Abstract
Similar to elderly humans, aged outbred Long Evans rats exhibit individual differences in memory abilities, including a subset of aged rats that maintain memory function on par with young adults. Such individuals provide a basis for investigating mechanisms of resilience to age-related decline. The present study examined hippocampal gene expression in young adults and aged rats with preserved memory function under behavioral task conditions well-established for assessing information processing central to the formation of episodic memory. While behavioral measures and hippocampal gene induction associated with neural activity and synaptic plasticity were similar across age groups, a marker for inhibitory interneuron function in the hippocampal formation was distinctively increased only in aged rats but not in young adults. Because heightened hippocampal neural activity is associated with age-related memory impairment across species, including rats, monkeys and humans, this finding may represent an adaptive homeostatic adjustment necessary to maintain neural plasticity and memory function in aging.
Keywords: Cognitive aging, Cue mismatch/double rotation, Hippocampal computation, Aging, Memory
1. Introduction
The brain is malleable over the course of a lifespan as it develops, incorporates new information, and adapts to an ever-changing environment well into old age. Although memory decline is common, it is not an inevitable consequence of aging. Many older adults maintain high performance throughout life (Duzel et al., 2011; Nyberg et al., 2012). Even in the presence of accumulating pathology that is associated with dementia, an apparent resilience is exhibited in a condition of asymptomatic Alzheimer’s Disease (AD) when cognitive function is maintained (Arnold et al., 2013; Driscoll & Troncoso, 2011). In that context, the study of elderly individuals with preserved cognitive function may yield important insights into mechanisms that could be broadly applicable to mitigate cognitive decline in aging, even in the presence of significant brain pathology.
Aged outbred rodents, similar to humans and non-human primates (Rapp & Amaral, 1991; Stark et al., 2010]), exhibit individual differences in aging for memory that is dependent on medial temporal lobe (MTL)-hippocampal circuitry (Gallagher et al., 1993; Koh et al., 2014). In the most extensively studied model for individual differences in aged rodents, approximately half of healthy aged Long Evans rats exhibit preserved cognitive function (Gallagher et al., 1993). These aged unimpaired (AU) rats perform on par with young adult (Y) rats and demonstrate test-retest reliability of intact memory for months after initial characterization (Gallagher & Burwell, 1989; Gallagher et al., 2006; Robitsek et al., 2008). Individual differences defined by performance in spatial memory in this model also extend to tests of hippocampal-dependent non-spatial memory (Gallagher & Burwell, 1989; Robitsek et al., 2008). For example, in assessing contributions of recollection and familiarity to recognition memory as defined by the dual-process model (Yonelinas, 2002), aged rats previously characterized with spatial memory impairment, similar to young adults with hippocampal damage (Fortin et al., 2004), have a selective deficit in recollection with intact item recognition based on familiarity (Fortin et al., 2004; Robitsek et al., 2008). In contrast, aged cohorts with preserved spatial memory exhibit intact recognition based on both recollection and familiarity on par with young adults.
As reviewed elsewhere (Leal & Yassa, 2015), an emerging understanding of an underlying basis for neurocognitive change within the hippocampal formation has also generalized across mammalian species as a basis for individual differences in aging outcomes. Specific circuits, including the dentate gyrus (DG) and CA3 hippocampal subfields, normally contribute to episodic memory by encoding representations tied to specific events and experiences in a manner that limits interference with similar experiences in the past. During in vivo recordings, unlike Y and AU rats, aged impaired (AI) rats exhibit heightened neural activity in these circuits and neurons fail to rapidly encode distinctive representations of new information (Wilson et al., 2005; Wilson et al., 2003). This overactivity has been identified as contributing to age-dependent memory impairment in affected rodents (Haberman et al., 2017; Koh et al., 2010) non-human primates (Thome et al., 2016) and human subjects (Yassa et al., 2011). In the case of activation in the DG/CA3 detected by functional magnetic resonance imaging (fMRI), greater activation is specifically associated with worse performance due to mnemonic interference in the elderly (Stark et al., 2013; Yassa et al., 2011). This feature, exhibited during aging with memory impairment in the mammalian brain across species, appears to worsen in elderly humans during the prodromal phase of AD (Bakker et al., 2015; Bakker et al., 2012)
In the healthy brain, homeostatic mechanisms normally stabilize neuronal excitability and firing properties through a variety of mechanisms, including the recruitment of inhibitory interneurons, to maintain synaptic function in an optimal range for plasticity. Recent evidence indicates that a failure of such homeostatic regulation could contribute to dysfunction in the aged brain as well as in AD (Styr & Slutsky, 2018; Xiao et al., 2017). Several studies have documented age-dependent reductions of interneuron markers (Stanley & Shetty, 2004; Vela et al., 2003; Spiegel et al., 2013) and function (Villanueva-Castillo et al., 2017) in rodents which, in some instances, correlate with impaired memory performance (Spiegel et al., 2013). In that context, a gain of inhibitory function in AU compared to AI rats is suggested by prior studies in the outbred rat model. Specifically, gene expression profiling of the hippocampal CA3 subfield indicated that induction of inhibitory pathway genes supports cognition in aged rats with intact memory (Haberman et al., 2013). After exposure to a new water maze environment, aged unimpaired rats have significantly increased expression, relative to aged memory-impaired rats, in a panel of genes related to inhibitory neurotransmission including the primary GABA synthesis enzyme, Gad1. In AU rats, such elevated expression of inhibitory genes occurred alongside the expected induction of synaptic plasticity-related genes. Those findings suggest that inhibitory neurotransmission is recruited to counter abnormal hyperactivity in the hippocampus of aged rats with preserved memory function but not in aged impaired subjects. This recruitment may contribute to normalization of neural excitability and resilience to cognitive decline in aging.
The current study examines this perspective by assessing gene expression measures related to excitation and inhibition in aged unimpaired rats and young adults in an independent protocol validated for hippocampal recruitment and well-studied for hippocampal computational processing. After determination of cognitive status by performance in a standardized Morris water maze protocol, we adapted a cue mismatch task (Knierim, 2002) that engages hippocampal functions critical for memory formation. Here we report that aged unimpaired rats show normal induction of gene markers for neural activation and plasticity in response to task contingencies, but unlike young adults, also exhibit elevated induction of markers for the function of inhibitory neurons in hippocampal circuits, consistent with an adaptive mechanism to control excitatory and inhibitory balance.
2. Materials and Methods
2.1. Subjects
Aged, male Long-Evans rats were obtained at 8-9 months of age from Charles River Laboratories (Raleigh, NC) and housed in a vivarium at Johns Hopkins University until 24-26 months of age. Young rats obtained from the same source were tested around 4-6 months of age. All rats were individually housed at 25°C and maintained on a 12 hr light/dark cycle. Food and water were provided ad libitum unless indicated otherwise. The rats were examined for health and pathogen-free status throughout the study, as well as necropsies at the time of sacrifice. Rats that showed impaired health or disabilities that could impact behavioral performance (e.g. poor eyesight, clinical evidence of renal impairment, pituitary or other tumors) were excluded from the study. All procedures were approved by the Johns Hopkins University Institutional Animal Care and Use Committee in accordance with the National Institutes of Health directive.
2.2. Behavioral Characterization
The aged Long Evans rat study population shows a larger range of individual differences in memory performance than younger adult control rats such that some older rats (aged unimpaired, AU) perform on par with the normative range of younger adult performance (Y) while others (aged impaired, AI) perform outside that range (Gallagher et al., 1993; Haberman et al., 2011). In order to specifically examine hippocampal properties that contribute to intact cognition in aging, we selected only memory unimpaired aged rats for testing alongside young adults in the current experiment. All rats were tested for hippocampal-dependent memory performance in a well-established Morris water maze protocol as described in further detail elsewhere (Gallagher et al., 1993). Training occurred during the light phase, consistent with the standard protocol. Briefly, the water maze consisted of a circular pool surrounded by curtains with large contrasting cues affixed to them. Rats were trained for eight days (three trials per day) to locate a camouflaged escape platform that remained at the same location throughout training. Every sixth trial consisted of a probe trial (no escape platform for the first 30s of the trial) that served to assess the development of a spatially localized search. A memory index was generated from the proximity of the rat to the escape platform during probe trials and was used to distinguish intact performance from memory impairment in the aged rats. The index is the sum of weighted proximity scores obtained during probe trials, with low scores reflecting a more accurate search. A learning index cutoff was used to segregate aged rats into unimpaired (AU, learning index < 240) and impaired (AI, learning index > 240) such that aged unimpaired rats fell within the range of young (Y) normative data collected over many years and exhibited by young rats in the current study. Each run cohort included both young and aged rats. Young and AU rats were selected for this experiment from several independent runs with a total of 18 aged unimpaired and 14 young rats with similar search error and learning indices. A repeated measures ANOVA confirmed all rats improved with training, (RM-ANOVA, trial; F(3,84) = 115.54, p = 0.0001), but there was no main effect of age (RM-ANOVA, age; F(1,28) = 1.353, p = 0.255) or interaction (RM-ANOVA, trial × age; F(3,84) = 1.036, p = 0.381) (Fig. 1B). There was no significant difference between learning index scores of Y and AU (1W-ANOVA; (F(1,28) = 0.104, p = 0.75) (Fig. 1C). Y and AU animals were pseudo-randomly assigned to one of two conditions (double rotation or no change, see below) such that memory index scores were similar across the double rotation and no change conditions.
