Abstract
EAU, an animal model for severe intraocular inflammatory eye diseases, is mediated by both Th1 and Th17 cells. Here, we examined the capacity of TMP778, a selective inhibitor of RORγt, to inhibit the development of EAU, as well as the related immune responses. EAU was induced in B10.A mice by immunization with interphotoreceptor retinoid-binding protein (IRBP). Treatment with TMP778 significantly inhibited the development of EAU, determined by histological examination. In addition, the treatment suppressed the cellular immune response to IRBP, determined by reduced production of IL-17 and IFN-γ, as well as lower percentages of lymphocytes expressing these cytokines, as compared to vehicle-treated controls. The inhibition of IFN-γ expression by TMP778 is unexpected in view of this compound being a selective inhibitor of RORγt. The observation was further confirmed by the finding of reduced expression of the T-bet (Tbx21) gene, the transcription factor for IFN-γ, by cells of TMP778-treated mice. Thus, these data demonstrate the capacity of TMP778 to inhibit pathogenic autoimmunity in the eye and shed new light on its mode of action in vivo.
Keywords: experimental autoimmune uveitis, RORγt, inhibition, Th17 cells, Th1 cells, TMP778
INTRODUCTION
Data collected over the past two decades have established the involvement of the Th17 lymphocyte population in the pathogenesis of cell-mediated autoimmune diseases, along with the previously identified Th1 cell population [1–4]. Further, in most systems, the Th17 population has been found to play the major pathogenic role in these conditions [4, 5]. Consequently, efforts are being made to identify or synthesize biologics and compounds with selective inhibitory capacity against Th17 cells. Thus, antibodies against IL-17 or biologic agents specific against the Th17 pathway have been reported to be beneficial for treatment of diseases such as psoriasis, rheumatoid arthritis, or ankylosing spondylitis [6–8]. Another approach for inhibition of pathogenic Th17 cells is by blocking the activity of the specific transcription factor that regulates the development of Th17 population, namely, RORγt, [9]. Two such molecules, digoxin and SR1001, have been reported to inhibit experimental autoimmune encephalomyelitis (EAE), experimental autoimmune uveitis (EAU) and experimental diabetes [10–13]. More recent publications reported the beneficial immunosuppressive activities of new synthetic molecules with the capacity to block RORγt activity and, consequently, to inhibit induction of psoriasis-like skin inflammation and EAE [14–16]. Here, we record the inhibitory effect of one of these molecules, TMP778, on the development of EAU, an animal model for uveitis in humans [17,18].
The term “uveitis” is used to define a family of eye conditions with intraocular inflammation, which includes diseases such as sympathetic ophthalmia, “birdshot” chorioretinopathy, sarcoidosis, Behcet’s disease and Vogt-Koyanagi-Harada disease [17, 18]. Cellular autoimmune processes are assumed to play major roles in the pathogenesis of these diseases, an assumption supported by the similarity between the pathological changes specific to these diseases and the typical ocular changes seen in EAU; an autoimmune disease mediated by immunopathogenic T-cells [18, 19]. In mice, EAU is induced by immunization with interphotoreceptor retinoid-binding protein (IRBP) [19]. We found here that treatment with TMP778 inhibited in mice the development of EAU, as well as the immune response against IRBP. Importantly, our data show that in addition to the expected suppression of Th17 response, the treatment of mice with TMP778 also inhibited the Th1 response.
RESULTS AND DISCUSSION
TMP778 inhibits the development of EAU.
