Abstract
Two studies were conducted to examine the effects of ice slushy ingestion (ICE) and cold water immersion (CWI) on thermoregulatory and sweat responses during constant (study 1) and self-paced (study 2) exercise. In study 1, 11 men cycled at 40–50% of peak aerobic power for 60 min (33.2 ± 0.3°C, 45.9 ± 0.5% relative humidity, RH). In study 2, 11 men cycled for 60 min at perceived exertion (RPE) equivalent to 15 (33.9 ± 0.2°C and 42.5 ± 3.9%RH). In both studies, each trial was preceded by 30 min of CWI (~22°C), ICE or no cooling (CON). Rectal temperature (Tre), skin temperature (Tsk), thermal sensation, and sweat responses were measured. In study 1, ICE decreased Tre-Tsk gradient versus CON (p = 0.005) during first 5 min of exercise, while CWI increased Tre-Tsk gradient versus CON and ICE for up to 20 min during the exercise (p<0.05). In study 2, thermal sensation was lower in CWI versus CON and ICE for up to 35–40 min during the exercise (p<0.05). ICE reduced thermal sensation versus CON during the first 20 min of exercise (p<0.05). In study 2, CWI improved mean power output (MPO) by ~8 W, compared with CON only (p = 0.024). In both studies, CWI (p<0.001) and ICE (p = 0.019) delayed sweating by 1–5 min but did not change the body temperature sweating threshold, compared with CON (both p>0.05). Increased Tre-Tsk gradient by CWI improved MPO while ICE reduced Tre but did not confer any ergogenic effect. Both precooling treatments attenuated the thermal efferent signals until a specific body temperature threshold was reached.
Introduction
During self-paced exercise with increased exogenous heat load, behavioral thermoregulation can be achieved through adjusting the work rate to manipulate metabolic heat production since heat dissipation is limited by involuntary mechanisms, i.e., sweating and cutaneous vasodilation [1]. Under this paradigm, skin and core temperature and thermal perception have been identified as controllers of thermoregulatory behavior [2–4]. These controllers may modify thermoregulatory behavior independently [3, 5]. Indeed, improved thermal sensation and greater work output have been observed following oral L-menthol mouth rinse [5], face cooling via fan or application of topical menthol gel [3], without changes in the skin and core temperature.
External cooling via whole body cold water immersion (CWI) and internal cooling via ingestion of ice slushy (ICE) have often been used as ergogenic aids before exercise in the heat [6]. The aim of precooling is to create a greater heat sink for subsequent metabolic heat production; however, the ergogenic mechanisms underlying precooling may be specific to different cooling methods. For example, whole body CWI involves direct contact with a large body surface area and thus resulting in reduced skin blood flow and increased core-to-skin temperature gradient, whereas ICE has a more direct effect on the core body temperature [7]. Recent meta-analyses have shown that CWI improves aerobic exercise performance in warm environments [6], with a lesser beneficial effect observed in moderate conditions (i.e., ambient temperatures between 18–26°C) [8]. During exercise in temperate environments, drastic muscle cooling during CWI has been shown to negatively impact delivery of oxygen (O2) and substrates to locomotive muscle as assessed by near-infrared spectroscopy (NIRS), resulting in increased anaerobic metabolism during subsequent exercise in temperate environments [9, 10]. It is worth noting that exercise in the heat has also been shown to impair muscle blood volume and tissue oxygenation assessed by NIRS [11].
ICE is logistically less challenging than CWI and has minimal muscle cooling effect, making this cooling method preferable to CWI. However, a recent meta-analysis showed that CWI and ICE may elicit different influences on the thermoregulatory behavior during exercise in the heat via changes in skin and core temperatures and thermal perception [6]. Specifically, while both ICE and CWI effectively reduced core temperature, but only CWI had a clear beneficial effect on exercise performance concomitant with reductions in skin temperature and thermal sensation. Additional benefits of CWI versus ICE include a greater body surface area being directly cooled during whole body immersion, a continued cooling effect after immersion [12], the practicality of ICE being limited by an optimal drinking volume required for significant core body cooling effect [13], and improved whole body fluid balance through lesser sweat loss [6]. Sweating during exercise is initiated by both thermal and non-thermal factors, with nitric oxide being identified as a non-neural regulating factor [14, 15]. ICE has been shown to activate the intra-abdominal thermoreceptors but minimally affect skin temperature and skin blood flow [16, 17]. Hence, sweating during ICE is predominantly regulated by thermal reflexes, whereas direct skin cooling during CWI is likely to regulate the sweat response via activation of the thermoreceptors and the nitric oxide pathway through blood flow reduction.
The present study aimed to examine the differences in some thermoregulatory parameters (i.e., core-to-skin temperature gradient, sweat response and thermal sensation) and muscle perfusion (NIRS parameters) during CWI and ICE versus a no cooling control condition (CON), and the consequential influence on thermoregulatory behavior as indicated by total work output during exercise in the heat. Accordingly, two studies were conducted. In the first study, the thermoregulatory parameters and muscle perfusion were assessed during 60 min of exercise at a fixed intensity following CWI, ICE and CON. In the second study, thermoregulatory behavior was investigated during 60 min of cycling at a fixed rating of perceived exertion (RPE). The RPE clamp protocol allows individuals to modify power output continually based on the regulatory role of perceived exertion in behavioral thermoregulation [18], and has been utilised to investigate the differential influences of thermal perception and temperature on thermoregulatory behavior [3, 5]. In the first study, it was hypothesized that CWI would result in a greater core-to-skin temperature gradient, but would attenuate the sweat responses and muscle perfusion when compared with ICE and CON. In the second study, we hypothesized CWI would improve mean power output and total work output relative to CON and ICE.
Materials and methods
Participants
The experimental procedures were approved by Edith Cowan University ethics committee for human research and were conducted according to the principles expressed in the Declaration of Helsinki. All participants were recruited locally via the institutional intranet portal and social media. All procedures and associated risks were made known to the participants before obtaining signed consent. For study 1, a priori analysis using whole body sweat loss data from a previous study [19] was performed using the calculated effect size of 0.78, an α of 0.05, and a β of 0.2. Minimum of 7 participants were required to identify significant difference in whole body sweat loss between conditions. Nevertheless, 13 men were recruited for study 1; however, only 11 men completed all trials (mean ± SD; age: 27 ± 6 y, body mass: 77.5 ± 10.5 kg, height: 177.1 ± 7.9 cm, sum of 4 skinfolds: 58.1 ± 25.7 mm, Peak O2 uptake (): 43.9 ± 11.4 mL·kg-1·min-1, peak aerobic power: 288 ± 64 W). One participant withdrew from the study due to personal reasons and another could not complete the exercise task. For study 2, power analysis was determined using an effect size of 0.80 based the work output data from a previous study using similar RPE clamp protocol [3]. A minimum of 7 participants were required to identify significant difference between conditions with an α of 0.05 and a β of 0.2. Eleven out of 13 participants completed the study (mean ± SD; age: 30 ± 6 y, body mass: 80.6 ± 12.8 kg, height: 1.8 ± 0.1 m, : 51.1 ± 8.2 mL.kg-1.min-1, sum of 7 skinfold: 131.2 ± 52.6 mm). Two participants did not complete the trials due to personal reasons. Thirteen participants were recruited for each study after taking into consideration attrition, missing data and inherent differences in the study designs. All participants were recreationally active who engaged in physical activity (e.g., cycling, running and soccer) for ≥3 times per week within the past two years, non-smokers and free from cardiovascular disease. All participants completed a preliminary visit and three experimental trials. Participants were asked to: 1) avoid strenuous exercise and alcohol consumption during the 24 h before each trial; 2) avoid caffeine during the 12 h before each trial; and 3) keep their diet, physical activity and sleep habits consistent before each trial, assisted by a 1-d dietary intake and physical activity record.
Preliminary measurements
Anthropometric measurements and were assessed during a preliminary session. The , defined as the highest 30-sec average, was assessed by an incremental cycling exercise test (Velotron Racermate, Seattle, WA, USA) starting at 70 W and increased by 35 W·min-1 until cadence dropped below 60 rpm. Minute ventilation, carbon dioxide production and was measured by a calibrated metabolic cart (TrueOne 2400, ParvoMedics, Utah, USA) during the incremental exercise test. Peak power output was prorated from the last completed stage plus the time in the last uncompleted stage multiplied by 35 W [20]. Heart rate ([HR], Polar S810i, Polar Electro Oy, Kempele, Finland) and RPE [21] were also assessed during the exercise. In study 2, participants performed a standardised familiarisation trial adapted from Lander et al. [22] after the incremental exercise test. The familiarisation trial began at RPE 11 for 4 min and increased to RPE 13 (3 min), RPE 15 (2 min) and ended at RPE 19 (1 min).