Figure 1. Performance in water maze behavioral characterization.
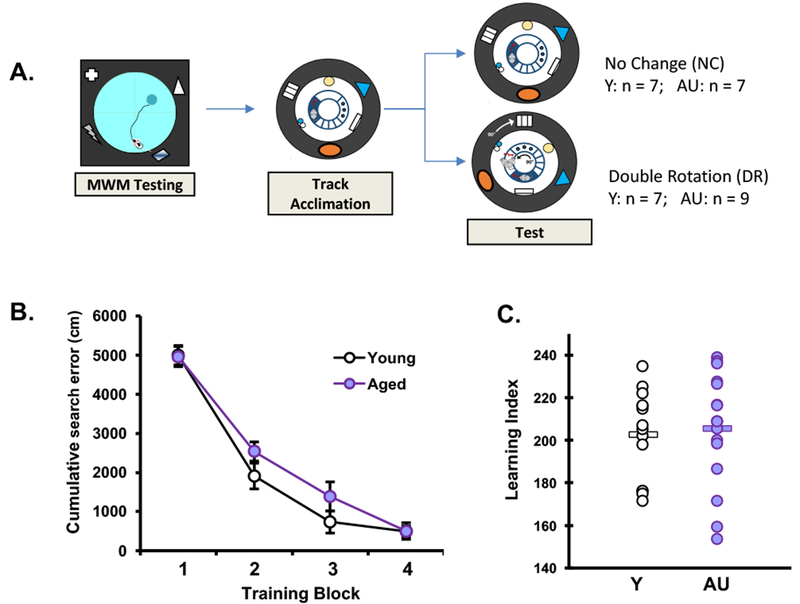
A. Schematic of Behavioral Procedures. Morris Water Maze testing was used to characterize hippocampal dependent learning ability in young and aged rats. This was followed by ten days of training on th a circular track with both local and global cues in a fixed position. On the 11th day, test day, animals in one condition were exposed to the track with cues located in the same position as during training (No Change condition), and in the other condition global cues were rotated 90 degrees clockwise and local cues rotated 90 degrees counterclockwise to create a 180 degree mismatch of position relative to training position (Double Rotation condition). B. Cumulative search error during water maze training, blocks of 5 trials each. This measure reflects the distance of the rat from the escape platform throughout its search, with higher numbers indicating worse performance. A repeated measures ANOVA confirmed both young and aged rats improved with training. Data points represent the average for blocks of five training trials ± SEM. C. Learning index scores were derived from proximity measures during probe trials interpolated throughout training as in Gallagher et. al. (1993), with lower scores indicating better performance. The graph illustrates that aged unimpaired rats perform within the range of young rats on this task, and groups means (horizontal bars) were not different. See methods section 2.2 for statistics.
2.3. Double Cue Rotation task
The double cue rotation protocol was adapted from that used originally by Knierim (2002). Following behavioral characterization, all rats were placed on a food restricted diet and weighed every day to maintain body weight at 85% of free-feeding weight. When rats reached stable body weight (average 19 days after beginning food deprivation), all Y and AU rats (Y, N=14; AU, N=18) were acclimated to run clockwise (CW) on a circular track (76-cm outer diameter, 10-cm width) to collect bacon crumble rewards placed at arbitrary locations on the track. This training occurred during the dark phase beginning at least 1 hour after the lights turned off. While running on the track, the rat was discouraged from turning around and moving counterclockwise (CCW) by blocking its path with a paper folder. By the time of test day, turning behavior occurred infrequently. The track was composed of four textured surfaces that served as local cues, each covering a quadrant of the track. The track was placed in a circular, curtained environment (2.7-m diameter) in which six distinct peripheral objects were present either on the floor or on the curtain, serving as global cues. Rats were run in 3 cohorts of approximately 12 rats each. For each day, the randomly chosen start point remained consistent for all rats in that cohort. Track acclimation occurred once a day for 10 days with a 1 day intermission after day 5. Each session lasted 20 minutes for days 1-5, after which each session was 20 min or 20 laps whichever occurred first. The number of laps completed in each 20-minute training session by each animal was recorded to ensure rats were adequately traversing the track and sampling the environment. The 20-lap limit was introduced to reduce potential differences in activity between young and aged rats, which tended to move more slowly around the track. However, very few rats met this criterion including 3 AU rats and 5 young rats completing 20 laps prior to 20 minutes by day 10. On the test day (day 11), rats were placed on the track with either the same cue orientation as during the acclimation period (no change, NC), or with cues rotated (double rotation, DR) such that local cues were rotated 90 degrees counter clockwise and global cues were rotated 90 degrees clockwise for a total of 180-degree mismatch. This results in a novel cue configuration where local cues are placed in maximum conflict with the global cues, a manipulation that has been previously shown to impact computational processing and hippocampal encoding (Knierim & Neunuebel, 2016; Lee et al., 2015; Lee et al., 2004; Neunuebel & Knierim, 2014).
2.4. Head scanning analysis
As a behavioral measure to indicate recognition of the cue mismatch in the double rotation condition, we examined head scans for all rats for final acclimation days 9 and 10 and the test day. Scans were manually counted from digital recordings from a camera mounted directly above the track. A head scan was counted when a rat paused in its locomotion around the track and rotated his head side to side such that his nose extended beyond the edge of the track on at least one side. We required that the rat proceed at least two steps forward before a second head scan could be recorded. Vertical head movements in the absence of side to side movements were not counted as they were difficult to discern given the camera location. Head scans were not counted if the rat was moving in the incorrect (counter-clockwise) direction on the track. To control for the variation in the amount of time rats were on the track, scanning rate was computed by dividing the total number of head scans for the session by the amount of time spent on the track during that session.
2.5. In situ hybridization and analysis.
Rats were perfused 2 hours from the beginning of each rat’s test day session with phosphate buffered saline followed by 4% paraformaldehyde. Brains were stored overnight in 4% paraformaldehyde then cryoprotected in 20% sucrose. Brains were cut in the coronal plane at 40 μm using a freezing microtome and stored in a 1 in 24 series in 4% paraformaldehyde until processed for mRNA by in situ hybridization. Quantitative in situ hybridization procedures and probe generation were performed as in (Haberman et al., 2011). Briefly, brain sections matched for number and location were hybridized overnight at 60°C in buffer containing a 35S-UTP labeled riboprobe generated using the Maxiscript kit (Ambion). In situ probe sequences were either PCR amplified from whole hippocampal cDNA with primers incorporating T7 and SP6 RNA polymerase binding sites or PCR-derived amplicons were cloned in pGEM plasmids and digested with appropriate restriction enzymes. Probe sequences were as follows: Gad1, nts 268 to 574 of Genbank sequence NM_017007.1; Zif268, nts 770-1143 from Genbank seq NM_012551.2; Tubg1, nts1252-1601 of Genbank seq NM_145778.2; cFos, nts 670 to 1043 from Genbank seq NM_022197.2. Nlgn1 and Camk2a probes were described previously (Haberman, Lee, Colantuoni, Koh, & Gallagher, 2008). All probes were verified for specificity by BLAST search of the fragment sequence. The specificity of Zif268 and Gad1 probes were further confirmed by competition assay with gene family members, Egr2 and Gad2 respectively. No cross competition was detected with these sequences. Mounted, dried sections were exposed in a phosphorimager cassette. Brain regions of interest were outlined by hand and matched for level along the anterior-posterior axis and quantified, blind to experimental conditions, using ImageQuant (GE Healthcare, PA). Radioactive standards exposed at the same time as the brain sections ensured that section intensity was within the linear range and all intensity values were normalized to these standards. Typically, 4 intensity measurements per animal for each area of interest were averaged to obtain a single score for each rat. Expression values for each rat of Zif268, Camk2a, Nlgn1, and Gad1 were normalized to Tubg1 levels, which shows no difference between Y and aged rats in either basal or activated conditions in previous work (Haberman et al., 2013; Haberman et al., 2011), or in the current study. The areas analyzed included whole dorsal hippocampus as well as CA3, CA1, and dentate gyrus (DG) hippocampal subfields (extending from approximately −2.8 mm to −4.16 mm relative to bregma).
