Abstract
The human airway epithelium is regenerated by basal cells. Thus, basal cell therapy has the potential to cure cystic fibrosis (CF) lung disease. We previously reported that the human basal cells repopulated the mouse airway epithelium after transplantation, and we estimated that 60 million cells would be needed to treat a human patient. To further develop cell therapy, we compared the proliferation potential of non‐CF and CF tissue‐derived bronchial basal cells. Three methods were used: regenerative cell frequency, burst size, and cell division frequency. Second, we used a serial passage strategy to determine if CF basal cells could be amplified to the estimated therapeutic dose. These studies evaluated that tissue‐derived bronchial basal cells and the basal cells that were recovered by brushing bronchial airways or the nasal respiratory epithelium. Finally, we used the limiting dilution method to isolate non‐CF and CF basal cell clones. The proliferation assays and the air‐liquid‐interface differentiation method were used to determine if cell amplification altered the proliferation and/or differentiation potential of clonal isolates. We demonstrate that: (a) non‐CF and CF basal cell proliferation is similar, (b) CF basal cells can be amplified to a therapeutic cell dose, and (c) amplified non‐CF and CF basal cell clones differentiate normally. Despite these encouraging findings, we also find that the cell amplification process depletes the regenerative basal cell pool. Analysis of basal cell clones indicates that serial passage selects for long‐lived basal cells and raise the possibility that prospective isolation of these stem‐like cells will improve the efficacy of cell replacement therapy. stem cells translational medicine 2019;8:225&235
Keywords: Airway, Basal cell, Cystic fibrosis, Epithelium, Stem, Progenitor
Significance Statement.
The present study supports the feasibility of autologous cell therapy for cystic fibrosis lung disease.
Introduction
Our long‐term goal is to develop a cell therapy for cystic fibrosis (CF) lung disease. CF is caused by mutations in the CF transmembrane conductance regulator (CFTR) gene, which results in abnormal chloride and bicarbonate secretion 1, 2. In the CF airway, CFTR mutation results in viscous respiratory secretions, chronic infection, inflammation, and tissue damage. The concept underlying our cell therapy initiative is that CF lung disease can be reversed through replacement of the CF airway epithelium with cells that express wild‐type CFTR.
The human conducting airway epithelium is maintained and regenerated by basal cells 3, 4, 5. Although there is controversy regarding the existence of an airway epithelial tissue stem cell, our work and that of others indicates that such a cell exists and that it is a basal cell subtype 3, 4, 5, 6, 7, 8, 9, 10. We reported a method to separate mouse airway epithelial tissue stem cells from other basal cell subtypes 6. However, methods to purify the human tissue stem cell require refinement 3. As a consequence, we refer to the mixture of tissue stem and progenitor cells using the collective term, “basal cells.”
We recently reported that human airway basal cells repopulate the conducting airway epithelium of immunocompromised mice 11. Airway epithelial chimerism was observed 14 days after cell delivery and persisted for at least 43 days. This transplantation study allowed us to estimate that 60 million cells will be needed each time a patient is treated with cell therapy. This large cell number makes it likely that therapeutic basal cells will be amplified in vitro prior to transplantation.
Optimally, cell therapy for CF lung disease will use autologous cells that have undergone correction of the CFTR gene mutation. The feasibility of this approach may be limited by disease‐related decrements in the mitotic and differentiation potential of CF basal cells 12 and the extensive selection and amplification that is required for successful gene editing 13, 14. The first goal of this study was to determine if the proliferation potential of CF basal cells was different from that of non‐CF basal cells. For these studies, bronchial tissue samples were recovered from non‐CF lung donors and F508del/F508del CF patients who were undergoing lung transplantation. We used enzymatic digestion to recover single cells and expanded the basal cell population using the modified conditional reprogramming culture (mCRC) technique 13.
Autologous cell therapy for CF lung disease will likely require a minimally invasive cell recovery technique such as airway epithelial brushing. This cell recovery method has the potential to compromise cell viability and may be more deleterious to the basal cell than enzymatic digestion of tissue explants. Our second objective was to determine if CF basal cells that are recovered using the brushing technique can be amplified to a therapeutic dose. Thus, we compared the amplification potential of tissue‐derived bronchial basal cells and those that were recovered by brushing the bronchial epithelium or the nasal respiratory epithelium. The donors were CF patients who were homozygous for the F508del CFTR mutation or were compound heterozygotes for the F508del CFTR mutation and a non‐F508del CFTR mutation. Basal cells were expanded using the mCRC method.
Cell therapy, in contrast with pharmaceutical treatments, has the potential to cure CF lung disease. However, we previously reported that basal cells have a finite life span 6 and others reported that basal cell differentiation decreased over time in vitro 15. These two parameters could limit the efficacy and durability of cell therapy. Thus, our third goal was to determine if basal cell proliferation and differentiation varied as basal cells were amplified in vitro. These studies used non‐CF and CF basal cells that were recovered from bronchial tissue segments and CF basal cells that were recovered by brushing the nasal respiratory epithelium or the bronchial epithelium. Basal cells were expanded as indicated above, and differentiation was evaluated using the air‐liquid‐interface (ALI) method 16. These studies included analysis of basal cell populations as well as clonal isolates.
Materials and Methods
Human Subjects
The Institutional Review Board at Nationwide Children's Hospital approved this study. Cells were collected after receiving written informed consent.
Donor Demographics
Bronchial tissue samples were obtained at the time of lung transplantation and included samples from the non‐CF donor and the CF recipient (Table 1). Bronchial and nasal brushing samples were obtained from stable CF patients who were undergoing clinically indicated sinus surgery and CF and healthy non‐CF persons who were undergoing research blood draws for surveillance of immune status at baseline (Table 2; Supporting Information Table S1).
Table 1.
Clinical data of organ donors and recipients
| Donor # | Age (yr) | Sex | Cause of death | Past smoking history | Weight (kg) | Height (cm) | Blood products during procurement | Microbiology | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 50 | M | Suicide, gunshot wound | >20 pack year | 95.25 | 170.18 | RBC 1,200 ml, FFP 946 ml, albumin 350 ml | None drawn | |
| 2 | 25 | M | Suicide, gunshot wound | None | 74.39 | 182.88 | RBC 300 ml, PLT 450 ml | Methicillin‐susceptible Staphylococcus aureus, Streptococcus pnuemoniae, Candida albicans | |
| 3 | 4 | M | Nonaccidental trauma | None | 20.87 | 104.14 | RBC 1,444 ml, FFP 910 ml, PLT 604 ml, Cryo 816 ml | Normal flora | |
| 4 | 37 | F | Stroke | None | 66.68 | 167.64 | PLT 1,000 ml | Normal flora | |
| 5 | 54 | F | Stroke | >20 pack year | 87.99 | 137.16 | Albumin 250 ml | Oropharyngeal microbes >10,000 cfu/ml | |
| 6 | 16 | M | Suicide, gunshot wound | None | 64.86 | 175.26 | RBC 4,433 ml, FFP 280, albumin 100 ml, PLT 270 ml, Cryo 130 ml | Sputum negative | |
| Recipient # | Age | Sex | CFTR genotype | Weight (kg) | Height (cm) | BMI (kg/m 2 ) | Pancreatic insufficiency | Orkambi | |
| 1 | 22 | M | F508del | F508del | 45.5 | 171.33 | 15.5 | Yes | No |
| 2 | 22 | M | F508del | F508del | 43.3 | 165.86 | 15.74 | Yes | No |
| 3 | 38 | F | F508del | F508del | 45.9 | 162.00 | 17.49 | Yes | No |
| 4 | 36 | M | F508del | F508del | 90.6 | 186.5 | 26.05 | Yes | No |
| 5 | 15 | F | F508del | F508del | 40.9 | 157.9 | 16.4 | Yes | Yes |
| 6 | 35 | F | F508del | F508del | 38.2 | 149.9 | 17.0 | Yes | No |
Abbreviations: BMI, body mass index; CFTR, cystic fibrosis transmembrane conductance regulator; Cryo, cryoprecipitate; F, female; FFP, fresh frozen plasma; M, male; PLT, plasmaLyte; RBC, red blood cells.