Experimental trials
In both studies, participants completed three experimental trials separated by at least 48 h, and the time of trials was kept within 2 h between sessions for each participant. All participants arrived 30 min before the experimental trials. Following urine specific gravity index (USG, Atago hand-held refractometer, Model Master-URC/Nα, Tokyo, Japan), nude body mass measurement (Model ID1, Mettler Toledo, Columbus, Ohio, USA) and self-insertion of a rectal thermistor, participants proceeded to a regulated climate chamber for at least 20 min for thermal equalisation and further instrumentation
In study 1, participants performed one of the three 30-min pre-exercise treatments in a randomized crossover manner: 1) ingestion of 1.25 g·kg-1·5 min-1 of an ice slushy mixture (-0.7 ± 0.1°C) with added orange flavoured syrup (Cottee’s Foods, NSW, Australia); 2) mid-sternal level CWI at 22.1 ± 0.1°C; and 3) passive rest on a chair beside the cycle ergometer during CON. During CWI and CON, participants consumed 1.25 g·kg-1·5 min-1 of warm fluid (36.3 ± 0.6°C) with added orange flavoured syrup. For all conditions, the mixed drinks contained 6% carbohydrate. At 9 min 9 sec ± 1 min 31 sec after the end of cooling, participants cycled for 60 min at 40–50% of peak aerobic power at 33.2 ± 0.3°C, 45.9 ± 0.5%RH. In study 2, participant completed three trials which involved 30 min of pre-exercise treatments. Before exercise, they consumed 1.25 g·kg-1·5 min-1 of ICE (0.1 ± 0.1°C) containing 0% carbohydrate, completed 30 min of CWI at 22.3 ± 0.2°C, and passive rest (CON) in a randomized, crossover manner. Warm water (35.8 ± 0.3°C) was consumed at 1.25 g·kg-1·5 min-1 during CWI and CON. For both studies, the ice slushy mixture (1:1 mixture of ice and liquid) was prepared using a commercially available food blender. All drinks consumed during precooling and exercise were stored in an insulated flask, and temperature of the drinks was measured immediately before serving.
In study 2, the experimental trials required participants to cycle for 60 min at a pace equivalent to 15 or ‘hard or heavy’ on the 15-point RPE scale [21] at 33.9 ± 0.2°C and 42.5 ± 3.9%RH. Participants had access to the elapsed time, but no other feedback or verbal encouragement was given. Exercise commenced at 10 min 22 sec ± 1 min 4 sec after the end of cooling. In both studies, thermal sensation [23] and RPE [21] were assessed every 5 min, and water consumption during the exercise was matched between trials for each participant.
Temperature and sweat measurements
Temperature and sweat data were logged at 0.2 Hz continuously during the experimental trials by a Squirrel data logger (Model 2040, Grant Instruments Ltd., Cambridge, UK). Weighted mean skin temperature was calculated from the measurement of four sites [24] over the sternum (Tst), forearm (Tarm), thigh (Tth) and calf (Tca) (YSI 409B thermistors, Dayton, OH, USA). Rectal temperature (Tre) was measured via a thermistor (Monatherm Thermistor 400 Series, Mallinckrodt Medical, St. Louis, MO, USA) self-inserted 10 cm past the anal sphincter. Mean body temperature (Tb) was calculated as (0.79 × Tre) + (0.21 × Tsk) [25].
Local sweat rate ([LSR], mg·cm-2·min-1) was measured using ventilated sweat capsules (5.31 cm2) attached to the left dorsal forearm 5 cm below the antecubital fossa (LSRarm), and the left thigh 15 cm above the superior border of the patella (LSRth). Dry air ventilated the capsules at 1.5 mL·min-1 and the water content of the effluent air was measured using capacitance hygrometers (HMP60, Vaisala, Helsinki, Finland). Onset of sweating in terms of exercise time was determined by fitting the 1-min averaged data using a one-phase exponential association model [26]. Thermoregulatory sweating threshold and sensitivity were determined by plotting LSR averaged from both sites against Tre and Tb as previously described [27]. Whole body sweat loss was determined from the change in body mass to the nearest 10 g, taking into consideration the total volume of fluid consumed.
NIRS measurements
A near-infrared spectroscopy system (Niromonitor NIRO-200, Hamamatsu Photonics, Japan) was used to measure tissue chromophore concentration changes in the right vastus lateralis at 10 Hz (Powerlab 16/30 ML 880/P, ADInstruments, New South Wales, Australia). The NIRS probe, with an interoptode distance of 4 cm, was secured to the right thigh with the centre of the probe 15 cm above the superior border of the patella towards the greater trochanter. A black polyethylene sheet (7 cm × 5 cm × 30 μm) was secured over the NIRS probe to minimise ambient light contamination, and participants placed their legs in a polyethylene bag (365 cm × 228 cm × 30 μm) to protect the measuring instruments from water damage during CWI.
Using the modified Beer-Lambert method, the NIRS system measures changes (μM·cm-1) in oxyhaemoglobin (OxyHb), deoxyhaemoglobin (Hb) and total haemoglobin (tHb) at 775, 810 and 850 nm from an arbitrary initial value. Simultaneously, tissue oxygenation index (TOI, %) is determined based on the spatially resolved spectroscopy method. Changes in the tHb and TOI reflect muscle blood volume and ratio between oxygenated haemoglobin and total haemoglobin, respectively [28]. The NIRS data were averaged over 1 min and expressed as absolute differences from the baseline values.
Heart rate, mean arterial pressure and skin perfusion
HR was recorded continuously during the experimental trials using the same Polar telemetric monitor. In study 1, skin perfusion was measured at the left forearm by laser Doppler flowmetry system (PeriFlux 5000 with thermostatic probe 457, Perimed AB, Järfälla, Stockholm, Sweden), expressed as arbitrary perfusion units (PU). However, due to probe displacement during the exercise, the authors decided not to include the PU signals in the data analysis. In study 2, skin PU was measured at the left thigh. Distance between the sweat capsule, skin thermistor and the laser Doppler probe were kept at 2 cm. Mean arterial pressure (MAP) was obtained from the blood pressure waveform recorded from a finger (Finapres NOVA, Finapres Medical Systems©, Amsterdam, The Netherlands). The PU and MAP signals were sampled at 10 Hz using the same data acquisition software as the NIRS signals. MAP was not recorded during the RPE clamp exercise (study 2) to minimise distraction to the participants. Cutaneous vascular conductance (CVC) was determined using the quotient between PU and MAP at baseline and at the end of cooling.
Statistical analysis
Statistical analyses were performed using the nlme and emmeans packages for R v3.5.0 (R Core Team, R Foundation for Statistical Computing, Vienna, Austria). Linear mixed effects modeling allowed inclusion of data sets with missing values during the exercise for Tre and the NIRS signals due to thermistor displacement and probe damage, respectively. The dependent variables were analyzed in separate linear mixed models fitted with restricted maximum likelihood. The initial models included a by-participant random intercept. Random effects for intercept and for slope with regards to condition, and covariance between intercepts and slopes were incorporated where indicated by minimising the values for Akaike information criteria. Marginal p-values were reported. Bonferroni correction was applied for multiple pairwise comparisons at a given time point. Significance level was accepted at p≤0.05. A total of 19 data sets from study 1 and 2 were included in the analysis of the sweating threshold and sensitivity. One participant partook in both studies and thus his data set was included only once. Additionally, two data sets from study 1 were excluded due to water damage during CWI and due to missing values >30 min during the exercise. All data are expressed as mean ± SD.
Results
Study 1
Baseline body mass and USG, physiological and perceptual responses during exercise
A significant effect was present for baseline body mass (p = 0.006) with post-hoc analysis revealing that baseline body mass was lower in CON versus ICE (p = 0.008), but no difference was observed between CWI and CON (p = 0.195) or between CWI and ICE (p = 0.979, CON: 77.3 ± 9.7 kg, CWI: 77.6 ± 10.2 kg, ICE: 77.8 ± 9.9 kg). USG before the exercise was similar between conditions (p = 0.494, CON: 1.013 ± 0.006 g·ml-1, CWI: 1.010 ± 0.007 g·ml-1, ICE: 1.011 ± 0.008 g·ml-1). Table 1 shows the mean physiological and perceptual responses during the 60 min of exercise. Whole body sweat loss was significantly lower in CWI compared with CON (p = 0.014) while there was no difference between ICE and CON (p = 0.161). Mean HR was lower in CWI relative to CON (p = 0.025), while differences between CWI and ICE did not reach statistical significance (p = 0.068). Thermal sensation, RPE, LSRarm and LSRth were not significantly different between conditions (Table 1).