2.6. Gad67 immunohistochemistry
Cryoprotected tissue sections from the same subjects used for in situ hybridization were matched for level and stained for Gad67 as in Spiegel et al (Spiegel et al., 2013) in a single run. Digital 5× images were inverted and the CA3 subfield and dentate granule layer (both dorsal and ventral blades) were manually outlined under blinded conditions using ImageJ to obtain overall intensity for the area of interest. As per in situ hybridization, 4 ROIs per animal covering the CA3 region or dentate granule cell layer were averaged to obtain a single score for each rat. Slide background levels were taken for each ROI and subtracted from mean intensity values. Data were analyzed by one-way ANOVA. The same CA3 ROIs were analyzed for the number of Gad67 positive cells with exhaustive counting using Stereo Investigator (MBF Bioscience, Williston, VT, USA). Using the optical fractionator process within Stereo Investigator, 100% of each ROI was counted using a 40× objective. The number of positive cells per ROI were averaged across 4 ROIs per subject. Tissue sections for two AU rats were unavailable for cell counting, and therefore for this experiment, group numbers were AU DR: n = 8, AU NC: n = 6, Y DR: N=7, Y NC: N=7. A 2W-ANOVA was used to assess main effects, followed by 1W-ANOVAs between conditions.
2.7. Experimental design and statistical analysis.
As the experiment was designed to assess hippocampal gene activation of AU rats under conditions intended to engage hippocampal computational processes, young rats were included as a comparison group to determine normative gene induction under these behavioral conditions. The NC behavioral condition was included to control for hippocampal mRNA modulation due to the physical performance of the task and exposure to the environment. Therefore, all gene measures were normalized to this condition within each age group. Figures illustrating gene expression are presented as percentage of NC average. To obtain these data, intensity values were normalized to the average intensity in the NC condition observed in the region of interest within each age group, and values are expressed as the mean ± SEM. For the behavioral measures, data are presented as within subject measures (change from baseline performance, see below).
For the behavioral analysis, we recorded the number of laps run during the acclimation period and test days (days 1-11), and head scans across days 9, 10, and test day. Two rats from the aged group in the NC condition failed to demonstrate adequate performance during acclimation, performing two standard deviations below the mean for both laps and scans when compared across all animals, as well as within the NC condition alone. Therefore, data for those animals were excluded from all analyses. This resulted in the following group sizes: Y: NC = 7, DR = 7; AU: NC = 7, DR = 9.
Test day behavioral measures were expressed as a percent of baseline, defined as each subject’s average performance on the last 2 acclimation days prior to test day. There were no differences in baseline measures of lap running or head scanning between age groups or behavioral conditions (data not shown). In addition, test day changes (relative to baseline) in the DR condition did not correlate with learning index for all subjects (Pearson, n=30; Laps: r = −0.1465, p = 0.44; Scans: r = −0.0662, p = 0.73; Scans/min: −0.0195. p =0.92) or independently for age group or behavioral measure. For all comparisons of behavioral measures between groups on test day, two-way ANOVAs (2W ANOVA) were run with age (Y and AU) and test condition (NC or DR) as between subject factors. Follow-up comparisons were conducted by either one-way ANOVA or paired t-tests, as appropriate. Levene’s test was used to determine if equal variances were assumed in t-test calculations.
Experiments for Zif268, Camk2a and Nlgnl in situ hybridization were run in two batches, while Gad1 and Tubgl were run in a single batch. Therefore, all intensity values were normalized to standards for each batch, then converted to z-scores within each batch prior to running statistical analyses for all genes. Expression levels of each gene were analyzed independently for each region of interest and each age group. For each gene assessed, the z-score for each animal in each region of interest was used as the dependent variable in a two-way ANOVA with age and test condition (NC, DR) as between subject factors. Pearson correlations were used to assess the relationship between variables with Fisher’s Z-transformation to test for differences between correlations. Statistical tests used to analyze each data set are noted in the corresponding results section, and statistical comparisons with p values of < 0.05 are considered significant. All statistical analysis was conducted using SPSS PASW Statistics (version 24.0, IBM, Chicago, IL, USA).
3. Results
3.1. Cognitively normal aged rats perform on par with young in the hippocampal-dependent cue-mismatch task
Memory index scores, a robust measure of water maze performance that reflects hippocampal integrity, did not differ between the young (n=14) and AU rats (n=16) used in this study (Fig 1 and see details in methods section 2.2). After the initial water maze characterization, we adapted a cue mismatch task (Knierim, 2002) that engages hippocampal functions critical to memory formation (schematically shown in Fig 1A, and details in methods section 2.3). All Y and AU rats were acclimated to run on a circular track for 10 days, followed by a test day in which rats experienced either a cue rearrangement (double rotation condition, DR), such that local cues (on the track) and distal cues (surrounding the track) were rotated in opposite directions (90° each for a 180° mismatch), or the familiar cue arrangement (no change condition, NC). This paradigm was designed and previously used to probe hippocampal computational functions (Knierim & Neunuebel, 2016; Knierim & Rao, 2003; Neunuebel & Knierim, 2014). Laps run and head scanning were monitored during the test day session relative to baseline at the end of track acclimation. Head scanning behavior in this task has previously provided a measure of investigatory behavior associated with hippocampal spatial encoding (Monaco et al., 2014).
As shown in Fig 2 and supplementary Fig 1, behavioral measures of performance, including number of laps run, head scans and scanning rate, differed by test condition for both age groups. During the test session, both young and aged DR animals responded to the cue mismatch with an increase in the number of laps run relative to NC (2W-ANOVA, condition: F(1,26) = 6.601, p = 0.016; age: F(1,26) = 0.007, p =0.934; condition × age: F(1,26) = 0.008, p = 0.929)(Fig. 2A), and relative to their baseline running behavior (Paired t-test: YDR: p = 0.053; AU DR: p = 0.014). In contrast, Y and AU rats in the NC condition ran similar numbers of laps during baseline and test (Paired t-test: YNC: p = 0.796; AUNC: p =0.832; (Fig. 2B). Cognitive engagement, indicated by a head scanning behavioral response, also significantly increased in both young and aged rats in the DR condition relative to NC (2W-ANOVA; condition: F(1,26) = 16.761, p = 0.0001; Age: F(1,26) = 0.017, p = 0.898; condition × age: F(1,26) = 0.687, p = 0.415) (Fig. 2C). Again, DR rats increased their scanning relative to baseline while animals in the NC condition did not (Paired t-test: YNC: p = 0.561; YDR: p = 0.009; AUNC: p = 0.746; AUDR: p = 0.0001) (Fig. 2D). Similar results were found for scanning rate (Fig. 2E) (2W-ANOVA: condition: F(1,26) = 12.825, p = 0.001; age: F(1,26) = 0.015, p = 0.904; condition × age: F(1,26) = 0.286, p = 0.597; Paired t-test: YNC: p = 0.482, YDR: 0.028; AUNC: p = 0.905, AUDR: p = 0.001) (Fig. 2F). Altogether, these data show that AU rats responded similarly to young rats during training and test, consistent with other studies demonstrating intact information processing and memory function in aged rats behaviorally characterized as unimpaired in this study population (Gallagher & Burwell, 1989; Haberman et al., 2013; Pereira et al., 2015; Robitsek et al., 2008). The equivalent behavioral response in this cue mismatch paradigm provides a basis to compare hippocampal gene expression signatures between Y and AU.
Figure 2. Double cue rotation task performance was similar in young and aged unimpaired rats.
