Abstract
We examined data and patterns in clinical islet transplant studies registered on ClinicalTrials.gov (CTgov) for treatment of type 1 diabetes (T1D), with a goal of extracting insights to apply in the design of a pluripotent stem cell‐derived islet therapy. Clinical islet transplantation, as a cell therapy (rather than solid organ transplant) is a unique precedent for stem cell‐based islet therapies. Registration activity shows that the field is not growing significantly, and newer registrations suggest that the reasons for stagnation include need for a more optimal site of infusion/transplantation, and especially a need for better immune protective strategies to advance a more effective and durable therapy for T1D. stem cells translational medicine 2019;8:209&214
Keywords: Hypoglycemia, Immunosuppression, Insulin, Pluripotent stem cells, Transplantation tolerance
Significance Statement.
Clinical experience with islet transplantation is small, limited by donor availability and regulatory approval in the U.S., but still provides an important precedent for companies developing stem cell‐based therapies for type 1 diabetes. This article reviews patterns in islet transplantation from the ClinicalTrials.gov registry, emphasizing lessons that should be applied to new stem cell‐based therapies. In particular, immunogenicity of allogeneic islets is an important problem, and optimal site for transplantation is still under investigation. Stem‐cell derived islets have potential to treat a broader range of patients with type 1 diabetes than can be treated with current clinical protocols.
Introduction
We used the search terms—“islet transplantation” and “type 1 diabetes” (T1D)—in reviewing the ClinicalTrials.gov (CTgov) database. The database is not curated, so there are likely overlapping trials and trials that represent continuations of earlier trials registered, and we did not contact centers to clarify these issues. Finally, we removed some studies for analysis in this review, if studies never got off the ground or had an “unknown” status after registration, or were not clearly focused on T1D and outcomes after islet transplantation. Islet auto‐transplantation studies are not part of this review, but islet xeno‐transplantation studies are, because of their (immunologic) relevance to allogeneic transplants. Islet allotransplants are only performed in the U.S. at centers Food and Drug Administration (FDA) approved to study islet transplantation for patients with T1D, and so they must register on CTgov. Consequently, some of the single center registrations are largely observational studies of local practice and results (although listed as phase I/II), rather than reporting interventional trials. Eighty percent of the CTgov listings are for single‐center studies. Another important reason that randomized control trials were not adopted for allogeneic islet transplantation is the unwillingness of patients to be assigned to a control group 1. Activity status of the clinical trial registries is shown in Table 1.
Table 1.
Activity status of islet transplantation CTgov registrations (June 2018)
| Status | Number of studies |
|---|---|
| Not yet recruiting (new) | 3 |
| Recruiting | 26 |
| Active, not recruiting | 12 |
| Enrolling by invitation | 2 |
| No longer available | 3 |
| Completed | 47 |
| Completed, has results | 11 (Note: 10 relevant) |
| Withdrawn | 9 |
| Terminated | 11 |
| Terminated, has results | 4 |
| Unknown | 13 |
Although the FDA issued reporting requirements for studies registered on CTgov in 2016—at that time only 23,000 of the 224,000 studies registered had results posted 2—our review suggests that reporting is still a problem. Access to actual results was major issue in reviewing the islet transplant experience on the CTgov website. Less than 1/5 of the “completed” trials we reviewed had results, a total of only 10 relevant studies. Part of the low reporting rate may be a reflection of the small number of islet transplants per center, which also limits statistical analysis of the CTgov database. We were able to uncover some data on completed studies for which the Principal Investigator (PI) had posted actual enrolment numbers, by searching PubMed, to extend the amount of information extractable from CTgov.
In 2016, 215 solid organ pancreas transplants were performed in the U.S., down from 1,500 performed in 2004 3. We did not include pancreas transplants in this analysis. The Collaborative Islet Transplant Registry (citregistry.org) supported by the NIH is also an important and extensive source of information approximately islet transplantation, and its most recent annual report (covering transplants done through 2013) was consulted for this review 4. Nonetheless, the CTgov database is the most up‐to‐date resource for examining trends in islet transplantation worldwide. CTgov also provides a unique history arc of clinical efforts in islet transplantation, including reasons for termination of studies, as well as studies registered after the latest Collaborative Islet Transplant Registry (CITR) report. Here, we review the database for patterns of activity that indicate factors in islet transplantation that need optimization, common and distinctive features of inclusion and exclusion criteria, and lessons from failed studies that can inform design of stem cell‐based islet transplantation clinical trials. Although the registry changes frequently, a review of past studies is a solid foundation to further build upon.
