Abstract
People generally spend more time indoors than outdoors resulting in a higher proportion of exposure to particulate matter (PM) occurring indoors. Consequently, indoor PM levels, in contrast to outdoor PM levels, may have a stronger relationship with lung function.
To test this hypothesis, indoor and outdoor PM2.5 and fungal spore data were simultaneously collected from the homes of forty-four asthmatic children aged 10 – 16 years. An optical absorption technique was utilized on the collected PM2.5 mass to obtain concentrations of black carbon (BC) and ultraviolet light absorbing particulate matter, (UVPM; a marker of light absorbing PM2.5 emitted from smoldering organics). Enrolled children completed spirometry after environmental measurements were made. Given the high correlation between PM2.5, BC, and UVPM, principal component analysis was used to obtain uncorrelated summaries of the measured PM. Separate linear mixed-effect models were developed to estimate the association between principal components of the PM variables and spirometry values, as well as the uncorrelated original PM variables and spirometry values.
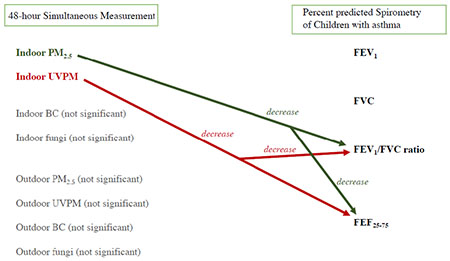
A one-unit increase in the first principal component variable representing indoor PM (predominantly composed of UVPM and PM2.5) was associated with 4.1% decrease (99% CI = −6.9, −1.4) in FEV1/FVC ratio. 11.3 μg/m3 increase in indoor UVPM was associated with 6.4% and 14.7% decrease (99% CI = −10.4, −2.4 and 99% CI = −26.3, −2.9, respectively) in percent predicted FEV1/FVC ratio and FEF25-75 respectively. Additionally, 17.7 μg/m3 increase in indoor PM2.5 was associated with 6.1% and 12.9% decrease (99% CI = −10.2, −1.9 and 99% CI = −24.9, −1.0, respectively) in percent predicted FEV1/FVC ratio and FEF25-75, respectively. Outdoor PM, indoor BC, and indoor fungal spores were not significantly associated with lung function.
The results indicate that indoor PM is more strongly associated with lung function in children with asthma as compared with outdoor PM.
Keywords: indoor particles, lung function, asthmatic children, outdoor particles, light absorbing particles
Graphical abstract
1.0. Introduction
While ambient air pollutants including particulate matter with aerodynamic diameter less than 2.5 μm (PM2.5) have generally declined in the United States over the past decade,(1) exposure to traffic-related air particles continue to be linked with respiratory health problems including reduced lung function, an indicator of respiratory health.(1–3) Elevated outdoor particulate matter (PM) levels are directly linked to a decrease in lung function in children.(4) Similarly, exposure to outdoor fungal spores is associated with an increased risk of hospital admissions and emergency department visits for asthma-related events in children.(5) Approximately 28% of indoor PM2.5 originate from outdoor sources.(6) This estimate can be 53-73% during low indoor activity,(7) and is modified by particle penetration efficiency(8) and geographical location.(9) Because indoor PM is composed of PM with indoor and outdoor origin, and people spend about 90% of their time indoors,(10) a substantial proportion of exposure to PM may occur indoors. Consequently, indoor PM is likely to have a stronger association with lung function than outdoor PM.
The elemental components of PM2.5 produced from indoor sources may differ from PM2.5 of outdoor origin.(6) Analysis performed by Habre et al. suggests that the elemental carbon (EC) components of PM2.5 from many indoor sources originate from smoldering combustion,(6) as found during cigarette smoking and using fireplaces. On the other hand, the EC components of PM2.5 from outdoor sources originate mainly from internal-combustion engine exhaust soot.(6) Given that light absorbing particles are emitted both from smoldering combustion and internal-combustion engine exhaust, distinguishing between the associated health effects from exposure to these different PM2.5 types is complex when light absorbance is measured with optical methods using a single wavelength. Many studies of light absorbing components of PM2.5 have been based on concentrations of light absorbing particles measured with a single wavelength.(11,12)
To obtain distinct measurements of airborne soot particles (black carbon, BC) and light absorbing PM2.5 emitted from smoldering organics such as burning cigarettes, light absorbance measured continuously from 350 nm to 1000 nm have been used to distinguish between BC and cigarette smoke, respectively.(13) We hypothesize that this technique can be utilized to obtain specific measurements of BC and cigarette smoke, and distinguish between the association of these PM types on lung function. Studying the association between UVPM, BC, PM2.5 and lung function will facilitate the understanding of safe indoor PM levels. Moreover, additional studies are needed to distinguish between the association of different combustion-related PM and respiratory health.(14) The objective of this study was two folds:
-
(1)
To differentiate between the association of BC and lung function, and ultraviolet light absorbing particulate matter, UVPM and lung function.
-
(2)
To compare the magnitude of association between outdoor PM and lung function, and indoor PM and lung function.
2.0. Methods
2.1. Study Overview
Between September 2015 to August 2017, 44 asthmatic children of 10 – 16 years old residing in 43 homes were recruited from an ongoing randomized intervention study conducted in the Cincinnati-Kentucky-Indiana tristate area.(15) Subjects were recruited from the Cincinnati Children’s Hospital Medical Center Asthma Clinic and the Cincinnati Childhood Allergy and Air Pollution Study.(16) A cross-over study design was used in the intervention study to test the efficiency of a portable high efficiency particulate air (HEPA) cleaner in reducing indoor particle concentrations (Figure S1).(15) In the current study, since we are studying the association between indoor PM2.5 mass (and its light-absorbing proportion) and lung function, only measurements conducted at the baseline (i.e., before the use of HEPA or placebo HEPA air cleaners) were used (Supplemental material 1.0).
The inclusion criteria in the intervention study were physician diagnosis of asthma and residing in a dwelling with an estimated outdoor level of ≥ 0.33 μg/m3 elemental carbon attributable to traffic, as determined from an earlier study.(17) Spirometry tests were conducted on subjects, and indoor measurements of PM2.5 mass and its light-absorbing proportion were made in the homes of subjects. Other factors including fungal spores,(5) pet dander(18), breathing/nasal medication use and socioeconomic status(19, 20) that may potentially affect lung function were also quantified. The study received ethical approval from the University of Cincinnati Institutional Review Board.