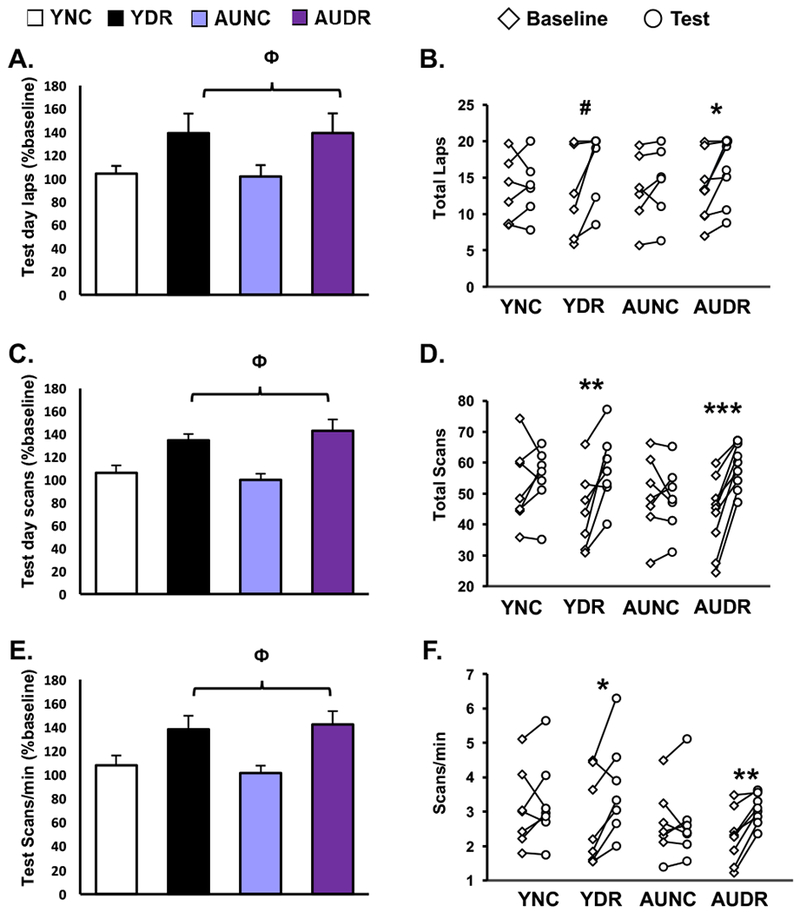
On test day, both Y and AU animals in the double rotation (DR) condition responded to the cue manipulation with enhanced exploratory behavior relative to animals in the NC condition. A.B. Number of laps run; C,D. Number of head scans; and E,F. Scanning rate (total scans per rat/duration of session(min). A, C, E show group averages as a percentage of baseline ± SEM; Φ, p<0.05, 2W-ANOVA main effect of behavioral condition; B, D, F: illustrate baseline performance (diamonds) and test day performance (circles) for each animal in each group, # p < 0.06, * p<0.05, ** p<0.01, *** p<0.001, paired t-test of baseline versus test day.
3.2. Young and AU rats show similar induction of neural activity and synaptic plasticity markers with cue rotation
Gene expression profiles induced in behavioral paradigms can reflect neurobiological processes representing neural activation and synaptic plasticity in which mRNA induction is required for subsequent maintenance and behavioral expression of memory. To investigate the relationship between the cue mismatch and hippocampal network activation, we measured hippocampal Zif268 mRNA, a gene induced by neural activity (Cole et al., 1989), by quantitative in situ hybridization. Zif268 expression (Fig. 3A-F, Supplemental Fig. 2) was elevated in whole hippocampus in animals that experienced the DR condition relative to animals in the NC condition (Fig. 3A; 2W-ANOVA: condition: F(1,26) = 24.01, p = 0.0001; age: F(1,26) = 0.128, p = 0.724; condition × age: F(1,26) = 0.668, p = 0.421). This difference was confirmed by analysis of cFos, a commonly used indicator of neural activity (Supplemental Fig 3)(Joo et al., 2016). Both Y-DR and AU-DR rats showed increased Zif268 expression in the principle cell layers of individual hippocampal subfields relative to NC (Fig 3B-D) (2W-ANOVA, condition: CA3: F(1,26) = 27.99, p = 0.0001; CA1: F(1,26) = 15, p = 0.001; DG: F(1,26) = F(1,26) = 12.031, p = 0.002, no main effect for age or condition × age interaction for any subfield). These differences were significant in all regions except Y in CA1 which represented a trend (1W-ANOVA; DG: Y: F(1,12) = 6.84, p = 0.023; AU: F(1,14) = 5.334, p = 0.037; CA3: Y: F(1,12) = 9.194, p = 0.01; AU: F(1,14) = 21.156, p = 0.0001; CA1: Y: F(1,12) = 4.012, p = 0.068; AU: F(1,14) = 4.012, p = 0.003). Further, hippocampal Zif268 expression correlated with the increase in scans (percent baseline) on test day for all subjects (Fig. 3E) (r = 0.423, p = 0.020) as well as within each age group (Y: r=0.536, p = 0.048; AU: r=0.559, p=0.024).
Figure 3. Elevation of activity and plasticity related gene expression in hippocampus following double cue rotation.
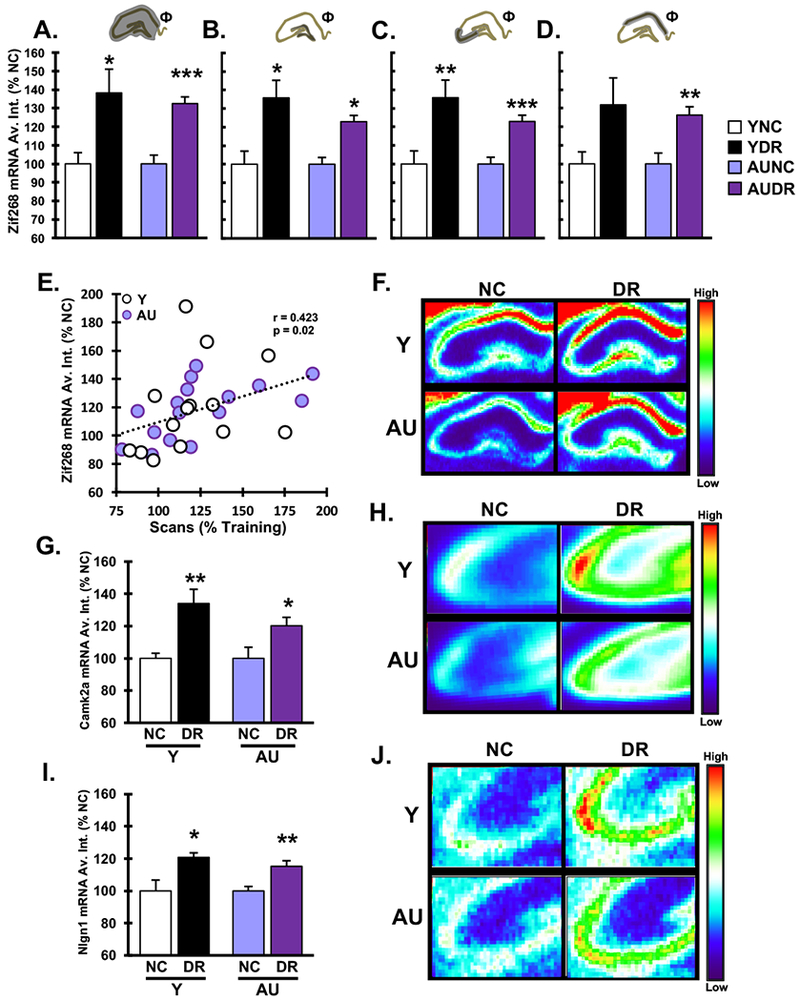
Quantification of Zif268 in situ hybridization signal intensity in the dorsal hippocampus showed increased expression of Zif268 in young and aged DR animals relative to NC controls when assessed in the A. Whole hippocampus B. Dentate gyrus C. CA3 and D. CA1 hippocampal subfields. Intensity measures are normalized to the average of NC condition for each age and region. E. Individual values for Zif268 expression for each animal (N=30, all NC and DR rats) in whole hippocampus is significantly correlated to the increase in scans on test day. Test day scans are shown as percent baseline, and Zif268 data are normalized to NC condition for each age group. F. Representative heat map images of Zif268 in situ hybridization as detected by phosphorimager with corresponding colormap. G. Expression intensity of Camk2a in situ hybridization was measured in the CA3 subfield and demonstrates higher expression in DR subjects relative to NC for both Y and AU groups. H. Representative heatmap of Camk2a expression. I. A similar increase in DR relative to NC was found for Nlgn1 expression in CA3. J. Representative heatmap of Nlgn1 expression. All bar graphs show average ± SEM, * p<0.05, ** p<0.01, *** p<0.001 for DR vs. NC, 2W-ANOVA main effect of behavioral condition, Φ.
We also examined the effect of cue mismatch on downstream synaptically-localized, plasticity related transcripts. In young adults, both Camk2a and Nlgn1 are increased in the CA3 subfield in response to spatial learning (Haberman et al., 2008). Consistent with this previous work, in situ hybridization assessment of CA3 showed increased expression of both CamK2a (Fig. 3 G, H: 2W-ANOVA, condition: F(1,26) = 17.203, p = 0.0001; age: F(1,26) = 0.05, p = 0.825; condition × age: F(1,26) = 0.337, p = 0.566), and Nlgn1 (Fig. 3I, J: 2W-ANOVA, condition: F(1,26) = 18.913, p = 0.0001; age: F(1,26) = 0.078 p = 0.782; condition × age: F(1,26) = 0.013, p = 0.908). These data show that, in both age groups, the double cue rotation condition engages not only markers of neural activity but also recruits mechanisms specifically involved in synaptic plasticity and maintenance in the CA3 subfield, both of which are critical to long term memory (Haberman et al., 2008).