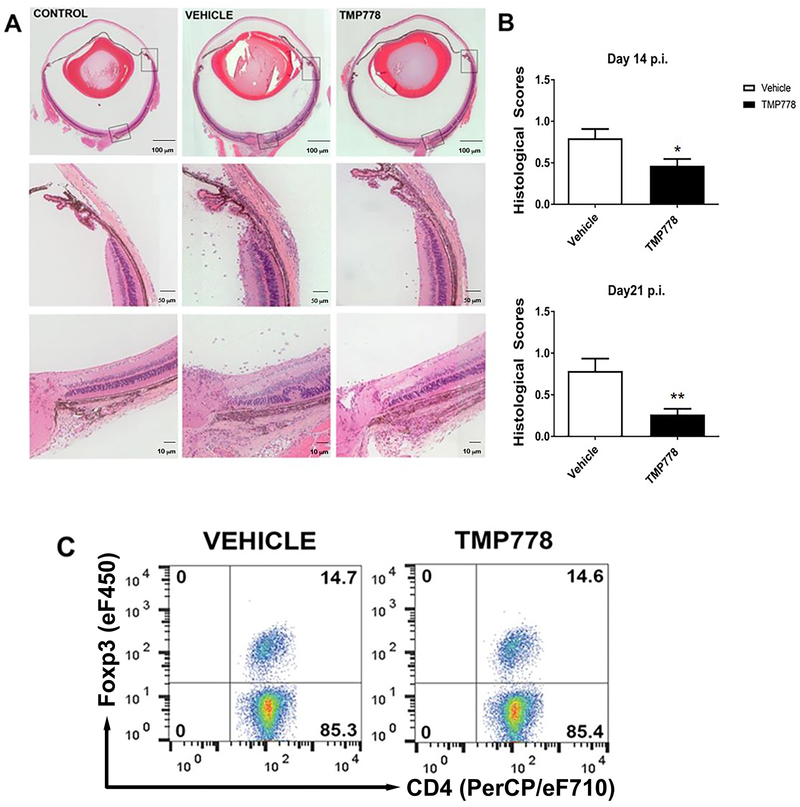
To test the capacity of TMP778 to inhibit the development of EAU, we immunized groups of B10.A mice with the uveitogenic antigen, IRBP, and treated them with TMP778, or the vehicle control, as detailed in the Materials and Methods. Development of EAU was determined by histological analysis of the mouse eyes, as detailed in the Materials and Methods section. Fig. 1A shows eye sections of (i) a normal mouse, used as a negative control, (ii) an immunized mouse treated with the vehicle (used to dissolve TMP778) and (iii) an immunized mouse treated with TMP778. The histopathological changes in the vehicle-treated mouse eye are scored at “1.5”, whereas those seen in the eye of the TMP778-treated mouse are scored at “0.5”. The specific histopathological changes in these mouse eyes are detailed in the Figure’s legend. Fig. 1B summarizes accumulated data of pathological changes in mouse eyes obtained in repeated experiments with mouse eyes collected on days 14 or 21 of treatment with TMP778, or with the vehicle control. The two panels of this Figure show significant inhibition of EAU development in the TMP778-treated mice, as compared to their controls, at the two tested time points.
Figure 1.
Treatment with TMP778 inhibits the development of EAU in mice. Groups of B10.A mice were immunized with IRBP and their eyes were collected as detailed in the Materials and Methods . The severity of pathological changes were scored as described elsewhere [24]. (A) Eye sections of a normal control mouse and of eyes of mice treated with the vehicle or TMP778. The histopathological changes in the vehicle treated mouse eye include accumulation of inflammatory cells in the limbal and the optic nerve head areas (the entry sites for the inflammatory cells), as well as inflammatory cells migrating into the retina and a typical retinal fold. In addition, inflammatory cells are visible throughout the vitreous (histopathological score: 1.5). Changes seen in the eye of the mouse treated with TMP778 are much less severe (score: 0.5). (B) Summary of scores are shown as +- SEM (n=4-5 mice in each group) and are pooled from five independent experiments for day 14 and three independent experiments for day 21. *p <0.05; **p <0.01 Mann-Whitney U test. (C) Treg cells expressing FoxP3 do not participate in the immunosuppressive effect of TMP778. Flow cytometric analysis of pooled spleen cells from mice treated with TMP778 or the vehicle, on day 14 of treatment. The mean ratio between percentages of FoxP3+ cells in spleens of mice treated with TMP778 or the vehicle-treated control was 0.96+/−0.02. Plots are from one experiment representative of four independent experiments, with n=4-5 mice in each group.
