Abstract
This national survey sought to determine the practices and policies pertaining to opioid and opioid substitution therapy (OST) use in the selection of liver transplant (LT) candidates. Of 114 centers, 61 (53.5%) responded to the survey, representing 49.2% of the LT volume in 2016. Only two programs considered chronic opioid (1 [1.6%]) or OST use (1 [1.6%]) absolute contraindications to transplant, while 63.9% and 37.7% considered either one a relative contraindication, respectively. The majority of programs did not have a written policy regarding chronic opioid use (73.8%) or OST use (78.7%) in LT candidates. Nearly half (45.9%) of centers agreed that there should be a national consensus policy addressing opioid and OST use. The majority of responding LT centers did not consider opioid or OST use in LT candidates to be absolute contraindications to LT, but there was significant variability in center practices. These surveys also demonstrated a lack of written policies in the assessment of the candidacy of such patients. The results of our survey identify an opportunity to develop a national consensus statement regarding opioid and OST use in LT candidates to bring greater uniformity and equity into the selection of LT candidates.
Keywords: liver transplantation, opioid analgesics, opioid substitution therapy, patient selection
1. INTRODUCTION
Significant resources have been allocated to combat the surging opioid epidemic and opioid- related deaths in the United States (US).1–3 The opioid epidemic is highly relevant to patients with cirrhosis and liver transplant (LT) candidates, as up to 79% of patients with cirrhosis and 77% of LT candidates report pain as a symptom.4,5 Prescription opioids are often used for this pain given the concerns for adverse effects from other analgesic medications in the setting of chronic liver disease.4–6 In addition to using opioids for chronic pain, persons who inject drugs, including opioids, have a high prevalence of hepatitis C infection, one of the most common indications for LT in the US.7,8 Opioid substitution therapy (OST), including methadone and buprenorphine, is a highly effective treatment for opioid use disorder, and many patients continue on OST maintenance for years or indefinitely due to concerns for relapse.9
Notably, pre- transplant prescription opioid use has been associated with an increased risk of complications, graft loss, and death after kidney transplantation.10,11 There is also evidence of hepatotoxicity and increased risk of liver graft rejection in animal models of opioid use,12,13 and a recent study demonstrated an association between high doses of opioid use and post- transplant mortality in LT recipients.14 This study also demonstrated an increasing prevalence of opioid prescriptions among LT recipients.14 However, the risks and benefits of opioids and OST in liver transplantation have not been fully explored, particularly for those patients taking lower doses of opioids. Moreover, the current practices and policies regarding these medications remain unknown.
The goal of evaluating LT candidates is to identify patients with acceptable perioperative and long- term complication risks who are most likely to receive a mortality benefit from the procedure. How prescription opioid and OST use impact transplant decisions remain unclear. Given the rising prevalence of opioids and OST use among patients with cirrhosis and the ongoing controversy regarding the role of these medications in patient selection for transplantation, we sought to characterize the practices and policies pertaining to opioid and OST use in the selection of candidates for LT among active transplant centers in the US.
2. EXPERIMENTAL PROCEDURES
2.1. Survey design
A working group of LT practitioners was assembled from the American Society of Transplantation (AST) Liver and Intestinal Community of Practice (LICOP). This working group developed a survey to assess center practices and policies surrounding opioid and OST use in relation to LT candidacy. Specifically, the following four objectives for this survey were established:
To identify the most common screening methods for the use of opioids and OST in LT candidates among LT centers.
To determine center practices regarding opioid and OST use in the LT selection process.
To evaluate the prevalence of formal center policies regarding opioid and OST use in LT candidates.
To assess stakeholder interest in a national consensus policy addressing opioid and OST use in LT candidates.
The survey was pilot- tested by members of the AST LICOP Education Subcommittee and revisions were made based on the respondents’ comments. The final survey was approved by the AST LICOP Executive Committee.