Brief History of Islet Transplantation (Context for this Analysis)
In 1977, the NIH convened a conference to review the potential for clinical islet transplantation 5 for treatment of T1D, resulting in a summary conclusion that clinical islet transplantation was biologically feasible. Islet transplantation was performed in a few centers for the decades following, but the modern islet transplant era really began with the publication of the Edmonton protocol 6, in which the major innovation was use of an immunosuppressive regimen without corticosteroids. An international study of 36 patients transplanted using the Edmonton protocol followed the initial report: in this study a cumulative islet “dose” of at least 10,000 islets/kg was delivered with ≥2 islet infusions into the portal vein, unless insulin independence was achieved by one infusion 7. Canada declared islet transplantation a standard clinical (nonexperimental) procedure in 2001, allowing government reimbursement for the procedure. Subsequently the U.K., Sweden, Switzerland, France, and Italy followed suit 8.
Although the Edmonton protocol led to improved clinical outcomes over early historic experience, sustained insulin independence is not guaranteed after islet transplantation (NCT00434811). A review of 347 allogeneic islet transplant recipients from the Collaborative Islet Transplant Registry, published in 2012 showed that 59% of islet recipients were free of severe hypoglycemic events and had HbA1c levels <6.5% at 1 year post‐transplant (a composite end‐point). Of these, 69% at 2 years, 54% at 3 years and 44% at 4 years sustained the positive end‐point outcome 1. A subsequent phase III CITR study confirmed effectiveness of islet transplantation in reducing severe hypoglycemic events in most recipients, with highly significant improvement in Clarke 9 and HYPO 10 scores. Median HbA1c levels decreased from 7.2% to 5.6% in the transplant cohort of 48 subjects from 8 academic centers 11. The most recent CITR study shows that insulin‐independence is achieved in just over 40% of islet recipients at 1 year but falls to half that at 5 years 4. Nonetheless, freedom from severe hypoglycemic events is an important clinical goal that can be achieved without complete insulin independence. It is important to note the small number of patients in these important multicenter reports, as small numbers are a problem with analyzing the CTgov registry critically, reflecting a reality of the field.
Results and Discussion
General Patterns
More than 140 clinical studies, either inactive (completed, withdrawn, terminated, or other/unknown status) or currently active (not yet recruiting, recruiting, enrolling by invitation, and active but not recruiting) were included in this analysis. The year of CTgov registration reflects the history of clinical islet transplantation. The very first trials were based at NIH and explored recipient eligibility. A peak of activity surrounds publication of the Edmonton protocol, and new trial registrations did not pick up after that time (2006–2008). However, the nature of the trials registered from 2011 to 2018 shifted toward exploration of novel implant sites (gastric submucosa, intrabone marrow, subcutaneous, anterior chamber of the eye for diabetic retinopathy, and omentum) and approaches to reducing graft rejection with new devices (physical barriers) or immune modulators (chemical barriers). The initial year of registration is shown in Figure 1.
Figure 1.
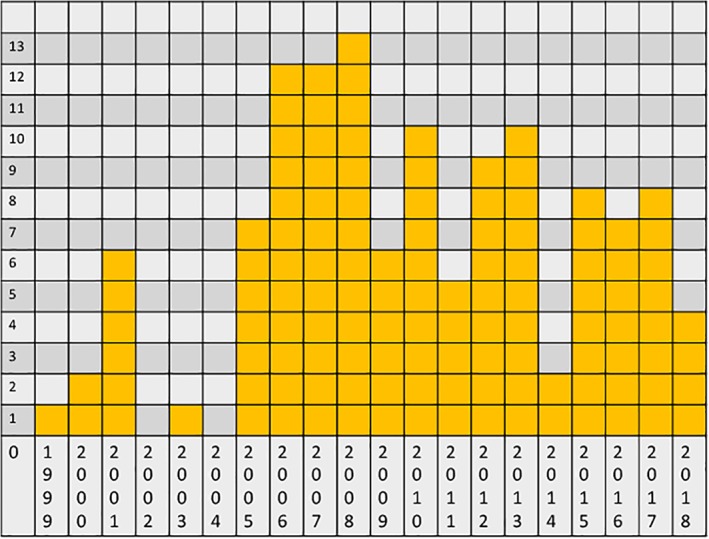
Number of new registrations for islet transplantation clinical trials for type 1 diabetes (y axis) for each year (x axis). Data were obtained by the “Date first posted” notation for each trial.