3.3. AU animals activate inhibitory gene, Gad1, alongside neural activity markers
While our results to this point support comparable behavioral responses and gene induction between young and AU rats, our previous work has demonstrated that AU animals exhibit gene expression signatures of elevated inhibitory control relative to impaired rats in the hippocampus during learning tasks (Haberman et al., 2013). To examine inhibitory activation in this protocol, we assessed Gad1 mRNA expression in the hippocampus in response to the cue mismatch (Fig. 4) and found that AU-DR rats displayed a striking increase over AU-NC, which was not observed in Y rats. In all three hippocampal subfields, there was an interaction between age and behavior but no main effect of behavior or age in any subfield (Fig. 4A-C). (2W-ANOVA, age × behavior condition, CA3: F(1,26) = 12.674, p = 0.001; DG: F(1,26) = 5.244, p = 0.03; p = 0.001; CA1: F(1,26) = 12.157, p = 0.002). Follow-up analyses showed that double cue rotation had no significant effect on Gad1 expression in young DR rats relative to NC in CA3 and DG and a small but significant decrease in CA1 (CA3: F(1,12)=2.379, p = 0.149; DG: F(1,12) = 0.234, p = 0.637; CA1: F(1,12) = 4.358, p = 0.03). In contrast, AU-DR rats showed a significant increase in Gad1 mRNA relative to NC across all three regions (CA3: F(1,15)=14.84, p=0.002; DG: F(1,15)=10.67, p=0.007; CA1: F(1,15) = 6.14, p = 0.027). In addition, CA3 Gad1 mRNA showed a strong positive correlation with test day increases in scanning behavior (Fig. 4D) in AU rats (Pearson r = 0.830; p = 0.0001) but not Y ( Pearson r = −0.269; p = 0.35). These correlations were significantly different from each other (Fisher’s Z-transformation: Z = 3.57, p = 0.0004). Similarly, Gad1 expression correlated with Zif268 expression (Fig 4E) in AU (Pearson r = 0.648, p = 0.007) but not Y (Pearson r = −0.182, p = 0.534) and were likewise significantly different from each other (Fisher’s Z-transformation: Z = 2.33, p = 0.020). Similar patterns were found for the CA1 and DG subfields, although not all AU correlations were significant (Supplemental Fig. 4). These data suggest that Gad1 mRNA is dynamically elevated in response to hippocampal engagement in AU but not Y rats. The CA3 correlation with a behavioral measure of cognitive engagement suggests Gad1 may be a key component of hippocampal spatial processing in aged unimpaired rats.
Figure 4. Aged unimpaired DR rats upregulate Gad1 mRNA.
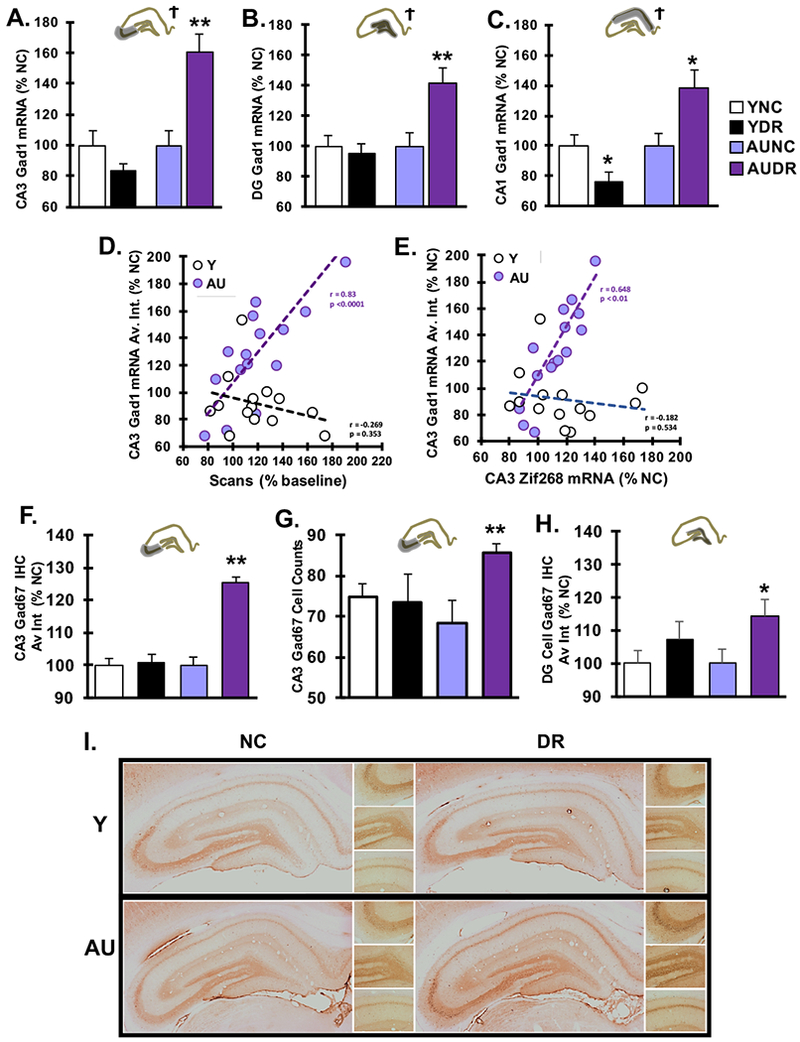
A. Quantification of Gad1 in situ hybridization signal intensity in CA3 demonstrates higher expression in AU DR animals relative to all other groups. Expression intensity of Gad1 mRNA measured in the B. dentate gyrus and in the C. CA1 subfield also show significantly higher expression in AU DR subjects relative to all other groups. In a 2W-ANOVA, there was no main effect of behavior condition or age for any subfield, but there was an interaction between age and behavior in all three subfields (Ϯ). Significance of subsequent, one-way ANOVAs are indicated by asterisks. D. Individual values for Gad1 mRNA expression in CA3 for each animal show a significant correlation with percent of baseline scans on test day for AU rats but not Y rats. Linear trendlines are based on values for each age group. Correlations for Y and AU were significantly different from each other. E. Individual values for Gad1 mRNA expression in CA3 for each animal show a significant correlation with CA3 Zif268 mRNA expression again only in AU rats. Linear trendlines are based on values for each age group and the correlations are significantly different. F. Immunohistochemical analysis for Gad67 protein in CA3 showed a near significant main effect of age. Subsequently, one-way ANOVAs within each age group showed that only AU animals displayed a significant increase in Gad67 in the DR condition. G. Counts of Gad67 positive cells in whole CA3 showed enhanced numbers of Gad67 expressing cells in the AU DR animals. H. Immunohistochemical analysis for Gad67 protein in DG cell layer showed a similar results as CA3. I. Representative images of Gad67 immunolabeled hippocampal sections for all groups. All bar graphs show average ± SEM; Ϯ, 2W-ANOVA interaction between behavioral condition and age; * p<0.05, ** p<0.01, *** p<0.001 for DR vs. NC post hoc tests.
To determine whether there was a corresponding increase in Gad67 protein, the product of the Gad1 gene, we performed immunohistochemical analysis of Gad67 on tissue sections from the same rats (Fig 4F-I). Although the time point for sacrifice was selected to optimize mRNA intensity, analysis of Gad67 showed trends similar to mRNA effects in CA3 and DG subfields (2W-ANOVA; CA3: Age: F(1,26) = 4.195, p=0.051; Behavior: F(1,26) = 2.664, p=0.115; Age × Behavior: F(1,26) = 2.403, p=0.133; DG: F(1,26) = 3.181, p=0.086; Behavior: F(1.26) = 1.752, p=0.197; Age × Behavior: F(1,26) = 3.181, p=0.086). Increased immunoreactivity was found in AU-DR rats relative to AU-NC in CA3 (1W-ANOVA: F(1,14) = 7.121, p = 0.018) and DG subfields (1W-ANOVA: F(1,14) = 7.07, p = 0.019). Consistent with the mRNA analysis, Y rats did not show any differences between DR and NC conditions in immunohistochemical measures (CA3: F(1,12) = 0.002, p = 0.961; DG: F(1,12) = 0.076, p = 0.788). The detected increase in Gad67 intensity appears to be due to an increase in the number of detectable neurons in AU DR animals relative to AU NC, as indicated by a near significant trend towards an age × behavior interaction (Fig. 4G; 2W-ANOVA: behavior: F(1,24) = 0.395, p = 0.536; Age: F(1,24) = 2.729, p = 0.112; age × behavior F(1,24) = 3.958, p = 0.058). Post hoc tests showed a significant difference between the number of cells in AU DR relative to AU NC (F(1,12) = 10.557. p = 0.007), but no difference between conditions in Y animals (F(1,12) = 0.042, p = 0.842). The consistency in trends between mRNA and protein measures in the AU rats support a functional consequence of the mRNA induction.