Table 1. Mean physiological and perceptual responses during 60 min of cycling at fixed exercise intensity (study 1), and during the RPE clamp exercise (study 2).
| CON | CWI | ICE | P-value | |
|---|---|---|---|---|
| Study 1: | ||||
| Whole body sweat loss (mL) | 1244 ± 374* | 1064 ± 343 | 1128 ± 329 | 0.016 |
| Thermal sensation (AU) | 6.0 ± 1.1 | 5.7 ± 0.6 | 5.8 ± 0.7 | 0.384 |
| RPE (AU) | 13.5 ± 1.9 | 13.4 ± 1.8 | 13.4 ± 1.6 | 0.982 |
| Heart rate (beats∙min-1) | 154 ± 14* | 149 ± 15 | 153 ± 14 | 0.018 |
| TreΔ (°C) (n = 8) | 1.3 ± 0.5# | 1.2 ± 0.6# | 1.6 ± 0.6 | <0.001 |
| LSRarm | 1.25 ± 0.75 | 1.10 ± 0.53 | 1.14 ± 0.44 | 0.470 |
| LSRth | 0.71 ± 0.46 | 0.74 ± 0.36 | 0.69 ± 0.35 | 0.772 |
| Study 2: | ||||
| Mean power output (W) | 130 ± 20* | 138 ± 18 | 129 ± 25 | 0.018 |
| Total work output (kJ) | 470 ± 74* | 498 ± 65 | 464 ± 90 | 0.018 |
| Whole body sweat loss (mL) | 1394 ± 381* | 1239 ± 367 | 1396 ± 119* | 0.004 |
| Heart rate (beats∙min-1) | 144 ± 20 | 141 ± 14 | 144 ± 15 | 0.679 |
| TreΔ (°C) | 1.4 ± 0.5# | 1.2 ± 0.6# | 1.7 ± 0.5 | <0.001 |
| LSRarm | 1.21 ± 0.35 | 1.21 ± 0.53 | 1.20 ± 0.60 | 0.994 |
| LSRth | 0.63 ± 0.19 | 0.68 ± 0.29 | 0.66 ± 0.27 | 0.592 |
CON, control; CWI, cold water immersion, ICE, ice slushy ingestion; RPE, rating of perceived exertion; TreΔ, magnitude of increase in rectal temperature during exercise; LSRarm, local sweat rate for the arm at the end of exercise; LSRth, local sweat rate for the thigh at the end of exercise
* p<0.05 versus CWI
# p<0.05 versus ICE.
Data are mean ± SD for n = 11 unless otherwise stated. See S5 Table for effect sizes (Cohen’s d) calculated from mean differences between conditions and pooled SD.
Tsk, Tre and Tre-Tsk gradient
One participant’s data for Tre were removed from the analysis due to water damage during CWI. A significant interaction effect was observed for Tre (p = 0.020). ICE decreased Tre by 0.3°C during the first 5 min of exercise when compared with CWI and CON (p<0.05, Fig 1A). However, the magnitude of increase in TreΔ during the exercise was higher in ICE relative to CON (p = 0.012) and CWI (p = 0.001, Table 1), resulting in similar Tre at the end of exercise (Fig 1). An interaction effect was observed for Tsk (p<0.001) such that it was significantly lower in CWI compared with ICE and CON during the exercise (Fig 1B). Tre-Tsk gradient exhibited an interaction effect (p<0.001) such that CWI increased the gradient during the first 15 min of exercise compared with CON and ICE (p<0.05, Fig 1C). ICE decreased Tre-Tsk gradient relative to CON during the first 5 min of exercise (p<0.05).
Fig 1.
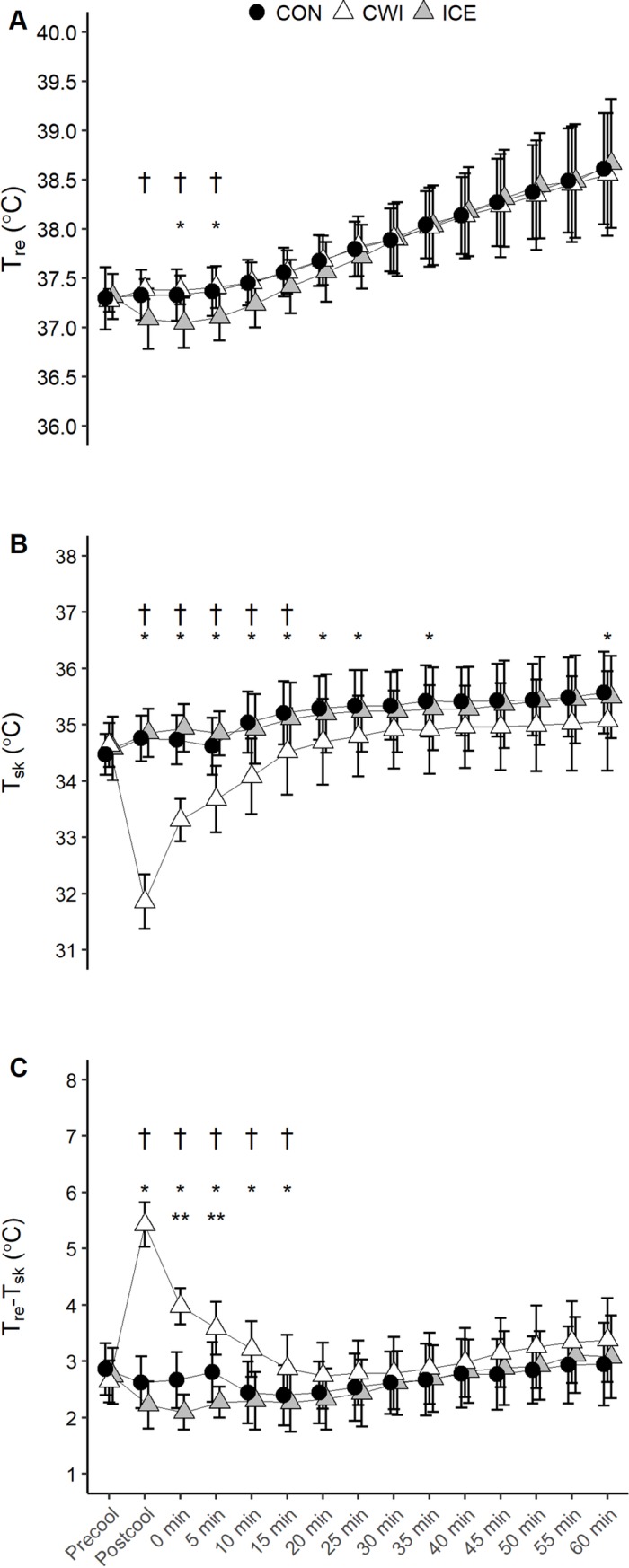
Tre (A), Tsk (B), and Tre-Tsk gradient (C) during 60 min of cycling at fixed intensity (study 1). CON, control; CWI, cold water immersion; ICE, ice slushy ingestion; * p<0.05 CWI versus CON; ** p<0.05 ICE versus CON; † p<0.05 CWI versus ICE. Data are mean ± SD for n = 11 for Tsk. Due to missing data at certain time points during the exercise, data for Tre and Tre-Tsk gradient are n = 10 during the first 10 min of exercise for all conditions and n = 8 or 9 thereafter (see S1 and S2 Tables for clarification).
NIRS data
An interaction effect was observed for tHb (p<0.001) whereby CWI significantly decreased tHb relative to CON and ICE during the first 20 min of exercise (Fig 2A). OxyHb exhibited an interaction effect (p<0.001) such that it was lower in CWI versus CON and ICE during the initial 10 min of exercise (p<0.05, Fig 2B). Hb showed an effect for time (p<0.001), but no main condition (p>0.05) or interaction effect (p = 0.350, Fig 2C) was observed. Although TOI demonstrated an effect for time (p<0.001), there was no condition (p>0.05) or interaction effect (p = 0.580, Fig 2D).
Fig 2.