Development of certain autoimmune processes is suppressed by the population of T-regulatory (Treg) cells [20, 21] and we examined here the possible involvement of these cells in the immunosuppressive effect of TMP778, by measuring the proportion of cells expressing the FoxP3 transcription factor, specific to Treg cells [20, 21], among the total spleen CD4 cells. Flow cytometric analysis of FoxP3 positive cells (Fig. 1C) revealed similar proportions of these cells in spleens of the TMP778-treated mice and their vehicle-treated controls. Thus, changes in Treg population do not seem to play a role in the immunosuppressive activity of TMP778 in our system.
Treatment with TMP778 affects both Th1 and Th17 cell populations.
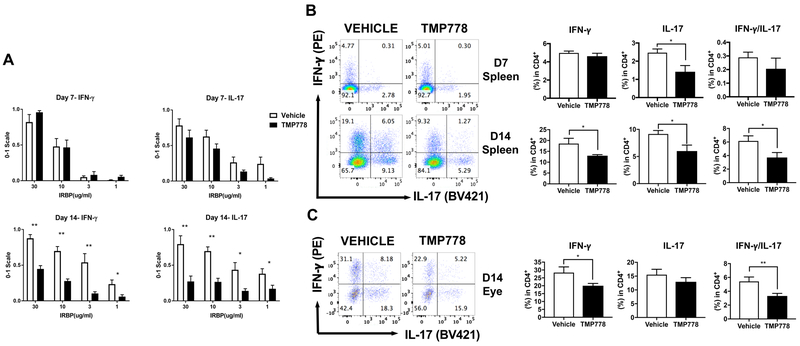
Previous studies revealed that the immunosuppressive capacity of TMP778 derives from its inhibition of RORγt, the specific transcription factor for the production of IL-17, the signature cytokine of Th17 lymphocytes. [9, 14–16]. To examine in our experimental system the effect of TMP778 treatment on Th17 and Th1 cells, the two major immunopathogenic lymphocyte populations [1, 2], we collected spleen cells of mice of the two treated groups and cultured them with the immunizing antigen, IRBP, at different concentrations. The cellular response was assessed by measuring in the culture supernatants the levels of IFN-γ and IL-17, the signature cytokines for Th1 and Th17 populations, respectively [1, 2]. The tested spleen cells were collected from mice following 7 or 14 days of treatment and data of 5 or 8 repeated experiments, respectively, are summarized in a normalized figure (Fig. 2A). When tested on day 7, spleen cells of mice treated with TMP778 released lower levels of IL-17 than their controls, but the differences did not reach significance. The differences in IL-17 production between the two groups reached significance, however, when the spleen cells were collected on day 14 post immunization. No differences were noted between the levels of IFN-γ in cultures of spleen cells of mice treated with TMP778 or the vehicle collected on day 7 post immunization. Unexpectedly, however, the IFN-γ levels in cultures of splenocytes collected on day 14 of treatment with TMP778 were significantly lower than those of the control cultures (Fig. 2A). Actual levels of IL-17 and IFN-γ secreted by spleen cells of the different groups of a representative experiment are recorded in supplemental Fig. S1
Figure 2.
Treatment with TMP778 suppresses the production of both IL-17 and IFN-γ by sensitized spleen and eye-infiltrating cells. (A) Spleen cells from B10.A mice immunized with IRBP and treated with TMP778, or the vehicle, were collected on days 7 or 14 post immunization and cultured with IRBP at the indicated concentrations. Supernatants were analyzed for the levels of IL-17 and IFN-γ, using ELISA kits. Collected data of repeated experiments (5 experiments for day 7 and 8 experiments for day 14) were normalized as detailed in the Materials and Methods section. Importantly, the figure shows that the treatment with TMP778 for 14 days reduced the response of both IL-17 (expected) and IFN-γ (unexpected). Treatment for 7 days had slight effect on IL-17 production and no effect on the levels of IFN-γ. Data are shown as +- SEM (n=4–5 mice in each group) and are pooled from 5 independent experiments for day 7 and 8 experiments for day 14). (B) Flow cytometric analysis of intracellular expression of IFN-γ and IL-17 by spleen cells of mice treated with TMP778, or the vehicle, for 7 or 14 days. Left: the scatterplots show data of representative experiments; right: the histogram bars show mean values of cells expressing IFN-γ or IL-17 among the total CD4 populations of repeated experiments. Data are shown as +- SEM (n=4–5 mice in each group of each experiment) and are pooled from 5 independent experiments with cells collected after 7 days of treatment, and from 10 experiments with cells collected after 14 days of treatment. (C) Flow cytometric analysis of intracellular expression of of IFN-γ and IL-17 by lymphoid cells collected from eyes of mice on day 14 of treatment with the vehicle (DMSO) or TMP778. The figure shows data collected in a representative experiment, while values of 4 repeated experiments are summarized in the histograms, as detailed above. * p<0.05, ** p<0.01; unpaired t test.
