‐related adverse events (AEs) reported for each study group as coded by the Common Terminology Criteria for Adverse Event classification. AEs were documented at each visit and described in terms of incidence, severity, and relatedness with intra‐articular infiltration. The secondary endpoint of the trial was efficacy, as assessed by the following validated clinical outcome scales: Western Ontario and Mc Master Universities Arthritis Index (WOMAC) Spanish validated version 27, Pain Visual Analog scale (VAS), Quality of life by the Short‐form 36 (SF‐36) questionnaire 28, Patient Global Assessment, and the Outcome Measures in Rheumatology Committee (OMERACT)‐Osteoarthritis Research Society International (OARSI) Responder Index Criteria 29. WOMAC was registered according to Likert Scale version using the following descriptors for each item: none (0), mild (1), moderate (2), severe (3), and extreme (4). Final scores are the sum of items in each subscale, ranging 0–20 for pain, 0–8 for stiffness, and 0–68 for physical function. Knee MRI assessments were performed and assessed blindly by a single radiologist at baseline, at 6 months, and at 12 months, according to the Whole‐Organ Magnetic Resonance Imaging Score (WORMS) 30.
Procedures
All injections were performed by two orthopedic surgeons in the study, blinded to the component being injected, in syringes equal in volume (3 cc) and external aspect, using an anterolateral approach at the medial joint line with 90° of knee flexion. Patients were encouraged to avoid physical activity for 48 hours after the procedure. Acetaminophen (1 g every 8 hours) was allowed as needed in case of pain.
Follow‐Up
Clinical outcome and trial assessments were evaluated at intervals of 1, 4, 8, 12, 24, 36, and 52 weeks by another orthopedic surgeon in the study, not involved in the treatment procedures and blinded to patient allocation.
Preparation and Characterization of UC‐MSC
The UC‐MSCs for this trial (Cellistem‐OA, Cells for Cells, Chile) were manufactured as previously described 23, and have been extensively characterized by our group 24. Briefly, treatments were processed in an International Organization for Standardization 9001:2015‐certified Good Manufacturing Practice (GMP)‐type Laboratory (Cells for Cells, Santiago, Chile), under GMP conditions according to the Food and Drug Administration Guidance for industry (current good tissue practice and additional requirements for manufacturers of human cells, tissues, and cellular and tissue‐based products). Umbilical cords were obtained from full‐term human placentas by cesarean section after informed consent, from healthy donors, and were aseptically stored in sterile phosphate‐buffered saline (PBS) supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin (Gibco, Grand Island, NY, U.S.). Within 3 hours of birth, the umbilical cord was sectioned and washed with PBS and antibiotics. Wharton's jelly was dissected into small fragments (1–2 mm), seeded in 100‐mm culture plates, and maintained in Minimum Essential Medium Eagle (MEM) Alpha Modifications (Alfa‐MEM) high glucose (Gibco, Grand Island, NY, U.S.) supplemented with 10% heat‐inactivated fetal bovine serum (Gibco, U.S.), 1% penicillin/streptomycin, and 2 mM L‐glutamine (Gibco, Grand Island, NY, U.S.). At 48 hours, nonadherent cells were removed and washed with PBS, and culture medium was replaced with fresh medium every 3 days. When the cell culture reached 70%–80% confluence, cells were detached by treatment with TrypLE TM Express (Gibco, Grand Island, NY, U.S.) and reseeded at a density of 2,500 cells per cm2 into 500‐cm2 flasks (Nunc, Denmark). At passage 3, UC‐MSC were characterized according to the International Society for Cellular Therapy Guidelines 30, harvested, and cryopreserved in Profreeze (Lonza, Walkersville, MD) following the manufacturer's instruction. Cell size and doubling time, senescence markers, paracrine and immunomodulatory activity, and migration capacity of UC‐MSCs as compared with BM‐MSCs have been described elsewhere (RIMECARD trial) 23, 24. The protocol is patented by Cells for Cells, request number 201702357.
For the trial, cells were thawed and expanded until passage 5 using Alfa‐MEM supplemented with 10% AB plasma. Cells were detached by treatment with TrypLE TM Express and washed twice with PBS prior to final suspension and packaging. The release criteria for clinical use of UC‐MSC comprised the absence of macroscopic clumps, contaminating pathogenic microorganisms (bacteria, mycoplasma, syphilis, hepatitis B virus, hepatitis C virus, HIV, cytomegalovirus, and fungi), or endotoxin (≤0.5 EU/mL); and a viability >80%, with an identity and purity pattern characterized by ≥95% positivity for CD73, CD90, and CD105, and negativity (≤2%) for the expression of CD45, CD34, CD14, and Human Leukocyte Antigen‐DR isotype (HLA‐DR). Cells (20 × 106) were suspended in a final volume of 3 mL (saline solution, 5% AB plasma) and dispensed in masked 5‐mL syringes to treat individual patients accordingly with the study design.
UC‐MSC Batch Selection
Several batches of UC‐MSCs from three different healthy donors were isolated and expanded under GMP conditions for testing of additional prespecified requirements (Fig. 2).
Figure 2.