Table 2.
Clinical data for nasal and bronchial brushing donors
| Donor # | Age (yr) | Sex | CFTR genotype | Weight (kg) | Height (cm) | BMI (kg/m2) | Pancreatic insufficiency | Kalydeco | Orkambi | FEV1 %PD | Nasal samples | Bronchial samples | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 27 | M | F508del | F508del | 104.2 | 180.4 | 32.08 | Yes | No | No | 81 | Yes | Yes |
| 2 | 15 | M | F508del | F508del | 53.5 | 169.5 | 18.66 | Yes | No | Yes | 93 | Yes | Yes |
| 3 | 13 | F | F508del | G551D | 61.6 | 163 | 23.23 | Yes | Yes | No | 94 | Yes | Yes |
| 4 | 21 | M | F508del | F508del | 86.7 | 182 | 26.23 | Yes | No | Yes | 133 | Yes | Yes |
| 5 | 1 | M | F508del | 390insT | 13.7 | 89.4 | 17.14 | Yes | No | No | ND | Yes | Yes |
| 6 | 6 | F | F508del | R1162X | 25.8 | 124.8 | 16.6 | Yes | No | No | 99 | Yes | Yes |
| 7 | 17 | M | F508del | F508del | 54.2 | 160 | 21.22 | Yes | No | No | 80 | Yes | No |
| 8 | 23 | F | F508del | EX2_3del | 46.9 | 151 | 20.61 | Yes | No | No | 81 | No | Yes |
| 9 | 20 | M | F508del | c.2215delG | 66.7 | 172.6 | 22.39 | Yes | No | No | 87 | Yes | No |
| 10 | 17 | M | F508del | R553X | 73.5 | 169.6 | 25.55 | Yes | No | No | 100 | Yes | Yes |
| 11 | 40 | F | F508del | F508del | 63 | 167.2 | 22.54 | Yes | No | No | 61 | Yes | Yes |
| 12 | 15 | F | F508del | F508del | 53.5 | 158.3 | 21.35 | Yes | No | Yes | 109 | Yes | No |
| 13 | 15 | F | F508del | F508del | 53.4 | 156.7 | 21.75 | Yes | No | Yes | 93 | Yes | Yes |
| 14 | 14 | M | F508del | 7T/9T variant | 39.6 | 160.6 | 15.35 | Yes | No | No | 75 | No | Yes |
| 15 | 15 | F | F508del | F508del | 50 | 164.6 | 18.45 | Yes | No | Yes | 103 | Yes | Yes |
| 16 | 2 | M | F508del | 3909C>G | 10.8 | 80 | 16.88 | Yes | No | No | ND | No | Yes |
| 17 | 16 | M | F508del | F508del | 60.1 | 176.7 | 19.25 | Yes | No | No | 93 | Yes | Yes |
Abbreviations: BMI, body mass index; CFTR, cystic fibrosis transmembrane conductance regulator; F, female; FEV1, forced expiratory volume in one second; M, male; ND, not determined; PD, predicted.
Airway Epithelial Cell Recovery
Bronchial tissue was digested with 0.15% pronase overnight at 4°C, and the cells were recovered as previously reported 17. The bronchial airway epithelium was sampled using a flexible fiberoptic bronchoscope and a protected bronchial brush. The sampling sites were the main bronchi including the lingula. The nasal respiratory epithelium was sampled as previously reported 13. Nasal and bronchial cells were recovered from the brushes as previously reported 13 and were treated with 14.3 μM 2‐mercaptoethanol to remove mucus.
Basal Cell Expansion
Nasal and bronchial epithelial cells were cultured using the mCRC method as previously reported 13. At passage 1, all cells in the culture were basal cells.
Clone‐Forming Cell Frequency assay
Regenerative basal cells were enumerated using the limiting dilution assay as previously reported 3.
Basal Cell Cloning
Passage 1 basal cells were seeded at five cells per well in 96‐well cell culture and cultured according to the mCRC method for 5–7 days. Wells containing a single colony were identified and verified after removal of the fibroblast feeder layer. The basal cells were then recovered by a second trypsinization and cultured in one well of a 12‐well plate.
Serial Passage
Cells were cultured at 6.67 × 103 cells per centimeter square and harvested at 80% confluence.
Basal Cell Differentiation
Basal cells were plated onto collagen‐coated 0.33 cm2 transwell membranes at 2 × 104 cells per membrane as previously described 13. At confluence, the medium was changed to half and half differentiation medium 18, which was composed of equal volumes of Wu differentiation medium 16 and Pneumacult Base Medium containing the ×10 supplement, heparin, and hydrocortisone (Stemcell Technologies, Vancouver, BC, Canada). On differentiation day 21, the cultures were fixed and stained. Nuclei were detected using 4′,6‐diamidino‐2‐phenylindole (DAPI), mucus cells were detected with rabbit‐anti‐MUC5b (1/100 19), and ciliated cells were detected with mouse‐anti‐acetylated tubulin (1/8,000, ACT 19). Differentiation was quantified using a serological method 13 that meets the American Thoracic Society standard for assessment of histological data sets 20.
Statistics
Statistical analysis was performed with GraphPad Prism version 7.00 for Windows, GraphPad Software, La Jolla, CA, USA, www.graphpad.com. Descriptive statistics for continuous variables were presented as means and standard deviations. Normally distributed data sets were evaluated by Student's t test, and data sets that exhibited non‐normal distributions were analyzed by the Mann‐Whitney test. A p value of ≤.05 was considered to be significant. Data sets containing multiple variables were analyzed by analysis of variance and a post hoc Tukey test. An adjusted p value of ≤.05 was considered to be significant. Linear regression analysis was conducted using the linear model.
Results
The Proliferation Potential of Non‐CF and CF Basal Cells Is Similar
To compare the proliferation potential of non‐CF and CF basal cells, bronchial tissue was recovered at the time of lung transplantation, digested with pronase, and the cells were cultured using the mCRC method. The first study evaluated the functional properties of basal cells from six non‐CF donors and six F508del/F508del CF donors (Table 1). Passage 2 was chosen for this study as this culture time point is commonly used for cell biology studies. A related set of studies evaluated proliferation potential across 10 passages. This study used four of the six non‐CF donors and four of the six CF donors that were used in the passage 2 study.