The survey was built with REDCap (Research Electronic Data Capture) software, which utilizes a web- based interface. The final survey included 12 questions and was designed with branch logic questioning, so that respondents were not required to answer questions that did not apply based on previous answers (Supporting information). Due to this, the number of responses for branch logic questions was expected to vary. The survey was approved by the IRBs at the Medical University of South Carolina and the University of Pittsburgh.
2.2. Survey participants and dissemination
The survey was sent electronically to physicians at all active U.S. LT centers using the United Network for Organ Sharing (UNOS) database on two separate occasions, 2 weeks apart. The vast majority of physicians contacted were medical directors of the LT program, with the minority being listed as staff transplant hepatologists or surgical directors. Subsequently, the survey was sent to the transplant pharmacists at each non- responding center twice, separated by 2 weeks, using listservs for the American College of Clinical Pharmacy Transplant and Immunology Practice and Research Network and the AST Community of Practice of Transplant Pharmacists. Finally, a member of the study team directly solicited personal contacts at remaining non- responding centers. The majority of these contacts were transplant hepatologists. The survey was open to responses from 21 October 2016 to 1 February 2017.
2.3. Data analysis
Data were exported from the REDCap system, and statistical analyses were performed using SPSS Statistics (IBM SPSS Statistics for Windows, Version 24.0; IBM Corp., Armonk, NY, USA). When multiple responses were provided from a single transplant center (n = 2), any inconsistencies between responses were addressed by emailing respondents to gain consensus responses.
Response frequencies were tabulated. High- volume (50 or more transplants in 2016) transplant center responses were compared to low- volume centers (less than 50 transplants in 2016). Dichotomous data were analyzed using Pearson’s chi-square or Fisher’s exact, where appropriate. Continuous data were analyzed using the Mann- Whitney U test.
3. RESULTS
There were 63 respondents, with two centers having multiple responses (2 responses each); both responses were merged into one consensus response for each of the two centers after any differences in responses to opioid and OST requirements were clarified using follow- up emails. There were 61 of 114 (53.5%) complete center- specific responses to the survey. These centers represented 49.2% (3856/7841) of the total LT volume in 2016.15 Respondents included transplant hepatologists (62.3%), transplant pharmacists (31.1%), transplant coordinators (3.3%), a transplant social worker (1.6%), and a transplant psychiatrist (1.6%).
3.1. Screening for opioid use
The vast majority of centers screened for opioid use by self- report (96.7%), toxicology screens (88.5%), and medical records review (85.2%). Less than half (40.9%) used a state prescription drug monitoring registry database for opioid use.
3.2. Practices regarding prescription opioid use
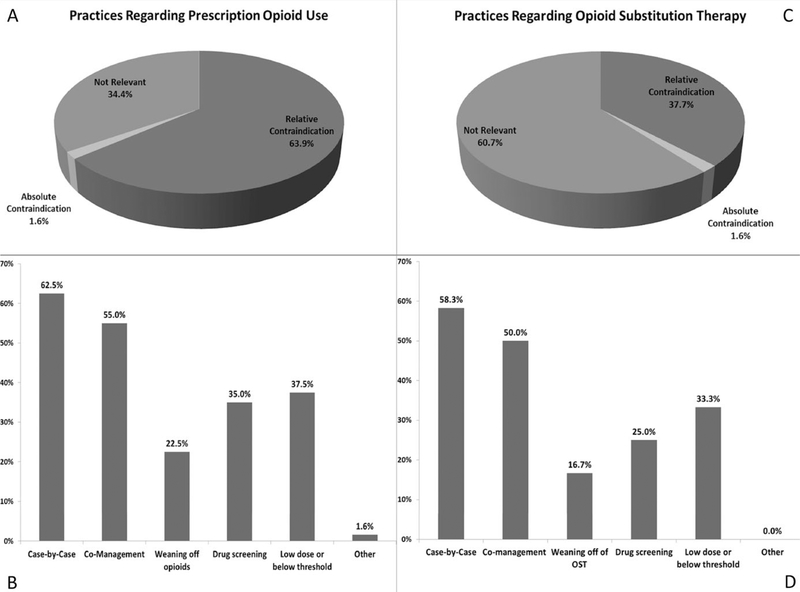
Only one center (1.6%) considered chronic opioid use to be an absolute contraindication to transplant. Most centers considered chronic opioid use a relative contraindication to transplant (63.9%), while 34.4% did not consider chronic opioid use as relevant to patient selection. Numerically, more large- volume centers indicated that they did not consider chronic opioid use to be relevant to patient selection than small- volume centers (42% vs 25%, P = 0.15, Table 3).