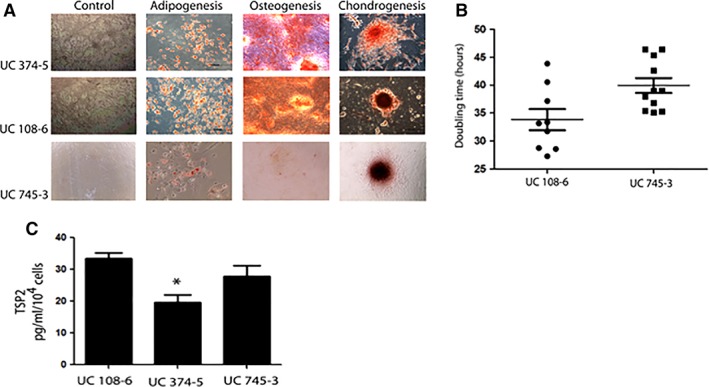
Selection of UC‐mesenchymal stromal cell (MSC) batch. (A): TSP2 secretion analysis from three UC‐MSCs batches of different donors. All data presented as mean ± SEM, n = 4, * p < .05. (B): Differentiation potential of different UC‐MSC batch tested. Scale bars 200 mm, n = 3. (C): UC‐MSC proliferation rate through the assessment of doubling times, p < .05, n = 3. Abbreviations: TSP2, thrombospondin‐2; UC, umbilical cord.
Differentiation Potential of UC‐MSCs
To ensure compliance of our cell source with the International Society for Cellular Therapy (ISCT) defined criteria for MSC identification 31, compliant good manufacturing process (cGMP) manufactured UC‐MSC batches were also evaluated for tri‐differentiation to mesodermic lineages. Cells were thawed, grown under specific culture conditions, and stained for detection of adipogenic (Oil Red O), osteogenic (Alizarin Red), and chondrogenic (Safranin O) differentiation (Fig. 2A).
Doubling Time of UC‐MSCs
UC‐MSC batches with satisfactory tri‐differentiation were further evaluated for proliferation rate, according to the calculated doubling times (DTs). The batch selected for the trial (CU 108–6) had the best DT values (33.8 vs. 39.9, 95% confidence interval −10.8 to −1.3, p = .01, compared with CU 745–3; Fig. 2B).
Paracrine Criteria
We assessed thrombospondin‐2 (TSP2) secretion as described by Jeong et al. given its role in chondrogenic differentiation 32. TSP2 enzyme‐linked immunosorbent assay was performed according to manufacturer instructions (DuoSet Human Thrombospondin‐2, R&D Systems, DY1635) with supernatants from the tested MSCs in passage 5. As indicated in Figure 2C, we selected the MSC source with higher TSP‐2 secretion to expand a single batch for all patients in the treatment arm of the trial.
Statistical Analysis
Sample description included the frequencies of each category for qualitative variables and mean plus SD for quantitative variables. To examine whether differences in qualitative variables were significant among treatment groups at baseline and during follow‐up, a Freeman–Halton extension of Fisher's exact test was performed for contingency tables of 2×3 and a Fisher's exact test for contingency tables of 2×2. A Kruskal‐Wallis one‐way analysis of variance‐by‐ranks was used to test significant differences in quantitative variables among treatment groups (HA, MSC‐1, MSC‐2) at baseline and during follow‐up. To detect statistical differences between treatments after Kruskal‐Wallis analysis was significant, we performed a multiple comparison of mean ranks. Significance level was set at 5% for all tests, and all statistical analyses were performed using the R platform (v3.4.1; R Development Core Team) in adherence to Good Statistical Practice in Clinical Research.
Results
Patient Characteristics
Mean age was 56 years, with a similar gender distribution. Demographics and clinical status were comparable among groups. Cell‐treated groups tended to have more severe baseline disease (although not significantly) as gauged by the total WOMAC score and the percent Kellgren‐Lawrence grade 3 patients in the MSC‐1 and MSC‐2 groups (50% and 40%) as opposed to the HA group (23%; Table 1).
Table 1.
Baseline patient characteristics
| Characteristics | HA group | UC‐MSC‐1 single‐dose group | UC‐MSC‐2 repeated‐dose group | Adjusted p value |
|---|---|---|---|---|
| Age, years | 54.8 ± 4.5 | 56.1 ± 6.8 | 56.7 ± 4.1 | .7 |
| Female, n (%) | 5 (55) | 6 (60) | 5 (50) | .99 |
| BMI (kg/m2) | 27.9 ± 3.4 | 27.6 ± 2.6 | 27.4 ± 2.6 | .99 |
| Kellgren grade, n (%) | ||||
| II | 7 (77) | 5 (50) | 6 (60) | .87 |
| III | 2 (23) | 5 (50) | 4 (40) | .78 |
| WOMAC, mean (SEM) | ||||
| Total | 28.9 ± 13.3 | 37.4 ± 12.8 | 35.6 ± 10.1 | .18 |
| A. Pain (0–20) | 7.0 ± 2.7 | 9.3 ± 3 | 8.1 ± 2.1 | .19 |
| B. Stiffness (0–8) | 3.2 ± 1.2 | 2.9 ± 1.1 | 2.8 ± 1.2 | .21 |
| C. Function (0–68) | 18.7 ± 10.9 | 25.3 ± 8.5 | 23.8 ± 9.2 | .15 |
| VAS 0–100, mm | 38.7 ± 19.4 | 44.8 ± 16.5 | 39.4 ± 21.4 | .57 |
| Global knee pain | ||||
| SF‐36 | ||||
| Physical scale | 51.3 ± 20.8 | 46.9 ± 16.5 | 60 ± 18.4 | .18 |
| Pain scale | 48.4 ± 19.4 | 48.9 ± 24 | 57.8 ± 19 | .36 |
| WORMS, 0–332 points | 30.9 ± 25.1 | 46.1 ± 18.1 | 40.1 ± 25.7 | .21 |
Data are presented as n (%) or mean ± SD.