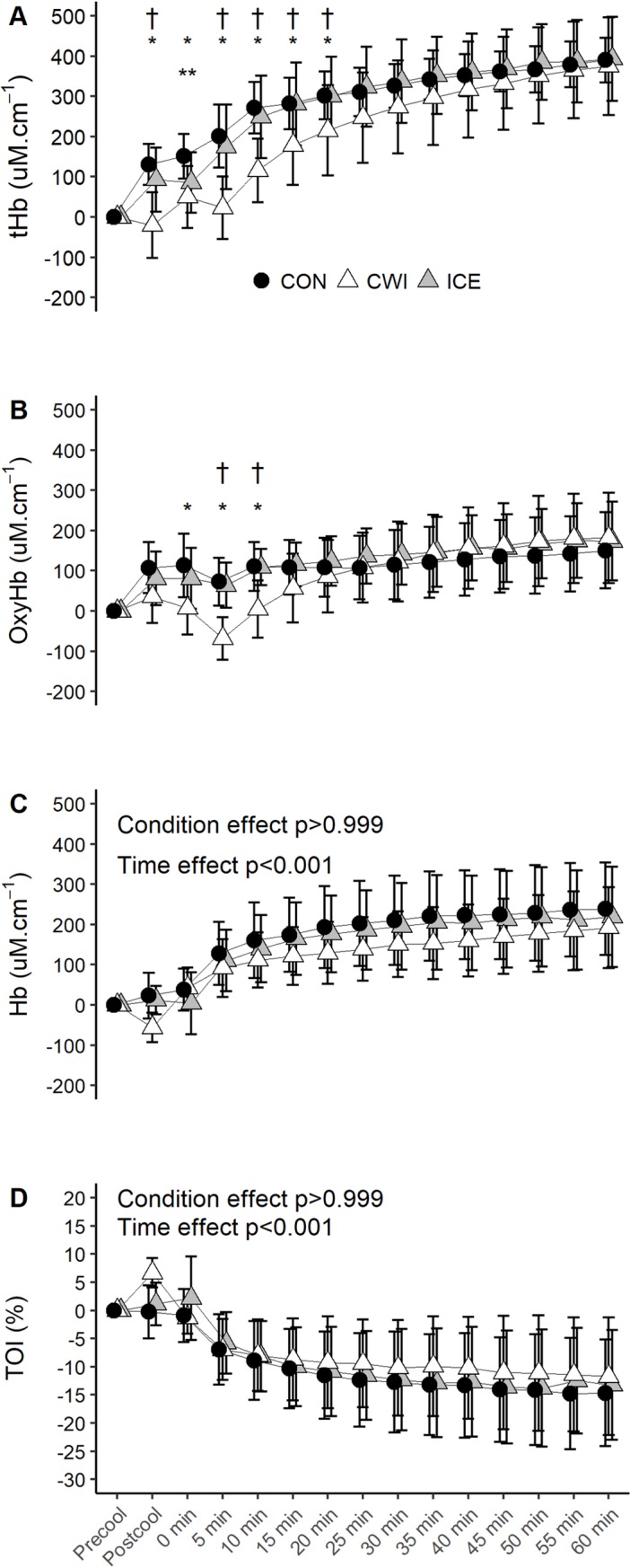
Changes in tHb (A), HbO2 (B), Hb (C) and TOI (D) during 60 min of cycling at fixed intensity (study 1). CON, control; CWI, cold water immersion; ICE, ice slushy ingestion; * p<0.05 CWI versus CON; ** p<0.05 ICE versus CON; † p<0.05 CWI versus ICE. Data are expressed as absolute changes from the baseline values and are mean ± SD for n = 11, except for the final 20 min of exercise during CON and the final 10 min of exercise during ICE where n = 10 due to probe damage.
Study 2
Baseline body mass and USG
There was no difference between conditions for baseline body mass (p = 0.498, CON: 80.2 ± 13.3 kg, CWI: 80.5 ± 12.9 kg, ICE: 80.3 ± 12.9 kg) and USG (p = 0.255, CON: 1.016 ± 0.009 g·ml-1, CWI: 1.017± 0.007 g·ml-1, ICE: 1.013 ± 0.008 g·ml-1). Four participants took up to 34 min 32 sec to ingest the given volume in the ICE trials.
MPO, total work output, HR and sweat responses
MPO in CWI was greater than CON (p = 0.024), but there was no difference between CON and ICE (p>0.999) or between CWI and ICE (p = 0.263, Table 1). Similarly, total work output in CWI was greater than CON (p = 0.024), whereas there was no difference between CON and ICE (p>0.999) or between CWI and ICE (p = 0.263, Table 1). There was no condition effect for the mean HR response during the exercise (p = 0.679, Table 1). CWI decreased whole body sweat loss relative to CON (p = 0.012) and ICE (p = 0.011); however, there was no condition effect for LSRarm (p = 0.994) or LSRth (p = 0.592).
Tsk, Tre, Tre-Tsk gradient and thermal sensation
Significant interaction effect was observed for Tre (p = 0.003) such that it was lower by ~0.3°C in ICE versus CWI and CON during the first 5–10 min of exercise (Fig 3A). However, the magnitude of increase in Tre during the 60 min of exercise was greatest in ICE compared with CON (p = 0.003) and CWI (p<0.001, Table 1). An interaction effect was observed for Tsk (p<0.001). CWI decreased Tsk for up to 15–20 min during the exercise compared with CON and ICE (p<0.05, Fig 3B), while ICE increased Tsk during the first 5 min of exercise compared with CON (p<0.05). Tre-Tsk gradient depicted an interaction effect (p<0.001) whereby it increased during the first 15–20 min of exercise in CWI relative to CON and ICE (p<0.05, Fig 3C). ICE decreased Tre-Tsk gradient during the first 5 min of exercise compared with CON (p<0.05). An interaction effect was observed for thermal sensation (p<0.001). Both CWI and ICE reduced thermal sensation during the exercise compared with CON (p<0.05), but final values were not different between conditions (p>0.05, Fig 3D). CWI decreased thermal sensation during the first 15 min of exercise and between 30 to 35 min during the exercise, when compared with ICE (p<0.05).
Fig 3.
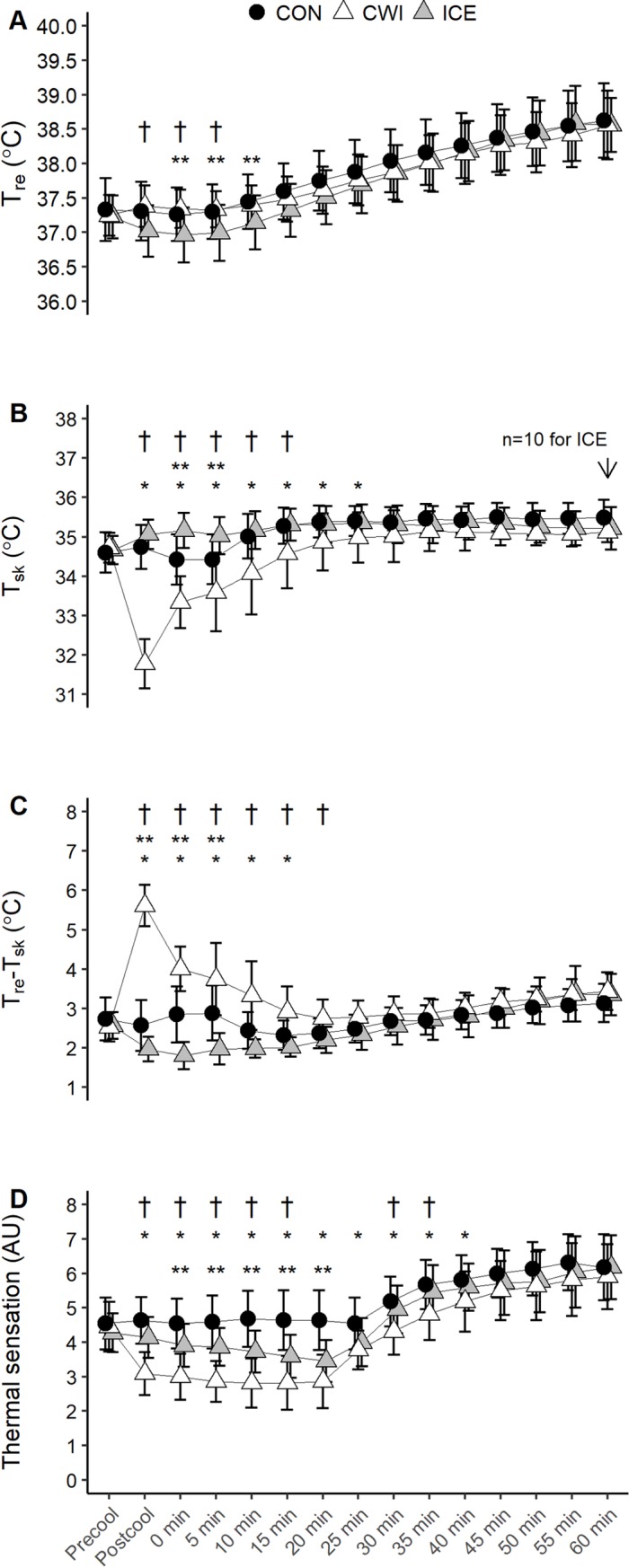
Tre (A), Tsk (B), and Tre-Tsk gradient (C), and thermal sensation (D) during 60 min of cycling at RPE 15 (study 2). CON, control; CWI, cold -water immersion; ICE, ice slushy ingestion; * p<0.05 CWI versus CON; ** p<0.05 ICE versus CON; † p<0.05 CWI versus ICE. Data are mean ± SD for n = 11 unless otherwise stated. Due to missing data at certain time points during the exercise, data for Tre and Tre-Tsk gradient are n = 11 during the first 25 min of exercise for all conditions, and n = 10 for CWI and ICE thereafter (see S3 and S4 Tables for clarification).