Table 3.
Comparison of survey responses in small- volume (<50 LT/year) and large- volume (50+ LT/year) programsa
| Small- volume (n = 28) |
Large- volume (n = 33) |
P-v alue | |
|---|---|---|---|
| Median number of transplants in 2016, median (range) |
28 (7, 49) | 84 (53, 159) | <0.001 |
| Screening methodsa | |||
| Self- report | 28 (100) | 31 (94) | 0.50 |
| Toxicology screens | 28 (100) | 26 (79) | 0.01 |
| Medical records | 26 (93) | 26 (79) | 0.16 |
| State registry | 11 (39) | 14 (42) | 0.80 |
| Other | 0 (0) | 1 (3) | 1.00 |
| Opioid use selection implicationsa | |||
| Relative contraindication | 20 (71) | 19 (56) | 0.26 |
| Absolute contraindication | 1 (4) | 0 (0) | 0.46 |
| Not relevant | 7 (25) | 14 (42) | 0.15 |
| Opioid requirements for eligibilitya | |||
| Case- by- case | 12 (57) | 13 (68) | 0.46 |
| Co- management with specialty service | 12 (57) | 10 (53) | 0.78 |
| Weaning off for period of time | 3 (14) | 6 (32) | 0.27 |
| Drug screening | 6 (29) | 8 (42) | 0.37 |
| Doses below certain threshold | 10 (48) | 5 (26) | 0.17 |
| Other | 1 (5) | 0 (0) | 1.00 |
| Written policy on opioids in candidatesa | |||
| Yes | 5 (18) | 4 (12) | 0.72 |
| No | 23 (82) | 22 (67) | 0.17 |
| Unsure | 0 (0) | 7 (21) | 0.01 |
| OST selection implicationsa | |||
| Relative contraindication | 13 (46) | 10 (30) | 0.20 |
| Absolute contraindication | 0 (0) | 1 (3) | 1.00 |
| Not relevant | 15 (54) | 22 (67) | 0.30 |
| OST requirements for eligibilitya | |||
| Case- by- case | 9 (69) | 5 (46) | 0.41 |
| Co- management with specialty service | 6 (46) | 6 (55) | 0.68 |
| Weaning off for period of time | 2 (15) | 2 (18) | 1.00 |
| Drug screening | 3 (23) | 3 (27) | 1.00 |
| Doses below certain threshold | 5 (39) | 3 (27) | 0.68 |
| Other | 0 (0) | 0 (0) | 1.00 |
| Written policy on OST in candidatesa | |||
| Yes | 1 (4) | 5 (15) | 0.21 |
| No | 25 (89) | 23 (70) | 0.06 |
| Unsure | 2 (7) | 5 (15) | 0.44 |
| Need national/central consensus policy?a | |||
| Yes | 12 (43) | 16 (49) | 0.66 |
| No | 12 (43) | 11 (33) | 0.44 |
| Unsure | 4 (14) | 6 (18) | 0.74 |
n (%). OST, opioid substitution therapy.
The bold values represent statistically significant P-values.
Thirty- nine of the centers considered chronic opioid use to be a relative or absolute contraindication to transplant and so had an opportunity to describe center requirements for candidacy. Of these centers, 62.5% did not have specific dosing and prescription management requirements for transplant, but considered each case independently in conjunction with other factors. Co- management with a specialty service (psychiatry/addiction medicine/pain management) was required by 55% (22 of 39) of centers, and 22.5% (9 of 39) required weaning off of prescription opioids for a certain period of time. Thirty- five percent (14 of 39) of centers performed surveillance drug screening in this population, and 37.5% (15 of 39) required that the opioid doses be below a certain threshold (Figure 1). The center that considered opioid use to be an absolute contraindication to LT, a small- volume center based on our definition above, indicated that patients could be transitioned to methadone or buprenorphine/naloxone to be eligible for transplant.