Abbreviations: BMI, body mass index; HA, hyaluronic acid; SF‐36, short‐form 36; UC‐MSC, umbilical cord‐derived mesenchymal stromal cells; VAS, visual analog scale; WOMAC, Western Ontario and Mc Master Universities Arthritis Index; WORMS, Whole‐Organ Magnetic Resonance Imaging Score.
Safety Profile
No serious AEs, deaths, permanent disability, neoplasia, or septic arthritis cases were registered during the trial. The most common adverse event related to intra‐articular injection was acute synovitis. One week after first injections, mild to moderate symptomatic knee effusion was present more often in cell‐treated groups than controls, but with no significant differences: At first injection, acute knee effusion was noted in 33% of cases in groups MSC‐1 and MSC‐2 versus only 22% for the HA group; p = .99. This occurred similarly with second injection in 44% of the MSC‐2 group patients versus 37.5% of the HA group patients (p = .99). Pain was the second most frequent AE without reaching statistical difference between groups. Both AEs were transient and responsive to rest and oral acetaminophen. No cases or controls required hospitalization or arthrocentesis (Table 2).
Table 2.
Safety profile
| Adverse Events | HA group | MSC‐1 single‐dose group | MSC‐2 repeated‐dose group | Adjusted p value |
|---|---|---|---|---|
| Injection‐related AE | ||||
| Synovitis after first injection | 2 (22%) | 3 (33%) | 3 (33%) | .99 |
| Synovitis after second injection | 3 (37.5%) | 4 (44%) | .99 | |
| Pain after first injection | 1 (12.5%) | 1 (11%) | 2 (22%) | .99 |
| Pain after second injection | 1 (12.5%) | 0 | 1 (11%) | .99 |
| Septic arthritis | 0 | 0 | 0 | — |
| Fever | 0 | 0 | 0 | — |
| Urticarial lesions | 0 | 0 | 0 | — |
| Bleeding | 0 | 0 | 0 | — |
| AE during follow‐up (%) | ||||
| Fatal | 0 | 0 | 0 | — |
| Serious | 0 | 0 | 0 | — |
| Nonserious | 0 | 0 | 0 | — |
Data are presented as n.
Abbreviations: AE, adverse event; HA, hyaluronic acid; MSC, mesenchymal stromal cells.
Efficacy Profile
Clinical Outcomes
Clinical outcomes are described in Table 3 for WOMAC components and VAS. The repeated dose MSC group exhibits a significant advantage in pain reduction. Thus, at 12 months follow‐up, the WOMAC‐A (pain subscale) reached significantly lower levels in the MSC‐2‐treated group (1.1 ± 1.3) as compared with the HA group (4.3 ± 3.5; p = .04). For total WOMAC, MSC‐2 also had significantly lower scores than HA at 12 months (4.2 ± 3.9 vs. 15.2 ± 11, p = .05). Similarly, VAS were significantly lower in the MSC‐2‐treated versus the HA group (2.4 ± 2.1 vs. 22.1 ± 9.8, p = .03). No changes in function subscale and SF‐36 were detected.
Table 3.
Outcomes for visual analog, pain, stiffness, and functional scales at 6 and 12 months follow‐up
| Outcomes | HA group | MSC‐1 group | MSC‐2 group | Difference between treatments a | Multiple comparison b |
|---|---|---|---|---|---|
| At 6 months | |||||
| WOMAC | |||||
| Total | 18.6 ± 14.7 | 13.8 ± 9.2 | 8.3 ± 5.1 | .2 | |
| A. Pain (0–20) | 4.2 ± 3.8 | 2.1 ± 1.4 | 2.4 ± 1.7 | .36 | |
| B. Stiffness (0–8) | 1.1 ± 1.8 | 1.4 ± 1.2 | 0.9 ± 0.9 | .54 | |
| C. Function (0–68) | 13.3 ± 6.9 | 10.3 ± 7.2 | 5 ± 3.1 | .07 | |
| VAS 0–100, mm | 28 ± 8.7 | 12 ± 7.5 | 10.8 ± 7.8 | .09 | |
| At 12 months | |||||
| WOMAC | |||||
| Total | 15.2 ± 11 | 14.9 ± 12.7 | 4.2 ± 3.9 | .04 | HA vs. MSC‐2 .05 |
| A. Pain (0–20) | 4.3 ± 3.5 | 3.7 ± 2.6 | 1.1 ± 1.3 | .04 | HA vs. MSC‐2 .04 |
| B. Stiffness (0–8) | 1.7 ± 1.4 | 1.7 ± 2.1 | 0.6 ± 0.8 | .14 | |
| C. Function (0–68) | 9.2 ± 9.4 | 9.5 ± 7.4 | 2.6 ± 2.3 | .08 | |
| VAS 0–100, mm | 22.1 ± 9.8 | 13.3 ± 8.4 | 2.4 ± 2.1 | .02 | HA vs. MSC‐2 .03 |
Data are presented as mean ± SD.
Bolded p values are statistically significant.
a p value of Kruskal‐Wallis one‐way analysis of variance‐by‐ranks.
b p value of multiple comparison of mean ranks.