Our previous studies demonstrated that some but not all basal cells formed colonies in vitro 3, 13. Consequently, basal cells that can generate a clone are referred to as “regenerative” cells. Regenerative basal cell number is quantified using the clone‐forming cell frequency (CFCF) assay, and the number of regenerative cells is reported as the CFCF × 1,000. If all test cells are regenerative cells, the CFCF × 1,000 is 1,000. Conversely, a CFCF × 1,000 of 500 indicates that half the cells are regenerative. We found that regenerative cell frequency does not vary between non‐CF and CF cells at passage 2 (p = .7539; Fig. 1A). Next, we evaluated CFCF in non‐CF and CF cells as a function of passage. We found that the CFCF did not vary between non‐CF and CF cells at any passage (Fig. 1B). We noted that the CFCF decreased with passage and that this change reached significance in non‐CF cells at passage 5 (p = .0081) and in CF cells at passage 6 (p = .0115). CFCF did not vary over passages 5 to 10 in non‐CF cells or over passages 6 to 10 in CF cells.
Figure 1.
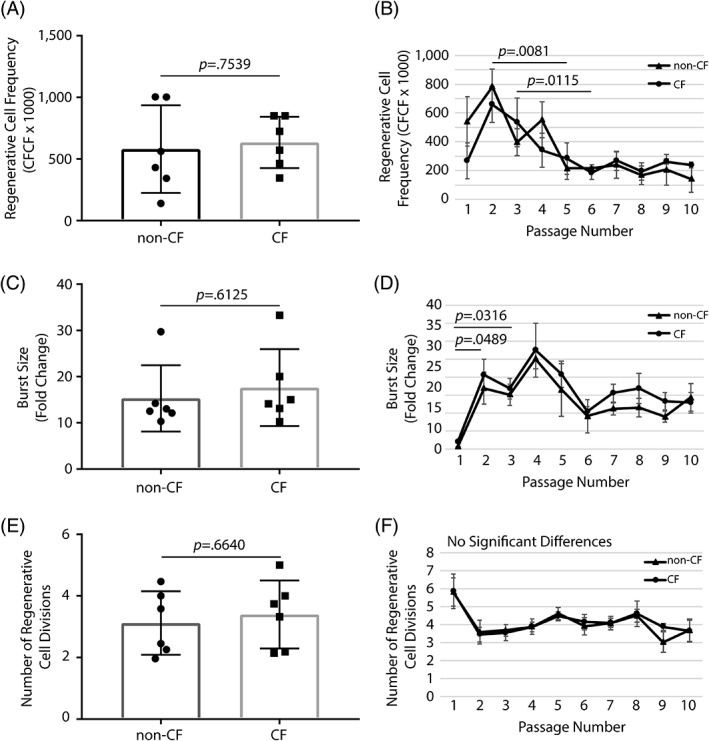
Proliferative properties of basal cells from non‐CF and CF donors. Basal cells were recovered from bronchial tissue at the time of lung transplantation. The donor demographics are summarized in Table 1. (A, C, E): Cells were recovered from six non‐CF donors and six CF donors. (B, D, F): Cells were recovered from four non‐CF donors and four CF donors. (A): Regenerative cell frequency was quantified at passage 2. (B): Changes in regenerative cell frequency were quantified over passages 1 to 10. (C): Burst size was quantified at passage 2. (D): Changes in burst size were quantified over passages 1 to 10. (E): The number of regenerative cell divisions was quantified at passage 2. (F): Changes in regenerative cell divisions were quantified over passages 1 to 10. Data are presented as the mean ± SD. Symbols in panels (A), (C), and (E) represent data from individual donors. Abbreviations: CF, cystic fibrosis; CFCF, clone‐forming cell frequency.
Burst size is defined as the number of cells recovered divided by the number of cells plated. This parameter can be used to determine if regenerative cell proliferation varies between samples. We found that burst size did not vary between non‐CF and CF cells at passage 2 (p = .6152; Fig. 1C). We also found that burst size did not vary between non‐CF and CF cells at any passage (Fig. 1D). However, we did observe a significant increase in burst size for non‐CF cells between passages 1 and 3 (p = .0316) and for CF cells between passages 1 and 2 (p = .0489). Burst size did not vary significantly over passages 2 to 10 in non‐CF or CF cells.
Somatic cells are typically constrained by the Hayflick limit, which is 40 to 50 population doublings 21. Thus, the number of times a regenerative cell divides can be used to determine the impact of cell amplification on progenitor cell life span. We found that the number of regenerative cell divisions did not vary between non‐CF and CF cells at passage 2 (p = .664; Fig. 1E). Similarly, we found that the number of regenerative cell divisions did not vary between non‐CF and CF cells at any passage (Fig. 1F). Collectively, these data indicate that the proliferative potential of non‐CF and F508del/F508del CF bronchial basal cells is similar when the cells are recovered by digestion and cultured using the mCRC method.
CFTR Genotype Does Not Alter Regenerative Basal Cell Frequency
Limitations of the previous study included the focus on F508del/F508del CF donors and use of tissue as a source of basal cells. To address these issues, we first determined if regenerative basal cell frequency varied in bronchial brushing samples recovered from F508del/F508del CF donors and those harboring a F508del allele and another CFTR mutant allele (Table 2). No significant differences in CFCF were identified (Supporting Information Fig. S1A). Similarly, a comparison of regenerative basal cell frequency in nasal brushing samples did not identify genotype‐dependent differences in CFCF (Supporting Information Fig. S1B). These data indicate that regenerative basal cell frequency did not vary between donors harboring one or two F508del alleles and justified pooling the data from CF donors irrespective of CFTR genotype.
Amplification of Human Basal Cells to the Estimated Therapeutic Dose
Our previous study suggested that non‐CF nasal basal cells could be amplified to the estimated therapeutic dose (60 million cells 11) within three passages of cell recovery 13. However, the decrease in regenerative basal cell frequency noted in Figure 1B indicated a need for empirical analysis of amplification potential. To address this issue, we evaluated basal cell amplification using bronchial airway tissue that was recovered from F508del/F508del CF donors at the time of lung transplantation (Table 1) and bronchial and nasal cells that were recovered from the airways CF donors with a spectrum of CFTR mutations (Table 2).
First, we determined if cells that were recovered by enzymatic digestion of explanted CF bronchial airway tissue could be amplified to the estimated therapeutic dose. We found that the total number of tissue digest‐derived CF bronchial basal cells was significantly greater than the estimated therapeutic dose at passage 3 (p = .0471; Fig. 2A). To determine the amount of time needed to amplify digest‐derived bronchial basal cells to the estimated therapeutic dose, we evaluated the duration of each passage. We found that the three‐passage amplification of CF brush‐derived basal cells required 16 days (range 12–17 days; Fig. 2B).
Figure 2.