Islet transplantation is international, but the number of active centers is very small: in the CTgov registry, 26 U.S. centers, 4 Canadian, 28 European, and 2 Asian centers are represented. By comparison, in the U.S., the United Network for Organ Sharing lists 256 centers with some transplant programs, so only approximately 10% of U.S. transplant centers offer islet transplantation. Another way of looking at these numbers: only ~1.5% of transplantation studies registered in the CTgov database are islet studies (141 out of 10,921). Of the U.S. centers in the database, at least two are no longer active. When viewed in the light of the small numbers of patients transplanted per center, these numbers represent a challenge for enrolling sufficient numbers of patients in clinical trials, and as noted below, poor or no enrolment is a common reason for clinical studies to be withdrawn. Furthermore, the site numbers suggest that finding centers with deep expertise in islet transplantation to host new stem cell based trials is not straightforward.
Outcomes in Completed Studies with Posted Results
Only 10 relevant completed studies were found with posted results (20% of completed studies). The total actual enrolment in these 10 studies was 86 but only 74 patients completed study. Overall, serious adverse events (SAE) were reported in 66% of patients. Of SAE that could be related to the immunosuppressive drug regimen, leukopenia was reported in 20 of 86 subjects and infection in 13. We looked at outcome and SAE data at 1 year whenever possible, but the data are not straightforward to pull from the records since each center had different primary and secondary endpoints; there was not a single primary or secondary end‐point in common posted by all these centers. For primary outcomes, the primary composite endpoint of normal range HbA1c level (e.g., HbA1c ≤ 6.5%) and elimination of hypoglycemia recommended by FDA 12 was not reported by most centers. The HbA1c goal of ≤6.5% reported as a secondary end‐point in 4 studies was reached by 23 of 30 subjects. The most commonly reported primary end‐point was insulin‐independence and at 1 year; 30 of 65 subjects met that end‐point. Secondary end‐points of hypoglycemia incidence were reported in various ways: percent subjects with NO hypoglycemic episodes was reported (by two centers); reduced number of hypoglycemic episodes, increased awareness of hypoglycemic episodes, or numbers of episodes per month were also ways of reporting; 23 of 30 subjects met one of these criteria at 1 year post‐transplant.
Learning from (Failure) Experience
We examined the 11 “Terminated” studies to determine reasons for premature closure of the trials. The primary reason given for termination was lack of enrolment in three studies. No reason was given for 3 studies, including one single‐center study that had an actual enrolment of 30 subjects; another closed because the PI left the university, and another due to “feasibility” issues. The closure of three studies points to the difficulty in achieving immune tolerance of allograft islets: one study (NCT00315614) in which donor CD34+ cells were given to three subjects was closed because tolerance was not achieved, and pharmacologic immunosuppression could not be withdrawn. Similarly, a B lymphocyte immunotherapy approach using the anti‐CD20 monoclonal antibody, rituximab, closed after two subjects were studied, for lack of efficacy. For “withdrawn” studies, the major reason for withdrawing the study was lack of enrollment or in one case, “university decision.”