Abbreviations: HA, hyaluronic acid; MSC, mesenchymal stromal cells; VAS, visual analog scale; WOMAC, Western Ontario and Mc Master Universities Arthritis Index.
When the evolution of symptoms throughout the study was followed in time for each group, only MSC‐treated patients showed significant improvements in pain and function from baseline, as opposed to the HA‐treated patients (Fig. 3A–3C). At 12 months, patients in the MSC‐2 group experienced 86% pain reduction and 89% disability reduction (p = .001) as opposed to 38% and 50% in the control HA group, respectively. Of note, our comparator HA group lost effect for pain, function, and total WOMAC at 6 months, only to regain improvement after receiving their second HA injection. The MSC‐1 group showed continued improvements throughout month 9, although reaching a level of symptoms that was similar to the control group at 12 months (after their second HA injection). In contrast, the MSC‐2 group showed steady improvement of both WOMAC and VAS until the study endpoint.
Figure 3.
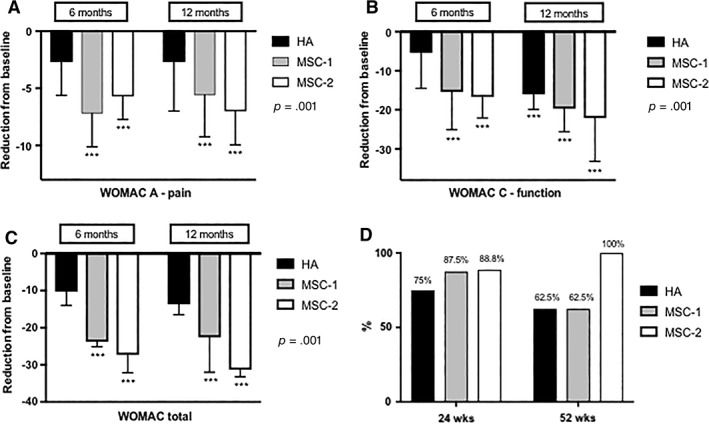
Efficacy outcomes. (A–C): Comparison with baseline in each group. (A): WOMAC‐A pain subscale. (B): WOMAC‐C function subscale. (C): Total WOMAC. (D): OMERACT‐OARSI Responder Index Criteria. Abbreviation: HA, hyaluronic acid; MSC, mesenchymal stromal cell; WOMAC, Western Ontario and Mc Master Universities Arthritis Index.
When comparing how often patients in the trial would achieve responder status at study endpoint, as defined by the OMERACT‐OARSI responder index criteria, all individuals in the repeated MSC group were found to be responders as opposed to 62.5% of patients in the control HA group, a tendency that did not reach significance (p = .08; Fig. 3D).
Structural Outcomes (MRI)
No evidence of chondral damage or intra‐articular calcifications was detected upon standardized MRI studies at either 24 or 48 weeks of follow‐up. Also, no change from baseline imaging or among groups was found in any of the 14 items composing the WORMS score (Table 4).
Table 4.
Structural assessment by magnetic resonance imaging
| HA group | MSC‐1 group | MSC‐2 group | p value | |
|---|---|---|---|---|
| WORMS at baseline | ||||
| Total (0–332) | 30.9 ± 25.1 | 46.1 ± 18.1 | 40.1 ± 25.7 | .21 |
| Articular cartilage | 16.5 ± 13.4 | 23.2 ± 10.9 | 21 ± 14 | .3 |
| Meniscal integrity | 1.7 ± 1.3 | 1.1 ± 1.2 | 2.7 ± 1.9 | .15 |
| WORMS at 6 months | ||||
| Total (0–332) | 33.2 ± 25.7 | 46.6 ± 18.1 | 40.6 ± 21.4 | .3 |
| Articular cartilage | 16.7 ± 14.5 | 22.4 ± 10.8 | 21.3 ± 14.1 | .28 |
| Meniscal integrity | 1.7 ± 1.6 | 0.9 ± 1.2 | 2.7 ± 2.1 | .13 |
| WORMS at 12 months | ||||
| Total (0–332) | 33.6 ± 26.3 | 41.5 ± 14.3 | 40.5 ± 23.9 | .15 |
| Articular cartilage | 16.8 ± 14.5 | 23.1 ± 10.2 | 21.3 ± 13.8 | .3 |
| Meniscal integrity | 1.7 ± 1.6 | 0.9 ± 1.2 | 2.7 ± 2.1 | .13 |
Data are presented as n (%) or mean ± SD.
Abbreviations: HA, hyaluronic acid; MSC, mesenchymal stromal cells; WORMS, Whole‐Organ Magnetic Resonance Imaging Score.
Discussion
This trial examined the safety and efficacy of both single and repeated injections of UC‐MSCs in patients with symptomatic knee OA as compared with intra‐articular HA. To the best of our knowledge, this is the first survey of UC‐MSCs in knee OA, and also the first trial of repeated intra‐articular MSC injections. No safety signals were detected in the experimental groups as compared with HA controls. At the end of the study follow‐up (12 months), the group with repeated UC‐MSC intra‐articular injections (20 × 106 every 6 months) experienced significant clinical changes in total WOMAC, pain component, and VAS when compared with HA (Table 3). Compared with baseline, only patients in the MSC groups experienced significant amelioration of pain and disability at 6 and 12 months of follow‐up as opposed to the control HA group (Fig. 3). Finally, no significant differences in the MRI scores were detected among the study groups (Table 4).