FIGURE 1.
Program practices regarding the relevancy of opioid use to selection is presented (A), as well as specific requirements by programs that indicated that opioid use was a relative or absolute contraindication to transplant (B). In (C), program practices regarding the relevancy of opioid substitution therapy use to selection is presented, followed by specific requirements by programs that indicated opioid substitution therapy was a relative or absolute contraindication to transplant (D)
The majority of transplant centers did not have a written policy on monitoring and management of chronic opioids use for pain in LT candidates (73.8%), while 15% did and 11% were unsure if such a policy existed. Specifically reported written policies are detailed in Table 1.
Table 1.
Reported written policies on opioid use in liver transplant candidates from survey respondents
| The opiates must be prescribed by addiction specialist or pain management. No street opiates are permitted. It must be a stable, non-escalating dose. We encourage, but do not require, a wean |
| Chronic opioids are acceptable provided that they're managed by the pain management service and are not abusing |
| The daily dose of the opioid must be <50 mg of methadone equivalent |
| Benzodiazepines and narcotics usage: one infraction after listing will be managed by a conversation with the patient to determine the cause of the drug use. If it is determined to be only temporary usage for an acceptable reason, then no further action is necessary. If the use is determined to be inappropriate, the patient will be made status 7 on the Unet transplant waiting list. The patient will need to establish drug and alcohol rehabilitation and/or counseling with negative random drug screens for a period of 3 months. Once the patient has completed this regimen, they are to be discussed by the liver selection committee to determine if they are eligible to be changed back to MELD/PELD status on the Unet transplant waiting list. A second infraction will remove patient from the waiting list for an indefinite period of time |
| If there is a history of opioid restriction, they must be evaluated by the substance abuse team. The total dose must be under the opioid equivalent of oxycodone 30 and they must undergo routine toxicology testing |
| All chronic opioid users will undergo a case-by-case assessment |
| Patients on chronic opioids must be followed in pain clinic with narcotics prescribed by only one physician |
| They cannot be taking chronic opioids unless there is a documented reason that “seems reasonable.” For example, if there is known cervical stenosis on MRI spine or chronic hemarthroses |
| After assessment by a licensed alcohol/drug counselor, as well as a transplant psychiatrist, individual recommendations will be made regarding the need to discontinue or decrease the use of opiate pain medications |
3.3. Practices regarding OST
Most centers did not consider OST use to be relevant to selection (60.7%), although 37.7% and 1.6% considered it to be a relative or absolute contraindication, respectively. Of the centers that considered OST use to be a relative or absolute contraindication to transplant (n = 24), most (58.3%) did not have specific dosing and prescription management requirements for transplant, but considered each potential candidate on a case- by- case basis in conjunction with other factors. Some requirements of these centers included co- management with psychiatry/addiction medicine/pain management in 50% (12 of 24), weaning off of OST for a certain period of time in 16.7% (4 of 24), drug screening in 25% (6 of 24), and lowering below a certain threshold in 33.3% (8 of 24) of centers (Figure 1)
The majority of transplant centers did not have a written policy on OST use for non- pain indications in LT candidates (78.7%), while 9.8% did, and 11.5% of respondents were unsure if a policy existed. Specifically reported written policies are detailed in Table 2.
Table 2.
