Abstract
Objective:
The relationships between frailty and body composition in older adults with HIV infection are poorly understood. We sought to describe associations between frailty and measures of body composition among adult men with HIV and without HIV
Design/Methods:
Men with and without HIV (age 50–69 years) in the Multicenter AIDS Cohort Study (MACS) Bone Strength Substudy were included if evaluated for frailty (by Fried phenotype) and body composition (body mass index [BMI], waist circumference [WC], abdominal visceral [VAT] and subcutaneous [SAT] adipose tissue, sarcopenia, and osteopenia/osteoporosis). All participants with HIV infection were on antiretroviral therapy. Multivariate multinomial logistic regression models were used to determine associations of frailty with body composition.
Results:
A total of 399 men, including 199 men with HIV and 200 men without HIV, both with median age 60 years, comprised our study population. Frailty prevalence was 16.0% (men with HIV) vs 8.0% (men without HIV). HIV serostatus was associated with a 2.43 times higher odds of frailty (p=0.01). Higher WC, VAT, sarcopenia, and femoral neck osteoporosis were associated with increased odds of frailty (aOR 4.18, 4.45, 4.15, and 13.6, respectively and all p <0.05); BMI and SAT were not. None of these measures presented a differential association with frailty by HIV serostatus (all p>0.20).
Conclusion:
Higher abdominal obesity and sarcopenia were associated with frailty among men with and without HIV. Assessment of these body composition parameters may help detect frailty in the clinical setting.
Keywords: visceral adipose tissue, HIV, aging, obesity, frailty, sarcopenia
Background:
Frailty is a clinical entity that is easy to recognize but difficult to define. It represents a vulnerability to adverse health outcomes and is the result of factors contributing to poor health and natural aging. Similarities between frailty and HIV infection have been observed since early in the HIV epidemic [1]. Prior to the use of effective antiretroviral therapy (ART), men with HIV in the Multicenter AIDS Cohort Study (MACS) were nine times more likely to have a frailty-related phenotype than men without HIV (13.9% vs 1.5% prevalence) [2]. Despite ART-induced viral suppression, adults with HIV infection continue to have higher rates of frailty than matched HIV-uninfected populations [3–8]. Furthermore, even in the presence of HIV viral suppression, chronic co-morbid illnesses (neurocognitive impairment, depression, co-infection with other viruses) and lifestyle factors common in people with HIV infection (e.g., smoking) are strongly associated with and likely contribute to the development of frailty [3, 8–10].
Since the advent of effective ART, the life expectancy of people with HIV has been extended dramatically [11]. However, the combination of aging, ART toxicities, and lifestyle, among other factors, has contributed to an increasing prevalence of obesity, declining skeletal muscle mass, and a high prevalence of osteopenia or osteoporosis in aging adults with HIV infection [12]. A recent AIDS Clinical Trials Group (ACTG) long-term study that followed persons up to seven years post-ART initiation found that, compared to people without HIV, those with HIV gained more trunk fat and lost more lean mass and more bone mineral density (BMD) at the lumbar spine [13, 14]. Another study reported that obese adults with HIV had a higher trunk-to-appendicular fat ratio (1.58 versus 1.32; p=0.05) and greater visceral fat mass (1.97 versus 1.60 kg, p=0.04) compared to HIV-uninfected with comparable body mass index (BMI) [15].
Associations between frailty and greater BMI, lower muscle mass, and BMD have been described among adults without HIV, and may contribute to pathogenesis or clinical manifestations of frailty [16–18]. Among people living with HIV, frailty has been associated with higher BMI, greater total fat mass, and trunk fat [19]. In other studies, frailty has been associated with low lean mass, low BMD [20], higher waist-to-hip ratio but not waist circumference (WC) [15], and obesity [21]. Whether the relationship between body composition and frailty differs in persons with HIV, among whom lipodystrophy, wasting, and osteoporosis are common, is not known.
We sought to determine associations between frailty and a diverse panel of measures of body composition that each capture and summarize different aspects of body composition among adult men with HIV and a comparison group of demographically similar men without HIV. These included summarized measures of overall adiposity (BMI), a clinical measure of abdominal obesity (WC), specific regions of adiposity (subcutaneous adipose tissue [SAT] and visceral adipose tissue [VAT]), presence of “sarcopenia” based upon lean mass-derived appendicular skeletal muscle index (ASMI), and lastly bone density by the presence of osteoporosis or osteopenia in the lumbar spine and femoral neck. We hypothesized that greater VAT and lower lean mass would be associated with frailty.
Methods:
Study Population
Our study population comprised men with and without HIV who participated in the MACS, and specifically within the Bone Strength Substudy (BOSS). The MACS is an ongoing study of men with or at risk for HIV infection at four sites in the United States: Los Angeles, CA; Pittsburgh, PA; Washington D.C/Baltimore, MD; and Chicago, IL. MACS participants return semi-annually for a standardized interview, clinical evaluation and laboratory tests. The BOSS Substudy included MACS participants aged 50–69 years and was initiated to investigate the contribution of aging, chronic HIV infection, and ART use on both skeletal and non-skeletal risk factors for fractures. BOSS participants underwent dual-energy X-ray absorptiometry (DXA), quantitative computed tomography (CT), and frailty phenotyping at enrollment into BOSS. MACS participants were excluded from BOSS if there was a history of bisphosphonate, denosumab, or teriparatide use or if they weighed >300lbs or had a BMI >35kg/m2. All men with HIV were on ART. Participants were included in the present analysis if they had completed a DXA, quantitative CT, and frailty phenotyping within 12 months of one another. The study period began in September 2012 and ended in April 2015. The institutional review boards at each site approved the study, and each participant provided written informed consent.
