Abstract
Background
Although Ureaplasma species are the most common organisms associated with prematurity, their effects on the maternal and fetal immune system remain poorly characterized.
Methods
Rhesus macaque dams at approximately 80% gestation were injected intra-amniotically with 107 colony-forming units of Ureaplasma parvum or saline (control). Fetuses were delivered surgically 3 or 7 days later. We performed comprehensive assessments of inflammation and immune effects in multiple fetal and maternal tissues.
Results
Although U. parvum grew well in amniotic fluid, there was minimal chorioamnionitis. U. parvum colonized the fetal lung, but fetal systemic microbial invasion was limited. Fetal lung inflammation was mild, with elevations in CXCL8, tumor necrosis factor (TNF) α, and CCL2 levels in alveolar washes at day 7. Inflammation was not detected in the fetal brain. Significantly, U. parvum decreased regulatory T cells (Tregs) and activated interferon γ production in these Tregs in the fetus. It was detected in uterine tissue by day 7 and induced mild inflammation and increased expression of connexin 43, a gap junction protein involved with labor.
Conclusions
U. parvum colonized the amniotic fluid and caused uterine inflammation, but without overt chorioamnionitis. It caused mild fetal lung inflammation but had a more profound effect on the fetal immune system, decreasing Tregs and polarizing them toward a T-helper 1 phenotype.
Keywords: chorioamnionitis, intrauterine infection, decidua, fetal immunology, regulatory T-cells
Prematurity is the leading cause of infant mortality and morbidity globally [1]. Intrauterine infection or inflammation is associated with 40% of preterm labor [2]. Its most common cause is Ureaplasma species [3, 4]. Lower genital colonization with Ureaplasma is correlated with preterm labor [5, 6]. In addition to preterm labor, intrauterine infection or inflammation due to Ureaplasma species is also associated with an increased incidence of chronic lung disease [7] and gastrointestinal [8] and neurologic complications in preterm infants [9]. However, there is considerable variation in clinical manifestations from intrauterine Ureaplasma infection, eliciting minimal inflammatory responses without preterm labor in some women while causing robust inflammation in others [4].
Similar to effects in humans, intra-amniotic injection of live Ureaplasma parvum in pregnant sheep also caused chorioamnionitis of variable severity and fetal inflammation [10]. However, there was no clear microbial factor that could explain the variability in host response, because U. parvum strains isolated from the amniotic fluid of sheep with severe versus mild chorioamnionitis failed to retain their initial virulence on repeated injection in pregnant sheep [11]. One possibility for the variable response is a variable host immune response, but this has not been systematically studied.
The most relevant animal model for the study of placenta and fetal immune responses is the rhesus monkey (Macaca mulatta), owing to close similarities to humans in immunologic and reproductive biologic characteristics [12–14] and the availability of reagents to perform in-depth immune studies. In addition, intra-amniotic injection of U. parvum serovar 1 to chronically catheterized rhesus monkeys caused efficient amniotic colonization, preterm delivery, histologic chorioamnionitis, a systemic fetal inflammatory response, and fetal pneumonitis [15]. The pathologic effects described in monkeys were more severe than those in sheep. However, immune responses were not characterized in depth. Therefore, to better understand maternal and fetal inflammation and immune responses to Ureaplasma infection, we injected U. parvum serovar 1 into the amniotic fluid of pregnant rhesus macaques to characterize immune responses to acute (3-day) and subacute (7-day) exposures to intra-amniotic U. parvum.