In addition to testing the cytokine release, we evaluated the inhibitory effects of TMP778 treatment by measuring by flow cytometry the proportions of spleen lymphocytes which selectively expressed intracellular IL-17, IFN-γ, or both cytokines. Fig. 2B shows data of representative flow cytometric analyses of spleen cells collected from mice on days 7 or 14 of treatment with TMP778 or with the vehicle. Data of repeated additional experiments are summarized in the histograms adjacent to the corresponding flow cytometric figures. Considerable variability was observed among the repeated experiments. The flow cytometric observations are in line with those obtained with the culture supernatants (Fig. 2A): clear reduction is seen in the proportion of IL-17 producing cells on both days 7 and 14 of treatment, whereas only minimal effect of the treatment on IFN-γ production is seen on day 7 of treatment, followed by substantial inhibition of this cytokine production on day 14 of treatment.
In addition to testing spleen cells from the experimental mice, we analyzed the effect of TMP778 treatment on the populations of Th cells that infiltrated the affected eyes. We collected these cells as detailed in the Materials and Methods section and determined by flow cytometry the proportions of the three cell subpopulations, expressing IFN-γ, IL-17, or IFN-γ/IL-17. The data of a representative experiment are shown in Fig. 2C and data of repeated experiments are summarized in the histograms adjacent to the flow cytometric figures. Similar to the observations with spleen cells collected on day 14 of treatment (Fig. 2B), treatment with TMP778 reduced the percentages of lymphocytes of all three subpopulations that infiltrated the eyes. It is of note that the percentage of cytokine-producing cells among total CD4 cells in the mouse eyes were remarkably higher than those in the mouse spleens (histograms C vs B).
Interestingly, analysis of flow cytometric data revealed that treatment with TMP778 particularly affected the population of “double stained” Th cells, that express both IL-17 and IFN-γ and were collected on both days 7 and 14 (Figs. 2B and 2C). Th cells expressing both IL-17 and IFN-γ (also named Th1*) were found to constitute a large proportion of Th cells collected from the CNS of mice developing EAE [9] and it is now believed that this subpopulation plays a major role in the pathogenesis of autoimmune diseases [22]. It is noticeable, therefore, that the IFN-γ /IL-17 subpopulation was highly susceptible to the suppressive effect of TMP778.
Treatment with TMP778 inhibits expression of both RORγt and T-bet
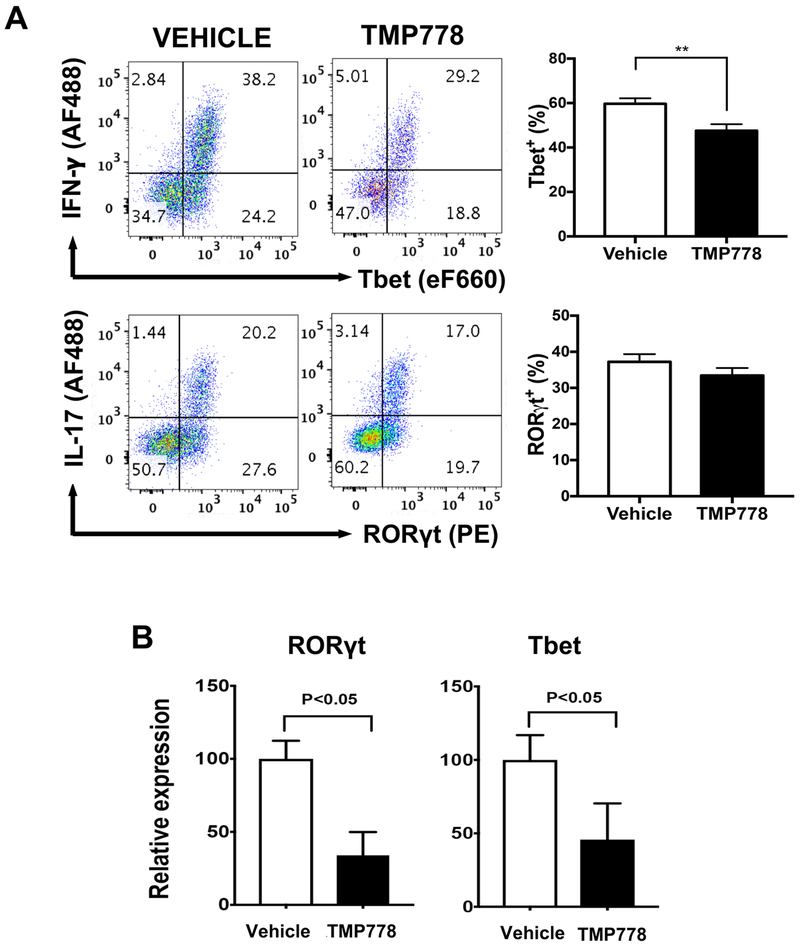