4. Discussion
The current study builds on previous findings demonstrating recruitment of inhibition in aged rats with preserved hippocampal-dependent cognitive function (Haberman et al., 2013). Here, we specifically examined gene expression measures related to inhibition, as well as neural activity and synaptic plasticity, in aged unimpaired rats in a cue mismatch paradigm modified to optimize gene expression of the targeted mRNAs. The cue mismatch paradigm was originally developed to investigate computational functions of the hippocampus through recording of single unit responses under varying degrees of cue rotation (Knierim, 2002). The cue mismatch generates a conflict between prior representations of a familiarized environment and the current environment. Prior studies in young animals have reported responses to cue mismatch including changes in neuronal firing rates and place field remapping such that new representations are encoded to minimize interference with previously encoded representations (Knierim & Neunuebel, 2016; Lee et al., 2015; Lee et al., 2004; Neunuebel & Knierim, 2014). When neural properties have been assessed in relation to aging and cognitive impairment in response to environmental to cue manipulation, aged impaired animals display distinct alterations in electrophysiological responses, while aged unimpaired respond similarly to young animals, suggesting intact encoding in AU (Tanila et al., 1997; Wilson et al., 2005; Wilson et al., 2003). Thus, this study focuses on AU animals in comparison to young to examine mechanisms of intact hippocampal encoding in aging.
As expected, aged unimpaired rats performed similarly to young adult rats on the three behavioral measures that differed according to the test condition (DR vs NC): laps, head scans and scanning rate. The similar behavioral response of young adult and aged unimpaired rats is consistent with many other studies demonstrating intact information processing and memory function in aged rats characterized as unimpaired by the standardized water maze protocol that has long been used in this study population. We have found that the water maze learning index measure robustly correlates with behavioral performance in other hippocampal-dependent tasks (Haberman et al., 2013; Pereira et al., 2015; Robitsek et al., 2008), and has high test/retest reliability over time (Gallagher et al., 1993; Gallagher & Burwell, 1989). Thus, it is not surprising that the behavior of AU rats was similar to young rats in the current study, with laps, head scans, and scan rate induced across both age groups in response to the double cue rotation manipulation. The increase in head scanning observed in the current study is particularly notable as recent data suggest head scanning at a particular position on the track is associated with the development of a place field at that location on the next traverse of the track in young rats (Monaco et al., 2014). Thus, head scanning represents an opportunity for the rat to encode features of the current environment in addition to serving as a behavioral indicator of information processing with reference to previously experienced spatial and environmental cues.
By comparing gene expression levels from the cue rotation group (DR condition) to a control group with a familiar cue orientation (NC condition), we found that induction of hippocampal gene expression was clearly tied to a change in the configuration of cues. The induction of Zif268 in both young and AU rats in response to the cue mismatch indicates a similar hippocampal activation in that condition across age groups. Such induction is consistent with multicellular recording evidence that both young and AU rats exhibit rapid encoding of new information when rats, familiarized in one environment, are exposed to a novel environment (Wilson et al., 2003). In addition to providing a marker for neural activity, Zif268 has a demonstrated role in induction of synaptic plasticity and long-term memory (Duclot & Kabbaj, 2017; Jones et al., 2001) and prompted a direct examination of genes whose products mediate synaptic plasticity.
In the context of hippocampal contributions to episodic memory, prior studies using the outbred rodent model have focused on basal and activated gene expression profiles in the CA3 subfield (Haberman et al., 2011; Haberman et al., 2017; Haberman et al., 2008). In young rats, a cluster of LTP/synaptic plasticity related genes, including both Camk2a and Nlgn1, is induced in CA3 in the setting of new spatial learning that occurs in a time frame similar to that used in the current study (Haberman et al., 2008). Induction of these genes contributes to the encoding and consolidation of memory as assessed by siRNA knock down during hidden platform water maze training. siRNA knock down of Nlgn1 in the CA3 subfield impaired performance on a probe test at 48 hours while rats injected with a control siRNA showed significant spatial bias for the trained platform location. These data suggest that behaviorally induced gene expression is required for the long-term memory of an event. The current study examined Nlgn1 along with CamK2a, and found that both mRNAs were induced with the cue mismatch procedure in both young and AU rats. The consistency of gene induction across Y and AU rats supports not only intact neural activation across age groups, but also similar mechanistic engagement and induction of relevant synaptic plasticity molecules tied to memory.
A notable finding in the current study was that Y and AU rats differed in the induction of Gad1 in response to the change in the environment in the DR test condition. The selective increase in inhibitory gene expression in AU rats is consistent with previous findings of gene induction in AU rats relative to aged impaired (AI) rats during a spatial memory task (Haberman et al., 2013). That study found not only an overall increased gene induction capacity in AU over AI subjects but greater induction specifically for a panel of genes associated with inhibitory function, including Gad1. Recent electrophysiological data has also provided evidence for augmented hippocampal inhibitory function in AU rats compared to both AI and Young (Tran et al., 2018), consistent with the earlier gene expression results. These studies, combined with the current findings, suggest inhibitory recruitment is unique to AU. Based on extensive work in aged impaired rats, including findings from electrophysiological recording studies, we would not expect a similar response from these subjects, as they consistently show blunted expression of inhibitory markers relative to AU (Haberman et al., 2013; Haberman et al., 2011; Spiegel et al., 2013), and altered neural responses to cue manipulation (Tanila et al., 1997; Wilson et al., 2003). In the current study, recruitment of inhibitory gene expression in AU rats occurs alongside gene induction typical of young rats that together may contribute to preserved memory performance in aging. Indeed, other recent research has directed attention to mechanisms for control of network excitability in human aging and early stages of AD in which recruitment of inhibition appears to represent an age-dependent resilience factor (Xiao et al., 2017).
The inhibitory gene induction by AU subjects observed in the current study is of particular interest based on hippocampal localization of elevated neural activity associated with age-related memory impairment identified across animal models (Simkin et al., 2015; Thome et al., 2016; Wilson et al., 2005) and detected in elderly humans by task-activated fMRI affecting the CA3/DG regions (Yassa et al., 2011; Yassa et al., 2010). Homeostatic regulation of neural activity in CA3 and dentate gyrus (DG) regions is critical for limiting interference between new representations and similar past representations by a process referred to as pattern separation. In aged individuals, increased hippocampal neuronal activity is tied to memory impairment in both aged rodents and humans by shifting computational processes such that the distinction between old and new representations is reduced (Stark et al., 2013; Wilson et al., 2005). Enhancement of inhibition in order to mitigate such heightened activity has a documented cognitive benefit in both preclinical animal and clinical human studies (Bakker et al., 2012; Koh et al., 2010; Sanchez et al., 2012) and improves performance of aged mice in a task designed to test hippocampal pattern separation (Guo et al., 2018). Thus, homeostatic regulation of E/I balance by greater engagement of inhibitory function, particularly under conditions that strongly engage hippocampal activation as demonstrated here, could be adaptive in the aged brain as distinct from young.
Previous research on therapeutics provides some evidence for age-dependent effects of agents that modulate inhibitory function. The use of GABAA α5 positive allosteric modulators to boost inhibitory function have shown preclinical efficacy in the context of age-related memory impairment (Koh et al., 2013). Based on the high expression of GABAA α5 receptors in the hippocampus, a novel class of GABAA α5 negative allosteric modulators (referred to as inverse agonists), which would heighten excitability, was previously reported to provide modest behavioral improvement in young adult rodents (e.g. (Atack et al., 2006; Ballard et al., 2009; Chambers et al., 2003; Collinson et al., 2006; Dawson et al., 2006). Those preclinical studies, however, failed in translational studies of age-related cognitive impairment (Atack, 2010). In Koh et al. (2013) the use of both negative and positive modulators of GABAA α5 in aged memory-impaired and young adult rats confirmed that negative modulation of GABAA α5 enhanced cognition in young animals, and conversely, positive modulation benefited cognition in the aged-impaired. Based on the existing evidence for deficits in hippocampal encoding associated with heightened neural activity in AI rats, and the current findings comparing Y and AU rats, further studies could test the hypothesis that recruitment of inhibitory function plays a distinctive role in the aging brain by comparing the effects of such agents in Y and AU rats. Indeed, the testing protocol used in the current study could be especially well-suited for such an analysis, allowing for behavioral and electrophysiological assessment of episodic encoding by hippocampal neurons (Monaco et al., 2014). Based on Koh et al. (2013) it would be predicted that positive allosteric modulation of GABAA α5 would improve the encoding properties of neurons in age-related memory impairment (AI rats), consistent with an inability to engage the additional inhibitory recruitment as demonstrated by AU animals. In contrast, given the beneficial effects of negative allosteric modulators in young adults, AU rats would likely be impaired by negative allosteric GABAA α5 modulation, consistent with recruitment of augmented inhibitory function as a naturally occurring mechanism contributing to resilience in the aging brain. In this context, the current study provides relevant support for further investigation of positive modulators of inhibition to mitigate cognitive decline in aging.