METHODS
Animals
Animal procedures were approved by the Institutional Animal Care and use Committee at the University of California, Davis. Animals were not colonized with Ureaplasma before inoculation based on nasal, rectal, and vaginal screening by culture and polymerase chain reaction (PCR). By ultrasound guided intra-amniotic injection, time-mated female rhesus macaques (n = 18) were given either 1 mL of saline solution (controls) or U. parvum serovar 1 (1 × 107 colony-forming units per animal, diluted in 1 mL of saline solution; kindly provided by K. B. W.) (Table 1). Hysterotomies were performed 3 or 7 days after U. parvum injection at about 130 days gestational age (80% term gestation). None of the animals displayed any overt preterm labor or sickness. Dams and fetuses were euthanized, and tissues collected at necropsy. For fetal alveolar wash, the left main stem bronchus was cannulated, and the lung was inflated to near total lung capacity with normal saline solution and aspiration of the fluid. The alveolar wash was repeated 3 times, and the fluid was pooled.
Table 1.
Ureaplasma parvum Infection and Inflammation
| Variable | Control (n = 8) |
U. parvum
Infecteda |
|
|---|---|---|---|
| 3 d (n = 5) | 7 d (n = 5) | ||
| Fetal weight, mean (SD) g | 331 (7.2) | 348 (44) | 341 (18) |
| Gestational age at delivery, mean (SD), d | 132 (0.8) | 132 (4.3) | 131 (1.2) |
| Histologic chorioamnionitis, No. | 1/8 | 0/5 | 1/5 |
| U. parvum quantitative culture, median (IQR), CFUs × 103/mL | |||
| Fetal membrane | 0 | 0 (0–0.2) | 0.2 (0–0.9) |
| Amniotic fluid | 0 | 0.1 (0.03–84) | 3900 (1875–9275) |
| U. parvum detection by PCR, No. | |||
| Fetal membrane | 0/8 | 4/5 | 4/5 |
| Amniotic fluidb | 0/5 | 5/5 | 5/5 |
| Maternal uterus | ND | 0/5 | 3/5 |
| Fetal lung | ND | 4/5 | 5/5 |
| Fetal spleen | ND | 0/5 | 2/5 |
| Fetal thalamus | ND | 1/5 | 0/5 |
Abbreviations: CFUs, colony-forming units; IQR, interquartile range; ND, not determined; PCR, polymerase chain reaction; SD, standard deviation; U. parvum, Ureaplasma parvum.
aU. parvum (1 × 107 CFUs) was given via intra-amniotic injection.
b Data from only 5 of 8 control animals.
Ureaplasma Culture and PCR
Samples of amniotic fluid, chorioamnion, fetal lung, spleen, uterus, and brain were received in the University of Alabama at Birmingham Diagnostic Mycoplasma Laboratory on dry ice after being stored frozen at −80°C in 10B broth. Quantitative cultures amniotic fluid and chorioamnion were performed using 10B broth and A8 agar [16]. Brown granular colonies grown on A8 agar after 1–3 days of incubation, typical of Ureaplasma species, were definitively identified as U. parvum by real-time PCR, as described by Xiao et al [17]. Amniotic fluid, chorioamnion, fetal lung, brain, spleen, and uterus were also analyzed by real-time PCR for direct U. parvum detection [17], using the gene target UU063, which encodes a highly conserved hypothetical protein found in all 4 U. parvum servovars but not in Ureaplasma urealyticum.
Cell Isolation and Culture
Fetal spleen and thymus were diced and dissociated with a pestle into cell suspensions, passed through 70-µm cell strainers, and washed in culture medium (Roswell Park Memorial Institute 1640 medium) containing 10% fetal calf serum, 100 IU/mL penicillin, 100 IU/mL streptomycin, and 2 mmol/L glutamine. Blood mononuclear cells were isolated from approximately 10 mL of heparinized fetal blood by means of Ficoll-Hypaque (GE Healthcare) gradient centrifugation within 3 hours of collection. In spleen samples, red blood cell lysis was performed with ammonium chloride–potassium carbonate–ethylenediaminetetraacetic acid (Lonza BioWhittaker).
Cells were rested overnight at 37°C in air with 5% carbon dioxide. After overnight culture, viability was consistently >90% (Trypan blue exclusion test). For cytokine measurements, cells were stimulated with 50 ng/mL phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich) and 750 ng/mL ionomycin (Calbiochem) for 5 hours, with 10 µg/mL brefeldin A (Sigma-Aldrich) and 1 µL/mL monensin ×1000 (eBioscience) added for the last 4 hours.