2.2. Assessment of Subject Characteristics
Housing characteristics and occupant activities that could potentially modify the lung function of study participants were documented with a questionnaire administered to the parent (mother or father). The questions included the presence of pets (cats and dogs), the presence of at least one smoker residing in the home, breathing/nasal medication use within the last 72 hours of spirometry, and annual household income as a surrogate for socioeconomic status. All responses were documented as categorical variables. Information on the frequency of subjects experiencing asthma symptoms were also collected via a questionnaire. A geographical information system (ArcGIS 9.0, Environmental Systems Research Institute, Inc., Redlands, CA) was used to calculate the distance of the study homes to the nearest highway or interstate.(17)
2.3. Assessment of Environmental Exposures
Two 48-hour measurements of PM2.5 and fungal spores were conducted outdoors and in the bedrooms (indoors) of 43 homes resided by the 44 study participants. The two measurements were spaced approximately 1-month apart from each other. PM2.5 samples were collected on 37-mm Teflon filters with a single-stage Personal Modular Impactor (SKC, Inc., PA) that had an aerodynamic particle cut-off of 2.5 μm at 3 L/min. For quality assurance, blank filter samples were deployed in parallel in 10% of PM2.5 samples. Filter samples were analyzed gravimetrically and blank corrected.(21) Using an integrating sphere with absorption measured continuously from 350 nm to 1000 nm, reflectance analysis was carried out on the collected PM2.5 mass to obtain concentrations of black carbon (BC) and ultraviolet light absorbing particulate matter, (UVPM, second-hand cigarette smoke).(13) The limit of detection (LOD) for BC was 1.4 ng/mm2 of the filter;(13) equivalent to an air concentration of 0.12 μg/m3 in this study. Furthermore, the LOD for UVPM was 0.7 ng/mm2 of the filter;(13) equivalent to 0.18 μg/m3 in this study.(13) Non-detectable measurements of indoor and outdoor BC and UVPM were replaced with LOD/2 as suggested by Hornung and Reed.(22)
Fungal spores were collected on 25-mm Teflon filters with 1-μm pore size (Merck Millipore, Billerica, MA) using a Button Inhalable Aerosol Sampler (SKC, Inc., PA) at 4 L/min. Utilizing mold specific quantitative PCR (MSQPCR) assays, 36 species were reported as spore equivalents (SE) per filter as described previously.(23–25) Results for each of the 36 species were summed per filter and then divided by the volume of air per sample yielding a concentration of summed MSQPCR-fungi in spore equivalents per cubic meter (SE/m3).
2.4. Assessment of Lung Function
Spirometry was conducted at the end of each 48-hour PM measurement using a commercially available spirometer (KoKo Sx 1000, nSpire Health, Longmont, CO) following the American Thoracic Society Standards (ATS).(26) Calibration check for the spirometer was performed with a 3 L syringe prior to each spirometry test. Volume verification was performed by discharging the 3 L syringe at three different flow rates varying between 0.5 and 12 L/s (analogous to the flow rate anticipated during subject testing). The observed volume at each flow rate was within the accuracy requirement of ± 3.5% as recommended by ATS.(26) Because age, height, gender, and race explain > 65% of the variability in lung function,(27) percent predicted spirometry values were used (100*observed value/predicted value). Since study participants were 10-16 years old, spirometry predictive values were based on Wang, et al. (28) (standards for 6-18-year olds) versus Hankinson, et al. (27) (8-80-year olds). Height of subjects was measured by subject standing against the wall with socks on. Gender and racial data were obtained by parent report. Data on percent predicted forced expiratory volume in one second (FEV1), forced vital capacity (FVC), FEV1/FVC ratio and forced expiratory flow between 25 and 75% of forced vital capacity (FEF25-75) were collected as these are commonly used in clinical and research settings to monitor obstructive lung disease, such as asthma. The spirometer was programmed to interprete spirometer readings based on an algorithm provided by McKay and Lockey.(29)
2.5. Statistical Analysis
Statistical analyses were performed with R statistical software.(30) To investigate the association between exposure to PM and percent predicted spirometry results, a linear mixed-effect model was utilized. Each subject and each home were assigned unique identifying numbers, and repeated measures were controlled for by having a unique intercept for each home and each subject, and the fixed effects of PM concentrations and other covariates on lung function were assessed. Because BC, and UVPM are produced from incomplete combustion and contribute to PM2.5, concentrations of these PM may be highly correlated.
To address collinearity in our exposure metrics, principal components of the PM variables were calculated after the investigation of high correlation between the PM variables. Because fungal spores were measured in spore equivalents per cubic meter (SE/m3) and other PM in μg/m3, the variables were normalized in order to have measurements on the same scale before applying principal component analysis (PCA). The principal components of the normalized PM variables (fungal spores, PM2.5, BC, and UVPM) were calculated with the “stats” package in R statistical software, then used in the linear mixed-effect model (equation S2). Separate linear mixed-effect models for indoor and outdoor measurements were developed with the “lmerTest” package in R statistical software. The presence of pets in the home, breathing/nasal medication use and annual household income were added as covariates. Equation S2 shows the model for analyzing the association between the principal components of indoor PM and percent predicted spirometry results.
In addition to PCA, we also explored the independent association of PM2.5, UVPM, BC, fungal spores and lung function using the normalized PM variables in linear mixed models. The normalized PM variables were used so that the regression estimates obtained can be comparable to the regression estimates from the models containing principal components. In cases where the normalized PM variables were highly correlated (Spearman r ≥ 0.7), only one of the correlated PM variables were included in the same model. Different models for studying the same percent predicted spirometry value were developed. The regression model containing all the PM variables is given in equation S3. For each spirometry variable there were 4 models developed using the PM variables as predictors of spirometry results. Therefore a Bonforreni correction was applied, and p values < 0.0125 (0.05/4) were considered statistically significant (equivalent to 99% CI).
Group comparisons of UVPM and PM2.5 concentrations in the homes of smokers and non-smokers were performed with analysis of variance (ANOVA), followed by a Tukey’s honestly significant difference (HSD) test to adjust for repeated measures with the “multcomp” package in R statistical software. Similarly, group comparisons of percent predicted spirometry results of subjects living in homes with and without smokers was performed. The Tukey’s HSD test was used to determine the significant difference between each group.
3.0. Results
We had a total of 44 subjects in 43 homes. Fourteen PM samples were lost due to pump failures and sample contamination. Furthermore, one spirometry observation was invalid because it did not meet the ATS acceptability criteria for spirometry. Thus, repeat measurements were obtained from 28 homes and single observations from 15 homes, making a total of 71 paired observations of lung function and PM concentrations. Of these, two observations on breathing/nasal medication use (Yes/No) within the last 72 hours were missing.
3.1. Study Participants
Table 1 shows the characteristics of the study participants. All 44 participants were reported being non-smokers with a mean age of 12.3 years (Table 1). Study participants included 33.3% females. The majority of the subjects (78.7%) resided in non-smoking homes and 21.3% in homes that had at least one smoker present. Over half of the study participants had parents with annual incomes > $50000. Over 40% of the subjects reported experiencing asthma symptoms at most once per month within the last 12 months. The median distance of a study home to a highway or interstate was 374 m (Table 1). In 38.9% of the 69 visits that had observations on breathing/nasal medication use, subjects took at least one medication within the last 72 hours. There were a variety of breathing/nasal medications taken; the most common was Albuterol (26%) (Figure S2).
Table 1:
Subject Characteristics (n=44)
| Mean (SD) or % | |
|---|---|
| Age (years) | 12.3 (2) |
| Sex (male) | 66.7% |
| Smoking (No) | 100.0% |
| Height (cm) | 164.1 (38.1) |
| Weight (kg) | 57.8 (16.8) |
| Caucasian | 48.9% |
| African-American | 36.2% |
| Hispanic | 14.9% |
| Smoking parents or other home occupants | 21.3% |
| Living with at least one dog/cat (Yes) | 68.0% |
| > $50000 household income | 51.1% |
| > $40000 - $50000 household income | 2.1% |
| > $30000 - $40000 household income | 10.6% |
| > $20000 - $30000 household income | 10.6% |
| > $10000 - $20000 household income | 12.8% |
| < $10000 household income | 12.8% |
| Subject experience of asthma symptoms/hospitalization due to asthma | |
| Never within the last 12 months | 6.5% |
| Rarely (less than once a month) | 34.8% |
| Sometimes (1 – 4 times a month) | 47.8% |
| Often (more than once a week but not daily) | 10.9% |
| Proximity of homes to road (highway or interstate) | Distance (m) |
| Minimum | 26 |
| First quartile (Q1) | 135 |
| Median | 374 |
| Mean | 520 |
| Third quartile (Q3) | 504 |
| Maximum | 3867 |
| SD | 683 |
3.2. Summary of Indoor and Outdoor PM
The mean concentrations of indoor BC, indoor UVPM, indoor PM2.5, and indoor fungal spores were 0.6 μg/m3, 5.8 μg/m3, 14.6 μg/m3 and 420 spore equivalents per cubic meter (SE/m3), respectively (Table 2). There was a strong correlation between indoor UVPM and indoor BC (r = 0.72) (Figure 1 A). Similarly, indoor PM2.5 and indoor BC (r = 0.70) as well as indoor UVPM and indoor PM2.5 (r = 0.80) were strongly correlated (Figures 1B and 1C). In contrast, indoor fungal spores were not correlated with PM2.5, BC, and UVPM (Figure 1D – 1F).