Inclusion/Exclusion Criteria
In order to compare results for stem cell based therapies to islet transplantation, inclusion and exclusion criteria (i.e., the patient phenotype) should be similar to those developed over time for islet transplantation. A general philosophy ruling eligibility of T1D patients for islet transplantation, because of the well‐known risks of life‐long immunosuppression, is that T1D must not be controllable using optimal intensive medical management. The vast majority of clinical trials require patients to have been diagnosed with T1D for at least 5 years, except the unusual trials examining islet transplantation specifically for newly diagnosed patients (NCT002505893 and NCT00807651). More than half the trials also specify that disease onset must have been before age 40 years. Age is usually specified between 18 and 65 years; patients aged 35 years and younger are less likely to achieve insulin‐independence or reduction of daily insulin requirement than older patients 4. All programs require that patients are experiencing symptomatic hypoglycemic episodes and/or unrecognized hypoglycemic episodes despite optimal intensive medical management. Most centers also require lack of c‐peptide production to confirm no residual islet function (with slight differences in how c‐peptide is assessed) and an abnormal HbA1c despite medical management. In the CTgov registry the HbA1c upper thresholds do vary considerably from center to center with a range of 7%–12%.
Cardiovascular disease is a common complication of T1D, so cardiac risk factors and cardiac functional status are often specified in exclusion criteria, with some variability between centers. For example, the upper limit of normal body mass index (BMI) specified ranges between 26 and 35 kg/m2 with a mean overall of 28.7 kg/m2 (and European study mean of 29 kg/m2). All three Chinese studies specify an upper BMI limit of 27 kg/m2. A maximum BMI criterion excludes patients who would require a larger mass of islets for transplant and potentially a more complicated procedure, and may help to exclude patients with insulin‐resistance. Several centers specify that eligible subjects must weigh at least 50 kg, possibly to ensure an adequate size of the portal vein.
Renal disease is also a common complication of T1D. Centers that perform islet transplantation may also perform simultaneous islet and kidney transplants, or islet transplants after kidney transplant. All studies exclude subjects with significant proteinuria (macroalbuminuria). Serum creatinine exclusion criteria cutoffs (above 1.5–1.6 mg/dl) are usually used in association with renal functional testing. Although glomerular filtration rate (GFR) is generally considered a better overall measure of renal function than creatinine clearance (CrCl), creatinine clearance criteria of renal function are used in approximately 1/3 of the clinical trial listings. Creatinine clearance tends to overestimate renal function compared with GFR 13, both measures tend to decline with age; we did not separate out CrCl or GFR criteria according to the precise methods used or otherwise try to normalize the reported renal function exclusion criteria. When CrCl is used to define kidney function inclusion criteria, the lowest acceptable value ranges from 50 to 80 ml/minute with a mean of 64.2 and median of 60 ml/minute. When GFR is used for inclusion criteria the lowest function for inclusion ranges from 40 (corresponding to moderate chronic kidney disease [CKD]) to 80 ml/minute (mild CKD), with an arithmetic mean of 67 and median of 70 ml/minute. The vast majority of programs therefore do not accept patients for islet transplantation when severity of kidney function has progressed to moderate (stage III) disease. This criteria is unlikely to change given the results of the latest international trial 11 in which GFR continued to decline in the year after islet transplantation despite 87.5% of subjects meeting the composite primary end‐point indicating good glycemic control. Additionally, immunosuppressive regimens, especially calcineurin inhibitors, may also contribute to further renal functional decline 14.
Marrow function is also evaluated for several reasons. First, immunosuppressive regimens are generally marrow suppressive. Second, bleeding complications of the surgical procedure are not uncommon. Platelet counts are routinely screened; five of 48 subjects had a serious bleeding adverse event in the recent international trial 11. Most often a platelet count above 100,000 per mm3 is required, with some centers setting less stringent (80,000 per mm3) and a few more stringent (150,000 per mm3) criteria. The hemoglobin criteria are surprisingly wide, with some centers accepting anemic patients (minimal hemoglobin, Hgb of 7 g/dl) and others accepting only (male) patients with Hgb > 13 g/dl. Hemoglobin for women and men are usually specified, with a lower number for women acceptable. The criteria for white blood cell counts are more uniform with most programs requiring a total white cell count of at least 3,000 per μl.
As in other clinical transplant programs, patients with active systemic infection or malignancy (except basal or squamous skin cancer) are excluded; significant end‐organ liver disease, neurologic or psychiatric disease are exclusion criteria. Particular to T1D, patients with untreated proliferative diabetic retinopathy are also excluded.