MSCs exhibit anti‐inflammatory properties in response to tissue damage and/or pro‐inflammatory states that lead to widespread suppressive effects on the maturation of dendritic cells, macrophages, Natural Killer (NK), and cytotoxic T lymphocytes 33. They have also been shown to promote a pool of endogenous cells to proliferate and contribute to chondrogenesis by renewing extracellular matrix and chondral synthesis of type II collagen 34. But despite the capacity of MSCs to differentiate into mesodermal cell lineages including cartilage, initial regenerative claims concerning therapeutic effects in OA have been revised given the more recent evidence suggesting that paracrine and anti‐inflammatory actions are crucial with respect to the tissue‐restoring effects of MSC treatments 35.
For example, Ichiseki et al. 36 have examined the role of MSCs in an enzymatic rat OA model. When cells were injected intra‐articularly, an increase in the expression of TNF‐α‐stimulated gene/protein 6 (TSG‐6) was observed in the joint cartilage, with no expression in the control group. A reduction of metalloprotease A disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS5) levels was evidenced as well. TSG‐6 has been reported as a mediator of the beneficial effect of MSCs in both cardiac and lung injury models of tissue damage. On the other hand, the expression of anti‐Calcitonin Gene‐Related Peptide in the C5 dorsal horn of the OA animals was also significantly decreased, indicating a suppressive effect on the central sensitization component of pain. Saulnier et al. 37 confirmed the reduced expression of metalloproteinases (1, 3, and 13) after MSC injection, but describe that MSC‐conditioned medium can also convey these anti‐inflammatory effects on OA synoviocytes. In sum, MSC treatments are known to induce potent anti‐inflammatory, tissue‐restoring, and analgesic effects that could explain the clinical findings described in recent trials as well as in the present phase I/II study.
Indeed, the size of the effect for clinical response of the VAS score in this trial reached 0.81 for cell therapy, evidencing a high symptomatic impact, leading to a number needed‐to‐treat of only 2.1. Such an effect surpasses the usual findings reported for intra‐articular placebo or HA according to recent meta‐analyses 38, 39, 40. Somehow underscoring these effects, the OARSI clinical responder status was achieved by all (100%) MSC‐2 group patients at 6 and 12 months. This is usually achieved only in 50%–60% of patients receiving intra‐articular steroids or HA during initial (3–6 months) follow‐up 41, 42. Our own data confirmed this trend, but without reaching significance (100% vs. 62%, p = .08; Fig. 3). Taken together, our findings suggest that clinical impact of UC‐MSC treatment is beyond the expected placebo effect described for most therapeutic approaches in knee OA 43, 44.
The past few years have witnessed increasing efforts in translating the benefits ascribed to cell therapy in a restricted—usually FCD—group of OA patients to the larger population with established nonfocal degenerative disease. Autologous cell‐based therapy with bone marrow (BM‐MSCs) and adipose tissue‐derived cells (ASCs) has been the aim of most of these series. Although MSC doses and follow‐up are variable between studies, all have used a single intra‐articular injection at baseline. Orozco et al. 19 demonstrated 70% pain relief with 40 × 106 autologous BM‐MSCs at 1 and 2 years of follow‐up, and subsequently, a 40% reduction of pain and 30% improvement in joint function in a more recent open label, single‐arm study of 15 patients 45. Jo et al. assessed ASCs in a proof of concept study at three different intra‐articular cell doses (10, 50, and 100 × 106), achieving a moderate decrease in WOMAC at 6 months only in the high‐dose group 20. However, in a phase I dose‐response trial by Pers et al. 21, preliminary evidence of clinical benefit at 6 months in terms of pain and disability was achieved only with the smallest cell dosing, of 2 × 106. The combination of MSC plus HA in a single injection has not added any clinical benefit so far 46.
Even if all such MSC‐based trials report a good safety record, and some degree of improvement of cartilage quality or chondral defects 20, 21, 45, autologous cell therapy does encounter limitations. When derived from bone marrow, a 100‐fold decline of precursor cells has been noted from birth to the average age of typical OA patients 47. Furthermore, reduced chondrogenic activity of MSCs has been described in cultures originating from individuals with advanced OA 48. In this regard, the allogeneic source provided in the present trial (UC‐MSC) was shown to express superior clonogenicity, migration, and paracrine capacities in vitro, as well as less senescence when compared with BM‐MSCs 23. Indeed, our cell source has been extensively characterized and tested for alloreactivity 23, immunoregulatory and paracrine effects 24, and tumor development in immunodeficient laboratory animals 23, 49. This underscores that allogeneic cell sources can be batch tested for quality, purity, or potency 32, and made accessible for “off the shelf” administration, with no need for delays or prior invasive harvesting procedures. In spite of these advantages, clinical testing of allogeneic cell sources in OA has been limited. In cases with established disease, Vega et al. 22 have recently published a trial with 40 × 106 allogeneic BM‐MSCs showing 10% reduction of pain by WOMAC and mild improvement in cartilage morphology by MRI T2 mapping. Gupta et al. 50, in a study also with allogeneic BM‐MSCs, found no significant differences when comparing several intra‐articular cell doses (25, 50, 75, and 150 million) with placebo. In our protocol, we chose a dose of 20 × 106 UC‐MSCs, considered intermediate, given the large variability (3 to 150 × 106 cells) reported in trials. Another advantage of our trial was to test repeated MSC injections, totaling 40 × 106 cells and allowing the comparison of single versus double MSC doses, with respect to HA administration, because the available preclinical data suggested periodic application of MSCs could inhibit OA progression in rats 51. In contrast with preclinical observations by Joswig et al. 52, in our case, repeated intra‐articular injection of UC‐MSCs in patients did not trigger more frequent or severe AEs. Although allogeneic Adipose Tissue (AT)‐MSCs have been shown to induce a humoral immune response in patients 53, our prior experience with the UC‐MSC source from this trial describes no emergence of donor reactivity in response to treatment 23.