Table 2:
Measured Levels of Indoor Particulate Matter in Study Homes (repeated measures, n=71)
| BC (μg/m3) | UVPM (μg/m3) | PM2.5 (μg/m3) | Fungi (SE/m3) | |
|---|---|---|---|---|
| Minimum | < LOD | < LOD | <LOD | 1 |
| Q1 | 0.2 | 0.8 | 5.3 | 84 |
| Median | 0.5 | 1.6 | 8.7 | 198 |
| Mean | 0.6 | 5.8 | 14.6 | 420 |
| Q3 | 0.8 | 4.8 | 14.5 | 599 |
| Maximum | 3.0 | 60.2 | 81.4 | 2346 |
| SD | 0.5 | 11.3 | 17.7 | 502 |
BC = black carbon, UVPM = ultraviolet absorbing particulate matter (light absorbing PM2.5 emitted from smoldering organics such as smoking cigarettes and burning fireplace wood), Fungi = fungal spore equivalents per cubic meter (SE/m3), Q1 = 25th percentile, Q3 = 75th percentile, LOD for BC = 0.12 μg/m3, LOD for UVPM = 0.18 μg/m3, LOD for PM2.5 = 0.3 μg/m3.
Figure 1:
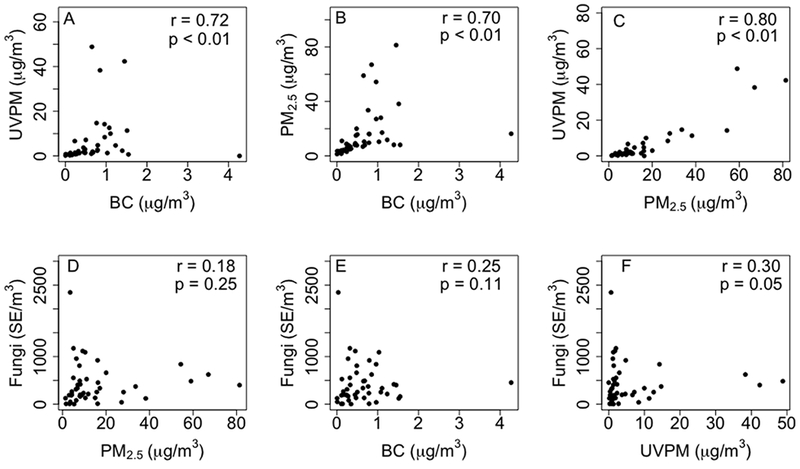
Correlation plots of the mean concentrations of measured indoor particulate matter in each home (n = 43).
r = Spearman correlation coefficient, UVPM = ultraviolet light absorbing particulate matter (light absorbing PM2.5 emitted from smoldering organics such as smoking cigarettes and burning fireplace wood), BC = black carbon, PM2.5 = particulate matter with aerodynamic diameter ≤ 2.5 μm, Fungi = fungal spore equivalents per cubic meter (SE/m3).
The mean concentrations of outdoor BC, outdoor UVPM, outdoor PM2.5, and outdoor fungal spores were 0.9 μg/m3, 2.0 μg/m3, 13.0 μg/m3, and 3965 SE/m3, respectively (Table S1). Outdoor UVPM and outdoor BC were weakly correlated (r = 0.39) (Figure S3A). Conversely, outdoor PM2.5 and outdoor BC were strongly correlated (r = 0.74) (Figure S3B). Without one influential observation, outdoor UVPM and outdoor PM2.5 were also strongly correlated (r = 0.80) (Figure S3C). Outdoor fungal spores were weakly correlated with PM2.5, BC, and UVPM (Figures S3D – S3F).
Indoor and outdoor PM were not strongly correlated (Figure S4) with the highest correlation observed between indoor and outdoor BC (r=0.49). Figure S5 shows the indoor/outdoor ratio of the measured PM from the 71 sampling periods. The median indoor/outdoor ratios for BC, UVPM, PM2.5 and fungal spores were 0.6, 0.9, 0.8 and 0.1, respectively. However, the indoor/outdoor ratios of UVPM and PM2.5 in the homes where a smoker resided were significantly higher than the levels in non-smoking homes (Figure 2). In the homes where smokers resided, median indoor/outdoor ratio for UVPM, and PM2.5 were 6.9 and 3.5, respectively (Figure 2). In contrast, in non-smoking homes, median indoor/outdoor ratio for UVPM and PM2.5 were 0.9 and 0.7, respectively.
Figure 2:
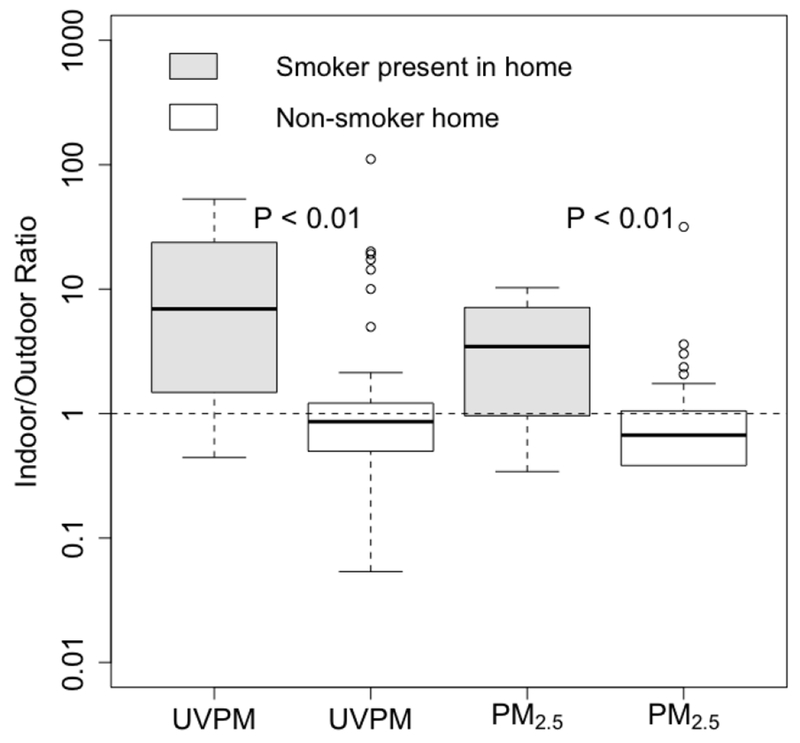
Indoor/outdoor ratios of UVPM and PM2.5 stratified by presence of smoker in the residence. P-values obtained from Tukey’s HSD test (controlling for repeated measures). From below, horizontal line in boxplot represents minimum (excluding outliers), 25th, 50th, 75th percentiles and maximum (excluding outliers); Circles = outliers. Smoker home (n=12) = a home where at least one occupant smokes at least one cigarette per day, non-smoker home (n=59) = a home where no occupant smoked.