Outcomes
Frailty was assessed using the Fried frailty phenotype [8] [22]. Unintentional weight loss was present if the participant answered “yes” to the question, “since your last visit, have you had an unintentional weight loss of at least ten pounds?”. Exhaustion was present if the participant answered “yes” to the question “during the past 4 weeks, as a result of your physical heath, have you had difficulty performing work or other activities (for example, it took extra effort)?”. Slowness was assessed by gait speed during a 4m walk test based on a study specific cut-off of the lowest 20th percentile among men without HIV: a speed of <1.03m/s for men ≤ 178cm tall and <1.06m/s for those >178cm tall met criteria for slowness. Low physical activity was present if the participant answered “yes, limited a lot” to the question “does your health limit you in vigorous activities, such as running, lifting heavy objects, participating in strenuous sports?”. Weakness was assessed by grip strength on a hand-held dynamometer, and defined by a study specific cut-off of the lowest 20th percentile among men without HIV with a grip strength of less than 32.7kg. Each component was given a score of “1” if present, and frailty was defined as a composite score of ≥3, pre-frail as a composite score of 1–2, and non-frail (or robust) as a composite score of 0. These procedures were standardized across all study sites.
Body Composition Measures
BMI was determined by dividing the body weight (kg) by height squared (m2). WC was measured in centimeters with participants in the standing position according to previously validated methods [23]. Quantitative CT using a single slice CT scan at the L4–L5 region was used to measure VAT and SAT area by methods previously described [24]. Whole body and site-specific (femoral neck and lumbar spine) dual energy X-ray absorptiometry (DXA) were used to assess lean mass and BMD, respectively. DXA scans were completed on a Lunar Prodigy (GE Medical Systems, Madison, WI) in conjunction with Encore 2002 software at the Pittsburgh site and Hologic 4500A machines with QDA4500A software version 9.03 (Hologic Inc., Waltham, MA) at the other sites. Scans were read centrally at Tufts Body Composition Analysis Center (Boston, MA).
BMI, WC, VAT, and SAT were categorized and analyzed in tertiles. ASMI was calculated as the sum of lean mass in the arms and legs, adjusted to height (kg/m2), with sarcopenia defined as ASMI ≤ 7.26 kg/m2 [25]. BMD at the lumbar spine and femoral neck was categorized per World Health Organization criteria as normal, osteopenic (T score <−1 but >−2.5), or osteoporotic (T score ≤ −2.5) [26].
Covariates:
Physical activity level was self-reported as low, moderate, or high, and measured using the International Physical Activity Questionnaire (IPAQ) [27]. Depressive symptoms were defined by a score of 16 or greater on the Center for Epidemiological Studies-Depression (CES-D) questionnaire [28], hepatitis B virus (HBV) infection was defined as the presence of a positive HBV surface antigen and hepatitis C virus (HCV) infection by the presence of serum viral RNA, diabetes mellitus (DM) was defined as a fasting glucose ≥ 126 mg/dL, or the self-reported diagnosis of diabetes with use of medications for glucose control, kidney disease was defined by estimated GFR < 60mL/min/1.73 m2 body surface area using the Modification of Diet in Renal Disease equation [29], hypertension was defined as systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg, or self-reported diagnosis of hypertension with use of anti-hypertensive medications. HIV-specific factors included current CD4+ T-lymphocyte count/mm3 (CD4), CD4 nadir, cumulative years of ART use, and cumulative years of use of specific antiretroviral classes or agents: protease inhibitors (PI); stavudine (D4T); zidovudine (AZT); and tenofovir disoproxil fumarate (TDF). Plasma HIV-1 RNA concentration was not included in the analysis due to the majority (90%) of men demonstrating virologic suppression (HIV-1 RNA <50 copies/mL).
Statistical Analysis:
Multinomial multivariate logistic regression models were used to estimate the crude (OR) and adjusted (aOR) odds ratios, and 95% confidence intervals (CI) for each measure of body composition with different levels of frailty as the outcome (i.e., non-frail, pre-frail and frail). Two different analyses were conducted on each body composition measure: 1) the entire cohort (both men with and without HIV) adjusted for covariates including HIV serostatus and 2) men with HIV adjusted for covariates including HIV-specific factors. The covariates that were used in the multivariate analysis were chosen based on statistically significant univariate association with frailty status and previously established scientific knowledge, including HIV infection, age, race, enrollment period, education, physical activity level, smoking status, alcohol use, injection drug use, depressive symptoms, viral hepatitis, diabetes, hypertension, and kidney disease as well as HIV-related factors (CD4, CD4 nadir, cumulative years of ART, PI, d4T, AZT. and TDF use). The interaction between each body composition measure and HIV serostatus with frailty was also evaluated in the adjusted model. A sensitivity analysis that was restricted to HIV-infected men with viral load < 50 copies/mL was conducted. The coefficients derived from the multinomial logistic regression model can be interpreted as either a relative risk ratio or conditional odds ratio, which is algebraically equivalent [30]. The association of each body composition component with frailty status here was reported as a conditional odds ratio (OR) with a 95% confidence interval (CI). Pearson test was used to assess correlation between WC and VAT. A p<0.05 guided interpretation of statistical significance. Statistical analyses were conducted using SAS software version 9.4 (Carey, NC, USA).
Results:
Of 399 men included in the study, 199 were men with HIV and 200 were men without HIV. As shown in Table 1, the groups were similar by age, education attained, physical activity level, alcohol and tobacco use. Men with HIV were significantly less likely to be white and were more likely to have an injection drug use history. Men with HIV had greater proportions of depressive symptoms, viral hepatitis, diabetes, kidney disease, and hypertension compared to men without HIV, although not all of the differences were statistically significant. Among men with HIV, median CD4 was 641cells/mm3 and median cumulative exposed time on ART was 12.5 years.
Table 1.