Flow Cytometry Analysis of Immune Cells
A cocktail of conjugated antibodies validated for the rhesus macaque was used (http://www.nhpreagents.org/NHP/clonelist.aspx?ID=15): anti-CD3 (SP34-2), anti-CD4 (L200), anti-CD45 (D058-1283), anti-Ki67 (B56) (BD Biosciences); anti–interferon (IFN) γ (B27), anti–forkhead box (FOX) P3 (PCH101), and anti-CD8α (RPA-T8) (eBioscience). Cells were treated with human IgG to block Fc receptors and stained for surface markers. Cells were then washed and fixed with fixation/permeabilization buffer followed by permeabilization buffer (eBioscience) before staining for intracellular markers and analysis (FACS Fortessa2; BD Biosciences). Live cells were gated based on forward- and side-scatter properties and the absence of fluorescence using the LIVE/DEAD Fixable Dead Cell Stain kit (Invitrogen).
To evaluate T-cell subsets in the decidua, cells were cultured overnight at 37°C and 5% carbon dioxide in Dulbecco's modified Eagle medium/F12 medium containing 10% fetal bovine serum, 100 IU/mL penicillin, 100 IU/mL streptomycin, and 2 mmol/L glutamine, because the dissociation/protease digestion can down-regulate CD4/CD8 receptors [18–20]. Data analysis was performed using FACSDiva (version 6.1.2; BD Biosciences) and FlowJo (version 9.9.4; Tree Star) software.
Immunohistochemistry
Immunohistochemistry was performed as described elsewhere [21]. Briefly, tissue sections were incubated with anti–connexin 43 (Abcam Ab11370, 1:750), anti-myeloperoxidase (MPO; Cell Marque 289A-75; 1:250), anti-CD3 (Dako Cytomation, A0452; 1:100), or anti-CD68 antibody (Dako Cytomation, M0814; 1:1000) in 4% normal goat serum at 4°C overnight, followed by incubation with anti-rabbit IgG secondary antibody diluted 1:200 in 2% serum for 2 hours at room temperature. After further washing, antigen-antibody complexes were visualized using a Vectastain ABC peroxidase kit (Vector Laboratories). Antigen detection was enhanced with nickel-diaminobenzidine, followed by incubation with Tris-cobalt. Slides were counterstained with Nuclear Fast Red for photomicroscopy. Blinded digital images of the uteri (5 nonoverlapping random fields per animal and 5 animals per group) were quantified for inflammatory cells, namely neutrophil (MPO+), monocyte/macrophage (CD68+), and T cells (CD3+). Connexin 43 staining intensity was also quantified based on color threshold and expressed as stained area relative to total area (Metamorph; Molecular Devices).
Histologic Evaluation of Fetal Membranes for Chorioamnionitis
Hematoxylin-eosin–stained sections of fetal membranes were scored in a blinded manner (by S. G. K.) for chorioamnionitis using criteria outlined by Redline et al [22], based on numbers and depth of neutrophil infiltration of the tissue.
RNA Isolation, Complementary DNA Generation, and Quantitative Reverse-Transcription PCR
Total RNA was extracted from snap frozen fetal lung, maternal uterus, and chorioamnion-decidua after homogenizing in TRIzol. RNA concentration and quality was measured with a Nanodrop spectrophotometer. Reverse-transcription (RT) of RNA was performed using Verso complementary DNA synthesis kits. Quantitative RT-PCR used a StepOnePlus Real Time PCR system following standard cycling conditions. Quantitative RT-PCR was performed with rhesus-specific TaqMan gene expression primers (Thermo-Fisher Scientific). The Eukaryotic 18S ribosomal RNA was the endogenous control for normalization of the target RNAs, and a sample from a control animal (administered intra-amniotic saline solution) was used as a calibrator.