The fractions of light absorbing PM (UVPM and BC) in the sampled PM2.5 mass were relatively low. The median BC and UVPM fractions of indoor PM2.5 mass were 0.04 and 0.23, respectively (Figures S6A and S6B), and the non-light absorbing fraction was 0.73 (Figure S6C). In the sampled outdoor PM2.5 mass, the median fractions of BC and UVPM were 0.10 and 0.23, respectively (Figures S6D and S6E).
3.3. Principal Components
The first three principal components (PC1 – PC3) of indoor PM from the study homes explained 98% of the variation in the data (BC, UVPM, PM2.5, and fungal spores). PC1 – PC3 were subsequently included as independent variables in the analysis of indoor PM and lung function. Similarly, the first three principal components of outdoor PM explained 86.2% of the variation in the data and were used in the regression models developed for the analysis of outdoor PM and lung function.
For the principal components derived for indoor PM, PC1 was predominantly made up of UVPM and PM2.5; the linear weights of UVPM and PM2.5 were the highest in comparison to the weights of BC and fungal spores (Table S2). Table S3 shows the linear weights for the principal components of outdoor PM. The first principal component of outdoor PM was predominantly composed of PM2.5, UVPM and BC (Table S3).
3.4. Spirometry Results
Figure 3 shows the variation in percent predicted spirometry results. The percent predicted FEV1 ranged from 48% – 163%, with a median of 100%. Percent predicted FVC values were less varied, ranging from 71% – 184%, with a median of 108%. Percent predicted FEV1/FVC ratio had a median of 92% (range = 54 – 115%), and percent predicted FEF25-75 had a median of 80% (range = 24 – 160%). Other summary statistics of subjects’ percent predicted spirometry results are given in Table S4. There was no significant difference between spirometry results from subjects residing in homes with occupants that smoked and subjects residing in non-smoking homes (Figure S7). Over 50% of the subjects had normal spirometry values (Table 3).
Figure 3:
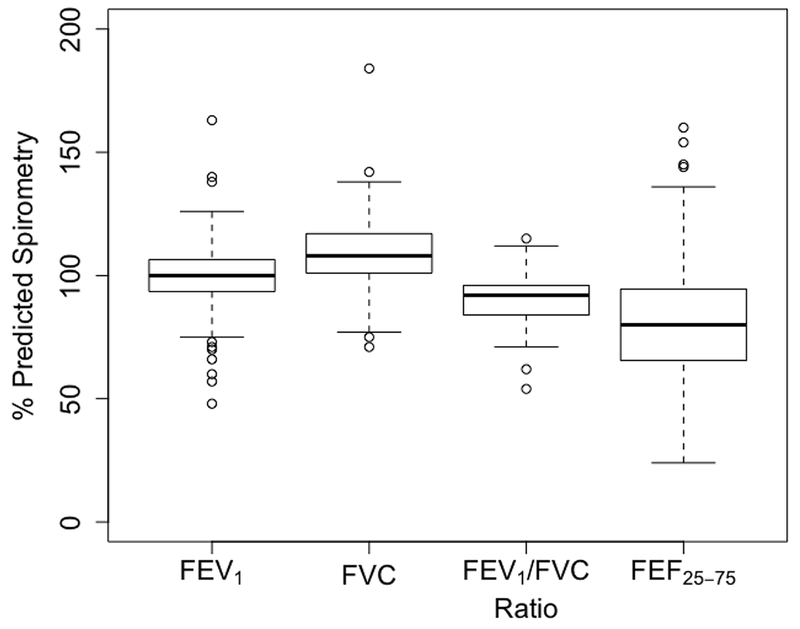
Boxplots of the spirometry results from the 71 observations.
FEV1 = Forced expiratory volume in 1 second, FVC = Forced vital capacity, FEV1/FVC ratio is the value obtained from dividing FEV1 by FVC and multiplying by 100, FEF25-75 = Forced expiratory flow at 25 – 75% of the FVC.
From below, horizontal line in boxplot represents minimum (excluding outliers), 25th, 50th, 75th percentiles and maximum (excluding outliers); Circles = outliers.
Table 3:
Subject spirometry interpretations
| Interpretations | Percentage of subjects |
|---|---|
| Normal spirometry values indicating absence of any significant degree of pulmonary impairment | 58.3% |
| FVC and FEV1 normal, and normal reduction in FEV1/FVC ratio that may occur in healthy individuals | 13.8% |
| Early obstructive pattern | 2.8% |
| Mild obstructive pattern | 16.7% |
| Moderate obstructive pattern | 1.4% |
| Moderately severe obstructive pattern | 1.4% |
| Mixed obstructive/restrictive pattern | 4.2% |
| Mild restrictive pattern | 1.4% |
Spirometry interpretations were obtained from the spirometer (KoKo Sx 1000, nSpire Health, Longmont, CO) automatically after a test was conducted. Manufacturers of the spirometer designed it to give interpretations of spirometry values based on McKay and Lockey.(29)
3.5. Change in Lung Function Associated with Exposure to PM
Tables S5 and S6 show the intercepts of the developed regression models, which represents the mean percent predicted spirometry results of the subjects in the absence of the independent variables. In addition, the percent change in FEV1, FVC, and the visualized estimates in Figures 4 and 5 (i.e., percent change in percent predicted FEV1/FVC ratio and FEF25-75) are given.
Figure 4:
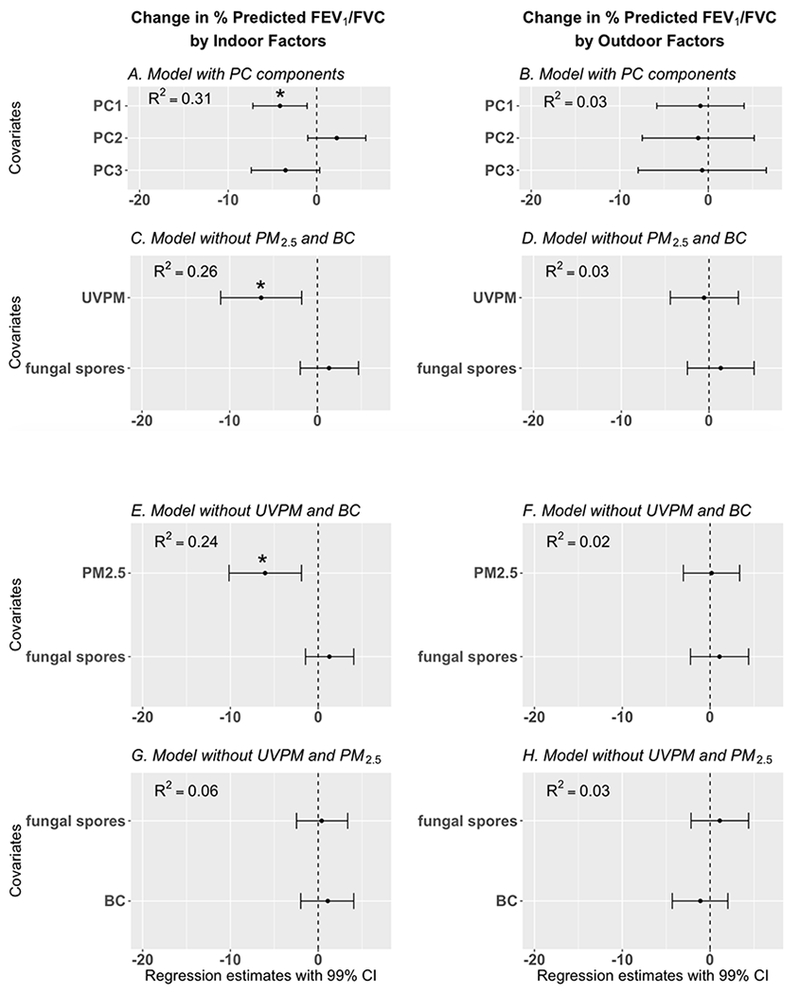
Results of regression models developed to explore the association between 48-hour measurements of particulate matter (indoor and outdoor simultaneously) and percent predicted FEV1/FVC ratio (adjusted for age, height, gender, and race).