Baseline Characteristics of the Study Population
| Characteristics Median (IQR) or N (%) |
Men without HIV N= 200 |
Men with HIV N=199 |
P-value | |
|---|---|---|---|---|
| Age (years) | 60.0 (55.8–64.9) |
60.1 (54.4–63.8) |
0.11 | |
| Race, white | 158 (79) |
137 (69) |
0.02 | |
| Frailty Status | 0.03 | |||
| Robust | 86 (43) |
70 (35) |
||
| Pre-Frail | 98 (49) |
97 (49) |
||
| Frail | 16 (8) |
32 (16) |
||
| Enrolled after 2001 | 35 (18) |
64 (32) |
<0.001 | |
| Alcohol use>= 14 drinks/week | 16 (8) |
15 (8) |
0.83 | |
| History of injection drug use | 14 (7) |
32 (16) |
<0.001 | |
| Tobacco use | 0.63 | |||
| Current | 33 (17) |
37 (19) |
||
| Former or never | 164 (83) |
163 (81) |
||
| High school education or less | 19 (10) |
28 (14) |
0.16 | |
| Physical activity (IPAQ) | 0.89 | |||
| Low | 35 (18) |
35 (18) |
||
| Moderate | 64 (32) |
59 (30) |
||
| High | 101 (50) |
104 (52) |
||
| Depression (CES-D ≥16) | 37 (19) |
49 (25) |
0.14 | |
| HBV or HCV infection | 9 (4) |
25 (13) |
<0.001 | |
| Diabetes | 19 (10) |
31 (17) |
0.06 | |
| Kidney disease | 18 (9) |
59 (30) |
<0.001 | |
| Hypertension | 103 (52) |
121 (62) |
0.05 | |
| HIV-specific factors | ||||
| Current CD4 (cells/mm3) | 865 (668–1099) |
641 (500–843) |
<0.001 | |
| CD4 (cells/mm3) nadir | 272 (165–392) |
|||
| Current HIV-1 RNA <50 cps/mL | 179 (90) |
|||
| Cumulative years on ART | 12.5 (9.1–15.3) |
|||
| Cumulative years on TDF | 5.5 (1.2–8.7) |
|||
| Cumulative years on PI | 7.6 (1.7–13) |
|||
| Cumulative years on ZDV or D4T | 6.9 (3.3–11.2) |
|||
| Body Composition Measurements by HIV Serostatus | ||||
| Marker of overall adiposity | BMI (kg/m2) | 25.3 (23.2–29.2) |
25.3 (22.8–28.4) |
0.240 |
| Clinical marker of abdominal adiposity | Waist circumference (cm) | 96.4 (89.9–106.5) |
96.2 (89.1–104.9) |
0.334 |
| Marker of targeted regions of adiposity | VAT area (cm2) | 113.4 (72.4–181.7) |
140.3 (85.7–194.6) |
0.007 |
| SAT area (cm2) | 225.6 (160.6–315.5) |
174.4 (117–252.2) |
<0.001 | |
| Marker of lean mass and sarcopenia | Appendicular Lean Mass/Height2 (kg/m2) | 7.6 (6.9–8.2) |
7.5 (6.9–8.3) |
0.494 |
| Marker of osteopenia/osteoporosis | Femoral Neck T-score | −0.5 (−1.3–0.5) |
−0.7 (−1.2–0.2) |
0.318 |
| Lumbar Spine T-score | −0.5 (−1.4–0.5) |
−0.6 (−1.4–0.4) |
0.744 | |
Abbreviations: IPAQ, international physical activities questionnaire; CES-D, Center for Epidemiologic Studies Depression Scale; HBV, hepatitis B virus; HCV, hepatitis C virus; HAART, highly active antiretroviral therapy; TDF, tenofovir disoproxil fumarate; PI, protease inhibitor; ZDV, zidovudine; D4T, stavudine; BMI, body mass index; VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue.
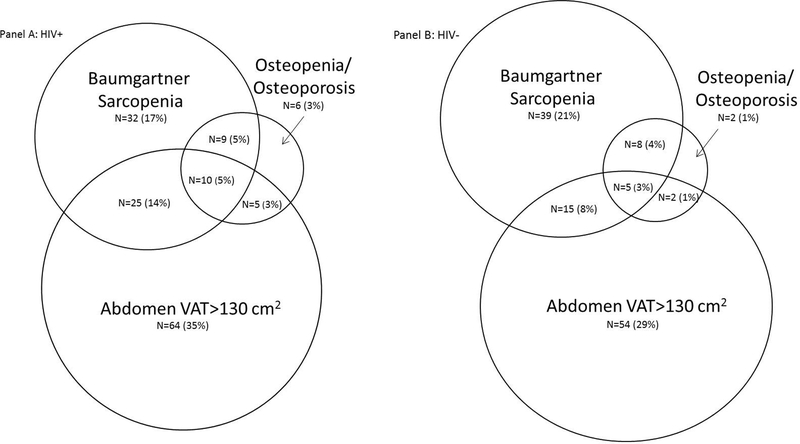
Among the men with HIV, 35% were non-frail, 49% were pre-frail, and 16% were frail. Among the men without HIV, 43% were non-frail, 49% were pre-frail, and 8.0% were frail (Table 1). As shown in Table 1, median measures of overall adiposity, abdominal adiposity, lean mass/sarcopenia, and bone density were similar between men with and without HIV. Measures of specific regions of adiposity were significantly different: VAT was higher among men with HIV and SAT was higher in men without HIV (p<0.05). As shown in Figure 1, of participants that had all three measurements, 39% were sarcopenic, 49% had high VAT (>130 cm2), 13% had osteopenia/osteoporosis, and 11% with both sarcopenia and high VAT. The percentage of men with sarcopenia was 41% (76/185) among men with HIV vs. 36% (67/186) (p=0.32) among men without HIV; VAT elevation was present in 56% (104/185) vs. 41% (76/186) (p=0.004) and osteopenia/osteoporosis among 16% (30/185) vs. 9% (17/186) (p=0.003), respectively.