Reported written policies on opioid substitution therapy use in liver transplant candidates from survey respondents
| Patients must provide information about their methadone maintenance, including contact information for the prescribing provider. This information will be vetted by social work and assessed for compliance |
| Patients must be on a stable dose and addiction specialists/pain management must be involved |
| OST is acceptable provided that they are managed by a specialty management service and are not abusing. Such patients will also receive extra scrutiny in the psychosocial evaluation |
| The daily dose of the opioid must be <50 mg of methadone equivalent |
| Patients can be taking methadone as part of a methadone maintenance program if they have been on a stable or lower doses of methadone for >2 years |
| If the OST use is longstanding and stable, it is acceptable. Prescribing must be performed in a relationship with a drug treatment facility or provider, irrelevant to listing |
OST, opioid substitution therapy.
3.4. Opinion on national consensus policy
Of the centers responding to the survey, 45.9% of centers believed that there should be a national or central consensus policy on opioid and OST use in LT candidates, while 37.7% believed this was unnecessary and 16.4% were not sure if such a policy should exist.
3.5. Comparison of small- volume and large- volume programs
The majority of the responses were similar between small- and large- volume LT centers, but significantly more large- volume centers respondents were unsure if a written policy on chronic opioid use in LT candidates existed (21% vs 0%, P = 0.01). Significantly more small- volume centers reported using toxicology screens to assess current use of opioids in LT candidates than large- volume centers (100% vs 79%, P = 0.01) (Table 3).
4. DISCUSSION
We report the results of the first comprehensive survey addressing the center- specific practices and policies regarding opioid and OST use surrounding the candidacy for LT. Our data indicate that there is significant center variability in practices and policies regarding opioid and OST use in transplant candidates.
Only two of the 61 centers considered either opioid or OST use to be an absolute contraindication to transplant (1 center for opioid and 1 center for OST). Over one-third of centers did not see prescription opioid use as relevant to selection at all. Similarly, the majority of programs did not consider OST use as relevant to selection.
Of the programs that considered opioid and OST use to be relevant to selection, approximately half required co-management of the opioids by a specialty service, such as pain management, transplant psychiatry or addiction medicine. This practice is somewhat lower than expected, considering the multidisciplinary approach in the transplant candidacy selection process in general and the recommendations for active roles of specialists in the management of post- transplant recipients with opioid use in published studies.10,11,14 Increased utilization of specialty services has the potential to reduce the risk of post- transplant complications associated with opioids.
Approximately 75% of centers reported that they did not have written policies on opioid or OST use. The lack of written policies in the s election process can contribute to inefficient and inconsistent decisions on candidacy for transplant, and has previously been identified as a recommendation for process improvement in LT evaluation.16 In a multicenter study that included observation of LT s election committees and interviews with members, Volk and colleagues identified psychosocial barriers as the most contentious and difficult topics addressed. Decisions were frequently unable to be clarified with written policies, and instead relied on judgment-based reasoning, leading to criticisms of the process in regard to inefficiency and inconsistency.16 With almost half of respondents indicating a desire for a national consensus policy, there appears to be a need for guidance regarding opioid and OST use.
The survey findings should be considered in the context of the limited available data regarding opioid use and transplant outcomes.14,17 These data suggest that the group of patients on the highest doses of pre-transplant opioids may represent a group at risk for deleterious transplant outcomes. However, it is unclear if this risk can be modified and whether the risk is mainly related to direct toxicity of the opioids or, more likely, the conditions leading to opioid use.