Regarding structural outcomes, we detected no change at 6‐ or 12‐month follow‐up in our patients, possibly given the mild baseline involvement upon imaging. Initial WORMS scores were low (46.1 and 40.1 out of total 332 in MSC‐1 and ‐2 groups), and indexes for the cartilage component showed only limited disease according to our inclusion criteria. Nonetheless, longer‐term—2 year—data from our series could be informative because Vangsness et al. 18 have noted a preventive effect of MSC cell‐based therapy in OA development, with increased (3.1×) odds of disease progression at 2‐year follow‐up in controls. The main limitations of this phase I/II, randomized, controlled, triple‐blind trial are related to the small number of patients per group and the mild‐to‐moderate OA, which was associated with a low clinical and radiological involvement.
Conclusion
In this phase I/II trial, a repeated UC‐MSC dose strategy led to a favorable safety profile and improved clinical result for the treatment of long‐term pain in knee OA patients.
Author Contributions
J.M. and D.A.: conception and design, provision of study material or patients, final approval of manuscript; M.O.: conception and design, provision of study material or patients, manuscript writing, data analysis and interpretation, final approval of manuscript; C.I.: collection and/or assembly of data, administrative support, data analysis and interpretation, final approval of manuscript; R.T.‐L.: provision of study material or patients, final approval of manuscript; M.I.C.: collection and/or assembly of data, final approval of manuscript; F.A.‐M.: provision of study material, final approval of manuscript; P.L.G.: provision of study material or patients, final approval of manuscript; E.M.: data analysis and interpretation, final approval of manuscript; M.K.: conception and design, final approval of manuscript; F.E.F. and F.E.: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
C.I., R.T.‐L., M.I.C., F.A.‐M., P.L.G., and M.K. have declared employment/leadership position with Cells for Cells. F.E. has declared employment/leadership position and intellectual property or patent holder with Cells for Cells. The other authors indicated no potential conflicts of interest.
References
- 1. Abbott JH, Usiskin IM, Wilson R et al. The quality‐of‐life burden of knee osteoarthritis in New Zealand adults: A model‐based evaluation. PLoS One 2017;12:e0185676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Losina E, Walensky RP, Reichmann WM et al. Impact of obesity and knee osteoarthritis on morbidity and mortality in older Americans. Ann Intern Med 2011;154:217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu Q, Niu J, Huang J et al. Knee osteoarthritis and all‐cause mortality: The Wuchuan Osteoarthritis Study. Osteoarthr Cartil 2015;23:1154–1157. [DOI] [PubMed] [Google Scholar]
- 4. Gore M, Tai KS, Sadosky A et al. Clinical comorbidities, treatment patterns, and direct medical costs of patients with osteoarthritis in usual care: A retrospective claims database analysis. J Med Econ 2011;14:497–507. [DOI] [PubMed] [Google Scholar]
- 5. McKenna MT, Michaud CM, Murray CJ et al. Assessing the burden of disease in the United States using disability‐adjusted life years. Am J Prev Med 2005;28:415–423. [DOI] [PubMed] [Google Scholar]
- 6. Minas T, Nehrer S. Current concepts in the treatment of articular cartilage defects. Orthopedics 1997;20:525–538. [DOI] [PubMed] [Google Scholar]
- 7. Ogura T, Mosier BA, Bryant T et al. A 20‐year follow‐up after first‐generation autologous chondrocyte implantation. Am J Sports Med 2017;45:2751–2761. [DOI] [PubMed] [Google Scholar]
- 8. Barry F, Murphy M. Mesenchymal stem cells in joint disease and repair. Nat Rev Rheumatol 2013;9:584–594. [DOI] [PubMed] [Google Scholar]
- 9. Le Blanc K. Mesenchymal stromal cells: Tissue repair and immune modulation. Cytotherapy 2006;8:559–561. [DOI] [PubMed] [Google Scholar]
- 10. François S, Bensidhoum M, Mouiseddine M, Mazurier C, Allenet B, Semont A, Frick J, Saché A, Bouchet S, Thierry D, Gourmelon P, Gorin NC, Chapel A Local irradiation not only induces homing of human mesenchymal stem cells at exposed sites but promotes their widespread engraftment to multiple organs: A study of their quantitative distribution after irradiation damage. Stem Cells 2006;24:1020–1029. [DOI] [PubMed] [Google Scholar]
- 11. Diekman BO, Wu CL, Louer CR et al. Intra‐articular delivery of purified mesenchymal stem cells from C57BL/6 or MRL/MpJ superhealer mice prevents posttraumatic arthritis. Cell Transplant 2013;22:1395–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Horie M, Choi H, Lee RH et al. Intra‐articular injection of human mesenchymal stem cells (MSCs) promote rat meniscal regeneration by being activated to express Indian hedgehog that enhances expression of type II collagen. Osteoarthritis Cartilage 2012;20:1197–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Toghraie F, Razmkhah M, Gholipour MA, Faghih Z, Chenari N, Torabi Nezhad S, Nazhvani Dehghani S, Ghaderi A. Scaffold‐free adipose‐derived stem cells (ASCs) improve experimentally induced osteoarthritis in rabbits. Arch Iran Med 2012;15:495–499. [PubMed] [Google Scholar]