Immunosuppression
The main concern with islet transplantation, beyond safety, continues to be long‐term survival of the graft. In spite of significant advancements in transplant immunosuppressive regimens and improved islet survival, current immunosuppression drug combinations have failed to significantly improve single‐donor graft success rates: the majority of patients still require two or more islet transplants (two or more donors) to achieve clinically significant improvement in disease. For this reason, the majority of the trials (64%, or 91 studies out of 141) recorded in CTgov, including currently active trials, continue to investigate the efficacy on islet survival of different combinations of induction (i.e., administered prophylactically at the time of transplant) and maintenance immune suppressive agents (i.e., post‐transplant long‐term regimen). Multidrug approaches involving agents with different mechanisms of action are typically being assessed in these studies to both reduce toxicities and improve outcomes. In addition to pharmacologic immunosuppressive regimen, several trials are currently exploring the use of regulatory T cells (Tregs) to induce immune tolerance by curbing the host autoimmunity and alloimmunity (NCT03444064 and NCT03162237). Tregs not only inhibit effector T cells, but regulate other lymphocytes and antigen presenting cells to turn down the immune response 15. Other centers are investigating immunomodulatory strategies that transiently deplete B lymphocytes to promote islet transplant engraftment and ultimately promote insulin independence (NCT00468442, NCT01049633, and NCT00434850). This approach, which demonstrated promising preclinical results 16, 17 has not yet been successful in small numbers of subjects and will require further optimization.
We also looked at the 10 relevant completed studies with posted results to determine the range of immunosuppressive regimens used. Four of the studies used the Edmonton protocol regimen (induction with daclizumab, maintenance with sirolimus + tacrolimus) and the rest were testing or using other regimens.
Optimization of the Implant Site
Islet transplantations are actually islet infusions into the portal vein, delivering islets to the liver. The liver has a unique two‐input blood supply via the portal vein and hepatic artery, with the portal vein draining the entire gastrointestinal tract and the hepatic artery supplying oxygen to the liver. Advantages of portal vein include its size with sufficient diameter for the islet infusion, and its accessibility for percutaneous cannulation and pressure monitoring. The portal venous blood also contains a rich trophic factor supply of gut hormones. Nonetheless, the liver is not an ideal environment for islet engraftment and survival. Unlike orthotopic solid organ transplants or hematopoietic stem cell transplants that are placed in or home to their normal environment, transplanted islets are expected to thrive in an alien environment. Upon infusion, the islets become trapped in an uncontrolled manner in the hepatic microvasculature and islets within such plugs must survive a period of relative hypoxia. These islets must actively migrate across the endothelial lining and subsequently establish residency within the hepatic parenchyma. It is estimated that less than half of transplanted islets survive the first 48 hours following infusion into portal vein 18.
Clinical Trial Design for Alternative Islet Sources and Reduced Pharmacologic Immune Suppression
Alternative sources of cadaveric donor islets in development are islets generated in the laboratory from human pluripotent stem cells (PSCs) including induced PSCs 19, human exocrine progenitor cells 20 and islets from neonatal piglet pancreata. The safety and efficacy of PSCs‐derived islets (NCT03162926, NCT02239354, NCT03163511, and NCT02939118) and porcine islets (NCT01736228, NCT03162237, and NCT01739829) are currently subjects of clinical trials. Alternatives to systemic immune suppression under study are encapsulation devices that provide a physical barrier between the graft and the host immune system (NCT02064309, NCT00940173, NCT01739829, and NCT01736228) as well as new pharmacologic and biologic approaches to induce transplant tolerance (NCT03444064, NCT03162237, NCT00468442, NCT01049633, and NCT00434850).
The factor that most dramatically influences clinical trial design and practice is elimination of long‐term systemic immune suppression. Removal of these agents could potentially enable enrolment of patients with (more significant) renal dysfunction, for example. In this case, a relevant clinical endpoint would be the observation of islet transplant on renal function in the absence of immune suppression to determine if the stabilization of diabetes (without potentially confounding nephrotoxic drugs) can also stabilize renal function. Removal of immune suppression from the clinical trial protocol also lowers the long‐term risk to patients, potentially extending the eligible pool of patients to those with less severe forms of diabetes, provided the risk/benefit ratio of encapsulated islets proves low.