Methadone maintenance treatment (MMT) has also been subject to very limited investigation in the context of LT.18–20 These case series and small cohort study demonstrated the potential success of LT in patients on MMT. These publications and rising public awareness of the effectiveness of OST for addiction treatment may have contributed to a change in perception of patients taking OST that was evident in our survey, as compared to a similar survey about MMT in 2001. In this prior survey, only about half (56%) of the responding programs accepted MMT patients for their LT program, but 32% required that patients discontinue methadone prior to transplant.21 Our survey shows a significant increase in the willingness to accept patients taking OST, with only 1.6% of programs considering OST use to be an absolute contraindication to LT. Further, the proportion of programs that require weaning off of OST has dropped considerably, with only 16.7% of programs requiring it in the current era. This is potentially due to shifting national policy and the increasing acceptance of OST use as a maintenance therapy to prevent relapse into intravenous or prescription drug misuse and abuse.9,22 Studies have demonstrated that the risk of relapse decreases with each year of OST treatment, and patients that taper off of OST are at a higher risk of relapse compared to chronic maintenance therapy.23,24 Additionally, the 2013 AASLD Practice Guideline for Evaluation for Liver Transplantation recommends that “methadone- maintained patients should not be denied transplantation based on methadone use alone, and expectations of methadone reduction or discontinuation should not be a requirement for transplant listing (1- B).”25 It is interesting that one-third of centers in our survey required that doses of OST be below a certain threshold, which contrasts with available knowledge on the need for dose individualization and increased effectiveness of higher doses of OSTs.26 Overall, the lack of consistent practice, despite scientific evidence and AASLD-based practice guidelines, illustrates the need for improved implementation of evidence- based practices across transplant programs.25,26
Our study adds to the existing literature on center practices that has previously focused only on “addictive substances” or methadone maintenance therapy.21,27 However, more work is required in this area to fully understand the role of opioid use and OST in transplantation and develop consensus policies. The results of our comprehensive assessment of center practices and policies surrounding opioid and OST demonstrates variability in practices. Opportunities to improve our understanding of the long- term impacts of opioid use and OST on outcomes of LT recipients, including further clarification of the subpopulation at greatest risk, may provide centers with more evidence to support consensus practices. There is a high prevalence of hepatitis C infection,28 depression, and other psychiatric disorders in patients on opioids and OST,29,30 and the impact of these and other comorbidities on the outcomes of these patients must be explored. In looking toward creating policies, it is also critical to determine whether it is appropriate to impose a threshold dose beyond which transplant is contraindicated, as several programs set such a threshold. In addition, the optimal means for screening for opioids and OST should be further investigated. The majority of transplant programs in our survey utilize unreliable screening methods, such as self- report and medical records reviews.31,32 There also is a large reliance on toxicology screens, which have limitations based on the timing between exposure and test and cross- reactivity of the ingested opioid’s metabolites and the target of the urinary drug test.33 Substantially fewer programs rely on state prescription drug monitoring programs, which may be the most accurate method of screening for prescriptions, and are operational in 49 of 50 states.34 Any policy put forth should consider recommending the use of these programs to comprehensively and objectively assess dosing of prescription opioids.
We acknowledge several limitations of this study, largely related to the survey- based methodology and sample size. This study includes a sample of 53% of all LT centers identified within UNOS, representing both large- and small- volume centers equally. However, it may still underrepresent small- volume centers given that there are more small than large centers.35 It is also possible that the centers that completed the surveys are those who are the most tolerant of opioid and OST use in LT candidates. Despite the limitations of sampling, the response rate to this survey was still better than the typical response rates for clinicians, which range from 27% to 54%, depending on the study, with a mean response rate of 35% to online surveys.36,37 Finally, surveys are limited by the accuracy of the respondents, as there was no validation of the answers provided. Many respondents reported a lack of knowledge about existence of policies. Despite these limitations, this survey represents the most comprehensive assessment of center practices and policies regarding opioid and OST use in LT candidate selection to date.
This study highlights the tolerance of opioid and OST use in LT candidates in the majority of the LT centers that responded to the survey, and demonstrates the variability in practices and lack of written policies in the assessment of the candidacy of such patients. The absence of written policies raises the possibility of significant variability in individual candidate selection even within a single center. Our data highlight an opportunity to develop a national consensus statement on opioid and OST use in LT candidates to bring greater uniformity and equity into selection of potential LT candidates.