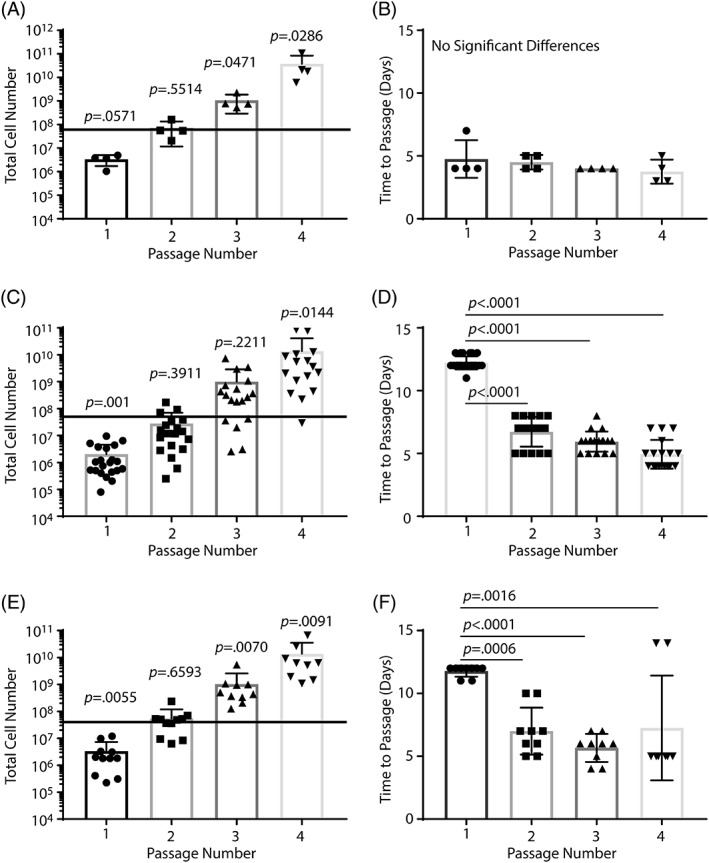
Amplification metrics for cystic fibrosis (CF) basal cells recovered from various airway regions. Basal cells were recovered from bronchial tissue at the time of lung transplantation or by brushing the bronchial airways or the nasal respiratory epithelium. Only cells from CF donors were studied. Donor demographics are presented in Tables 1 and 2 and supporting information table S1. (A, B): Tissue‐derived bronchial basal cell amplification as a function of passage (A) and time to passage (B). n = 4 donors. (C, D): Brush‐derived bronchial basal cell amplification as a function of passage (C) and time to passage (D). n = 9 donors. (E, F): Brush‐derived nasal basal cell amplification as a function of passage (E) and time to passage (F). n = 11 donors. The black line in panels (A), (C), and (E) indicates the estimated therapeutic dose. Data are presented as the mean ± SD. Symbols represent data from different donors.
Next, we determined if cells that were recovered by brushing the bronchial airways of CF patients could be amplified to the estimated therapeutic dose. We found that the total number of brush‐derived CF bronchial basal cells was significantly greater than the estimated therapeutic dose at passage 4 (p = .0144; Fig. 2C). To determine the amount of time needed to amplify brush‐derived bronchial basal cells to the estimated therapeutic dose, we evaluated the duration of each passage (Fig. 2D). We found that the time to passage at passage 1 was significantly longer than at passage 2 (p < .0001), passage 3 (p < .0001), and passage 4 (p < .0001). Overall, amplification of CF brush‐derived basal cells to the estimated therapeutic dose required 29.8 days (range 25–36 days; Fig. 2D).
Finally, we determined if CF nasal respiratory epithelial basal cells could be amplified to the estimated therapeutic dose. We found that total nasal basal cell number was significantly greater than the estimated therapeutic dose at passage 3 (p = .007; Fig. 2E). To determine the amount of time needed to amplify nasal basal cells to the estimated therapeutic dose, we evaluated the duration of each passage (Fig. 2F). We found that the time to passage at passage 1 was significantly longer than at passage 2 (p = .0006), passage 3 (p < .0001), and passage 4 (p = .0016). Overall, amplification of CF brush‐derived basal cells to the estimated therapeutic dose required 24.4 days (range 20–29 days; Fig. 2F).
Although these studies indicate that number of passages and time needed to reach the estimated therapeutic dose varies with airway region and sampling method, an analysis of data for nasal and bronchial brushing samples did not identify region‐dependent differences in regenerative basal cell frequency (Supporting Information Fig. S2). We conclude that the estimated therapeutic dose can be generated using basal cells that are recovered by digestion of explanted bronchial tissue or through brushing the bronchial or nasal airways.
Regenerative Basal Cell Attrition
The study presented in Figure 1B suggested that regenerative basal cell number decreased as digest‐derived bronchial basal cells were passaged. To further evaluate this observation, we determined if regenerative basal cell frequency varied between passages 2 and 10 in the bronchial‐digest‐derived basal cell samples (Fig. 3A). This study demonstrated a significant decrease in regenerative basal cell frequency for both non‐CF (p = .0286) and CF (p = .0286) cells. To evaluate the rate of regenerative basal cell loss, the data were evaluated by linear regression. Over passages 2 to 6, regenerative basal cell frequency decreased by 131.7 ± 44.6 regenerative cells per passage in non‐CF cells and 120.6 ± 12.5 regenerative cells per passage in CF cells. This decrease was significant for both non‐CF (p = .049) and CF (p = .0024) cells. Regenerative cell frequency did not vary significantly between passages 6 and 10 for non‐CF (p = .5667) or CF cells (p = .3125). These data indicated that the tissue digest‐derived bronchial regenerative basal cell pool decreased rapidly over passages 2 to 6 and then stabilized over passages 6 to 10.
Figure 3.
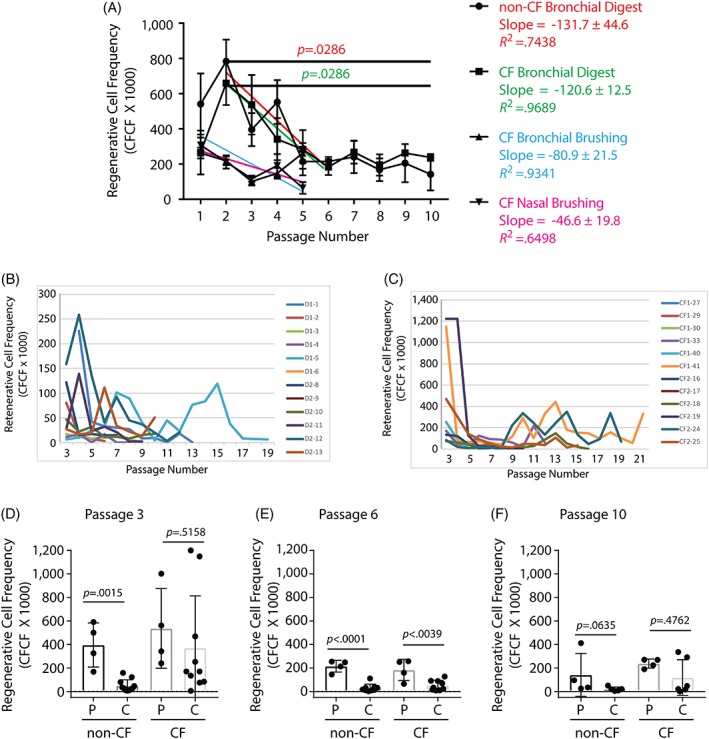
Regenerative cell frequency decreases in serially passaged human basal cell populations and clonal isolates. Basal cells were recovered from bronchial tissue at the time of lung transplantation (non‐CF and CF), by brushing the bronchial airways (CF only), or brushing the nasal respiratory epithelium (CF only). Donor demographics are presented in Tables 1 and 2 and supporting information table S1. (A): Regenerative basal cells are lost in two phases: rapid and slow. Data are presented as the mean ± SD (n = 4 non‐CF bronchial tissue digest samples, n = 4 CF bronchial digest samples, n = 14 bronchial brushing samples, and n = 14 nasal brushing samples). The colored trend lines correspond to the statistical analyses presented to the right of the graph. (B, C): Regenerative basal cell frequency in basal cell clones that were derived from digests of non‐CF (B) and CF (C) bronchial tissue. Data are presented as the mean for each clone at each passage. n = 12 non‐CF clones and n = 12 CF clones. (D–F): Comparison of regenerative basal cell frequency in populations (P) and clonal isolates (C) at passage 3 (D), passage 6 (E), and passage 10 (F). n = 4 non‐CF populations, n = 12 non‐CF clones, n = 4 CF populations, and n = 12 CF clones. Data are presented as the mean ± SD. Symbols represent the data from individual populations or clones. Abbreviations: CF, cystic fibrosis; CFCF, clone‐forming cell frequency.