Advancing the Field
Islet transplantation remains challenging with enormous resources mobilized to yield positive results that do not “cure” disease and are associated with significant risk and side‐effects. With maturation of stem cell biology and tissue engineering, we are optimistic that a safe and effective PSCs‐based therapy will be developed along with engineered immune barriers that obviate need for pharmacologic immunosuppression. Figure 2 summarizes our conclusions approximately how the treatment of T1D can be improved beyond the use of cadaveric islet grafts with a stem cell‐based strategy.
Figure 2.
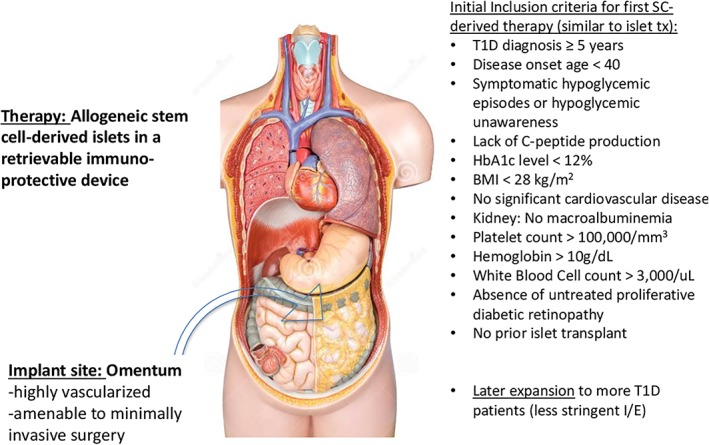
A vision for the future of islet transplantation. Stem cell‐derived islets, although allogeneic, have the advantages over cadaveric islets of being prequalified during product development, and the transplantation of these islets can be timed to suit the recipient. Because pluripotent stem cell‐derived islets are allogeneic (and will be for the near future because of the expense of generating multiple lines) they will require encapsulation in a device that protects them from the host immune system. The goal of encapsulation is elimination of the need for pharmacologic immunosuppression. The omentum is an optimal site for transplantation because of its rich vascularity and it is amenable to minimally invasive surgery for both implantation and retrieval. Initially inclusion/exclusion criteria for stem cell‐derived islets will be similar to those for cadaveric islet transplantation, until the risks and benefits are better understood. Demonstrated safety and efficacy with stem cell‐derived islets is likely to lead to islet transplantation offered to a larger population of patients with type 1 diabetes than currently treated with cadaveric islets. Source: Copyright free from https://www.dreamstime.com/confirm.php?changeuname=1.
Conclusion
An examination of the CTgov registrations for islet transplantation highlights current bottlenecks to broader use of islet transplantation for treatment of T1D. These bottlenecks include poor islet survival and significant side‐effects, many associated with obligate pharmacologic immunosuppression. Current inclusion criteria emphasize the most severe forms of T1D because the risk of lifelong pharmacologic immunosuppression is high and because the therapy does not reliably result in insulin‐independence. The most striking improvement in outcomes of islet transplantation came with adaptation of the Edmonton protocol, further highlighting the importance of finding an optimal and safe method to avoid immune destruction of islet grafts. Newer CTgov registrations emphasize novel approaches to promoting islet survival: T regulatory cells to promote graft tolerance, physical barriers to immune cell graft invasion, stem cell sources of islets, and extrahepatic sites for engraftment. Because of the small numbers of islet transplantations performed and regulatory hurdles inherent in first‐in‐human trials, these promising therapies will take time to evaluate, but all represent potential major improvements toward more optimal use of islets for treatment of T1D.
Author Contributions
C.A.W.: conception and design, data collection, analysis and interpretation, manuscript writing; W.L.R.: data analysis and interpretation, manuscript writing; M.C.: conception and design, data collection, analysis and interpretation, manuscript writing; C.A.W., W.L.R., and M.C.: final approval of manuscript.
Disclosure of Potential Conflicts of Interest
C.A.W. discloses employment with R&D stage company. W.L.R. discloses employment and inventor/patent holder with R&D stage company. M.C. discloses Consultant/Advisory role with Seraxis, Inc.