Much has been learned in research on individual differences concerning the alterations that are most closely associated with impairment in neurocognitive aging (Haberman et al., 2017; Leal et al., 2017; Wilson et al., 2006). At the same time, attention directed to the study of resilience in individuals with preserved cognitive function may yield important insights into mechanisms that contribute to preserved function in the context of aging. Such understanding may point in new directions for therapeutics that optimize healthy brain aging, and perhaps mitigate cognitive decline even in the presence of significant brain pathology.
Supplementary Material
Highlights:
-
-
Aged memory unimpaired rats behave similarly to young on a cue mismatch task
-
-
Cue mismatch similarly induced hippocampal activity and synaptic genes across ages
-
-
Cue mismatch induced inhibitory gene expression only in aged unimpaired hippocampus
-
-
Inhibitory induction may counteract impairment-associated hyperactivity in aging
Acknowledgements:
This work was supported by NIA grant P01AG009973-21A1 to M.G and J.J.K. and NIH postdoctoral training grant 1T32AG027668-01A1 to A.B. The authors would like to thank Karen Bandeen-Roche for statistical assistance, Rob McMahan for assistance with behavioral experiments, and Geeta Rao for advice in animal training and detection of head scans.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest:
M.G. is the founder of AgeneBio Incorporated, a biotechnology company that is dedicated to discovery and development of therapies to treat cognitive impairment. She has a financial interest in the company. The authors (M.G. and R.A.H.) are inventors on Johns Hopkins University intellectual property that is licensed to AgeneBio. Otherwise, M.G. has had no consulting relationships with other public or private entities in the past three years and has no other financial holdings that could be perceived as constituting a potential conflict of interest. All conflicts of interest are managed by Johns Hopkins University. A.B., A.M., G.B., and J.J.K. have no conflicts of interests to declare.
References:
- Arnold SE, Louneva N, Cao K, Wang LS, Han LY, Wolk DA, … Bennett DA (2013). Cellular, synaptic, and biochemical features of resilient cognition in Alzheimer’s disease. Neurobiol Aging, 34(1), 157–168. doi: 10.1016/j.neurobiolaging.2012.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atack JR (2010). Preclinical and clinical pharmacology of the GABAA receptor alpha5 subtype-selective inverse agonist alpha5IA. Pharmacol Ther, 125(1), 11–26. doi: 10.1016/j.pharmthera.2009.09.001 [DOI] [PubMed] [Google Scholar]
- Atack JR, Bayley PJ, Seabrook GR, Wafford KA, McKernan RM, & Dawson GR (2006). L-655,708 enhances cognition in rats but is not proconvulsant at a dose selective for alpha5-containing GABAA receptors. Neuropharmacology, 51(6), 1023–1029. doi: 10.1016/j.neuropharm.2006.04.018 [DOI] [PubMed] [Google Scholar]
- Bakker A, Albert MS, Krauss G, Speck CL, & Gallagher M (2015). Response of the medial temporal lobe network in amnestic mild cognitive impairment to therapeutic intervention assessed by fMRI and memory task performance. Neuroimage Clin, 7, 688–698. doi: 10.1016/j.nicl.2015.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Krauss GL, Albert MS, Speck CL, Jones LR, Stark CE, … Gallagher M (2012). Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron, 74(3), 467–474. doi: 10.1016/j.neuron.2012.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard TM, Knoflach F, Prinssen E, Borroni E, Vivian JA, Basile J, … Hernandez MC (2009). RO4938581, a novel cognitive enhancer acting at GABAA alpha5 subunit-containing receptors. Psychopharmacology (Berl), 202(1–3), 207–223. doi: 10.1007/s00213-008-1357-7 [DOI] [PubMed] [Google Scholar]
- Chambers MS, Atack JR, Broughton HB, Collinson N, Cook S, Dawson GR, … MacLeod AM (2003). Identification of a novel, selective GABA(A) alpha5 receptor inverse agonist which enhances cognition. J Med Chem, 46(11), 2227–2240. doi: 10.1021/jm020582q [DOI] [PubMed] [Google Scholar]
- Cole AJ, Saffen DW, Baraban JM, & Worley PF (1989). Rapid increase of an immediate early gene messenger RNA in hippocampal neurons by synaptic NMDA receptor activation. Nature, 340(6233), 474–476. doi: 10.1038/340474a0 [DOI] [PubMed] [Google Scholar]
- Collinson N, Atack JR, Laughton P, Dawson GR, & Stephens DN (2006). An inverse agonist selective for alpha5 subunit-containing GABAA receptors improves encoding and recall but not consolidation in the Morris water maze. Psychopharmacology (Berl), 188(4), 619–628. doi: 10.1007/s00213-006-0361-z [DOI] [PubMed] [Google Scholar]
- Dawson GR, Maubach KA, Collinson N, Cobain M, Everitt BJ, MacLeod AM, … Atack JR (2006). An inverse agonist selective for alpha5 subunit-containing GABAA receptors enhances cognition. J Pharmacol Exp Ther, 316(3), 1335–1345. doi: 10.1124/jpet.105.092320 [DOI] [PubMed] [Google Scholar]
- Driscoll I, & Troncoso J (2011). Asymptomatic Alzheimer’s disease: a prodrome or a state of resilience? Curr Alzheimer Res, 8(4), 330–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclot F, & Kabbaj M (2017). The Role of Early Growth Response 1 (EGR1) in Brain Plasticity and Neuropsychiatric Disorders. Front Behav Neurosci, 11, 35. doi: 10.3389/fnbeh.2017.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duzel E, Schutze H, Yonelinas AP, & Heinze HJ (2011). Functional phenotyping of successful aging in long-term memory: Preserved performance in the absence of neural compensation. Hippocampus, 21(8), 803–814. doi: 10.1002/hipo.20834 [DOI] [PubMed] [Google Scholar]
- Fortin NJ, Wright SP, & Eichenbaum H (2004). Recollection-like memory retrieval in rats is dependent on the hippocampus. Nature, 431(7005), 188–191. doi : 10.1038/nature02853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, & Burchinal M (1993). Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci, 107(4), 618–626. [DOI] [PubMed] [Google Scholar]
- Gallagher M, & Burwell RD (1989). Relationship of age-related decline across several behavioral domains. Neurobiol Aging, 10(6), 691–708. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Colantuoni C, Eichenbaum H, Haberman RP, Rapp PR, Tanila H, & Wilson IA (2006). Individual differences in neurocognitive aging of the medial temporal lobe. Age (Dordr), 28(3), 221–233. doi: 10.1007/s11357-006-9017-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo N, Soden ME, Herber C, Kim MT, Besnard A, Lin P, … Sahay A (2018). Dentate granule cell recruitment of feedforward inhibition governs engram maintenance and remote memory generalization. Nat Med, 24(4), 438–449. doi: 10.1038/nm.4491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberman RP, Branch A, & Gallagher M (2017). Targeting Neural Hyperactivity as a Treatment to Stem Progression of Late-Onset Alzheimer’s Disease. Neurotherapeutics, 14(3), 662–676. doi: 10.1007/s13311-017-0541-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberman RP, Colantuoni C, Koh MT, & Gallagher M (2013). Behaviorally activated mRNA expression profiles produce signatures of learning and enhanced inhibition in aged rats with preserved memory. PLoS One, 8(12), e83674. doi: 10.1371/journal.pone.0083674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberman RP, Colantuoni C, Stocker AM, Schmidt AC, Pedersen JT, & Gallagher M (2011). Prominent hippocampal CA3 gene expression profile in neurocognitive aging. Neurobiol Aging, 32(9), 1678–1692. doi: 10.1016/j.neurobiolaging.2009.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberman RP, Koh MT, & Gallagher M (2017). Heightened cortical excitability in aged rodents with memory impairment. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2016.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberman RP, Lee HJ, Colantuoni C, Koh MT, & Gallagher M (2008). Rapid encoding of new information alters the profile of plasticity-related mRNA transcripts in the hippocampal CA3 region. Proc Natl Acad Sci U S A, 105(30), 10601–10606. doi: 10.1073/pnas.0804292105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW, Errington ML, French PJ, Fine A, Bliss TV, Garel S, … Davis S (2001). A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat Neurosci, 4(3), 289–296. doi: 10.1038/85138 [DOI] [PubMed] [Google Scholar]