NIRS data and skin PU
An interaction effect was evident for tHb (p<0.001) in which it decreased during CWI and for up to 10–15 min during the exercise compared with CON and ICE (p<0.05, Fig 4A). Significant interaction effect was observed for OxyHb (p = 0.005) whereby it decreased during CWI for up to 15–20 min during the exercise compared with CON and ICE (p<0.05, Fig 4B). OxyHb was also lower at the end of cooling during ICE compared with CON (p = 0.034). Hb increased during the exercise (p<0.001) but there was no main condition (p>0.999) or interaction effect (p = 0.546, Fig 4C). No effect for condition (p>0.05) or interaction (p>0.05) was observed for TOI, although it decreased during the exercise (p<0.001, Fig 4D).
Fig 4.
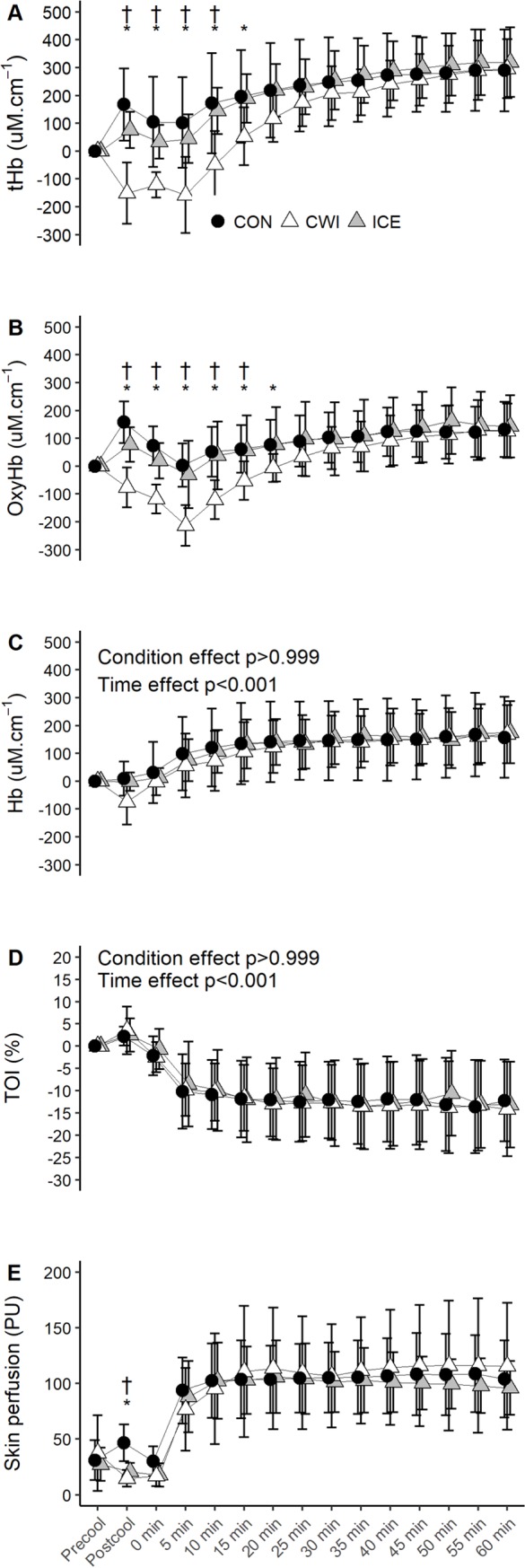
Changes in tHb (A), OxyHb (B), Hb (C) TOI (D), and skin PU (E) during 60 min of cycling at RPE 15 (study 2). CON, control; CWI, cold water immersion; ICE, ice slushy ingestion; * p<0.05 CWI versus CON; ** p<0.05 ICE versus CON; † p<0.05 CWI versus ICE. Data are expressed as absolute changes from the baseline values and are mean ± SD for n = 11, except for the final 30 min of exercise during CWI where n = 10 due to probe damage.
Skin PU depicted an interaction effect (p = 0.005) whereby both CWI (p = 0.012) and ICE (p = 0.044) decreased PU by the end of cooling relative to CON (Fig 4E). An interaction effect was evident for CVC (p = 0.001). CVC was not different between conditions at baseline (p>0.05, CON: 0.39 ± 0.17 PU·mmHg-1, CWI: 0.49 ± 0.40 PU·mmHg-1, ICE: 0.37 ± 0.23 PU·mmHg-1). At the end of cooling, CWI (p<0.001) and ICE (p = 0.001) decreased CVC relative to CON (CON: 0.54 ± 0.20 PU·mmHg-1, CWI: 0.19 ± 0.11 PU·mmHg-1, ICE: 0.22 ± 0.10 PU·mmHg-1). When expressed as percentage of the baseline values, the changes in CVC were +15%, -61% and -40% for CON, CWI and ICE, respectively.
Sweat threshold and sweat sensitivity from studies 1 and 2
CWI (p<0.001) and ICE (p = 0.019) delayed sweat recruitment in terms of exercise time compared with CON (Table 2). Additionally, CWI delayed sweating onset by ~4 min relative to ICE (p<0.001). Sweating occurred at a lower Tre in ICE versus CON (p = 0.007) and CWI (p<0.001), while CWI resulted in an elevated Tre threshold for sweating compared with CON (p = 0.007, Table 2). However, there was no difference between conditions for the Tb threshold (p = 0.973). Tre sweat sensitivity was increased following CWI compared with CON (p = 0.007) and ICE (p = 0.009). There was a significant effect for Tb sweat sensitivity (Table 2). CWI resulted in a higher sweat sensitivity compared with CON (p = 0.037), while the difference between CON and ICE did not reach statistical significance (p = 0.107).
Table 2. Tre and Tb at the onset of sweating and slopes of regression lines determined after plotting average LSR against Tre and Tb during exercise at fixed intensity (study 1) and during the RPE clamp exercise (study 2).
| CON | CWI | ICE | P-value | |
|---|---|---|---|---|
| Onset of sweating (min) | 1.8 ± 1.8* # | 6.4 ± 2.2# | 2.7 ± 1.6* | <0.001 |
| Tre sweat threshold (°C) | 37.0 ± 0.3* # | 37.1 ± 0.2# | 36.8 ± 0.3* | <0.001 |
| Tb sweat threshold (°C) | 36.5 ± 0.3 | 36.5 ± 0.3 | 36.5 ± 0.4 | 0.973 |
| Tre sweat sensitivity (mg∙cm-2∙min-1∙°C-1) | 1.51 ± 0.61* | 1.89 ± 0.82 | 1.42 ± 0.68* | 0.001 |
| Tb sweat sensitivity (mg∙cm-2∙min-1∙°C-1) | 1.18 ± 0.42* | 1.36 ± 0.55 | 1.39 ± 0.63 | 0.007 |
CON, control; CWI, cold water immersion; ICE, ice slushy ingestion; Tre, rectal temperature; Tb, weighted mean body; LSR, average local sweat rate for arm and thigh
* p<0.05 versus CWI
# p<0.05 versus ICE. Data are mean ± SD for n = 19 from study 1 and 2. See S5 Table for effect sizes (Cohen’s d) calculated from mean differences between conditions and pooled SD.