References
- 1. Tiwari JL, Schneider B, Barton F et al. Islet cell transplantation in type 1 diabetes: An analysis of efficacy outcomes and considerations for clinical trial designs. Am J Transplant 2012;12:1898–1907. [DOI] [PubMed] [Google Scholar]
- 2. Zarin DA, Tse T, Williams RJ et al. Trial reporting in ClinicalTrials.gov—The Final Rule. N Engl J Med 2016;375:1998–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kandaswamy R, Stock PG, Gustafson SK et al. OPTN/SRTR 2016 annual data report: Pancreas. Am J Transplant 2018;18:114–171. [DOI] [PubMed] [Google Scholar]
- 4. Collaborative Islet Transplant Registry (CITR) , The Emmes Corporation, 9th Annual Report, December 8, 2016. Available at https://citregistry.org/content/reports-publications-presentations
- 5. Lacy PE. Workshop on pancreatic islet cell transplantation in diabetes sponsored by the National Institute of Arthritis, Metabolism, and Digestive Diseases and held at the National Institutes of Health in Bethesda, Maryland, on November 20 and 30, 1977. Diabetes 1978;27:427–429. [DOI] [PubMed] [Google Scholar]
- 6. Shapiro AM, Lakey JR, Ryan EA et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid‐free immunosuppressive regimen. N Engl J Med 2000;343:230–238. [DOI] [PubMed] [Google Scholar]
- 7. Shapiro AM, Ricordi C, Hering BJ et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006;355:1318–1330. [DOI] [PubMed] [Google Scholar]
- 8. McCall M, Shapiro AMJ. Update on islet transplantation. Cold Spring Harb Perspect Med 2012;2:a007823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clarke WL, Cox DJ, Gonder‐Frederick LA et al. Reduced awareness of hypoglycemia in adults with IDDM: A prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care 1995;18:517–522. [DOI] [PubMed] [Google Scholar]
- 10. Ryan EA, Shandro T, Green K et al. Assessment of the severity of hypoglycemia and glycemic lability in type 1 diabetic subjects undergoing islet transplantation. Diabetes 2004;53:955–962. [DOI] [PubMed] [Google Scholar]
- 11. Hering BJ, Clarke WR, Bridges ND et al. Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care 2016;39:1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. FDA . Guidance for industry. Consideration for allogeneic pancreatic islet cell products. 2009.
- 13. Van Acker BA, Koomen MC, Koopman MG et al. Creatinine clearance during cimetidine administration for measurement of glomerular filtration rate. Lancet 1992;340:1326–1329. [DOI] [PubMed] [Google Scholar]
- 14. Posselt AM, Szot GL, Frassetto LA et al. Islet transplantation in type 1 diabetic patients using calcineurin inhibitor‐free immunosuppressive protocols based on T‐cell adhesion or costimulation blockade. Transplantation 2010;90:1595–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perdigoto AL, Chatenoud L, Bluestone JA et al. Inducing and administering Tregs to treat human disease. Front Immunol 2016;6:654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu C, Noorchashm H, Sutter JA et al. B lymphocyte‐directed immunotherapy promotes long‐term islet allograft survival in non‐human primates. Nat Med 2007;13:1295–1298. [DOI] [PubMed] [Google Scholar]
- 17. Kang HK, Wang S, Dangi A et al. Differential role of B cells and IL‐17 versus IFN‐γ during early and late rejection of pig islet xenografts in mice. Transplantation 2017;101:1801–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bruni A, Gala‐Lopez B, Pepper AR et al. Islet cell transplantation for the treatment of type 1 diabetes: Recent advances and future challenges. Diabetes Metab Syndr Obes 2014;7:211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Southard SM, Kotipatruni RP, Rust WL. Generation and selection of pluripotent stem cells for robust differentiation to insulin‐secreting cells capable of reversing diabetes in rodents. PLoS ONE 2018;13:e0203126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nichols RJ, New C, Annes JP. Adult tissue sources for new beta‐cells. Transl Res 2014;163:418–431. [DOI] [PMC free article] [PubMed] [Google Scholar]