Next, we determined if regenerative basal cell frequency varied with passage in CF cells that were recovered by brushing the bronchial or nasal epithelium. A linear regression analysis of passages 1 to 5 suggested that regenerative basal cell frequency decreased by 80.9 ± 21.51 cells per passage in the bronchial samples and by 46.6 ± 19.8 cells per passage in the nasal samples. However, this decrease was not significant for either non‐CF (p = .1653) or CF (p = .0994) cells. Collectively, these data indicate that regenerative basal cell loss is a characteristic of cell preparations which have a high initial CFCF, that CFCF stabilizes at ∼200, and that this pattern is not altered by sampling site or cell recovery method.
To determine if regenerative basal cell attrition was a characteristic of all basal cells within a population, we cloned basal cells from populations of non‐CF and CF basal cells that were recovered by digestion of bronchial tissue (Table 1). Cloning occurred at passage 1. The parental population and six clones per parental population were serially passaged using the mCRC culture method (Table 3). Culture of one non‐CF parental isolate was discontinued at passage 20, and the other parental isolate terminated at passage 15. Among the 12 non‐CF clones, 3 terminated by passage 6, 5 terminated between passages 6 and 10, and 4 survived more than 10 passages. Within the latter group, culture of one clone was discontinued at passage 18. Culture of both parental CF isolates was discontinued at passage 24. Among the 12 CF clones, 4 terminated between passages 3 and 6, 3 terminated between passages 6 and 10, and 5 survive more than 10 passages. The latter group contained two clones that survived to at least passages 19 or 24.
Table 3.
Serial passage history of regenerative basal cell clones.
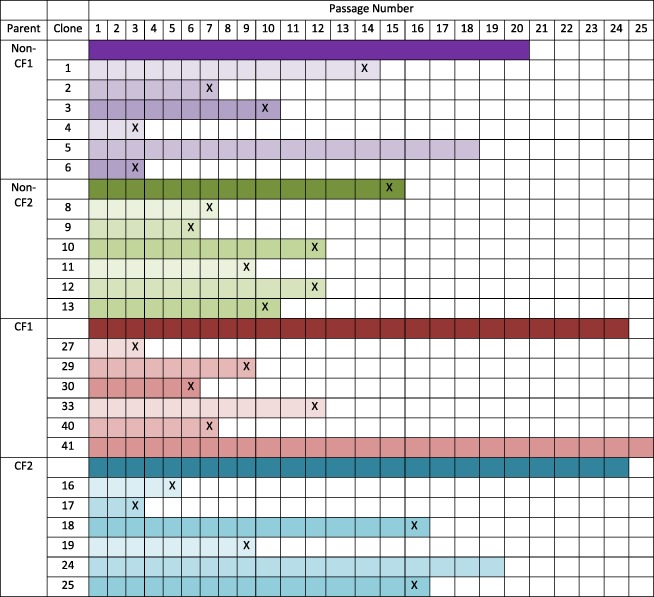
|
Abbreviations: CF, cystic fibrosis; X, culture terminated at the indicated passage.
We next used the CFCF assay to determine regenerative cell number in non‐CF and CF clonal isolates over passages 3 to 21. We found that all human basal cell clones, like their mouse counterparts 3, 6, 22, lose regenerative basal cells over time (Fig. 3B, 3C). Linear regression analysis did not identify a relationship between CFCF and life span for either the non‐CF or the CF clones (data not shown). Similar results were obtained from analysis of two additional non‐CF donors whose basal cells were recovered by digestion of bronchial tissue and cloned at passage 1 (Supporting Information Fig. S3A) and two additional CF donors whose basal cells were recovered by brushing their nasal respiratory epithelium and cloned at passage 2 (Supporting Information Fig. S3B).
Finally, we compared regenerative basal cell frequency in parental and clonal isolates at passage 3 (the first passage at which clones were analyzed), passage 6, and passage 10 (Fig. 3D, 3F). At passage 3 (Fig. 3D), regenerative basal cell frequency was significantly greater for the non‐CF parental population relative to the non‐CF clones (p = .0015); however, no statistically significant differences were identified between the CF parental population and the CF clones (p = .5158). At passage 6 (Fig. 3E), regenerative basal cell frequency was greater for both the non‐CF (p < .0001) and CF parental populations (p = .0039) relative to the clones. At passage 10 (Fig. 3F), regenerative basal cell frequency was similar for the non‐CF parental population and non‐CF clones (p = .0635) and for the CF parental population and the CF clones (p = .4762).
In general, these data indicate that life span of regenerative basal cells varies in vitro, that regenerative basal cells are lost over time in culture, and that CFCF is not predictive of life span. However, identification of five clones with atypically high CFCF and four unusually long‐lived clones suggests that the regenerative basal cell population is functionally heterogeneous.
Basal Cell Differentiation
Loss of regenerative basal cells raised the concern that amplified basal cells would be unable to differentiate into secretory and ciliated cells. To address this issue, we evaluated the differentiation potential of the basal cell clones presented in Figure 3 and Supporting Information Figure S3. The clonal isolates were differentiated using the ALI method at various passages. On differentiation day 21, total cell density was evaluated by staining nuclei with DAPI (Fig. 4A; Supporting Information Figs. S4 and S5), mucus cells were detected by MUC5b staining (Fig. 4B; Supporting Information Figs. S4 and S5), and ciliated cells were detected by ACT (Fig. 4C; Supporting Information Figs. S4 and S5) staining. Statistically significant differences in cell density, mucus cell differentiation, and ciliated cell differentiation potential were not detected. Although regenerative cell number decreases over time, these data indicate that the differentiation potential of the remaining regenerative cells is normal.
Figure 4.