- Joo JY, Schaukowitch K, Farbiak L, Kilaru G, & Kim TK (2016). Stimulus-specific combinatorial functionality of neuronal c-fos enhancers. Nat Neurosci, 19(1), 75–83. doi: 10.1038/nn.4170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ (2002). Dynamic interactions between local surface cues, distal landmarks, and intrinsic circuitry in hippocampal place cells. J Neurosci, 22(14), 6254–6264. doi:20026608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ, & Neunuebel JP (2016). Tracking the flow of hippocampal computation: Pattern separation, pattern completion, and attractor dynamics. Neurobiol Learn Mem, 129, 38–49. doi: 10.1016/j.nlm.2015.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ, & Rao G (2003). Distal landmarks and hippocampal place cells: effects of relative translation versus rotation. Hippocampus, 13(5), 604–617. doi: 10.1002/hipo.10092 [DOI] [PubMed] [Google Scholar]
- Koh MT, Haberman RP, Foti S, McCown TJ, & Gallagher M (2010). Treatment strategies targeting excess hippocampal activity benefit aged rats with cognitive impairment. Neuropsychopharmacology, 35(4), 1016–1025. doi: 10.1038/npp.2009.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh MT, Rosenzweig-Lipson S, & Gallagher M (2013). Selective GABA(A) alpha5 positive allosteric modulators improve cognitive function in aged rats with memory impairment. Neuropharmacology, 64, 145–152. doi: 10.1016/j.neuropharm.2012.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh MT, Spiegel AM, & Gallagher M (2014). Age-associated changes in hippocampal-dependent cognition in Diversity Outbred mice. Hippocampus, 24(11), 1300–1307. doi: 10.1002/hipo.22311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal SL, Landau S, Bell R, & Jagust W (2017). Hippocampal activation is associated with longitudinal amyloid accumulation and cognitive decline. eLife, In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal SL, & Yassa MA (2015). Neurocognitive Aging and the Hippocampus across Species. Trends Neurosci, 38(12), 800–812. doi: 10.1016/j.tins.2015.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Wang C, Deshmukh SS, & Knierim JJ (2015). Neural Population Evidence of Functional Heterogeneity along the CA3 Transverse Axis: Pattern Completion versus Pattern Separation. Neuron, 87(5), 1093–1105. doi: 10.1016/j.neuron.2015.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Yoganarasimha D, Rao G, & Knierim JJ (2004). Comparison of population coherence of place cells in hippocampal subfields CA1 and CA3. Nature, 430(6998), 456–459. doi: 10.1038/nature02739 [DOI] [PubMed] [Google Scholar]
- Monaco JD, Rao G, Roth ED, & Knierim JJ (2014). Attentive scanning behavior drives one-trial potentiation of hippocampal place fields. Nat Neurosci, 17(5), 725–731. doi: 10.1038/nn.3687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neunuebel JP, & Knierim JJ (2014). CA3 retrieves coherent representations from degraded input: direct evidence for CA3 pattern completion and dentate gyrus pattern separation. Neuron, 81(2), 416–427. doi: 10.1016/j.neuron.2013.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L, Lovden M, Riklund K, Lindenberger U, & Backman L (2012). Memory aging and brain maintenance. Trends Cogn Sci, 16(5), 292–305. doi: 10.1016/j.tics.2012.04.005 [DOI] [PubMed] [Google Scholar]
- Pereira TI, Gallagher M, & Rapp PR (2015). Head west or left, east or right: interactions between memory systems in neurocognitive aging. Neurobiol Aging, 36(11), 3067–3078. doi: 10.1016/j.neurobiolaging.2015.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PR, & Amaral DG (1991). Recognition memory deficits in a subpopulation of aged monkeys resemble the effects of medial temporal lobe damage. Neurobiol Aging, 12(5), 481–486. [DOI] [PubMed] [Google Scholar]
- Robitsek RJ, Fortin NJ, Koh MT, Gallagher M, & Eichenbaum H (2008). Cognitive aging: a common decline of episodic recollection and spatial memory in rats. J Neurosci, 28(36), 8945–8954. doi: 10.1523/JNEUROSCI.1893-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez PE, Zhu L, Verret L, Vossel KA, Orr AG, Cirrito JR, … Mucke L (2012). Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer’s disease model. Proc Natl Acad Sci U S A, 109(42), E2895–2903. doi: 10.1073/pnas.1121081109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkin D, Hattori S, Ybarra N, Musial TF, Buss EW, Richter H, … Disterhoft JF (2015). Aging-Related Hyperexcitability in CA3 Pyramidal Neurons Is Mediated by Enhanced A-Type K+ Channel Function and Expression. J Neurosci, 35(38), 13206–13218. doi: 10.1523/JNEUROSCI.0193-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel AM, Koh MT, Vogt NM, Rapp PR, & Gallagher M (2013). Hilar interneuron vulnerability distinguishes aged rats with memory impairment. J Comp Neurol, 521(15), 3508–3523. doi: 10.1002/cne.23367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley DP, & Shetty AK (2004). Aging in the rat hippocampus is associated with widespread reductions in the number of glutamate decarboxylase-67 positive interneurons but not interneuron degeneration. J Neurochem, 89(1), 204–216. doi: 10.1111/j.1471-4159.2004.02318.x [DOI] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Lacy JW, & Stark CE (2013). A task to assess behavioral pattern separation (BPS) in humans: Data from healthy aging and mild cognitive impairment. Neuropsychologia, 51(12), 2442–2449. doi: 10.1016/j.neuropsychologia.2012.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, & Stark CE (2010). Individual differences in spatial pattern separation performance associated with healthy aging in humans. Learn Mem, 17(6), 284–288. doi: 10.1101/lm.1768110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styr B, & Slutsky I (2018). Imbalance between firing homeostasis and synaptic plasticity drives early-phase Alzheimer’s disease. Nat Neurosci, 21(4), 463–473. doi: 10.1038/s41593-018-0080-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanila H, Shapiro M, Gallagher M, & Eichenbaum H (1997). Brain aging: changes in the nature of information coding by the hippocampus. J Neurosci, 17(13), 5155–5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome A, Gray DT, Erickson CA, Lipa P, & Barnes CA (2016). Memory impairment in aged primates is associated with region-specific network dysfunction. Mol Psychiatry, 21(9), 1257–1262. doi: 10.1038/mp.2015.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TT, Gallagher M, & Kirkwood A (2018). Enhanced postsynaptic inhibitory strength in hippocampal principle cells in high-performing aged rats. Neurobiol Aging, 70, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vela J, Gutierrez A, Vitorica J, & Ruano D (2003). Rat hippocampal GABAergic molecular markers are differentially affected by ageing. J Neurochem, 85(2), 368–377. [DOI] [PubMed] [Google Scholar]
- Villanueva-Castillo C, Tecuatl C, Herrera-Lopez G, & Galvan EJ (2017). Aging-related impairments of hippocampal mossy fibers synapses on CA3 pyramidal cells. Neurobiol Aging, 49, 119–137. doi: 10.1016/j.neurobiolaging.2016.09.010 [DOI] [PubMed] [Google Scholar]
- Wilson IA, Gallagher M, Eichenbaum H, & Tanila H (2006). Neurocognitive aging: prior memories hinder new hippocampal encoding. Trends Neurosci, 29(12), 662–670. doi: 10.1016/j.tins.2006.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, Gallagher M, Eichenbaum H, & Tanila H (2005). Age-associated alterations of hippocampal place cells are subregion specific. J Neurosci, 25(29), 6877–6886. doi: 10.1523/JNEUROSCI.1744-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, McMahan RW, Gallagher M, Eichenbaum H, & Tanila H (2003). Place cell rigidity correlates with impaired spatial learning in aged rats. Neurobiol Aging, 24(2), 297–305. [DOI] [PubMed] [Google Scholar]
- Xiao MF, Xu D, Craig MT, Pelkey KA, Chien CC, Shi Y, … Worley PF (2017). NPTX2 and cognitive dysfunction in Alzheimer’s Disease. eLife, 6. doi: 10.7554/eLife.23798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, & Stark CE (2011). Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus, 21(9), 968–979. doi: 10.1002/hipo.20808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, & Stark CE (2010). High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic Mild Cognitive Impairment. Neuroimage, 51(3), 1242–1252. doi: 10.1016/j.neuroimage.2010.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP (2002). The nature of recollection and familiarity: a review of 30 years of research. Journal of Memory and Language, 46(3), 441–517. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