Dots= regression estimates; error bars = 99% CI = 99% confidence interval of regression estimate (adjusted for presence/absence of pets and family income).
* indicates statistically significant finding, BC = black carbon, UVPM = ultraviolet light absorbing particulate matter, PC components = principal components of BC, UVPM, PM2.5 and fungal spores. In Figures 4C – 4H, one standard deviation increase in BC, UVPM, PM2.5, and fungal spores are associated with the corresponding regression estimates in the Figures. Four different models are presented to avoid multicollinearity of UVPM, BC, and PM2.5.
Figure 5:
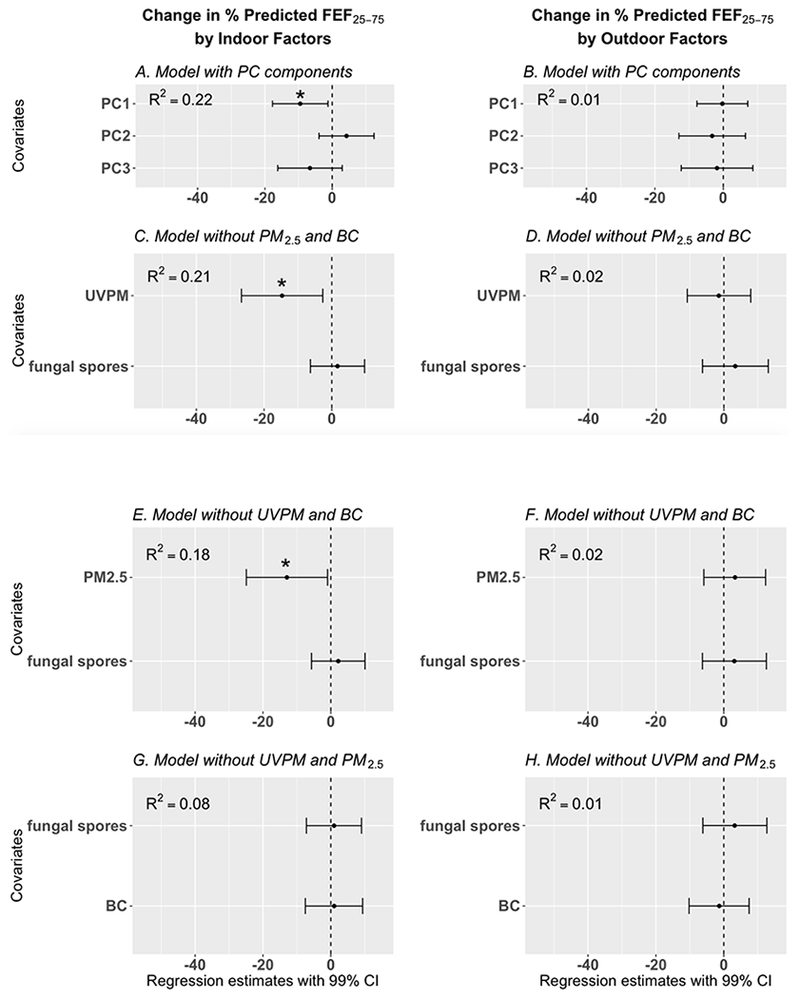
Results of regression models developed to explore the association between 48-hour measurements of particulate matter (indoor and outdoor) and percent predicted forced expiratory flow between 25 – 75% of the forced vital capacity (FEF25-75) [adjusted for age, height, gender, and race], regression estimates are adjusted for adjusted for presence/absence of pets and family income. In Figures 5C – 5H, one standard deviation increase in BC, UVPM, PM2.5, and fungal spores are associated with the corresponding regression estimates in the Figures. See Figure 4 footnotes for additional figure descriptions.
None of the outdoor PM variables were significantly associated with lung function. Furthermore, there were no significant associations between indoor and outdoor PM variables (BC, UVPM, PM2.5, fungal spores) and percent predicted FEV1 and percent predicted FVC (Tables S5 and S6). In contrast, indoor PM2.5 and indoor UVPM were significantly associated with percent predicted FEV1/FVC ratio and FEF25-75.
Figures 4 and 5 show that indoor PM was significantly associated with reduced percent predicted FEV1/FVC ratio. Principal component 1 (Table S3), predominantly composed of indoor UVPM and indoor PM2.5, was significantly associated with reduced percent predicted FEV1/FVC ratio (Figure 4A, β = −4.1, 99% CI = −6.9, −1.4). A one standard deviation increase in indoor UVPM (11.3 μg/m3) was significantly associated with reduced percent predicted FEV1/FVC ratio (Figure 4C, β = −6.4, 99% CI = −10.4, −2.4). Similarly, one standard deviation increase in indoor PM2.5 (17.7 μg/m3) was also associated with significant decrease in percent predicted FEV1/FVC ratio (Figure 4C, β = −6.1, 99% CI = −10.2, −1.9). In contrast, indoor BC was not significantly associated with percent predicted FEV1/FVC ratio (Figure 4G).
Indoor PM was significantly associated with reduced percent predicted FEF25-75. Principal component 1 (Table S3), predominantly composed of indoor UVPM and indoor PM2.5, was significantly associated with reduced percent predicted FEF25-75 (Figure 5A, β = −9.5, 99% CI = −17.7, −1.3). A one standard deviation increase in indoor UVPM (11.3 μg/m3) was significantly associated with reduced percent predicted FEF25-75 (Figure 5C, β = −14.7, 99% CI = −26.3, −2.9). Similarly, one standard deviation (17.7 μg/m3) increase in indoor PM2.5 was significantly associated with reduced percent predicted FEF25-75 (Figure 4C, β = −12.9, 99% CI = −24.9, −1.0). Indoor BC was not significantly associated with percent predicted FEF25-75 (Figure 4G).
Among the covariates, the association between family income and lung function was not significant, but having pets (dogs/cats) were significantly associated with a decrease in percent predicted FEV1/FVC ratio and percent predicted FEF25-75, when compared to not having pets. In a sensitivity analysis on the 69 observations of breathing/nasal medications use within the last 72 hours of spirometry (Yes/No), lung function measurements, PM concentrations and other covariates, breathing/nasal medications use was not significant (results not shown).
4.0. Discussion
Our results show that 48-hour average concentration of indoor PM, but not outdoor PM were associated with reduced lung function in children with asthma. Increase in indoor UVPM and indoor PM2.5, but not indoor BC and indoor fungal spores were associated with reduced percent predicted FEV1/FVC ratio and percent predicted FEF25-75. Of all the measured combustion-related PM, UVPM had the strongest inverse relationship (highest β) with percent predicted FEV1/FVC ratio and percent predicted FEF25-75. The association between exposure to light absorbing PM2.5 emitted from smoldering organics such as burning cigarettes (UVPM) and adverse respiratory outcome may be stronger in contrast to exposure to BC and adverse respiratory outcome.