Cytokine Measurements
Cytokine/chemokine concentrations in the amniotic fluid and alveolar wash fluid were determined using Luminex technology with Non-Human Primate Multiplex kits (Millipore). Concentrations were calculated from standard curves for recombinant proteins.
Statistical Analyses
GraphPad Prism software (version 7.01) was used to graph and analyze statistical significance. Values were expressed as mean (standard deviation) or median (interquartile range). Statistical differences between groups were analyzed using nonparametric Mann–Whitney U tests. Results were considered significantly different at P ≤ .05. Considering the limited number of animals per group, we also report trends (0.05 < P < .10).
RESULTS
Intra-amniotic Injection of U. parvum Caused Minimal Inflammation in the Amniotic Cavity
The amniotic fluids from all the U. parvum–injected animals were U. parvum positive by PCR, whereas U. parvum was not detected in the amniotic fluid or fetal membranes from any of the control animals. The amniotic fluid U. parvum titer increased by 4 logs from 3 to 7 days after intra-amniotic injection, with wide variation for both the 3- and 7-day U. parvum groups (Table 1). In marked contrast to its robust growth in the amniotic fluid, U. parvum grew poorly from the fetal membranes. Although the PCR was positive in fetal membranes from 4 of 5 animals in each group, U. parvum could be cultured from only 1 of 5 fetal membranes from the 3-day group, and 2 of 5 from the 7-day group.
Histologic chorioamnionitis was present in only 1 of 5 animals in the 7-day U. parvum group (Table 1). Compared with controls, CCL2 (monocyte chemoattractant protein [MCP]) was the only chemokine increased in the amniotic fluid of the 7-day U. parvum group, although all measured cytokines and chemokines were numerically higher at 7 days (Table 2). Neutrophils were not detected in the amniotic fluid of either the control or U. parvum–infected animals (both 3- and 7-day groups). The messenger RNA (mRNA) expression of several cytokines and chemokines (interleukin 1β [IL-1β], interleukin 6, CXCL8, TNF-α, and CCL2) were not increased in the fetal membranes (chorion-amnion decidua) at either 3 or 7 days of intra-amniotic U. parvum infection (Supplementary Table 1). Moreover, there were no changes in the frequency of macrophages, neutrophils, natural killer (NK) cells, NKT cells, T cells, B cells, or regulatory T cells (Tregs) in the dissociated chorio-decidua cells either 3 or 7 days after intra-amniotic U. parvum (Supplementary Table 2), further confirming the histologic scoring for chorioamnionitis.
Table 2.
Amniotic Fluid Cytokine Levels
| Study Groupa | Cytokine Level, Median (IQR), ng/mL |
||||||
|---|---|---|---|---|---|---|---|
| IL-1β | IL-6 | CXCL8 | TNF-α | CCL2 | IL-1ra | IL-10 | |
| Control (n = 5) | 0.4 (0.01–0.4) | 396 (301–893) | 138 (67–946) | 0.1 (0.1–21) | 954 (521–1152) | 208 (142–289) | 0.1 (0.1–3) |
| U. parvum infected, 3 d (n = 5) | 0.4 (0.01–0.4) | 360 (210–859) | 147 (119–700) | 0.1 (0.1–6.4) | 673 (512–878) | 90 (27–213) | 0.1 (0.1–0.1) |
| U. parvum infected, 7 d (n = 5) | 0.6 (0.6–1.2) | 532 (426–1006) | 635 (323–1486) | 2.5 (5.8–55.9) | 2231 (1416–2541)b | 257 (172–644) | 0.1 (0.1–27.6) |
Abbreviations: IL-1β, interleukin 1β; IL-1ra, interleukin 1ra; IL-6, interleukin 6; IL-10, interleukin 10; IQR, interquartile range; TNF, tumor necrosis factor; U. parvum, Ureaplasma parvum.
aU. parvum (1 × 107 colony-forming units) was given via intra-amniotic injection.
bP < .05 vs control.