- 14. Murphy JM, Fink DJ, Hunziker EB et al. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum 2003;48:3464–3474. [DOI] [PubMed] [Google Scholar]
- 15. ter Huurne M, Schelbergen R, Blattes R, Blom A, de Munter W, Grevers LC, Jeanson J, Noël D, Casteilla L, Jorgensen C, van den Berg W, van Lent PLEM Antiinflammatory and chondroprotective effects of intraarticular injection of adipose‐derived stem cells in experimental osteoarthritis. Arthritis Rheum 2012;64:3604–3613. [DOI] [PubMed] [Google Scholar]
- 16. Saw KY, Anz A, Merican S et al. Articular cartilage regeneration with autologous peripheral blood progenitor cells and hyaluronic acid after arthroscopic subchondral drilling: A report of 5 cases with histology. Arthroscopy 2011;27:493–506. [DOI] [PubMed] [Google Scholar]
- 17. Saw KY, Anz A, Siew‐Yoke Jee C et al. Articular cartilage regeneration with autologous peripheral blood stem cells versus hyaluronic acid: A randomized controlled trial. Arthroscopy 2013;29:684–694. [DOI] [PubMed] [Google Scholar]
- 18. Vangsness CT, Farr J, Boyd J et al. Adult human mesenchymal stem cells delivered via intra‐articular injection to the knee following partial medial meniscectomy: A randomized, double‐blind, controlled study. J Bone Joint Surg Am 2014;96:90–98. [DOI] [PubMed] [Google Scholar]
- 19. Orozco L, Munar A, Soler R, Alberca M, Soler F, Huguet M, Sentís J, Sánchez A, García‐Sancho J. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: Two‐year follow‐up results. Transplantation 2014;97:e66–e68. [DOI] [PubMed] [Google Scholar]
- 20. Jo CH, Lee YG, Shin WH et al. Intra‐articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof‐of‐concept clinical trial. Stem Cells 2014;32:1254–1266. [DOI] [PubMed] [Google Scholar]
- 21. Pers YM, Rackwitz L, Ferreira R, Pullig O, Delfour C, Barry F, Sensebe L, Casteilla L, Fleury S, Bourin P, Noël D, Canovas F, Cyteval C, Lisignoli G, Schrauth J, Haddad D, Domergue S, Noeth U, Jorgensen C, on behalf of the ADIPOA Consortium .Adipose mesenchymal stromal cell‐based therapy for severe osteoarthritis of the knee: A phase I dose‐escalation trial. Stem Cells Translational Medicine 2016;5:847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vega A, Martín‐Ferrero MA, Del Canto F et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: A randomized controlled trial. Transplantation 2015;99:1681–1690. [DOI] [PubMed] [Google Scholar]
- 23. Bartolucci J, Verdugo FJ, González PL et al. Safety and efficacy of the intravenous infusion of umbilical cord mesenchymal stem cells in patients with heart failure: A phase 1/2 randomized controlled trial (RIMECARD trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy]). Circ Res 2017;121:1192–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. González PL, Carvajal C, Cuenca J, Alcayaga‐Miranda F, Figueroa FE, Bartolucci J, Salazar‐Aravena L, Khoury M. Chorion mesenchymal stem cells show superior differentiation, immunosuppressive, and angiogenic potentials in comparison with haploidentical maternal placental cells. Stem Cells Translational Medicine 2015;4:1109–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Park YB, Ha CW, Lee CH et al. Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood‐derived mesenchymal stem cells and hyaluronate hydrogel: Results from a clinical trial for safety and proof‐of‐concept with 7 years of extended follow‐up. Stem Cells Translational Medicine 2017;6:613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lohan P, Treacy O, Griffin MD et al. Anti‐donor immune responses elicited by allogeneic mesenchymal stem cells and their extracellular vesicles: Are we still learning? Front Immunol 2017;8:1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Escobar A, Quintana JM, Bilbao A et al. Validation of the Spanish version of the WOMAC questionnaire for patients with hip or knee osteoarthritis. Clin Rheumatol 2002;21:466–471. [DOI] [PubMed] [Google Scholar]
- 28. Alonso J, Prieto L, Anto JM. The Spanish version of the SF‐36 health survey (the SF‐36 health questionnaire): An instrument for measuring clinical results [in Spanish]. Med Clin (Barc) 1998;104:771–776. [PubMed] [Google Scholar]
- 29. Dougados M, Leclaire P, van der Heijde D et al. Response criteria for clinical trials on osteoarthritis of the knee and hip: A report of the Osteoarthritis Research Society International Standing Committee for Clinical Trials response criteria initiative. Osteoarthritis Cart 2000;8:395–403. [DOI] [PubMed] [Google Scholar]
- 30. Peterfy CG, Guermazi A, Zaim S, Tirman PFJ, Miaux Y, White D, Kothari M, Lu Y, Fye K, Zhao S, Genant HK Whole‐Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage 2004;12:177–190. [DOI] [PubMed] [Google Scholar]
- 31. Dominici M, Le Blanc K, Mueller I et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315–317. [DOI] [PubMed] [Google Scholar]
- 32. Jeong SY, Kim DH, Ha J et al. Thrombospondin‐2 secreted by human umbilical cord blood‐derived mesenchymal stem cells promotes chondrogenic differentiation. Stem Cells 2013;31:2136–2148. [DOI] [PubMed] [Google Scholar]