Discussion
The present study compared the effects of precooling internally via ICE and externally via CWI on thermoregulatory responses (e.g., Tsk and Tre) during steady state exercise (study 1) and thermoregulatory behavior (i.e., power output and total work output) during the RPE clamp protocol (study 2). These cooling methods were utilised for their distinct influences on Tsk and Tre. As hypothesised, study 1 demonstrated that ICE resulted in a narrower Tre-Tsk gradient via a reduction in Tre without influencing Tsk, and CWI increased the Tre-Tsk gradient via reduction in Tsk. Additionally, CWI significantly reduced whole body sweat loss and muscle blood volume (i.e., tHb) but did not impair O2 utilisation as inferred from the TOI and Hb responses. The main findings from study 2 showed that CWI increased MPO and total work output during 60 min of cycling regulated at RPE 15 when compared with CON only. Additionally, both precooling strategies improved thermal sensation compared with CON, with a longer and larger effect observed following CWI. These changes occurred at similar HR response, muscle O2 utilisation (TOI and Hb) and skin PU between conditions. The LSR data from both studies showed that CWI and ICE delayed sweating by 1–5 min relative to CON. Furthermore, CWI resulted in a higher Tre threshold for sweating whereas ICE resulted in a lower Tre threshold for the effector response; however, sweating occurred at similar Tb between conditions.
ICE decreased Tre by ~0.3°C while CWI significantly decreased Tsk compared with CON and ICE in both studies (Figs 1 and 3). In study 2, the higher work output observed during the CWI trials was in agreement with the importance of Tsk as controller of thermoregulatory behavior during exercise in the heat [4, 29]. Notably, manipulating Tsk via different ambient temperatures (18°C, 26°C, 34°C, and 42°C) did not influence Tre but resulted in impaired exercise capacity at a higher Tsk [29]. Similarly, self-selected work rate during 60 min of cycling has also been shown to parallel the changes in Tsk rather than Tre [4]. Indeed, Tsk appeared to have a greater influence on thermoregulatory behavior than Tre since the reduction in Tre via ICE did not influence total work output when compared with CON in study 2. Nevertheless, this current observation should be viewed with the understanding that Tre as an index of inner body temperature is confounded by its delayed response.
Tsk and thermal perception have been identified as independent controllers for thermoregulatory behavior [1]. However, study 2 showed that decreased thermal sensation in ICE did not have any beneficial effect on the work capacity when RPE was clamped at 15. Our findings also contradicted previous works which demonstrated that L-Menthol mouth rinse and face cooling improved thermal perception and increased time to exhaustion during a RPE clamp exercise without concomitant changes in the physical thermal state (i.e., Tsk and Tre) [3, 5]. It should be noted that maximum exercise duration within these two studies was less than 30 min, which paralleled the time course of changes in thermal sensation between ICE and CON in study 2 (Fig 3D). Taken together, the current findings do not refute the importance of thermal perception as controller of thermoregulatory behavior but suggest that the psychophysiological effect of ICE and other cooling methods are most important during shorter trials. A larger heat storage capacity conferred by ICE as a precooling strategy is negated by a lower evaporative heat loss [30], which helps to explain the lack of a clear ergogenic effect of ICE on endurance performance herein and within the literature [6]. In contrast, CWI significantly improved thermal sensation when compared with CON and ICE for up to 35–40 min during the RPE clamp exercise (study 2), supporting the importance of Tsk and thermal perception for mediating thermoregulatory behavior.
In line with our initial hypothesis, ICE decreased the Tre-Tsk gradient whereas CWI significantly increased the Tre-Tsk gradient (Figs 1 and 3). A high skin temperature (>35°C) and a concerted decrease in the Tre-Tsk gradient increase skin blood flow demand for heat dissipation [31]. CWI may alleviate cardiovascular strain by reducing reliance on cutaneous vasodilation for heat loss [7]. Indeed, study 1 showed that CWI resulted in a marginal decrease of ~5 bpm in mean HR compared with CON during the exercise, consistent with others who observed a transient decrease in the HR response to exercise following CWI at 17.7°C x 30 min [32]. However, a narrower Tre-Tsk gradient during early exercise stages following ICE did not affect the HR response compared with CON, which may be due to a greater increase in Tre, causing the Tre-Tsk gradient in the ICE trials to increase rapidly to levels similar to the CON trials. Another interesting observation was that although ICE and CWI decreased skin PU before the exercise, there were no differences between all conditions during the RPE clamp exercise in study 2 (Fig 4). For the ICE trials, this was concurrent with a higher Tsk during the first 5 min of exercise compared with CON (Fig 3), which may be related to the narrow temperature gradient between the environment, core body and skin [33], and attenuated evaporative heat loss secondary to a delayed sweating onset (Table 2). The cooling effect of CWI on the Tsk and Tre-Tsk gradient was observed for up to 15–20 min during the exercise (Fig 3), thereby maintaining the convective heat flux from the core body to the skin during early exercise phases. However, as the environmental temperature was much higher than Tsk following CWI, convective heat flux to the environment was most likely reversed until Tsk approximated the environmental temperature, which may help to explain the lack of differences between conditions in skin PU during the exercise.
ICE purportedly improves gross efficiency and has a glycogen sparing effect related to the core body cooling effect [34]. However, no differences in the NIRS parameters were observed in both studies (Figs 2 and 4), which suggested that ICE did not affect muscle metabolism. Conversely, CWI decreased muscle blood volume and limited O2 delivery to locomotive muscle during the precooling period and early exercise stages in both studies. It has been previously demonstrated that 5–15 min of CWI at 10°C impaired muscle blood volume and local tissue oxygenation [9, 10], resulting in greater anaerobic contribution during intermittent high-intensity exercise in temperate environments [10]. Contrary to these earlier studies, the present study provided evidence that CWI-induced decrease in muscle blood volume did not adversely affect O2 utilisation during the steady state exercise (study 1) or the RPE clamp exercise (study 2) as inferred from the changes in the TOI and Hb, attributable to a more thermally comfortable CWI at 22°C versus CWI at 10°C. Importantly, a higher work output was achieved during the CWI trials at muscle O2 utilisation and HR response similar to CON, consistent with the propositions that relative exercise intensity was maintained secondary to alleviated cardiovascular strain [35], and that individuals paced themselves such that metabolic homeostasis was maintained [22]. It is also possible that a certain magnitude of the increase in muscle blood volume during exercise in the no-cooling trials is related to elevated heat stress and not to metabolic demand per se. In support, it has been shown that heat stress at rest and during exercise increases blood flow to the muscle vasculature related to a direct thermal response, an increase in arterial plasma adenosine triphosphate and/or modulation by the muscle sympathetic vasoconstrictor activity [36]. Regardless, the present results are delimited to submaximal exercise, and do not refute the possible effects that CWI may have on muscle metabolic inertia during high-intensity exercise [10].
Both CWI and ICE delayed sweating response in terms of exercise time, in agreement with previous precooling studies [32, 37, 38]. We found that sweat recruitment occurred at a lower Tre in ICE and at a higher Tre in CWI compared with CON (Table 2), whereas others observed unchanged Tre sweating threshold [32], or lower Tb sweating threshold determined from weighted Tsk and esophageal temperature [39] after cooling. While the disparity may be due to the different core temperature indexes or different cooling techniques (cold air versus CWI), it is more likely attributable to the influence that skin and core temperatures have on the sweat response. Indeed, sweating occurred at similar Tb for all conditions and the differences in sweat gain between conditions were attenuated when plotted against Tb (Table 2). Because ICE primarily activates the sudomotor response via the abdominal thermoreceptors [16], it is not surprising that the sweat gain against Tb and Tre are similar, and the difference in sweat sensitivity between CON and ICE did not reach statistical significance. Conversely, CWI modifies the sweat responses via the cutaneous afferent signals and the nitric oxide pathway [14]. Because Tre was similar between CWI and CON in studies 1 and 2, we suggest that the higher sweat gain following CWI reflects the rapid increase in Tsk during the exercise. Taken together, our data demonstrate that precooling attenuates the thermal afferent signals such that the efferent signals start firing when a specific Tb threshold is attained, but CWI modifies the efferent signals by the changes in Tsk.