Intra-amniotic U. parvum Infection Induced Modest Fetal Lung Inflammation
Consistent with aspiration of the infected amniotic fluid, U. parvum was detected in the fetal lung of all U. parvum–inoculated animals except 1 at 3 days. CXCL8 (interleukin 8) (8-fold) and IL-1ß (4-fold) mRNAs increased at 7 days after inoculation with no change at 3 days, compared with control animals (Figure 1A). Similarly, alveolar wash protein levels for TNF-α (6-fold), CXCL8 (interleukin 8) (15-fold), and CCL2 (MCP-1) (14-fold) increased from control levels 7 days after intra-amniotic inoculation with U. parvum (Figure 1B). Unlike the mRNA expression, there were no significant changes in IL-1ß protein in the alveolar wash fluid. Bronchoalveolar lavage fluid did not contain neutrophils or macrophages in either the controls or U. parvum–inoculated animals at 3 days. Only 1 in 5 fetuses in the U. parvum 7-day group had a few neutrophils and monocytes in the bronchoalveolar lavage fluid. There were no changes in mRNA expression for surfactant proteins A, B, C, and D (data not shown).
Figure 1.
Intra-amniotic Ureaplasma parvum–induced modest fetal lung inflammation. A, Messenger RNAs (mRNAs) were isolated from the right lower lobe of the fetal lung. Quantitative polymerase chain reaction was performed using rhesus-specific TaqMan probes. The values in box-and-whisker plots were first internally normalized to the endogenous 18S RNA, and the resultant values for the experimental animals were shown as fold increase compared with the mean value for the controls. B, Box-and-whisker plots show cytokine protein levels in alveolar wash fluid from controls and U. parvum–infected animals at 3 and 7 days. Boxes represent 25th–75th percentiles; horizontal lines, medians; and the whiskers, represent 5th and 95th percentile values (n = 5 per group); *P < .05 (Mann–Whitney U test). A strong trend toward significance was observed for IL-6 protein levels at 7 days, CXCL8 protein levels at 3 days, and CCL2 protein levels at 3 days in alveolar wash fluid (all P = .06 vs controls; Mann–Whitney U test). Abbreviations: IL-1β, interleukin 1β; IL-6, interleukin 6; TNF, tumor necrosis factor.
Intra-amniotic U. parvum Infection Did Not Induce Fetal Brain Inflammation
U. parvum was detected with PCR in only 1 of 5 fetal brains (thalamus) 3 days after exposure and none of 5 7 days after exposure. Brain inflammation was measured by quantitative RT-PCR for IL-1β, CCL2, TNF-α, interleukin 10, cyclooxygenase 2, and prostaglandin E synthase 2 in the cortex, periventricular white matter, thalamus, hippocampus, and cerebellum. Exposure to intra-amniotic U. parvum did not increase the mRNA expression of proinflammatory cytokines in any of these brain areas (data not shown). Similarly, none of the cytokines increased in the cerebrospinal fluid (data not shown). Immunohistology demonstrated that there was no infiltration of CD3+ or CD4+ lymphocytes, nor any cellular changes in microglia (Iba-1), oligodendrocytes (Olig2), astrocytes (GFAP), neurons (NeuN), myelin basic protein, or apoptosis (caspase 3) in any of the brain areas (data not shown) (methods for brain analysis provided in Supplementary information).
Intra-amniotic U. parvum Infection Decreased Tregs Proportion in Thymus and Peripheral Lymphoid Organs, and Activated a Th1-Type Profile in the Fetal Tregs
Systemic dissemination of the intra-amniotic U. parvum in the fetus was evident in only 2 of 5 fetal spleens at 7 day and none at 3 days. We previously reported that exposure to chorioamnionitis (from IL-1ß or lipopolysaccharide [LPS]) rapidly decreased Treg proportions in fetal lymphoid organs in preterm rhesus monkey [23, 24]. We thus analyzed the effect of U. parvum exposure on immune cells and subsets. Compared with control animals, U. parvum exposure did not change the absolute number of fetal thymocytes or the proportions of the major thymocyte subsets (ie, progenitors, double-negative, double-positive, or single-positive CD4+ or CD8+ thymocytes; data not shown). However, the proportion of FOXP3+ thymic Tregs within the single-positive CD4+ thymocytes was significantly decreased 3 days after U. parvum infection, with a variable nonsignificant decrease at 7 days (Figure 2C).