- 33. Kyurkchiev D, Bochev I, Ivanova‐Todorova E et al. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J Stem Cells 2014;6:552–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang X, Wu S, Naccarato T et al. Regeneration of hyaline‐like cartilage in situ with SOX9 stimulation of bone marrow‐derived mesenchymal stem cells. PLoS One 2017;12:e0180138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vizoso F, Eiro N, Cid S et al. Mesenchymal stem cell secretome: Toward cell‐free therapeutic strategies in regenerative medicine. Int J Mol Sci 2017;18:1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ichiseki T, Shimazaki M, Ueda Y et al. Intraarticularly‐injected mesenchymal stem cells stimulate anti‐inflammatory molecules and inhibit pain related protein and chondrolytic enzymes in a monoiodoacetate‐induced rat arthritis model. Int J Mol Sci 2018;19:E203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Saulnier N, Viguier E, Perrier‐Groult E et al. Intra‐articular administration of xenogeneic neonatal mesenchymal stromal cells early after meniscal injury down‐regulates metalloproteinase gene expression in synovium and prevents cartilage degradation in a rabbit model of osteoarthritis. Osteoarthritis Cartilage 2015;23:122–133. [DOI] [PubMed] [Google Scholar]
- 38. Xing D, Wang B, Liu Q et al. Intra‐articular hyaluronic acid in treating knee‐osteoarthritis: A PRISMA‐compliant systemic review of overlapping metanalysis. Sci Rep 2016;6:32790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bellamy N, Campbell J, Robinson V et al. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev 2006;2:CD005321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang W, Robertson J, Jones AC et al. The placebo effect and its determinants in osteoarthritis: Meta‐analysis of randomized controlled trials. Ann Rheum Dis 2008;67:1716–1723. [DOI] [PubMed] [Google Scholar]
- 41. Rutjes AW, Jüni P, da Costa BR et al. Viscosupplementation for osteoarthritis of the knee: A systematic review and meta‐analysis. Ann Intern Med 2012;157:180–191. [DOI] [PubMed] [Google Scholar]
- 42. Leighton R, Akermark C, Therrien R et al. NASHA hyaluronic acid vs. methylprednisolone for knee osteoarthritis: A prospective, multi‐centre, randomized, non‐inferiority trial. Osteoarthritis Cartilage 2014;22:17–25. [DOI] [PubMed] [Google Scholar]
- 43. Mandl LA, Losina E. Relative efficacy of knee osteoarthritis treatments: Are all placebos created equal?. Ann Intern Med 2015;162:71–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Strand V, Baraf HS, Lavin PT et al. A multicenter, randomized controlled trial comparing a single intra‐articular injection of Gel‐200, a new cross‐linked formulation of hyaluronic acid, to phosphate buffered saline for treatment of osteoarthritis of the knee. Osteoarthritis Cartilage 2012;20:350–356. [DOI] [PubMed] [Google Scholar]
- 45. Soler R, Orozco L, Munar A et al. Final results of a phase I‐II trial using ex vivo expanded autologous mesenchymal stromal cells for the treatment of osteoarthritis of the knee confirming safety and suggesting cartilage regeneration. Knee 2016;23:647–654. [DOI] [PubMed] [Google Scholar]
- 46. Lamo‐Espinosa JM, Mora G, Blanco JF et al. Intra‐articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: Multicenter randomized controlled clinical trial (phase I/II). J Transl Med 2016;14:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Caplan A. Why are MSCs therapeutic? New data: New insight. J Pathol 2009;217:318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Murphy JM, Dixon K, Beck S et al. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum 2002;46:704–713. [DOI] [PubMed] [Google Scholar]
- 49. Kim JY, Jeon HB, Yang YS et al. Application of human umbilical cord blood‐derived mesenchymal stem cells in disease models. World J Stem Cells 2010;2:34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gupta PK, Chullikana A, Rengasamy M et al. Efficacy and safety of adult human bone marrow‐derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel®): Preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Res Ther 2016;18:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ozeki N, Muneta T, Koga H, Nakagawa Y, Mizuno M, Tsuji K, Mabuchi Y, Akazawa C, Kobayashi E, Matsumoto K, Futamura K, Saito T, Sekiya I. Not single but periodic injections of synovial mesenchymal stem cells maintain viable cells in knees and inhibit osteoarthritis progression in rats. Osteoarthritis Cartilage 2016;24:1061–1070. [DOI] [PubMed] [Google Scholar]
- 52. Joswig AJ, Mitchell A, Cummings KJ et al. Repeated intra‐articular injection of allogeneic mesenchymal stem cells causes an adverse response compared to autologous cells in the equine model. Stem Cell Res Ther 2017;8:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Panés J, García‐Olmo D, Van Assche G et al. Expanded allogeneic adipose‐derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn's disease: A phase 3 randomised, double‐blind controlled trial. Lancet 2016;388:1281–1290. [DOI] [PubMed] [Google Scholar]