The lymphocyte populations that infiltrated the mouse eyes were further analyzed by flow cytometry for their expression of T-bet and RORγt, the transcription factors for IFN-γ and IL-17, respectively. Cells expressing the transcription factors were identified by co-staining for the corresponding cytokines (Fig. 3A). In line with the expression profiles of lymphocytes expressing IFN-γ or IL-17, more eye-infiltrating cells expressed T-bet than RORγt. In accord with data reported above, treatment of mice with TMP778 significantly inhibited the proportions of T-bet expressing cells, while the reduction in cells expressing RORγt did not reach significance (Fig. 3A).
Figure 3.
Treatment with TMP778 inhibits the expression of both RORγt and T-bet. (A) Flow cytometric analysis of intracellular expression of T-bet and RORγt by lymphoid cells collected from eyes of mice on day 14 post-immunization. Representative flow plots displaying the frequency of T-bet+ Th1 cells or RORγt+ Th17 cells in the eyes. Data are shown as +- SEM (n=4) and are from one experiment representative of four independent experiments. ** p<0.01; unpaired t test. (B) Quantitative PCR analysis of TMP778 treatment effect on transcript expression of transcription factor genes. Spleen cells of TMP778 treated mice and of their vehicle-treated controls were processed as detailed elsewhere [23]. Data are shown as +- SEM (n=3) and are pooled from three independent experiments. Please note that T-bet transcript expression is suppressed in the TMP778-treated mice, similarly to RORγt transcript. * p <0.05; two-tailed t test.
The proportions of cells expressing T-bet or RORγt among spleen cells of the experimental mice were too low to be accurately measured by flow cytometry and we used the qPCR method for this purpose. Data of three experiments are summarized in Fig. 3B. In line with the suppressive effect of TMP778 treatment on the differentiation of both Th17 and Th1, the expression of both Rorgt and T-bet were markedly lower in splenocytes from treated mice than in their vehicle-treated controls.
The observation of reduced expression of T-bet in TMP778-treated mice thus further establishes the unexpected finding that treatment with TMP778 reduces the proportion of Th1 cells in the in vivo system.
Importantly, unlike the observations made in the current study in mice, showing the suppressive effects of TMP778 treatment on both Th17 and Th1 populations, the selective suppressive effect of TMP778 only on the production of IL-17 [9] was confirmed in experiments in vitro. Thus, adding TMP778 to cultures of naïve CD4 cells stimulated with anti-CD3/CD28 antibodies selectively inhibited the production of IL-17, but essentially had no effect on IFN-γ production (Supplementary Fig. S2).