Limitations
The NIRS parameters derived using the modified Beer-Lambert method are known to be affected by skin blood flow, whereas TOI is based on the spatially-resolved spectroscopy and has been shown to be affected by a lesser degree [28, 40]. It is important to note that the aforementioned studies examined the NIRS signals during heat stress at rest or a brief bout of single joint exercise. In contrast, skin blood flow has been shown to have a lesser influence on the NIRS signal during CWI [41]. If the cutaneous interference did contribute to the NIRS signal, it seems logical to have both parameters change similarly by cooling or heating. Moreover, skin and muscle blood flow increase drastically during prolonged exercise in the heat, and muscle blood flow has been shown to increase during local heating [42]. Therefore, while the contribution of skin blood flow to the NIRS signals during exercise cannot be dismissed, it remains challenging to distinguish between thermoregulatory and metabolic demands from the cutaneous interference.
CVC is often expressed as percentage of maximum cutaneous vasodilation achieved by local heating and/or administration of sodium nitroprusside. However, the reliability of baseline CVC, expressed as absolute values or relative to maximum CVC, remains poor with coefficients of variation ranged from 25–30% [43]. Improved reliability has been observed as CVC increases during local heating [43] or during exercise [44]. Moreover, our pilot observation showed that it took more than 40 min to achieve a plateau in CVC after the exercise. As such, skin PU data are expressed in absolute values.
The difference in power output between CON and CWI in study 2 is considered as small effect (Cohen’s d = 0.42, mean difference of 8 W). The modest beneficial effect from CWI may be related to the training status of the present cohort ( <55 mL.kg-1.min-1), as well-trained athletes have been shown to have a greater beneficial effect from precooling maneuvers [8]. Additionally, the present results are based on a male sample. Future research should explore the possible gender effect on precooling and the resultant physiological responses, as it has been shown that females have a lower evaporative heat loss and blunted sweating thermosensitivity than males during exercise at a fixed rate of metabolic heat production after controlling for menstrual cycle, body mass, and relative to lean muscle mass [45].
Conclusions
To conclude, significant skin cooing by CWI resulted in a greater reduction in thermal sensation and improved thermoregulatory behavior (i.e., higher MPO and total work output). Additionally, although CWI decreased muscle blood volume during early exercise stages, it did not limit muscle oxygenation during steady state exercise or RPE clamp exercise in the heat. Conversely, a reduction of ~0.3°C in Tre by ICE decreased thermal sensation but did not confer any ergogenic effect on the thermoregulatory behavior during 60 min of exercise. As such, the duration of events should be taken into consideration when planning for cooling strategies prior to exercise in hot conditions. ICE and CWI delayed sweat recruitment in terms of exercise time and attenuated the thermal efferent signals until a specific Tb threshold was attained, but the efferent signals were modified by the changes in Tsk in CWI.
Supporting information
CON, control; CWI, cold water immersion, ICE, ice slushy ingestion.
(PDF)
CON, control; CWI, cold water immersion, ICE, ice slushy ingestion.
(PDF)
CON, control; CWI, cold water immersion, ICE, ice slushy ingestion.
(PDF)
CON, control; CWI, cold water immersion, ICE, ice slushy ingestion.
(PDF)
CON, control; CWI, cold water immersion, ICE, ice slushy ingestion.
(PDF)
CON, control; CWI, cold water immersion, ICE, ice slushy ingestion.
(XLSX)
Acknowledgments
The authors would like to express our gratitude to the participants who took part in the study.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The study was funded by the School of Medical and Health Sciences, Edith Cowan University. At the time of the study was conducted, HCC and RNOM were supported by the International Postgraduate Research Scholarship and Edith Cowan University. João Lopes-Silva (88881.132395/2016-01) was supported by CAPES Scholarship. The other authors have no support or funding to report.
References
- 1.Schlader ZJ, Stannard SR, Mündel T. Human thermoregulatory behavior during rest and exercise—A prospective review. Physiol Behav. 2010;99(3):269–75. 10.1016/j.physbeh.2009.12.003 [DOI] [PubMed] [Google Scholar]
- 2.Flouris AD, Cheung SS. Human conscious response to thermal input is adjusted to changes in mean body temperature. Br J Sports Med. 2008;43(3):199–203. 10.1136/bjsm.2007.044552 [DOI] [PubMed] [Google Scholar]
- 3.Schlader ZJ, Simmons SE, Stannard SR, Mundel T. The independent roles of temperature and thermal perception in the control of human thermoregulatory behavior. Physiol Behav. 2011;103(2):217–24. WOS:000290011300013. 10.1016/j.physbeh.2011.02.002 [DOI] [PubMed] [Google Scholar]
- 4.Schlader ZJ, Simmons SE, Stannard SR, Mundel T. Skin temperature as a thermal controller of exercise intensity. Eur J Appl Physiol. 2011;111(8):1631–9. WOS:000293980000009. 10.1007/s00421-010-1791-1 [DOI] [PubMed] [Google Scholar]
- 5.Flood TR, Waldron M, Jeffries O. Oral L-menthol reduces thermal sensation, increases work-rate and extends time to exhaustion, in the heat at a fixed rating of perceived exertion. Eur J Appl Physiol. 2017;117(7):1501–12. 10.1007/s00421-017-3645-6 [DOI] [PubMed] [Google Scholar]
- 6.Choo HC, Nosaka K, Peiffer JJ, Ihsan M, Abbiss CR. Ergogenic effects of precooling with cold water immersion and ice ingestion: A meta-analysis. Eur J Sport Sci. 2017;18(2):170–81. 10.1080/17461391.2017.1405077 [DOI] [PubMed] [Google Scholar]
- 7.Bongers CC, Hopman MT, Eijsvogels TM. Cooling interventions for athletes: An overview of effectiveness, physiological mechanisms, and practical considerations. Temperature. 2017;4(1):60–78. 10.1080/23328940.2016.1277003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wegmann M, Faude O, Poppendieck W, Hecksteden A, Frohlich M, Meyer T. Pre-cooling and sports performance: A meta-analytical review. Sports Med. 2012;42(7):545–64. Epub 2012/05/31. 10.2165/11630550-000000000-00000 . [DOI] [PubMed] [Google Scholar]
- 9.Ihsan M, Watson G, Lipski M, Abbiss CR. Influence of postexercise cooling on muscle oxygenation and blood volume changes. Med Sci Sports Exerc. 2013;45(5):876–82. 10.1249/MSS.0b013e31827e13a2 [DOI] [PubMed] [Google Scholar]
- 10.Stanley J, Peake JM, Coombes JS, Buchheit M. Central and peripheral adjustments during high-intensity exercise following cold water immersion. Eur J Appl Physiol. 2014;114(1):147–63. 10.1007/s00421-013-2755-z [DOI] [PubMed] [Google Scholar]
- 11.Périard JD, Thompson MW, Caillaud C, Quaresima V. Influence of heat stress and exercise intensity on vastus lateralis muscle and prefrontal cortex oxygenation. Eur J Appl Physiol. 2013;113(1):211–22. 10.1007/s00421-012-2427-4 [DOI] [PubMed] [Google Scholar]
- 12.Proulx CI, Ducharme MB, Kenny GP. Safe cooling limits from exercise-induced hyperthermia. Eur J Appl Physiol. 2006;96(4):434–45. 10.1007/s00421-005-0063-y [DOI] [PubMed] [Google Scholar]