4.1. Interpretation of Results
The results indicate that indoor PM level is a better metric for PM exposure assessment than outdoor PM levels. However, the relationship between outdoor PM and respiratory health must not be underemphasized, given the weighty evidence of the association between outdoor PM and respiratory health.(1, 2, 4) Our results suggest that greater statistical power is needed to identify a significant association with outdoor PM and lung function, than with indoor PM and lung function. Sample size calculations using the regression estimates of outdoor PM and equations for calculating sample size for longitudinal studies with correlated repeated measures(31, 32) revealed that a required sample size of > 20 per subject is needed. Some cross-sectional and longitudinal studies on the relationship between outdoor PM and lung function were based on the exposure of 5,921 children to annual averages of outdoor PM,(4) 11,000 children followed up for 8 years,(1, 33) daily averages of PM collected over three months and daily assessments of respiratory health.(34) In other studies with smaller sample size, PM2.5 in addition to traffic-related gaseous pollutants were measured and included in their models.(2, 34–41) In our study, despite a short monitoring time (48 hours) and relatively small sample size (71), we demonstrated an association between indoor PM and reduced lung function.
Unlike other studies, we did not observe a significant association between indoor BC and lung function. In comparison to indoor UVPM and indoor PM2.5, the variation of BC was low, making an association difficult to detect with our sample size. It is also possible that optical methods used to assess exposure to BC in previous studies may be confounded by other particles such as UVPM if only a single wavelength was used. Consequently, outcomes made about exposure to BC in such studies, may also be associated with UVPM. The lack of a significant association between fungal spores and respiratory health in the current study is consistent with results from another cohort of Cincinnati children, as reported by Lierl and Hornung.(42) Conversely, Atkinson et al. demonstrated a positive relationship between thirty different taxa of fungal spores outdoors and asthma-related hospital visits in London.(5) The fungal species analyzed in the current study and those analyzed by Atkinson et al. are different. Further research into fungal spore exposure and lung function may aid in understanding the differences in the studies.
4.2. How do our results compare with prior knowledge?
The finding of an association between indoor PM2.5 and respiratory health is consistent with results from previous studies. McCormack et al. showed an association between PM2.5 and asthma symptoms such as wheezing,(43) and Delfino et al. demonstrated a relationship between increased indoor PM2.5 and reduced FEV1 among children with asthma.(44) Airway obstruction results in decreased airflow and decreases in FEV1 and FEF25-75. Therefore, the FEV1/FVC ratio and FEF25-75 are used as markers for airway obstruction.(45) We observed a reduction in FEV1/FVC ratio that was associated with increased PM2.5 and UVPM levels. Values of FEF25-75 give suggestions on the state of the bronchus and therefore asthma status.46, 47) Our data suggest that increase in PM2.5 and UVPM was associated with bronchial constriction and airway obstruction in the subjects studied. Previous studies suggest that this relationship may not exist in non-asthmatic children(48, 49) indicating a stronger correlation of PM2.5 and lung function in children with asthma.(50) While UVPM and PM2.5 may be associated with airway obstruction, the data suggest that the level of obstruction may not be clinically severe, given that the relationships between FEV1 (value used to measure severity of obstructive diseases)51, 52) and all the PM variables were not significant.
Analysis performed by Habre et al. shows that during the winter, the odds of wheezing and coughing among children with asthma exposed to indoor PM2.5 of outdoor origin is greater when compared to the odds of wheezing and coughing from exposure to outdoor PM2.5.(53) In the same population during fall and spring, the odds of wheezing and coughing from exposure to indoor PM2.5 emitted from indoor sources was greater when compared to the odds of wheezing and coughing from exposure to outdoor PM2.5.(53) Results in the current study are similar to that of Habre et al. showing a stronger association between indoor PM2.5 and lung function in contrast to outdoor PM2.5 and lung function.
The spirometry values of the subjects in the current study are comparable to spirometry values of other children with asthma.(49) Using percent predicted FEV1/FVC of ≤ 70% as the threshold for airway obstruction,(54) majority of the subjects in the current study had relatively good spirometry results. Only 2.8% of subjects had percent predicted FEV1/FVC of ≤ 70%. Similarly, average indoor levels of PM2.5 (median indoor = 8.7 μg/m3) and BC (median indoor = 0.5 μg/m3) in the current study are comparable to levels reported in other studies.(55–57) On the other hand, measured outdoor PM2.5 and BC in the current study (mean = 13.0 μg/m3 and 0.9 μg/m3, respectively) are higher than the levels reported in some earlier studies.(2, 4)
4.3. Secondary Results
In the current study, the covariates accounted for in the regression estimates for the PM variables were the presence/absence of pets and family income. Presence of pets was associated with reduced percent predicted FEV1/FVC ratio and percent predicted FEF25-75, but the association between socioeconomic status (as determined from family income) and lung function was not significant. Exposure to pet dander may reduce lung function in children sensitized to pet or food allergens.(18) Furthermore, sensitization to mite dust is associated with reduced lung function.(58) As the prevalence of sensitization in children with asthma is relatively high,(58) there may be an increased chance of finding significant associations between pets and lung function in children with asthma.
The median indoor/outdoor ratio of BC in the current study (0.6) indicate that on the average, infiltration was a major source of indoor BC in the studied homes. The substantial difference in indoor/outdoor ratio of UVPM and PM2.5 in the homes of smokers (6.9 and 3.5, respectively) when compared to non-smoking homes (0.9 and 0.7, respectively) suggests that tobacco smoke was a major source of indoor UVPM and indoor PM2.5 in the study homes. In the current study, parents of the subjects were asked about the smoking status of people residing in the home, and second-hand cigarette smoke was assessed using UVPM as a surrogate. The results indicate that some parents may have misclassified the smoking status of home occupants. Ultraviolet light absorbing PM (UVPM) is an objective assessment of second-hand cigarette smoke, and PM2.5 is also emitted from smoking cigarettes. Expectedly, measurements of UVPM and PM2.5 were significantly higher in the homes where at least one smoker resided. Furthermore, indoor PM2.5 and UVPM were inversely associated with percent predicted FEV1/FVC ratio and FEF25-75. However, the difference in percent predicted FEV1/FVC and FEF25-75 was not significant among children in the homes of smokers versus non-smokers. To solve the potential problem of subject recall bias, direct measures of tobacco smoke exposure such as cotinine levels in hair(59) and UVPM should be used to assess exposure, but our study was not specifically designed to answer this question.
5.0. Limitations
Since reduced lung function can be potentially affected by unstudied pollutants, such as NO2 and O3, results of our investigation should be taken with caution. This study can be improved upon by examining the association between PM concentrations and lung function in a larger set of subjects for longer periods. Furthermore, adjusting for seasonal differences of particulate matter concentrations will improve upon our study. Nevertheless, we were able to show significant associations between 48-hour averages of indoor PM concentrations and lung function in 44 subjects.
6.0. Conclusions and Implications
Indoor PM is a better predictor of lung function in comparison to outdoor PM. Both indoor UVPM (a surrogate of light absorbing PM2.5 from smoldering organics such as burning cigarettes) and indoor PM2.5 significantly reduce FEV1/FVC ratio and FEF25-75. The association between exposure to UVPM and reduced lung function may be stronger than that of PM2.5, BC and reduced lung function. Exposure to low levels of BC indoors (mean = 0.6 μg/m3) may not significantly reduce lung function. Our results suggest that indoor PM (in comparison to outdoor PM) is associated with decreased lung function in children with asthma. We recommend that children with asthma avoid indoor environments with PM emitted from smoldering organics such as burning cigarettes, wood or incense.
Supplementary Material
Highlights.
Reduced lung function (FEV1/FVC ratio and FEF25-75) is more strongly associated with indoor particles than outdoor particles.
Lung function is more strongly associated with UVPM than PM2.5 or black carbon.