The finding that treatment of mice with TMP778 inhibits remarkably the production of IFN-γ and reduces the proportion of Th1 cells among total CD4 cells is unexpected, since this compound is a selective inhibitor of RORγt, the transcription factor responsible for the production of IL-17 [9]. We hypothesize that the reduction in Th1 cell proportion and IFN-γ production in TMP778-treated mice could be attributed to the plasticity of Th17 cells, that readily switch phenotype and acquire Th1 features [23, 24]. Thus, it is conceivable that the reduction in proportion of Th17 cells at the early period of treatment with TMP778 reduces the population of Th17 cells available for the switch to the Th1 phenotype, as determined at the later time points. In other words, we hypothesize that as their proportions decrease in the TMP778 treated mice at the early phase of treatment, fewer Th17 cells remain available to undergo the phenotype switch at the later phase, yielding lower proportions of Th1 cell levels (Figs. 2B and 2C), as well as lower levels of IFN-γ (Fig. 2A) than those in the vehicle-treated control mice.
CONCLUDING REMARKS
Data reported here provide new information on two issues: (i) treatment with the synthetic compound TMP778 significantly inhibits the development of EAU, an animal model for uveitis, a potentially blinding condition in humans. This finding thus supports the notion that specific inhibitors of RORγt could be considered as immunosuppressive agents for treatment of autoimmune conditions. (ii) An unexpected finding in this study is that treatment with TMP778, a selective inhibitor of RORγt, substantially reduced in mice the proportions of Th1 cells and the levels of IFN-γ. To our knowledge, this is the first report describing this observation. We hypothesize that the decrease in Th1 cells in TMP778-treated mice is due to the known switch of Th17 cells to the Th1 phenotype during the pathogenic process [23, 24].
MATERIALS AND METHODS
Mice
Female B10.A mice, at 7–10 weeks of age, were provided by Jackson Labs, Bar Harbor, ME. The mice were housed in a specific pathogen free facility and all procedures were carried out in compliance with the GlaxoSmithKline Policy on the Care, Welfare and Treatment of Laboratory Animals, as well as with the NIH Resolution on the Use of Animals in Research, under protocol NEI-624, approved by the National Eye Institute Animal Care and Use Committee.
Induction and Scoring of EAU
Groups of 4–5 mice were immunized by injection of 0.2 ml emulsion containing 50 μg of bovine IRBP in complete Freund’s adjuvant, administered by subcutaneous injections. Pertussis toxin, 0.2 μg (List Biological Laboratories, Campbell, CA), or 1 μg (Sigma, St Louis, MO) was injected intraperitoneally, concurrently with the immunization. The mice were euthanized on days 14 or 21 post immunization and eyes were collected for histological analysis, using a scale of 0–4 in half points increments, as detailed elsewhere [25].
Treatment with TMP778
TMP778 was dissolved in 3% dimethylacetamide, 10% Solutol, and 87% saline (“solvent/vehicle”) and injected subcutaneously twice daily at abdomen sites, at 20 mg/kg. In certain experiments, as indicated, the compound was dissolved in DMSO. Control mice were similarly injected with the corresponding vehicle.
Measurements of cellular immune responses: cytokine release
Mouse spleens and eyes were collected upon euthanasia. Suspensions of spleen cells or inflammatory cells that infiltrated affected eyes were prepared as described in Ref. 26. Spleen cell suspensions were cultured in RPMI-1640 medium containing HL-1 (Lonza, Walkersville, MD), antibiotics, L-glutamine and 2-ME at 50 μM. Cytokine production was measured in culture supernatants collected after 48 hrs of incubation with IRBP at different concentrations, as indicated (the method is detailed in Refs. 23, 25).
Measurement of cellular immune response: intracellular expression of cytokines and transcription factors
Mouse spleen and eye infiltrating cell cultures were prepared and their intracellular expression of IL-17, IFN-γ, Τ−bet, or RORγt were determined as detailed elsewhere [23, 26]. Briefly, the cells were incubated with ionomycin (500 ng/ml), PMA (50 ng/ml) and Brefeldin A (GolgiPlug, BD Biosciences), followed by staining with conjugated anti-CD4 monoclonal antibody (RM4–5, Biolegend). After 4% paraformaldehyde fixation and permeabilization (BD bioscience or ebioscience), cells were intracellularly stained for T-bet (4B10), RORγt (AFKJS-9), IFN-γ (XMG1.2) and IL-17A (TC11–18H10.1). The staining patterns were analyzed by a MACSQuant Analyzer 10 (Miltenyi Bioitec. Cambridge, MA) and processed by FlowJo (Tree Star, Ashland, OR).