- 13.Siegel R, Laursen PB. Keeping your cool: Possible mechanisms for enhanced exercise performance in the heat with internal cooling methods. Sports Med. 2012;42(2):89–98. 10.2165/11596870-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 14.Fujii N, McGinn R, Halili L, Singh MS, Kondo N, Kenny GP. Cutaneous vascular and sweating responses to intradermal administration of ATP: A role for nitric oxide synthase and cyclooxygenase? J Physiol. 2015;593(11):2515–25. 10.1113/JP270147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shibasaki M, Crandall CG. Mechanisms and controllers of eccrine sweating in humans. Front Biosci (Scholar Ed). 2010;2:685–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris NB, Bain AR, Cramer MN, Jay O. Evidence that transient changes in sudomotor output with cold and warm fluid ingestion are independently modulated by abdominal, but not oral thermoreceptors. J Appl Physiol. 2014;116(8):1088–95. 10.1152/japplphysiol.01059.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris NB, Coombs G, Jay O. Ice slurry ingestion leads to a lower net heat loss during exercise in the heat. Med Sci Sports Exerc. 2016;48(1):114–22. 10.1249/MSS.0000000000000746 [DOI] [PubMed] [Google Scholar]
- 18.Tucker R, Marle T, Lambert EV, Noakes TD. The rate of heat storage mediates an anticipatory reduction in exercise intensity during cycling at a fixed rating of perceived exertion. J Physiol. 2006;574(3):905–15. 10.1113/jphysiol.2005.101733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siegel R, Maté J, Watson G, Nosaka K, Laursen PB. Pre-cooling with ice slurry ingestion leads to similar run times to exhaustion in the heat as cold water immersion. J Sports Sci. 2012;30(2):155–65. 10.1080/02640414.2011.625968 [DOI] [PubMed] [Google Scholar]
- 20.Kuipers H, Verstappen F, Keizer H, Geurten P, Van Kranenburg G. Variability of aerobic performance in the laboratory and its physiologic correlates. Int J Sports Med. 1985;6(04):197–201. 10.1055/s-2008-1025839 [DOI] [PubMed] [Google Scholar]
- 21.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–81. [PubMed] [Google Scholar]
- 22.Lander PJ, Butterly RJ, Edwards AM. Self-paced exercise is less physically challenging than enforced constant pace exercise of the same intensity: Influence of complex central metabolic control. Br J Sports Med. 2009;43(10):789–95. 10.1136/bjsm.2008.056085 [DOI] [PubMed] [Google Scholar]
- 23.Young AJ, Sawka MN, Epstein Y, DeCristofano B, Pandolf KB. Cooling different body surfaces during upper and lower body exercise. J Appl Physiol. 1987;63(3):1218–23. 10.1152/jappl.1987.63.3.1218 [DOI] [PubMed] [Google Scholar]
- 24.Ramanathan NL. A new weighting system for mean surface temperature of the human body. J Appl Physiol. 1964;19(3):531–3. [DOI] [PubMed] [Google Scholar]
- 25.Colin J, Timbal J, Houdas Y, Boutelier C, Guieu JD. Computation of mean body temperature from rectal and skin temperatures. J Appl Physiol. 1971;31(3):484–9. 10.1152/jappl.1971.31.3.484 [DOI] [PubMed] [Google Scholar]
- 26.Kenefick RW, Cheuvront SN, Elliott LD, Ely BR, Sawka MN. Biological and analytical variation of the human sweating response: Implications for study design and analysis. Am J Physiol Regul Integr Comp Physiol. 2012;302(2):R252–R8. 10.1152/ajpregu.00456.2011 [DOI] [PubMed] [Google Scholar]
- 27.Cheuvront SN, Bearden SE, Kenefick RW, Ely BR, DeGroot DW, Sawka MN, et al. A simple and valid method to determine thermoregulatory sweating threshold and sensitivity. J Appl Physiol. 2009;107(1):69–75. 10.1152/japplphysiol.00250.2009 [DOI] [PubMed] [Google Scholar]
- 28.Messere A, Roatta S. Influence of cutaneous and muscular circulation on spatially resolved versus standard Beer–Lambert near‐infrared spectroscopy. Physiol Rep. 2013;1(7):e00179 10.1002/phy2.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cuddy JS, Hailes WS, Ruby BC. A reduced core to skin temperature gradient, not a critical core temperature, affects aerobic capacity in the heat. J Therm Biol. 2014;43:7–12. 10.1016/j.jtherbio.2014.04.002 [DOI] [PubMed] [Google Scholar]
- 30.Jay O, Morris NB. Does cold water or ice slurry ingestion during exercise elicit a net body cooling effect in the heat? Sports Med. 2018:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sawka MN, Cheuvront SN, Kenefick RW. High skin temperature and hypohydration impair aerobic performance. Exp Physiol. 2012;97(3):327–32. 10.1113/expphysiol.2011.061002 [DOI] [PubMed] [Google Scholar]
- 32.Wilson TE, Johnson SC, Petajan JH, Davis SL, Gappmaier E, Luetkemeier MJ, et al. Thermal regulatory responses to submaximal cycling following lower-body cooling in humans. Eur J Appl Physiol. 2002;88(1–2):67–75. 10.1007/s00421-002-0696-z [DOI] [PubMed] [Google Scholar]
- 33.Taylor NA. Challenges to temperature regulation when working in hot environments. Ind Health. 2006;44(3):331–44. [DOI] [PubMed] [Google Scholar]
- 34.Zimmermann MR, Landers GJ, Wallman KE, Saldaris J. The effects of crushed ice ingestion prior to steady state exercise in the heat. Int J Sport Nutr Exerc Metab. 2017:1–21. 10.1123/ijsnem.2017-0057 [DOI] [PubMed] [Google Scholar]
- 35.Périard JD, Cramer MN, Chapman PG, Caillaud C, Thompson MW. Cardiovascular strain impairs prolonged self‐paced exercise in the heat. Exp Physiol. 2011;96(2):134–44. 10.1113/expphysiol.2010.054213 [DOI] [PubMed] [Google Scholar]
- 36.Pearson J, Low DA, Stöhr E, Kalsi K, Ali L, Barker H, et al. Hemodynamic responses to heat stress in the resting and exercising human leg: Insight into the effect of temperature on skeletal muscle blood flow. Am J Physiol Regul Integr Comp Physiol. 2011;300(3):R663–R73. 10.1152/ajpregu.00662.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimmermann MR, Landers GJ, Wallman KE. Crushed ice ingestion does not improve female cycling time trial performance in the heat. Int J Sport Nutr Exerc Metab. 2017:1–23. Epub 2016/07/28. 10.1123/ijsnem.2016-0028 . [DOI] [PubMed] [Google Scholar]
- 38.Morrison SA, Cheung S, Cotter JD. Importance of airflow for physiologic and ergogenic effects of precooling. J Athl Train. 2014;49(5):632–9. 10.4085/1062-6050-49.3.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olschewski H, Bruck K. Thermoregulatory, cardiovascular, and muscular factors related to exercise after precooling. J Appl Physiol. 1988;64(2):803–11. 10.1152/jappl.1988.64.2.803 [DOI] [PubMed] [Google Scholar]
- 40.Tew GA, Ruddock AD, Saxton JM. Skin blood flow differentially affects near-infrared spectroscopy-derived measures of muscle oxygen saturation and blood volume at rest and during dynamic leg exercise. Eur J Appl Physiol. 2010;110(5):1083–9. 10.1007/s00421-010-1596-2 [DOI] [PubMed] [Google Scholar]
- 41.Choo HC, Nosaka K, Peiffer JJ, Ihsan M, Yeo CC, Abbiss CR. Peripheral blood flow changes in response to post-exercise cold water immersion. Clin Physiol Funct Imaging. 2016;38(1):46–55. 10.1111/cpf.12380 [DOI] [PubMed] [Google Scholar]
- 42.Heinonen I, Brothers RM, Kemppainen J, Knuuti J, Kalliokoski KK, Crandall CG. Local heating, but not indirect whole body heating, increases human skeletal muscle blood flow. J Appl Physiol. 2011;111(3):818–24. 10.1152/japplphysiol.00269.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tew GA, Klonizakis M, Moss J, Ruddock AD, Saxton JM, Hodges GJ. Reproducibility of cutaneous thermal hyperaemia assessed by laser Doppler flowmetry in young and older adults. Microvasc Res. 2011;81(2):177–82. 10.1016/j.mvr.2010.12.001 [DOI] [PubMed] [Google Scholar]
- 44.Choo HC, Nosaka K, Peiffer JJ, Ihsan M, Yeo CC, Abbiss CR. Reliability of laser Doppler, near-infrared spectroscopy and Doppler ultrasound for peripheral blood flow measurements during and after exercise in the heat. J Sports Sci. 2016;35(17):1715–23. 10.1080/02640414.2016.1235790 [DOI] [PubMed] [Google Scholar]
- 45.Gagnon D, Kenny GP. Sex modulates whole‐body sudomotor thermosensitivity during exercise. J Physiol. 2011;589(24):6205–17. 10.1113/jphysiol.2011.219220 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CON, control; CWI, cold water immersion, ICE, ice slushy ingestion.
(PDF)
CON, control; CWI, cold water immersion, ICE, ice slushy ingestion.
(PDF)
CON, control; CWI, cold water immersion, ICE, ice slushy ingestion.
(PDF)
CON, control; CWI, cold water immersion, ICE, ice slushy ingestion.
(PDF)
CON, control; CWI, cold water immersion, ICE, ice slushy ingestion.
(PDF)
CON, control; CWI, cold water immersion, ICE, ice slushy ingestion.
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.