Figure 1:
Venn diagram showing overlap between different body composition abnormalities by HIV serostatus (not drawn exactly to scale). Panel A details men living with HIV and percentages are out of N=185. Panel B details men without HIV and percentages are out of N= 186. Of note, 34 (18%) of men with HIV and 61 (33%) of men without HIV didn’t belong to any of the three groups and are not included in the Venn diagram. Abbreviation: VAT, visceral adipose tissue. Osteopenia is described as T score <−1 but >−2.5 and osteoporosis as T score ≤ −2.5. Baumgartner’s sarcopenia defined as ASMI ≤ 7.26 kg/m2.
Associations between Frailty and HIV
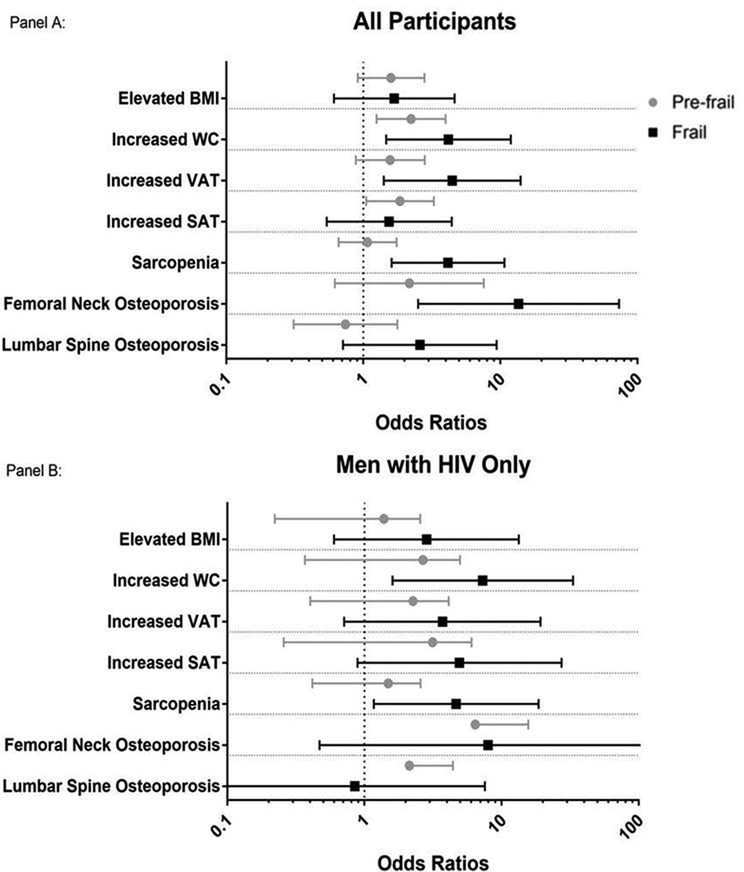
HIV infection was associated with a 2.43-fold (95% CI: 1.23, 4.79) increase in the odds of frailty, but was not significantly associated with increased risk of pre-frailty (aOR=1.19, 95% CI: 0.78, 1.82), in adjusted analyses (reviewed in the footnote of Figure 2).
Figure 2:
Odds Ratio (with Confidence Intervals) of Frail vs Non-Frail and Pre-Frail vs Non-Frail for Different Body Composition Measures. Panel A, Overall cohort adjusted for HIV serostatus and Panel B, men with HIV only, additionally adjusted for HIV-specific variables. All models were adjusted for age, cohort, race/ethnicity, education, physical activity level, smoking, alcohol and other substance use, depression, viral hepatitis (HBV/HCV), diabetes, kidney disease, and hypertension. The HIV-specific model also included CD4, CD4 nadir, cumulative exposure to ART, PI, D4T, AZT, and/or TDF. *BMI, WC, VAT and SAT are reported as odds of being in the highest tertile compared to the middle tertile. The tertile cut points are as follows: BMI: lst ≤23.7, 2nd 23.7–27.7, 3rd>27.7 (kg/m2), WC: 1st ≤91.9, 2nd 91.9–101.5, 3rd >101.5 (cm), VAT: lst ≤94.6, 2nd 94.6–172.2, 3rd >172.2 (cm2), SAT: lst ≤160.4, 2nd 160.4–245.6, 3rd >245.6 (cm2).
Associations between Frailty and Adiposity in Multivariate Analyses
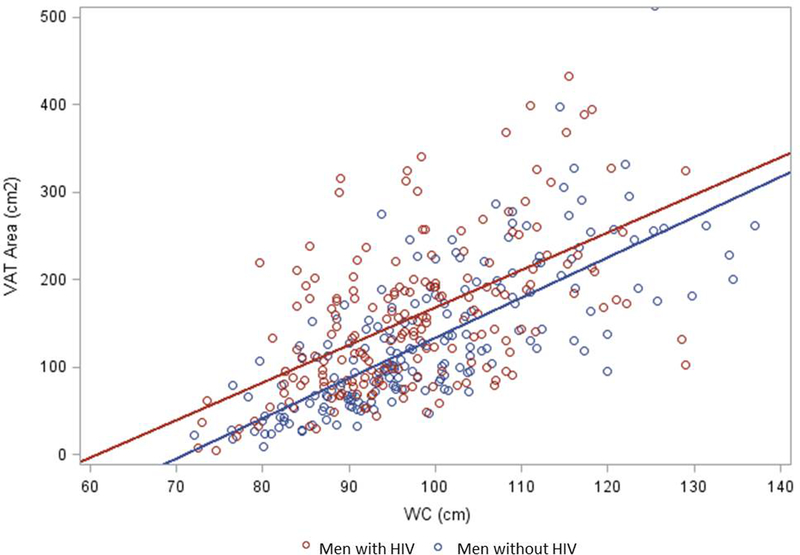
In adjusted models, there were no significant associations between BMI and pre-frailty or frailty among the entire cohort or the group of men with HIV (all p >0.05, Figure 2). The highest WC tertile was associated with increased odds of both pre-frailty (aOR=2.23 [95% CI 1.25, 3.98]) and frailty (aOR=4.18 [95% CI 1.47, 11.9]) in the entire cohort, and with frailty among the group of men with HIV (aOR=7.28 [95% CI 1.6, 33.21]). In the entire cohort, the highest tertile of VAT was associated with a 4.45-fold greater odds of frailty (95% CI 1.41, 14.04) in relation to the middle and lower tertile of VAT, Figure 2. A similar odds of frailty with increased VAT was observed in the group of men with HIV but did not meet significance (aOR=3.71 [95% CI 0.71, 19.24]). The highest tertile of SAT was associated with pre-frailty (aOR=1.85 [95% CI 1.05, 3.27]) but not frailty in the entire cohort (aOR=1.54 [95% CI 0.54, 4.42]); SAT was not associated with frailty (aOR=4.92 [95% CI 0.89, 27.37]) or pre-frailty (aOR=2.26 [95% CI 0.8, 6.36]) among men with HIV. As shown in Figure 3, there was a positive correlation between WC and VAT in both the entire cohort (r=0.62, p<0.0001) and the men with HIV (r=0.56, p<0.0001).