Measurement of cellular immune response: intracellular expression of cytokines and transcription factors
Mouse spleen and eye infiltrating cell cultures were prepared and their intracellular expression of IL-17, IFN-γ, Τ−bet, or RORγt were determined as detailed elsewhere [23, 26]. Briefly, the cells were incubated with ionomycin/PMA and Brefeldin A (GolgiPlug, BD Biosciences), followed by staining with conjugated anti-CD3 monoclonal antibody (145–2C11, BD Biosciences), anti-CD4 monoclonal antibody (RM4–5, Biolegend) and anti-CD45 monoclonal antibody (30-F11, Biolegend). After 4% paraformaldehyde fixation and permeabilization (BD bioscience) cells were intracellularly stained for T-bet (4B10, Biolegend), RORγt (AFKJS-9, BD Bioscience), IFN-γ (XMG1.2, Biolegend) and IL-17A (TC11–18H10.1, ebioscience). The staining patterns were analyzed by a MACSQuant Analyzer 10 (Miltenyi Bioitec. Cambridge, MA) and processed by FlowJo (Tree Star, Ashland, OR). Dead cells were excluded using ghost red 780 viability dye (Tonbo biosciences, San Diego, CA) and only single cells were gated for further analysis using FSC-A and FSC-H. Gating strategies are shown in supplementary Fig. S3 and Fig. S4.
Expression of FoxP3 by spleen cells
Spleen cell suspensions prepared as described above were tested for their expression of FoxP3 using flow cytometry, following the instructions of the manufacturer’s protocol (eBioscience). In short, splenocytes were treated with the fixation/permeabilization buffer before their being stained with allophycocyanin-conjugated anti-FoxP3 antibody. Cells were acquired and data were analyzed as detailed above. The gating strategy is shown in Fig. S3.
Quantitative (q)PCR analysis of gene expression
The method is detailed in Ref. 23. Briefly, total RNA was extracted from spleen cells with TRIzol (Invitrogen-Life Technologies, Camarillo, CA). RNA (5 μg), Superscript III Reverse Transcriptase (Invitrogen Technologies) and oligo(dT)12–16 were used for first-strand cDNA synthesis. Primer-probe sets for qPCR for T-bet (Tbx21) and Rorγt were purchased from Applied Biosystems. PCR parameters were as recommended for the TaqMan Universal PCR Master Mix kit (Applied Biosystems).
Statistical analysis
Where non-normal distributions were compared, the Mann-Whitney U test was used (Fig. 1B). To allow comparison of ELISA data across multiple experiments (Fig.2A), the results were normalized 0 to 1 with feature scaling [27], where:
Statistical significance was determined using unpaired t-tests with P ≤ 0.05, as determined by the Holm-Sidak method. Comparison of intracellular expression of molecules by flow cytometric analysis was analyzed by the unpaired t-test (Figs. 2B, 2C and 3A), whereas qPCR data were analyzed by the two-tailed t-test (Fig. 3B).
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the Intramural Program of the National Eye Institute (NEI), National Institutes of Health. We thank the NEI Cores of Flow Cytometry and of Histology for superb technical support and the NIH Bldg 10A/4 Animal Facility for taking outstanding care of the mice.
Footnotes
Conflict of interest: Mercedes Lobera and Jianfei Yang are employees of GlaxoSmithKline, as indicated in the affiliations. All other authors declare no conflict of interest.