Low levels of black carbon indoors (0.6 μg/m3) may not be associated with lung function.
Acknowledgement
The United States Department of Housing and Urban Development (Grant OHHHU0027-14) supported this study. K.I received assistantship from the Department of Environmental Health University of Cincinnati Graduate Scholarship. Support was also received from the National Institutes of Health (Grant P30ES009089).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Notice
The U.S. Environmental Protection Agency (EPA) through its Office of Research and Development collaborated in the research described here. It has been subjected to the Agency’s peer review and has been approved as an EPA publication. Mention of trade names or commercial products does not constitute endorsement or recommendation by the EPA for use. The findings and the conclusions in this report are those of the authors and do not necessarily represent the views of the US EPA.
References
- 1.Chen Z, Salam MT, Eckel SP, Breton CV, and Gilliland FD: Chronic effects of air pollution on respiratory health in Southern California children: findings from the Southern California Children’s Health Study. Journal of Thoracic Disease 7(1): 46 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rice MB, Ljungman PL, Wilker EH, Gold DR, Schwartz JD, Koutrakis P et al. : Short-term exposure to air pollution and lung function in the Framingham Heart Study. American Journal of Respiratory and Critical Care Medicine 188(11): 1351–1357 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cotes JE, Chinn DJ, and Miller MR: Lung function: Physiology, Measurement and Application in Medicine: John Wiley & Sons, 2009. [Google Scholar]
- 4.Gehring U, Gruzieva O, Agius RM, Beelen R, Custovic A, Cyrys J et al. : Air pollution exposure and lung function in children: the ESCAPE project. Environmental Health Perspectives 121(11-12): 1357 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atkinson RW, Strachan DP, Anderson H, Hajat S, and Emberlin J: Temporal associations between daily counts of fungal spores and asthma exacerbations. Occupational and Environmental Medicine 63(9): 580–590 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Habre R, Coull B, Moshier E, Godbold J, Grunin A, Nath A et al. : Sources of indoor air pollution in New York City residences of asthmatic children. Journal of Exposure Science and Environmental Epidemiology 24(3): 269 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Kopperud RJ, Ferro AR, and Hildemann LM: Outdoor versus indoor contributions to indoor particulate matter (PM) determined by mass balance methods. Journal of the Air & Waste Management Association 54(9): 1188–1196 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Abt E, Suh HH, Catalano P, and Koutrakis P: Relative contribution of outdoor and indoor particle sources to indoor concentrations. Environmental Science & Technology 34(17): 3579–3587 (2000). [Google Scholar]
- 9.Meng QY, Turpin BJ, Korn L, Weisel CP, Morandi M, Colome S et al. : Influence of ambient (outdoor) sources on residential indoor and personal PM 2.5 concentrations: analyses of RIOPA data. Journal of Exposure Science and Environmental Epidemiology 15(1): 17 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Klepeis NE, Nelson WC, Ott WR, Robinson JP, Tsang AM, Switzer P et al. : The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. Journal of Exposure Science and Environmental Epidemiology 11(3): 231 (2001). [DOI] [PubMed] [Google Scholar]
- 11.Pan L, Wu S, Li H, Xu J, Dong W, Shan J et al. : The short-term effects of indoor size-fractioned particulate matter and black carbon on cardiac autonomic function in COPD patients. Environment International 112: 261–268 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Dons E, Panis LI, Van Poppel M, Theunis J, and Wets G: Personal exposure to black carbon in transport microenvironments. Atmospheric Environment 55: 392–398 (2012). [Google Scholar]
- 13.Yan B, Kennedy D, Miller RL, Cowin JP, Jung K.-h., M. Perzanowski et al. : Validating a nondestructive optical method for apportioning colored particulate matter into black carbon and additional components. Atmospheric Environment 45(39): 7478–7486 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization: Health Effects of Black Carbon: The WHO European Centre for Environment and Health, Bonn, 2012. [Google Scholar]
- 15.Jennie Cox KI, Ryan Patrick, Grinshpun Sergey, Yermakov Michael, Desmond Colleen, Jandarov Roman, Vesper Stephen, Ross James, Chillrud Steven, Dannemiller Karen, Reponen Tiina: Effectiveness of a portable air cleaner in removing traffic related aerosol particles in homes of asthmatic children. Indoor Air (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LeMasters GK, Wilson K, Levin L, Biagini J, Ryan P, Lockey JE et al. : High prevalence of aeroallergen sensitization among infants of atopic parents. The Journal of Pediatrics 149(4): 505–511 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryan PH, LeMasters GK, Levin L, Burkle J, Biswas P, Hu S et al. : A land-use regression model for estimating microenvironmental diesel exposure given multiple addresses from birth through childhood. Science of the Total Environment 404(1): 139–147 (2008). [DOI] [PubMed] [Google Scholar]
- 18.Lowe LA, Woodcock A, Murray CS, Morris J, Simpson A, and Custovic A: Lung function at age 3 years: effect of pet ownership and exposure to indoor allergens. Archives of Pediatrics & Adolescent Medicine 158(10): 996–1001 (2004). [DOI] [PubMed] [Google Scholar]
- 19.Blanc P, Yen I, Chen H, Katz P, Earnest G, Balmes J et al. : Area-level socioeconomic status and health status among adults with asthma and rhinitis. European Respiratory Journal 27(1): 85–94 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Demissie K, Ernst P, Hanley JA, Locher U, Menzies D, and Becklake MR: Socioeconomic status and lung function among primary school children in Canada. American Journal of Respiratory and Critical Care Medicine 153(2): 719–723 (1996). [DOI] [PubMed] [Google Scholar]
- 21.Zhang T, Chillrud SN, Ji J, Chen Y, Pitiranggon M, Li W et al. : Comparison of PM2. 5 Exposure in Hazy and Non-Hazy Days in Nanjing, China. Aerosol and Air Quality Research 17(9): 2235–2246 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hornung RW, and Reed LD: Estimation of average concentration in the presence of nondetectable values. Applied Occupational and Environmental Hygiene 5(1): 46–51 (1990). [Google Scholar]
- 23.Haugland R, and Vesper S: Method of identifying and quantifying specific fungi and bacteria: Google Patents, 2002.