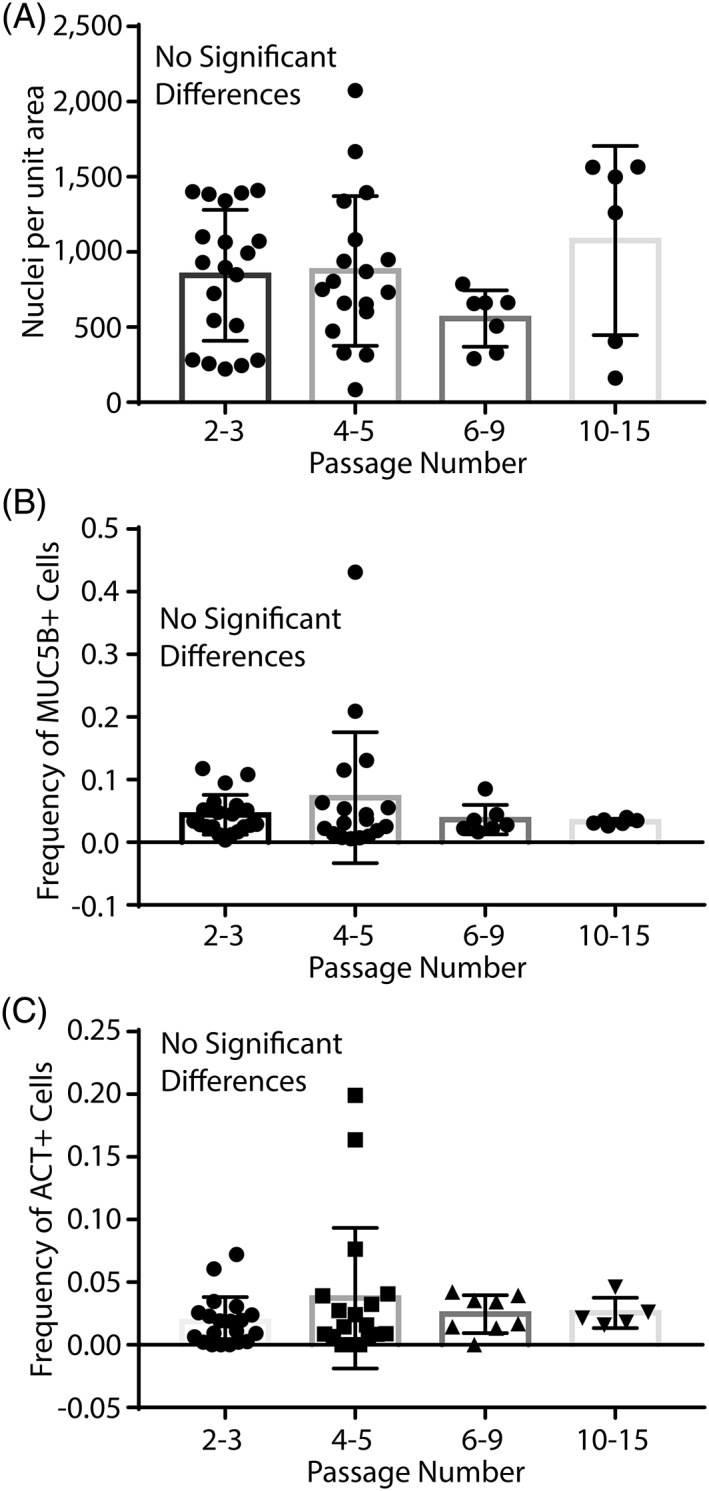
Differentiation potential of basal cell clones. Basal cells were recovered from bronchial tissue at the time of lung transplantation (non‐cystic fibrosis [CF] and CF), by brushing the bronchial epithelium (CF only), or brushing the nasal respiratory epithelium (CF only). Donor demographics are presented in Tables 1 and 2 and Supporting Information Table S1. (A): Cell density as a function of passage number was assessed by quantification of DAPI‐positive nuclei. (B): The frequency of MUC5B‐positive mucus cells was quantified as a function of passage. (C): The frequency of ciliated cells was assessed by quantification of cells expressing multiple ACT positive apical cilia. Data are presented as the mean ± SD, n = 22 clones. Symbols represent data from different clones. Abbreviation: ACT, acetylated tubulin.
Discussion
Both Non‐CF and CF Basal Cells Can Be Amplified to a Therapeutic Dose
We report that CF basal cell proliferation is comparable to that of non‐CF cells (Fig. 1) and that CF basal cells can be amplified to the estimated therapeutic dose (Fig. 2). The time required for amplification depends on how the cells are recovered and is much shorter if cells are isolated by enzymatic digestion of explanted lung tissue than by brushing the airway epithelium.
Although the primary goal of cell therapy is to treat CF patients, a secondary goal is to prevent allograft dysfunction after lung transplantation. Within 3–14 days of lung transplantation 25%–29% of patients develop primary graft dysfunction which is manifested by dyspnea and sloughing of the bronchial epithelium 23. We show that basal cells can be recovered from donor or recipient tissue that is trimmed from the anastomotic site and that these cells can be amplified to a therapeutic dose within 16 days. These data raise the possibility that autologous or allogeneic cell therapy can be used to treat primary graft dysfunction after lung transplantation.
Therapeutic Potential of Basal Cells that Are Expanded Using the mCRC Method
We previously reported that mouse airway basal cells have a finite life span 6. The present study extends this finding to human basal cells from both non‐CF and CF donors. Importantly, regenerative cell frequency is decreased to ∼20% by the time a therapeutic dose is generated (Figs. 1, 2, 3). Despite this decline in regenerative basal cell number, we find that the remaining regenerative basal cells can differentiate to mucus and ciliated cells and that differentiation potential does not vary from passages 2 to 15 (Fig. 4). Thus, our data indicate that the residual regenerative cells have therapeutic potential.
Our demonstration of multilineage differentiation in passages 9 to 15 cells is in contrast with reports indicating that differentiation potential decreases with passage 15. Although our study and that by Gentzsch and colleagues used variations of the conditional reprogramming method to expand human basal cells, there were also significant differences in the method used to induce differentiation and to quantify mucus and ciliated cell frequency. Furthermore, our analysis of differentiation used clonal isolates rather than populations of basal cells. Additional studies are needed to reconcile differences in these results.
Therapeutic Cell Selection
Given that CF basal cells will also require CFTR gene correction, our results further suggest that prospective identification of long‐lived clones (Table 3) would improve both the gene editing process and therapeutic potential of CF basal cells. An additional benefit of clone selection would be to decrease the frequency of non‐regenerative basal cells. The fate of the nonregenerative basal cell pool is not known; however, one possibility is that nonregenerative basal cells undergo terminal differentiation to a cell type that can no longer proliferate. If this is the case, a cell therapy that uses high passage basal cells could generate a replacement epithelium that persists only as long as the terminally differentiated cells survive. Several studies indicate that ciliated and secretory cells live approximately 200 days in vivo 24, 25. An alternative fate of nonregenerative cells is senescence. As these cells do not proliferate or differentiate, they would not contribute to epithelial regeneration or to improvement of airway epithelial function. Furthermore, senescent basal cells could compete with regenerative basal cells for space within the epithelium. Collectively, our data indicate that further advancement of the cell therapy initiative also requires analysis of nonregenerative basal cells and their fate.
Is Regenerative Cell Loss due to Tissue Stem Cell Depletion?