REFERENCES
- 1.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*). Annual review of immunology 2010;28:445–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dardalhon V, Korn T, Kuchroo VK, Anderson AC. Role of Th1 and Th17 cells in organ-specific autoimmunity. Journal of autoimmunity 2008;31:252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annual review of immunology 2009;27:485–517. [DOI] [PubMed] [Google Scholar]
- 4.Luger D, Silver PB, Tang J, et al. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. The Journal of experimental medicine 2008;205:799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh RP, Hasan S, Sharma S, et al. Th17 cells in inflammation and autoimmunity. Autoimmunity reviews 2014;13:1174–1181. [DOI] [PubMed] [Google Scholar]
- 6.Genovese MC, Van den Bosch F, Roberson SA, et al. LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: A phase I randomized, double-blind, placebo-controlled, proof-of-concept study. Arthritis and rheumatism 2010;62:929–939. [DOI] [PubMed] [Google Scholar]
- 7.Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. The New England journal of medicine 2012;366:1190–1199. [DOI] [PubMed] [Google Scholar]
- 8.Patel DD, Lee DM, Kolbinger F, Antoni C. Effect of IL-17A blockade with secukinumab in autoimmune diseases. Annals of the rheumatic diseases 2013;72 Suppl 2:ii116–123. [DOI] [PubMed] [Google Scholar]
- 9.Ivanov II, McKenzie BS, Zhou L, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 2006;126:1121–1133. [DOI] [PubMed] [Google Scholar]
- 10.Huh JR, Leung MW, Huang P, et al. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORgammat activity. Nature 2011;472:486–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hinshaw SJ, Ogbeifun O, Wandu WS, et al. Digoxin Inhibits Induction of Experimental Autoimmune Uveitis in Mice, but Causes Severe Retinal Degeneration. Investigative ophthalmology & visual science 2016;57:1441–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solt LA, Burris TP. Action of RORs and their ligands in (patho)physiology. Trends in endocrinology and metabolism: TEM 2012;23:619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solt LA, Banerjee S, Campbell S, Kamenecka TM, Burris TP. ROR inverse agonist suppresses insulitis and prevents hyperglycemia in a mouse model of type 1 diabetes. Endocrinology 2015;156:869–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skepner J, Ramesh R, Trocha M, et al. Pharmacologic inhibition of RORgammat regulates Th17 signature gene expression and suppresses cutaneous inflammation in vivo. Journal of immunology 2014;192:2564–2575. [DOI] [PubMed] [Google Scholar]
- 15.Xiao S, Yosef N, Yang J, et al. Small-molecule RORgammat antagonists inhibit T helper 17 cell transcriptional network by divergent mechanisms. Immunity 2014;40:477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skepner J, Trocha M, Ramesh R, et al. In vivo regulation of gene expression and T helper type 17 differentiation by RORgamma t inverse agonists. Immunology 2015;145:347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nussenblatt RB, Whitcup SM. Uveitis: Fundamentals and Clinical Practice. Fourth Edition. Mosby Elsevier; 2016. [Google Scholar]
- 18.Forrester JV, Klaska IP, Yu T, Kuffova L. Uveitis in mouse and man. International reviews of immunology 2013;32:76–96. [DOI] [PubMed] [Google Scholar]
- 19.Caspi RR. A look at autoimmunity and inflammation in the eye. The Journal of clinical investigation 2010;120:3073–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell 2008;133:775–787. [DOI] [PubMed] [Google Scholar]
- 21.Shevach EM. Biological functions of regulatory T cells. Advances in immunology 2011;112:137–176. [DOI] [PubMed] [Google Scholar]
- 22.Stockinger B, Omenetti S, The dichotomous nature of T helper 17 cells. Nature Reviews Immunology, 2017; 17:535–544. [DOI] [PubMed] [Google Scholar]
- 23.Shi G, Cox CA, Vistica BP, Tan C, Wawrousek EF, Gery I. Phenotype switching by inflammation-inducing polarized Th17 cells, but not by Th1 cells. Journal of immunology 2008;181:7205–7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harbour SN, Maynard CL, Zindl CL, Schoeb TR, Weaver CT. Th17 cells give rise to Th1 cells that are required for the pathogenesis of colitis. Proceedings of the National Academy of Sciences of the United States of America 2015;112:7061–7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wandu WS, Tan C, Ogbeifun O, et al. Leucine-Rich Repeat Kinase 2 (Lrrk2) Deficiency Diminishes the Development of Experimental Autoimmune Uveitis (EAU) and the Adaptive Immune Response. PloS one 2015;10:e0128906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chong WP, van Panhuys N, Chen J, et al. NK-DC crosstalk controls the autopathogenic Th17 response through an innate IFN- γ-IL-27 axis. Journal of Experimental Medicine 2015; 212:1739–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siebert JC, Inokuma M,, Waid DM,, et al. An analytical workflow for investigating cytokine profiles.Cytometry A. 2008; 73: 289–298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.