- 24.Vesper S, McKinstry C, Haugland R, Wymer L, Bradham K, Ashley P et al. : Development of an environmental relative moldiness index for US homes. Journal of Occupational and Environmental Medicine 49(8): 829–833 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Haugland RA, Brinkman N, and Vesper SJ: Evaluation of rapid DNA extraction methods for the quantitative detection of fungi using real-time PCR analysis. Journal of Microbiological Methods 50(3): 319–323 (2002). [DOI] [PubMed] [Google Scholar]
- 26.Crapo RO, Hankinson JL, Irvin C, MacIntyre NR, Voter K, Wise R et al. : Standardization of spirometry: 1994 update. American Journal of Respiratory and Critical Care Medicine 152(3): 1107–1136 (1995). [DOI] [PubMed] [Google Scholar]
- 27.Hankinson JL, Odencrantz JR, and Fedan KB: Spirometric reference values from a sample of the general US population. American Journal of Respiratory and Critical Care Medicine 159(1): 179–187 (1999). [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Dockery DW, Wypij D, Gold DR, Speizer FE, Ware JH et al. : Pulmonary function growth velocity in children 6 to 18 years of age. American Journal of Respiratory and Critical Care Medicine 148(6): 1502–1508 (1993). [DOI] [PubMed] [Google Scholar]
- 29.McKay R, and Lockey J: Pulmonary function testing: guidelines for medical surveillance and epidemiological studies. Occupational Medicine (Philadelphia, Pa.) 6(1): 43–57 (1991). [PubMed] [Google Scholar]
- 30.RStudio: Integrated Development for R. Boston, MA: RStudio, Inc., 2016. [Google Scholar]
- 31.Liu G, and Liang K-Y: Sample size calculations for studies with correlated observations. Biometrics: 937–947 (1997). [PubMed] [Google Scholar]
- 32.Diggle P, Diggle PJ, Heagerty P, Heagerty PJ, Liang K-Y, and Zeger S: Analysis of Longitudinal Data: Oxford University Press, 2002. [Google Scholar]
- 33.Urman R, McConnell R, Islam T, Avol EL, Lurmann FW, Vora H et al. : Associations of children’s lung function with ambient air pollution: joint effects of regional and near-roadway pollutants. Thorax: thoraxjnl-2012-203159 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee J-T, Son J-Y, and Cho Y-S: The adverse effects of fine particle air pollution on respiratory function in the elderly. Science of the Total Environment 385(1-3): 28–36 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Cakmak S, Dales R, Leech J, and Liu L: The influence of air pollution on cardiovascular and pulmonary function and exercise capacity: Canadian Health Measures Survey (CHMS). Environmental Research 111(8): 1309–1312 (2011). [DOI] [PubMed] [Google Scholar]
- 36.Korrick SA, Neas LM, Dockery DW, Gold DR, Allen GA, Hill LB et al. : Effects of ozone and other pollutants on the pulmonary function of adult hikers. Environmental Health Perspectives 106(2): 93 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Son J-Y, Bell ML, and Lee J-T: Individual exposure to air pollution and lung function in Korea: spatial analysis using multiple exposure approaches. Environmental Research 110(8): 739–749 (2010). [DOI] [PubMed] [Google Scholar]
- 38.Alexeeff SE, Litonjua AA, Suh H, Sparrow D, Vokonas PS, and Schwartz J: Ozone exposure and lung function: effect modified by obesity and airways hyperresponsiveness in the VA normative aging study. Chest 132(6): 1890–1897 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Chang Y-K, Wu C-C, Lee L-T, Lin RS, Yu Y-H, and Chen Y-C: The shortterm effects of air pollution on adolescent lung function in Taiwan. Chemosphere 87(1): 26–30 (2012). [DOI] [PubMed] [Google Scholar]
- 40.Schindler C, Kunzli N, Bongard J-P, Leuenberger P, Karrer W, Rapp R et al. : Short-term variation in air pollution and in average lung function among never-smokers: the Swiss Study on Air Pollution and Lung Diseases in Adults (SAPALDIA). American Journal of Respiratory and Critical Care Medicine 163(2): 356–361 (2001). [DOI] [PubMed] [Google Scholar]
- 41.Lagorio S, Forastiere F, Pistelli R, Iavarone I, Michelozzi P, Fano V et al. : Air pollution and lung function among susceptible adult subjects: a panel study. Environmental Health 5(1): 11 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lierl MB, and Hornung RW: Relationship of outdoor air quality to pediatric asthma exacerbations. Annals of Allergy, Asthma & Immunology 90(1): 28–33 (2003). [DOI] [PubMed] [Google Scholar]
- 43.McCormack MC, Breysse PN, Matsui EC, Hansel NN, Williams DA, Curtin-Brosnan J et al. : In-home particle concentrations and childhood asthma morbidity. Environmental Health Perspectives 117(2): 294–298 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delfino RJ, Quintana PJ, Floro J, Gastañaga VM, Samimi BS, Kleinman MT et al. : Association of FEV1 in asthmatic children with personal and microenvironmental exposure to airborne particulate matter. Environmental Health Perspectives 112(8): 932 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barreiro TJ, and Perillo I: An approach to interpreting spirometry. American family physician 69(5): 1107–1116 (2004). [PubMed] [Google Scholar]
- 46.Boutin B, Koskas M, Guillo H, Maingot L, La Rocca M-C, Boulé M et al. : Forced expiratory flows’ contribution to lung function interpretation in schoolchildren. European Respiratory Journal 45(1): 107–115 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Simon MR, Chinchilli VM, Phillips BR, Sorkness CA, Lemanske RF, Szefler SJ et al. : Forced expiratory flow between 25% and 75% of vital capacity and FEV1/forced vital capacity ratio in relation to clinical and physiological parameters in asthmatic children with normal FEV1 values. Journal of Allergy and Clinical Immunology 126(3): 527–534.e528 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neas LM, Dockery DW, Ware JH, Spengler JD, Ferris BG Jr, and Speizer FE: Concentration of indoor particulate matter as a determinant of respiratory health in children. American Journal of Epidemiology 139(11): 1088–1099 (1994). [DOI] [PubMed] [Google Scholar]
- 49.Trenga CA, Sullivan JH, Schildcrout JS, Shepherd KP, Shapiro GG, Liu L-JS et al. : Effect of particulate air pollution on lung function in adult and pediatric subjects in a Seattle panel study. Chest 129(6): 1614–1622 (2006). [DOI] [PubMed] [Google Scholar]
- 50.Guarnieri M, and Balmes JR: Outdoor air pollution and asthma. The Lancet 383(9928): 1581–1592 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jakeways N, McKeever T, Lewis S, Weiss S, and Britton J: Relationship between FEV1 reduction and respiratory symptoms in the general population. European Respiratory Journal 21(4): 658–663 (2003). [DOI] [PubMed] [Google Scholar]
- 52.Johnson JD, and Theurer WM: A stepwise approach to the interpretation of pulmonary function tests. Am Fam Physician 89(5): 359–366 (2014). [PubMed] [Google Scholar]
- 53.Habre R, Moshier E, Castro W, Nath A, Grunin A, Rohr A et al. : The effects of PM 2.5 and its components from indoor and outdoor sources on cough and wheeze symptoms in asthmatic children. Journal of Exposure Science and Environmental Epidemiology 24(4): 380 (2014). [DOI] [PubMed] [Google Scholar]
- 54.Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS et al. : Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1: the Lung Health Study. Jama 272(19): 1497–1505 (1994). [PubMed] [Google Scholar]
- 55.Karottki DG, Bekö G, Clausen G, Madsen AM, Andersen ZJ, Massling A et al. : Cardiovascular and lung function in relation to outdoor and indoor exposure to fine and ultrafine particulate matter in middle-aged subjects. Environment International 73: 372–381 (2014). [DOI] [PubMed] [Google Scholar]
- 56.Coombs KC, Chew GL, Schaffer C, Ryan PH, Brokamp C, Grinshpun SA et al. : Indoor air quality in green-renovated vs. non-green low-income homes of children living in a temperate region of US (Ohio). Science of the Total Environment 554: 178–185 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baxter LK, Clougherty JE, Laden F, and Levy JI: Predictors of concentrations of nitrogen dioxide, fine particulate matter, and particle constituents inside of lower socioeconomic status urban homes. Journal of Exposure Science and Environmental Epidemiology 17(5): 433 (2007). [DOI] [PubMed] [Google Scholar]
- 58.ULRIK CS, and Backer V: Markers of impaired growth of pulmonary function in children and adolescents. American Journal of Respiratory and Critical Care Medicine 160(1): 40–44 (1999). [DOI] [PubMed] [Google Scholar]
- 59.Brunst KJ, Ryan PH, Lockey JE, Bernstein DI, McKay RT, Khurana Hershey GK et al. : Unraveling the relationship between aeroallergen sensitization, gender, second-hand smoke exposure, and impaired lung function. Pediatric Allergy and Immunology 23(5): 479–487 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