Previous studies suggested that regenerative basal cell loss could be as a result of depletion of the tissue stem cell pool 26. As tissue stem cells are typically characterized as long‐lived cells, basal cell clones that can be passaged many times have the potential to be tissue stem cells. In this study, we identified basal cell clones that could be passaged for more than 18 passages (Table 3; Fig. 3; Supporting Information Fig. S3). We identified three long‐lived clones out of 49 non‐CF and CF clonal isolates. The frequency of these long‐lived clones is 6.1% and is much lower than the published frequency of human airway epithelial tissue stem cells which is 43%–100% of basal cells 4, 5. In contrast, if human tissue stem cell frequency is similar to that of the mouse, which is ∼1% 6, 22, then serial passage may select for the stem cell subset of human basal cells. Additional studies are needed to clarify this potential mechanism.
We also considered the possibility that our serial passage parameters might result in dilution of the tissue stem cell pool by non‐stem‐cell basal cells. To address this idea, we first modeled a situation in which all basal cells are stem cells. Based on tissue stem cell theory, we assumed that each tissue stem cell would self‐renew and generate a regenerative basal cell. Using empirically determined regenerative basal cell frequency (Fig. 1A) and burst size (Fig. 1B), we found that serial passage of 5 × 104 cells would result in loss of the stem cell by passage 4. If the initial tissue stem cell frequency was less than 100%, our model predicts culture termination prior to passage 4. As most of our basal cell clones could be passaged more than 4 times, we suggest that tissue stem cell depletion is not the simplest explanation for regenerative basal cell attrition.
Study Limitations
Within the CF donor pool, we found that regenerative basal cell number did not vary between cells that were homozygous or heterozygous for the F508del CFTR mutation (Supporting Information Fig. S1). However, the heterozygous pool contained the F508del CFTR mutation and eight other CFTR mutations. This variation prevents us from drawing conclusions about the specific impact of non‐F508del mutations on basal cell function. Donor age may also impact regenerative basal cell number and function. The present study evaluated basal cells from CF donors ranging in age from 1 to 40 years (Tables 1 and 2). Although we did not detect any differences in regenerative basal number or function, it is likely that the study is underpowered to detect an effect of age. Thus, additional studies are needed to evaluate age as a determinant of therapeutic potential. A prerequisite for autologous cell replacement therapy is reversion of the CFTR mutation. As gene editing in primary basal cell isolates is under development, the present studies did not evaluate the impact of CFTR gene editing on basal cell function. Once the gene editing methods have been perfected, we will need to compare regenerative basal cell function in nonedited and edited cells. Finally, we report that non‐CF and CF basal cells are functionally similar in vitro. The strength of this conclusion is limited to the culture conditions we tested and may not predict the behavior of non‐CF and CF cells under the conditions they are likely to encounter during acute and chronic infection or after transplantation. Our future studies can address this critical question using the quantitative assays presented in this study.
Conclusion
Both non‐CF and CF basal cells can be amplified to the estimated therapeutic dose. The time needed for basal cell amplification is sufficiently rapid to allow treatment of acute lung disease such as primary graft dysfunction after lung transplantation. However, we also find that regenerative basal cells are lost as the cells are amplified and that this loss exhibits two phases. Short‐lived basal cells are depleted during the early phase, resulting in late‐phase cultures that are enriched for long‐lived cells that can undergo multilineage differentiation to mucus and ciliated cells.
Author Contributions
D.H.: conception and design, acquisition of clinical samples, interpretation of data, manuscript writing, final approval of manuscript; B.T.K.: acquisition of clinical samples, final approval of manuscript; C.L.H.: analysis of laboratory samples, final approval of manuscript; S.W.L.: analysis of laboratory samples, final approval of manuscript; C.M.S.: interpretation of data, final approval of manuscript; M.T.: analysis of laboratory samples, final approval of manuscript; A.A.: analysis of laboratory samples, final approval of manuscript; S.D.R.: conception and design, analysis of laboratory samples, interpretation of data, drafting of the manuscript, financial support, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Supporting information
Supplemental Figure 1. Impact of CFTR genotype on regenerative basal cell frequency. Basal cells were recovered by brushing the bronchial airways or the nasal respiratory epithelium. Donor demographics are presented in Table 2 and STable 1.
A. Regenerative basal cell frequency in bronchial samples derived from donors who were homozygous (blue bar, circles) or heterozygous (red bar, squares) for the F580del CFTR mutation. Comparisons were done at each of 5 passages (P).
B. Regenerative basal cell frequency in nasal samples derived from donors who are homozygous (blue bar, circles) or heterozygous (red bar, squares) for the F580del CFTR mutation. Comparisons were done at each of 3 passages (P).
Data are presented as the mean ± SD. Symbols represent the data from individual samples.
Supplemental Figure 2: Impact of airway region on regenerative basal cell frequency. Basal cells were recovered by brushing the nasal respiratory epithelium (green bars, circles) or the bronchial airways (purple bars, squares). Donor demographics are presented in Table 2 and STable 1. Comparisons were done at each of 3 passages (P). Data are presented as the mean ± SD. Symbols represent the data from individual samples.
Supplemental Figure 3. Regenerative basal cell frequency decreases in serially‐passaged human basal cell populations and clonal isolates. Basal cells were recovered from bronchial tissue at the time of lung transplantation (non‐CF) or by brushing the nasal respiratory epithelium (CF). Donor demographics are presented in Tables 1 and 2 and STable 1.
A. Regenerative basal cell frequency in basal cell populations and clones that were derived from digests of non‐CF bronchial tissue. Data are presented as the mean for each population or clone at each passage. n = 2 populations and n = 12 clones.
B. Regenerative basal cell frequency in basal cell populations and clones that were recovered by brushing the nasal respiratory epithelium (CF). Data are presented as the mean for each population or clone at each passage. n = 2 populations and n = 13 clones.
Supplemental Figure 4. Dual immunofluorescence analysis of basal cell clone differentiation at passages 3 and 5. Clones were differentiated using the air‐liquid‐interface method. Cellular differentiation was assayed by immunostaining for acetylated tubulin, a ciliated cell marker, MUC5B, a mucus cell marker, and DAPI, a nuclear marker. Data are presented as single color images and a merge of the three channels.
Supplemental Figure 5. Dual immunofluorescence analysis of basal cell clone differentiation at passages 9 and 15. Clones were differentiated using the air‐liquid‐interface method. Cellular differentiation was assayed by immunostaining for acetylated tubulin, a ciliated cell marker, MUC5B, a mucus cell marker, and DAPI, a nuclear marker. Data are presented as single color images and a merge of the three channels.
Supplemental Table 1. Microbiology and Mycology Analysis of Nasal and Bronchial Brushing Donors.
Acknowledgments
This work was supported by a research grant from the CF Foundation and seed funds from the Nationwide Children's Hospital Cell Based Therapeutics program.