Figure 3:
Visceral Adipose Tissue and Waist Circumference Correlation by HIV Serostatus. Men with HIV (red dots) had less robust correlation (R=0.56; P<0.001) than men without HIV (blue dots; R=0.74; P<0.001).
Associations between Frailty and Sarcopenia in Multivariate Analyses:
Sarcopenia was associated with an increased odds of frailty in both the entire cohort (aOR=2.68 [95% CI 1.11, 6.45]) and men with HIV (aOR=4.08 [95% CI 1.01, 16.41]), Figure 2. There was no interaction between VAT and sarcopenia (aOR=0.79 [95% CI 0.07, 8.79]).
Associations between Frailty and Bone Density:
Frailty but not pre-frailty was strongly related to femoral neck osteoporosis in the entire cohort (aOR=13.6 [95% CI 2.51, 73.57], Figure 2a). When restricted to only those with HIV infection, the point estimate was large, but the confidence interval was quite wide (aOR=7.96 [95% CI 0.47, 133.99], Figure 2b), likely due to the low numbers with osteoporosis. Lumbar spine BMD was not associated with pre-frailty or frailty in either the entire cohort or the group of men with HIV (full multivariate results are shown in Supplementary Table 1).
Effect of HIV Serostatus and Viral Suppression:
In separate models, the effect of HIV serostatus on the relationship between body composition and frailty or pre-frailty was evaluated: no body composition measures demonstrated a differential association with either pre-frail or frailty by HIV serostatus (see Supplementary Table 1; all p values for interaction >0.20). In a sensitivity analysis restricted to HIV-infected men with HIV-1 RNA <50 copies/mL, the direction and magnitude of effects of body composition on frailty remained consistent compared to results estimated among the group of men with HIV.
Discussion
In a cohort of well-characterized men with and without HIV, we found that sarcopenia and central adiposity (either VAT or WC) were strongly associated with frailty. In contrast, BMI and SAT were not associated with frailty. Although HIV was associated with increased frailty, the effect of these body composition measurements on frailty did not differ by HIV serostatus [3–5, 31, 32]. In contrast to prior studies linking frailty to low CD4 count and a history of AIDS [8, 33, 34], we did not find an association between frailty and HIV-specific factors, likely reflective of low rates of clinical AIDS and high CD4 T-cell counts.
We found robust associations between frailty with central adiposity and sarcopenia. To the best of our knowledge, the association between frailty and VAT area in adult men with HIV has not been previously reported. One small prior study of people with HIV found that greater trunk fat but not lean mass was associated with frailty [19], in contrast to the strong association we found between sarcopenia and frailty among both men with and without HIV. In a previously reported case-control study of middle-aged, adults with HIV, we similarly found that low-functioning adults had greater sarcopenia (by ASMI) than high-functioning controls [20]. Also similar to this previously published study we found no association between BMI and frailty, perhaps due to the relatively small range of BMIs observed in our cohort or the restricted upper range of BMI [20]. The lack of an identifiable association between frailty and BMI is likely multifactorial. First, the relationship between BMI and frailty may be not be linear; many prior studies of both people living with and without HIV, have identified both low and high BMI as risk factors for development of frailty [5, 16, 19, 35–37]. Second, BMI is not an accurate assessment of adiposity [38]: Low BMI is likely a marker of loss in muscle mass or fat, and is often seen in conjunction with comorbidities common in frailty (e.g., chronic obstructive pulmonary disease, chronic kidney disease). A higher BMI could be a marker of increased lean mass or increased adipose tissue, with the latter associated with increased levels of inflammation and the presence of comorbid diseases that impede function (arthritis, coronary artery disease, DM)[39–41]. Thus, separate measures of fat and lean mass describe the components of body composition associated with frailty more accurately than the “composite” measure of BMI.
Underlying mechanisms that link central adiposity, sarcopenia, and frailty are likely due, in part, to chronic levels of underlying inflammation and immune activation. Adipose tissue, and in particular VAT, is a metabolically active tissue that contributes to heightened inflammation and immune activation [40]. In addition to adipokines, many markers of inflammation are associated with VAT, including serum levels of interleukin(IL)-6, tumor necrosis factor alpha, and C-reactive protein [42–44]. Inflammation has also been implicated as a major causative factor in the development of sarcopenia [45]. Though no HIV-specific causality for frailty has been established among adults with HIV, an enhanced underlying inflammatory state associated with central adiposity and sarcopenia (“sarcopenic obesity”) may contribute. For example, elevated IL-6 has been associated with both VAT and sarcopenia in adults with HIV [42], and elevated IL-6 has also been associated with frailty in adults with HIV [46–48]. However, frailty is a dynamic process and frailty itself likely contributes to increased VAT and sarcopenia through many potential mechanisms including reduced physical activity.