Supplementary Material
ACKNOWLEDGEMENTS
This manuscript is a work product of the American Society of Transplantation’s Liver and Intestinal Community of Practice. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Footnotes
CONFLICT OF INTERESTS
No authors have any conflict of interests related to this manuscript.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
REFERENCES
- 1.Warner M, Trinidad JP, Bastian BA, Minino AM, Hedegaard H. Drugs most frequently involved in drug overdose deaths: United States, 2010–2014. Natl Vital Stat Rep. 2016;65:1–15. [PubMed] [Google Scholar]
- 2.Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372:241–248. [DOI] [PubMed] [Google Scholar]
- 3.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1–49. [DOI] [PubMed] [Google Scholar]
- 4.Rogal SS, Bielefeldt K, Wasan AD, et al. Inflammation, psychiatric symptoms, and opioid use are associated with pain and disability in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13:1009–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madan A, Barth KS, Balliet WE, et al. Chronic pain among liver trans-plant candidates. Prog Transplant. 2012;22:379–384. [DOI] [PubMed] [Google Scholar]
- 6.Chandok N, Watt KD. Pain management in the cirrhotic patient: the clinical challenge. Mayo Clin Proc. 2010;85:451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amon JJ, Garfein RS, Ahdieh-Grant L, et al. Prevalence of hepatitis C virus infection among injection drug users in the United States, 1994– 2004. Clin Infect Dis. 2008;46:1852–1858. [DOI] [PubMed] [Google Scholar]
- 8.Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2015 Annual Data Report: liver. Am J Transplant. 2017;17(s1):174–251. [DOI] [PubMed] [Google Scholar]
- 9.Schuckit MA. Treatment of opioid- use disorders. N Engl J Med. 2016;375:357–368. [DOI] [PubMed] [Google Scholar]
- 10.Lentine KL, Yuan H, Tuttle-Newhall JE, et al. Quantifying prognostic impact of prescription opioid use before kidney transplantation through linked registry and pharmaceutical claims data. Transplantation. 2015;99:187–196. [DOI] [PubMed] [Google Scholar]
- 11.Lentine KL, Lam NN, Xiao H, et al. Association of pre- transplant prescription narcotic use with clinical complications after kidney transplantation. Am J Nephrol. 2015;41:165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bekheet SH. Morphine sulfate induced histopathological and histo-chemical changes in the rat liver. Tissue Cell. 2010;42:266–272. [DOI] [PubMed] [Google Scholar]
- 13.Rafati A, Taj SH, Azarpira N, Zarifkar A, Noorafshan A, Najafizadeh P. Chronic morphine consumption increase allograft rejection rate in rat through inflammatory reactions. Iran Biomed J. 2011;15:85–91. [PMC free article] [PubMed] [Google Scholar]
- 14.Randall HR, Alhamad T, Schnitzler MA, et al. Survival implications of opioid use before and after liver transplantation. Liver Transpl. 2016;23:305–314. [DOI] [PubMed] [Google Scholar]
- 15.Scientific Registry of Transplant Recipients. Program-specific reports. https://www.srtr.org/reports-tools/program-specific-reports/. Accessed January 18, 2017. [DOI] [PubMed]
- 16.Volk ML, Biggins SW, Huang MA, Argo CK, Fontana RJ, Anspach RR. Decision making in liver transplant selection committees. Ann Intern Med. 2011;155:503–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rogal S, Mankaney G, Udawatta V, et al. Association between opioid use and readmission following liver transplantation. Clin Transplant. 2016;30:1222–1229. [DOI] [PubMed] [Google Scholar]
- 18.Kanchana TP, Kaul V, Mazarbeitia C, et al. Liver transplant for patients on methadone maintenance. Liver Transpl. 2002;8:778–782. [DOI] [PubMed] [Google Scholar]
- 19.Liu LU, Schiano TD, Lau N, et al. Survival and risk of recidivism in methadone- dependent patients undergoing liver transplantation. Am J Transplant. 2003;3:1273–1277. [DOI] [PubMed] [Google Scholar]
- 20.Weinrieb RM, Barnett R, Lynch KG, DePiano M, Atanda A, Olthoff KM. A matched comparison study of medical and psychiatric complications and anesthesia and analgesia requirements in methadone- maintained liver transplant recipients. Liver Transpl. 2004;10:97–106. [DOI] [PubMed] [Google Scholar]