References
- 1. Elborn JS. Cystic fibrosis. Lancet 2016;388:2519–2531. [DOI] [PubMed] [Google Scholar]
- 2. Adam D, Roux‐Delrieu J, Luczka E et al. Cystic fibrosis airway epithelium remodelling: Involvement of inflammation. J Pathol 2015;235:408–419. [DOI] [PubMed] [Google Scholar]
- 3. Ghosh M, Ahmad S, Jian A et al. Human tracheobronchial basal cells. Normal versus remodeling/repairing phenotypes in vivo and in vitro. Am J Respir Cell Mol Biol 2013;49:1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Engelhardt JF, Schlossberg H, Yankaskas JR et al. Progenitor cells of the adult human airway involved in submucosal gland development. Development 1995;121:2031–2046. [DOI] [PubMed] [Google Scholar]
- 5. Rock JR, Onaitis MW, Rawlins EL et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA 2009;106:12771–12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ghosh M, Helm KM, Smith RW et al. A single cell functions as a tissue‐specific stem cell and the in vitro niche‐forming cell. Am J Respir Cell Mol Biol 2011;45:459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Borthwick DW, Shahbazian M, Krantz QT et al. Evidence for stem‐cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol 2001;24:662–670. [DOI] [PubMed] [Google Scholar]
- 8. Schoch KG, Lori A, Burns KA et al. A subset of mouse tracheal epithelial basal cells generates large colonies in vitro. Am J Physiol Lung Cell Mol Physiol 2004;286:L631–L642. [DOI] [PubMed] [Google Scholar]
- 9. Hegab AE, Ha VL, Darmawan DO et al. Isolation and in vitro characterization of basal and submucosal gland duct stem/progenitor cells from human proximal airways. Stem Cells Translational Medicine 2012;1:719–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hegab AE, Ha VL, Gilbert JL et al. Novel stem/progenitor cell population from murine tracheal submucosal gland ducts with multipotent regenerative potential. Stem Cells 2011;29:1283–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ghosh M, Ahmad S, White CW et al. Transplantation of airway epithelial stem/progenitor cells: A future for cell‐based therapy. Am J Respir Cell Mol Biol 2017;56:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Voynow JA, Fischer BM, Roberts BC et al. Basal‐like cells constitute the proliferating cell population in cystic fibrosis airways. Am J Respir Crit Care Med 2005;172:1013–1018. [DOI] [PubMed] [Google Scholar]
- 13. Reynolds SD, Rios C, Wesolowska‐Andersen A et al. Airway progenitor clone formation is enhanced by Y‐27632‐dependent changes in the transcriptome. Am J Respir Cell Mol Biol 2016;55:323–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chu HW, Rios C, Huang C et al. CRISPR‐Cas9‐mediated gene knockout in primary human airway epithelial cells reveals a proinflammatory role for MUC18. Gene Ther 2015;22:822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gentzsch M, Boyles SE, Cheluvaraju C et al. Pharmacological rescue of conditionally reprogrammed cystic fibrosis bronchial epithelial cells. Am J Respir Cell Mol Biol 2017;56:568–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu R. Culture of normal human airway epithelial cells and measurement of mucin synthesis and secretion. Methods Mol Med 2000;44:31–39. [DOI] [PubMed] [Google Scholar]
- 17. You Y, Huang T, Richer EJ et al. Role of f‐box factor foxj1 in differentiation of ciliated airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 2004;286:L650–L657. [DOI] [PubMed] [Google Scholar]
- 18.Malleske DT, Hayes D, Jr., Lallier SW et al. Regulation of human airway epithelial tissue stem cell differentiation by beta‐catenin, P300, and CBP. Stem Cells 2018. [DOI] [PubMed]
- 19. Seibold MA, Smith RW, Urbanek C et al. The idiopathic pulmonary fibrosis honeycomb cyst contains a mucocilary pseudostratified epithelium. PloS One 2013;8:e58658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hsia CC, Hyde DM, Ochs M et al. An official research policy statement of the American Thoracic Society/European Respiratory Society: Standards for quantitative assessment of lung structure. Am J Respir Crit Care Med 2010;181:394–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hayflick L. The cell biology of aging. J Invest Dermatol 1979;73:8–14. [DOI] [PubMed] [Google Scholar]
- 22. Ghosh M, Smith RW, Runkle CM et al. Regulation of trachebronchial tissue‐specific stem cell pool size. Stem Cells 2013;31:2767–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Whitson BA, Prekker ME, Herrington CS et al. Primary graft dysfunction and long‐term pulmonary function after lung transplantation. J Heart Lung Transplant 2007;26:1004–1011. [DOI] [PubMed] [Google Scholar]
- 24. Saunders CJ, Reynolds SD, Finger TE. Chemosensory brush cells of the trachea. A stable population in a dynamic epithelium. Am J Respir Cell Mol Biol 2013;49:190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rawlins EL, Hogan BL. Ciliated epithelial cell lifespan in the mouse trachea and lung. Am J Physiol Lung Cell Mol Physiol 2008;295:L231–L234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yim HW, Slebos RJ, Randell SH et al. Smoking is associated with increased telomerase activity in short‐term cultures of human bronchial epithelial cells. Cancer Lett 2007;246:24–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Impact of CFTR genotype on regenerative basal cell frequency. Basal cells were recovered by brushing the bronchial airways or the nasal respiratory epithelium. Donor demographics are presented in Table 2 and STable 1.
A. Regenerative basal cell frequency in bronchial samples derived from donors who were homozygous (blue bar, circles) or heterozygous (red bar, squares) for the F580del CFTR mutation. Comparisons were done at each of 5 passages (P).
B. Regenerative basal cell frequency in nasal samples derived from donors who are homozygous (blue bar, circles) or heterozygous (red bar, squares) for the F580del CFTR mutation. Comparisons were done at each of 3 passages (P).
Data are presented as the mean ± SD. Symbols represent the data from individual samples.
Supplemental Figure 2: Impact of airway region on regenerative basal cell frequency. Basal cells were recovered by brushing the nasal respiratory epithelium (green bars, circles) or the bronchial airways (purple bars, squares). Donor demographics are presented in Table 2 and STable 1. Comparisons were done at each of 3 passages (P). Data are presented as the mean ± SD. Symbols represent the data from individual samples.
Supplemental Figure 3. Regenerative basal cell frequency decreases in serially‐passaged human basal cell populations and clonal isolates. Basal cells were recovered from bronchial tissue at the time of lung transplantation (non‐CF) or by brushing the nasal respiratory epithelium (CF). Donor demographics are presented in Tables 1 and 2 and STable 1.
A. Regenerative basal cell frequency in basal cell populations and clones that were derived from digests of non‐CF bronchial tissue. Data are presented as the mean for each population or clone at each passage. n = 2 populations and n = 12 clones.
B. Regenerative basal cell frequency in basal cell populations and clones that were recovered by brushing the nasal respiratory epithelium (CF). Data are presented as the mean for each population or clone at each passage. n = 2 populations and n = 13 clones.
Supplemental Figure 4. Dual immunofluorescence analysis of basal cell clone differentiation at passages 3 and 5. Clones were differentiated using the air‐liquid‐interface method. Cellular differentiation was assayed by immunostaining for acetylated tubulin, a ciliated cell marker, MUC5B, a mucus cell marker, and DAPI, a nuclear marker. Data are presented as single color images and a merge of the three channels.
Supplemental Figure 5. Dual immunofluorescence analysis of basal cell clone differentiation at passages 9 and 15. Clones were differentiated using the air‐liquid‐interface method. Cellular differentiation was assayed by immunostaining for acetylated tubulin, a ciliated cell marker, MUC5B, a mucus cell marker, and DAPI, a nuclear marker. Data are presented as single color images and a merge of the three channels.
Supplemental Table 1. Microbiology and Mycology Analysis of Nasal and Bronchial Brushing Donors.