The interplay between frailty, muscle mass, and bone density is well-recognized, and our findings in the overall cohort were consistent with those previously published among populations without HIV [17, 18, 20], even in the absence of statistical significance. The prevalence of osteopenia and osteoporosis was lower than that seen in prior studies of HIV cohorts, which may have decreased power to attain statistical significance. Additionally, other HIV-specific factors likely to contribute to low BMD including CD4 cell nadir and long-term use of TDF; adjustment for these factors may have explained a greater proportion of low BMD than frailty.
Lastly, we assessed associations between WC and VAT. WC is a validated surrogate for VAT in HIV-uninfected persons [49, 50]. Recently published clinical guidelines on obesity and lipodystrophy in adults with HIV recommend measurement of annual WC [51], based on growing evidence that central adiposity, particularly VAT, is associated with adverse health outcomes. We found a strong, positive correlation between WC and VAT in both the entire cohort (r=0.62, p<0.0001) and the men with HIV (r=0.56, p<0.0001). Our findings corroborate a prior study that showed self-reported waist gain and WC correlated with DXA- and CT-derived measures of abdominal fat, including VAT, among adults with HIV [52]. Furthermore, the strong association between WC and frailty emphasizes the clinical importance of WC measurement, and suggests that WC may be an easy, clinically accessible marker for adults with HIV at increased risk for frailty.
The strengths of this study include the control group of men without but at risk for HIV, similar demographically to the group of men with HIV who were studied. Body composition measurements from DXA and CT scans allowed for more thorough and precise comparisons than BMI or DXA alone. Further, all men with HIV were prescribed ART and the vast majority of the participants were virologically suppressed. This study had several limitations: the cross-section design restricted our ability to establish temporality between the independent and dependent variables. The results may not be applicable to more racially-diverse population or to women, especially with respect to hormonal changes associated with aging and differences in body composition. The exclusion of participants with a BMI of >35mg/kg2 may have introduced a bias that precluded finding an association between BMI and frailty although this represents less than 2% of the MACS. Lastly, more contemporary definitions of sarcopenia as described by the The European Working Group on Sarcopenia in Older People [53] and the International Working Group on Sarcopenia [54], focus on both loss of muscle mass and function (gait speed or grip strength). As these measures of function are also components the outcome of frailty, we did not use these definitions.
In summary, in this study of nearly 400 older men with and without HIV, frailty was associated with HIV infection as well as with central adiposity, sarcopenia and femoral neck osteoporosis. The association of frailty with central adiposity, and the strong correlation between a simple clinical measure of central adiposity (WC) and CT-based VAT assessment, provide additional clinical rationale supporting the annual assessment of WC for both metabolic risk and for identifying frailty risk. Lastly, the high degree of overlap in central adiposity, sarcopenia, and femoral osteoporosis supports the probable existence of a common mechanistic pathway for these conditions; interventions with beneficial effects on all three outcomes may have the greatest potential to prevent, delay, or improve frailty. Longitudinal studies can further ascertain the potential causal role of these body composition changes in the pathway to frailty, and to evaluate the risks and benefits of interventions such as cardiovascular and resistance exercise, hormone-based therapies, or dietary interventions on frailty that may be mediated through changes in body composition.
Supplementary Material
Acknowledgements
Author’s contributions: K.L.H, K.M.E, T.T.B designed the study. L.Z. was the primary statistical analyst, under the guidance of D.K.N. K.L.H prepared the first draft of the manuscript. All authors contributed to interpretation of results and critically reviewed the manuscript.
We thank the study volunteers who participated in the BOSS Substudy of the MACS, all of the MACS clinical units that enroll and follow participants, and the MACS.
Funding:The MACS Bone Strength Substudy (BOSS) was supported by NIH (NIAID) R01AI095089 (TTB). The Multicenter AIDS Cohort Study is supported by the National Institute of Allergy and Infectious Diseases, with additional supplemental funding from the National Cancer Institute (grants UO1-AI-35042, UL1-RR025005, UM1-AI-35043, UO1-AI-35039, UO1-AI-35040, and UO1-AI-35041). Additional support by the National Heart, Lung, and Blood Institute, the National Institute of Immunology, Allergy, and Infectious Disease and the National Institute of Aging of the National Institutes of Health under award numbers T32HL116276 to University of Colorado; K24 AI120834 to TTB; NIA K23 AG050260 and R01AG054366 to KME; K23 AI110532 to JEL. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures:
KME received research funding and consultant fee (both paid to the University of Colorado) from Gilead Sciences, and received a consultant fee (paid to the University of Colorado) from Theratechnologies and EMD-Serono. FJP has served as a consultant and or provided lectures for Gilead, Janssen, Merck, ViiV and Bristol Myers Squibb. TTB has served as a consultant for BMS, Merck, Abbott, Gilead, Theratechnologies, and EMD-Serono. JEL has received research funding and consultancy fees from Gilead Sciences, and consultancy fees from Merck. KNA has received consultancy fees from TrioHealth, Inc, and Gilead Sciences. The remaining authors have no conflicts of interest to disclose.