- 21.Koch M, Banys P. Liver transplantation and opioid dependence. JAMA. 2001;285:1056–1058. [DOI] [PubMed] [Google Scholar]
- 22.Nielsen S, Larance B, Degenhardt L, Gowing L, Kehler C, Lintzeris N. Opioid agonist treatment for pharmaceutical opioid dependent people. Cochrane Database Syst Rev. 2016;5:CD011117. [DOI] [PubMed] [Google Scholar]
- 23.Clark RE, Baxter JD, Aweh G, O’Connell E, Fisher WH, Barton BA. Risk factors for relapse and higher costs among Medicaid members with opioid dependence or abuse: opioid agonists, comorbidities, and treatment history. J Subst Abuse Treat. 2015;57:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiellin DA, Schottenfeld FS, Cutter CJ, Moore BA, Barry DT, O’Connor PG. Primary care- based buprenorphine taper vs maintenance therapy for prescription opioid dependence: a randomized clinical trial. JAMA Intern Med. 2014;174:1947–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin P, DiMartini A, Feng S, Brown R, Fallon M. Evaluation for liver transplantation in adults: 2013 Practice Guideline by the AASLD and American Society of Transplantation. https://www.aasld.org/publications/practice-guidelines-0. Accessed August 7, 2017. [DOI] [PubMed]
- 26.Farre M, Mas A, Torrens M, Moreno V, Cami J. Retention rate and il-licit opioid use during methadone maintenance interventions: a meta- analysis. Drug Alcohol Depend. 2002;65:283–290. [DOI] [PubMed] [Google Scholar]
- 27.Levenson JL, Olbrisch ME. Psychosocial evaluation of organ transplant candidates: a comparative survey of process, criteria, and outcomes in heart, liver, and kidney transplantation. Psychosomatics. 1993;34:314–323. [DOI] [PubMed] [Google Scholar]
- 28.Weaver MF, Cropsey KL, Fox SA. HCV prevalence in methadone maintenance: a self- report versus serum test. Am J Health Behav. 2005;29:387–394. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z, Du J, Zhao M, Page K, Xiao Z, Mandel JS. Hepatitis C virus infection is independently associated with depression among methadone maintenance treatment heroin users in China. Asia Pac Psychiatry. 2013;5:191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Batki SL, Canfield KM, Ploutz-Snyder R. Psychiatric and substance abuse disorders among methadone maintenance patients with chronic hepatitis C infection: effects on eligibility for hepatitis C treatment. Am J Addict. 2011;20:312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monte AA, Anderson P, Hoppe JA, Weinshilboum RM, Vasiliou V, Heard KJ. Accuracy of electronic medical record medication reconciliation in emergency department patients. J Emerg Med. 2015;49: 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reedy AB, Yeh JY, Nowacki AS, Hickner J. Patient, physician, medical assistant, and office visit factors associated with medication list agreement. J Patient Saf. 2016;12:18–24. [DOI] [PubMed] [Google Scholar]
- 33.Reisfield GM, Salazar E, Bertholf RL. Rational use and interpretation of urine drug testing in chronic opioid therapy. Ann Clin Lab Sci. 2007;37:301–314. [PubMed] [Google Scholar]
- 34.National Alliance for Model State Drug Laws. http://www.namsdl.org/library/CAE654BF-BBEA-211E-694C755E16C2DD21/. Accessed February 18, 2017.
- 35.Reese PP, Yeh H, Thomasson AM, Shults J, Markmann JF. Transplant center volume and outcomes after liver re- transplantation. Am J Transplant. 2009;9:309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flanigan TS, McFarlane E, Cook S. Conducting survey research among physicians and other medical professionals – a review of current literature. 2008 AAPOR: Section on Survey Research Methods. ww2.amstat.org/sections/srms/Proceedings/y2008/Files/flanigan.pdf. Accessed August 14, 2017. [Google Scholar]
- 37.Cunningham CT, Quan H, Hemmelgarn B, et al. Exploring physician specialist response rates to web- based surveys. BMC Med Res Methodol. 2015;15:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.