References
- 1.Margolick JB, Chopra RK. Relationship between the immune system and frailty: pathogenesis of immune deficiency in HIV infection and aging. Aging (Milan, Italy) 1992; 4(3):255–257. [DOI] [PubMed] [Google Scholar]
- 2.Desquilbet L, Jacobson LP, Fried LP, Phair JP, Jamieson BD, Holloway M, et al. HIV-1 Infection Is Associated With an Earlier Occurrence of a Phenotype Related to Frailty. J Gerontol A Biol Sci Med Sci 2007; 62(11):1279–1286. [DOI] [PubMed] [Google Scholar]
- 3.Gustafson DR, Shi Q, Thurn M, Holman S, Minkoff H, Cohen M, et al. Frailty and Constellations of Factors in Aging HIV-infected and Uninfected Women--The Women’s Interagency HIV Study. J Frailty Aging 2016; 5(1):43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brothers TD, Kirkland S, Guaraldi G, Falutz J, Theou O, Johnston BL, et al. Frailty in people aging with human immunodeficiency virus (HIV) infection. J Infect Dis 2014; 210(8):1170–1179. [DOI] [PubMed] [Google Scholar]
- 5.Kooij KW, Wit FW, Schouten J, van der Valk M, Godfried MH, Stolte IG, et al. HIV infection is independently associated with frailty in middle-aged HIV type 1-infected individuals compared with similar but uninfected controls. AIDS 2016; 30(2):241–250. [DOI] [PubMed] [Google Scholar]
- 6.Piggott DA, Erlandson KM, Yarasheski KE. Frailty in HIV: Epidemiology, Biology, Measurement, Interventions, and Research Needs. Curr HIV/AIDS Rep 2016; 13(6):340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding Y, Lin H, Liu X, Wong FY, Sun YV, Marconi VC, et al. Higher Prevalence of Frailty Among a Sample of HIV-Infected Middle-aged and Older Chinese Adults Is Associated With Neurocognitive Impairment and Depressive Symptoms. J Infect Dis 2017; 215(5):687–692. [DOI] [PubMed] [Google Scholar]
- 8.Althoff KN, Jacobson LP, Cranston RD, Detels R, Phair JP, Li X, et al. Age, comorbidities, and AIDS predict a frailty phenotype in men who have sex with men. J Gerontol A Biol Sci Med Sci 2014; 69(2):189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kallianpur KJ, Sakoda M, Gangcuangco LM, Ndhlovu LC, Umaki T, Chow D, et al. Frailty Characteristics in Chronic HIV Patients are Markers of White Matter Atrophy Independently of Age and Depressive Symptoms: A Pilot Study. Open Med 2016; 3:138–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piggott DA, Muzaale AD, Mehta SH, Brown TT, Patel KV, Leng SX, et al. Frailty, HIV infection, and mortality in an aging cohort of injection drug users. PloS One 2013; 8(1):e54910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV 2017; 4(8):e349–e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McComsey GA, Moser C, Currier J, Ribaudo HJ, Paczuski P, Dube MP, et al. Body Composition Changes After Initiation of Raltegravir or Protease Inhibitors: ACTG A5260s. Clin Infect Dis 2016; 62(7):853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grant PM, Kitch D, McComsey GA, Collier AC, Bartali B, Koletar SL, et al. Long-term body composition changes in antiretroviral-treated HIV-infected individuals. AIDS 2016; 30(18):2805–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grant PM, Kitch D, McComsey GA, Collier AC, Koletar SL, Erlandson KM, et al. Long-term Bone Mineral Density Changes in Antiretroviral-Treated HIV-Infected Individuals. J Infect Dis 2016; 214(4):607–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koethe JR, Grome H, Jenkins CA, Kalams SA, Sterling TR. The metabolic and cardiovascular consequences of obesity in persons with HIV on long-term antiretroviral therapy. AIDS 2016; 30(1):83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hubbard RE, Lang IA, Llewellyn DJ, Rockwood K. Frailty, body mass index, and abdominal obesity in older people. J Gerontol A Biol Sci Med Sci 2010; 65(4):377–381. [DOI] [PubMed] [Google Scholar]
- 17.Cesari M, Leeuwenburgh C, Lauretani F, Onder G, Bandinelli S, Maraldi C, et al. Frailty syndrome and skeletal muscle: results from the Invecchiare in Chianti study. Am J Clin Nutr 2006; 83(5):1142–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cook MJ, Oldroyd A, Pye SR, Ward KA, Gielen E, Ravindrarajah R, et al. Frailty and bone health in European men. Age Ageing 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah K, Hilton TN, Myers L, Pinto JF, Luque AE, Hall WJ. A new frailty syndrome: central obesity and frailty in older adults with the human immunodeficiency virus. J Am Geriatr Soc 2012; 60(3):545–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erlandson KM, Allshouse AA, Jankowski CM, MaWhinney S, Kohrt WM, Campbell TB. Functional impairment is associated with low bone and muscle mass among persons aging with HIV infection. J Acquir Immune Defic Syndr (1999) 2013; 63(2):209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erlandson KM, Wu K, Koletar SL, Kalayjian RC, Ellis RJ, Taiwo B, et al. Association Between Frailty and Components of the Frailty Phenotype With Modifiable Risk Factors and Antiretroviral Therapy. J Infect Dis 2017; 215(6):933–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56(3):M146–156. [DOI] [PubMed] [Google Scholar]
- 23.Body Measurements (Anthropometry). Rockville, MD; 1988. [Google Scholar]
- 24.Palella FJ Jr., McKibben R, Post WS, Li X, Budoff M, Kingsley L, et al. Anatomic Fat Depots and Coronary Plaque Among Human Immunodeficiency Virus-Infected and Uninfected Men in the Multicenter AIDS Cohort Study. Open Forum Infect Diss 2016; 3(2):ofw098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998; 147(8):755–763. [DOI] [PubMed] [Google Scholar]
- 26.Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organization technical report series 1994; 843:1–129. [PubMed] [Google Scholar]
- 27.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Medicine and science in sports and exercise 2003; 35(8):1381–1395. [DOI] [PubMed] [Google Scholar]
- 28.Radloff L The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measur 1977; 1:385–401. [Google Scholar]
- 29.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130(6):461–470. [DOI] [PubMed] [Google Scholar]
- 30.O’Halloran S, and I.. Econometrics. “Lecture 10. Logistical regression II. Multinomial data.”. In; Retrieved on August 1 (2008): 2008.
- 31.Piggott DA, Muzaale AD, Varadhan R, Mehta SH, Westergaard RP, Brown TT, et al. Frailty and Cause-Specific Hospitalization Among Persons Aging With HIV Infection and Injection Drug Use. J Gerontol A Biol Sci Med Sci 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Almansouri AY, Abdulfatah ME, Baaqil OH, Bakheet AA, Turki SA, Kotb MM, et al. Serum Sclerostin Levels in Patients with Human Immunodeficiency Virus Infection and Their Association with Bone Turnover Markers and Bone Mineral Densitometry. J Bone Metab 2016; 23(1):16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terzian AS, Holman S, Nathwani N, Robison E, Weber K, Young M, et al. Factors associated with preclinical disability and frailty among HIV-infected and HIV-uninfected women in the era of cART. J Womens Health (2002) 2009; 18(12):1965–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Desquilbet L, Margolick JB, Fried LP, Phair JP, Jamieson BD, Holloway M, et al. Relationship between a frailty-related phenotype and progressive deterioration of the immune system in HIV-infected men. J Acquir Immune Defic Syndr (1999) 2009; 50(3):299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheehan KJ, O’Connell MD, Cunningham C, Crosby L, Kenny RA. The relationship between increased body mass index and frailty on falls in community dwelling older adults. BMC Geriatr 2013; 13:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Onen NF, Agbebi A, Shacham E, Stamm KE, Onen AR, Overton ET. Frailty among HIV-infected persons in an urban outpatient care setting. J Infect 2009; 59(5):346–352. [DOI] [PubMed] [Google Scholar]
- 37.Pathai S, Gilbert C, Weiss HA, Cook C, Wood R, Bekker LG, et al. Frailty in HIV-infected adults in South Africa. J Acquir Immune Defic Syndr 2013; 62(1):43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smalley KJ, Knerr AN, Kendrick ZV, Colliver JA, Owen OE. Reassessment of body mass indices. Am J Clin Nutr 1990; 52(3):405–408. [DOI] [PubMed] [Google Scholar]
- 39.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009; 9(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006; 444(7121):881–887. [DOI] [PubMed] [Google Scholar]
- 41.Schols AM, Soeters PB, Dingemans AM, Mostert R, Frantzen PJ, Wouters EF. Prevalence and characteristics of nutritional depletion in patients with stable COPD eligible for pulmonary rehabilitation. Am Rev Respir Dis 1993; 147(5):1151–1156. [DOI] [PubMed] [Google Scholar]
- 42.Langkilde A, Petersen J, Henriksen JH, Jensen FK, Gerstoft J, Eugen-Olsen J, et al. Leptin, IL-6, and suPAR reflect distinct inflammatory changes associated with adiposity, lipodystrophy and low muscle mass in HIV-infected patients and controls. Immun Ageing 2015; 12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsuriya D, Morita H, Morioka T, Takahashi N, Ito T, Oki Y, et al. Significant correlation between visceral adiposity and high-sensitivity C-reactive protein (hs-CRP) in Japanese subjects. Internal medicine (Tokyo, Japan) 2011; 50(22):2767–2773. [DOI] [PubMed] [Google Scholar]
- 44.Hamdy O, Porramatikul S, Al-Ozairi E. Metabolic obesity: the paradox between visceral and subcutaneous fat. Curr Diabetes Rev 2006; 2(4):367–373. [DOI] [PubMed] [Google Scholar]
- 45.Bano G, Trevisan C, Carraro S, Solmi M, Luchini C, Stubbs B, et al. Inflammation and sarcopenia: A systematic review and meta-analysis. Maturitas 2017; 96:10–15. [DOI] [PubMed] [Google Scholar]
- 46.Erlandson KM, Ng D, Jacobson LP, Margolick JB, Dobs AS, Palella FJ Jr., et al. Inflammation, Immune Activation, Immunosenescence, and Hormonal Biomarkers in the Frailty-Related Phenotype of Men with or at Risk for HIV. J Infect Dis 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piggott DA, Varadhan R, Mehta SH, Brown TT, Li H, Walston JD, et al. Frailty, Inflammation, and Mortality Among Persons Aging With HIV Infection and Injection Drug Use. J Gerontol A Biol Sci Med Sci 2015; 70(12):1542–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Margolick JB, Bream JH, Martinez-Maza O, Lopez J, Li X, Phair JP, et al. Frailty and Circulating Markers of Inflammation in HIV+ and HIV-Men in the Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr (1999) 2017; 74(4):407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lemieux S, Prud’homme D, Bouchard C, Tremblay A, Despres JP. A single threshold value of waist girth identifies normal-weight and overweight subjects with excess visceral adipose tissue. Am J Clin Nutr 1996; 64(5):685–693. [DOI] [PubMed] [Google Scholar]
- 50.Despres JP, Lamarche B. Effects of diet and physical activity on adiposity and body fat distribution: implications for the prevention of cardiovascular disease. Nutr Res Rev 1993; 6(1):137–159. [DOI] [PubMed] [Google Scholar]
- 51.Lake JE, Stanley TL, Apovian CM, Bhasin S, Brown TT, Capeau J, et al. Practical Review of Recognition and Management of Obesity and Lipohypertrophy in Human Immunodeficiency Virus Infection. Clin Infect Dis 2017; 64(10):1422–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhagwat P, Ofotokun I, McComsey GA, Brown TT, Moser C, Sugar CA, et al. Changes in abdominal fat following antiretroviral therapy initiation in HIV-infected individuals correlate with waist circumference and self-reported changes. Antivir Ther 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010; 39(4):412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 2011; 12(4):249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.