Figure 2.
Intra-amniotic Ureaplasma parvum infection decreased fetal regulatory T cell (Treg) proportion. Cell suspensions from the thymus, spleen and, blood mononuclear cells were analyzed by flow cytometry. Box-and-whisker plots show frequency of Tregs (defined as forkhead box [FOX] P3+) within total CD4+CD3+ T cells in cord blood mononuclear cells (A), spleen (B), and total CD4 single-positive thymocytes (C), defined as CD34−CD7+CD3+CD4+CD8a− cells. Boxes represents 25th–75th percentiles; horizontal line, medians; and whiskers, 5th and 95th percentile values (n = 5–6 per group). *P < .05 vs controls (Mann–Whitney U test).
In the periphery (cord blood, spleen), U. parvum exposure had no effect on total CD8 and CD4 T-cell frequency and absolute counts, or on NK cells (CD3−CD8−NKG2A+). Similarly, compared with controls, the frequency of monocytes (CD14+), plasmacytoid dendritic cells (HLA-DR+CD11c−CD123+), and myeloid dendritic cells (HLA-DR+CD11c+CD123−), as well as their activation status (CD40, CD80, CD86 expression), was unchanged in the blood or lymphoid organs of U. parvum–exposed fetuses (data not shown). However, as in the thymus, the U. parvum–exposed animals at 3 days also had significantly lower proportion of CD4+FOXP3+ blood and spleen Tregs (Figure 2A and 2B; gating strategy in Supplementary Figure 1A). Absolute numbers of splenic Tregs in U. parvum animals were also significantly decreased compared to control animals (×106/g)(5.2 [2.2] vs 2.5 [1.9]; P = .03, mean [SD]). Similar decreases in Tregs were also detected in the mesenteric lymph nodes 3 days after intra-amniotic U. parvum infection (data not shown). Again, as in the thymus, peripheral Tregs started to rebound 7 days after inoculation (Figure 2).
We next investigated whether U. parvum changed the expression profile of cytokines (IFN-γ and interleukin 17 [IL-17], 2, and 22) in fetal T and NK cells after a short PMA/ionomycin ex vivo challenge. The percentage of IFN-γ–expressing CD4+ splenic T cells was significantly increased at day 3 (Figure 3A; gating strategies in Supplementary Figure 1B), and similar increases were observed in the blood and mesenteric lymph nodes (data not shown). This activation was selective for IFN-γ, because the frequency of cells expressing interleukin 2, IL-17, and interleukin 22 did not change (data not shown). In the U. parvum 7-day group, the proportion of IFN+CD4+ T cells tended to remain high, although the difference from controls was not significant (Figure 3A). We previously showed that fetal Tregs express effector cytokines, and the percentage of these multifunctional Tregs increases with inflammation [24]. Therefore, we analyzed which subsets contribute to the enhanced IFN-γ expression. Within the CD4+ population, up-regulation of IFN-γ expression was restricted to the FOXP3+ cells (Figure 3B). Similarly, expression of IFN-γ by CD8+ T or NK cells was unchanged (data not shown). These IFN-γ+FOXP3+ cells had increased cell cycling compared with their IFN-γ− counterparts, as indicated by their enhanced expression of Ki67 (Figure 3C ; gating strategy in Supplementary Figure 1C).
Figure 3.
Intra-amniotic Ureaplasma parvum activated a T-helper (Th) 1–type profile in fetal CD4+ forkhead box (FOX) P3+ regulatory T cells (Tregs). Cell suspensions were prepared from spleen and stimulated with PMA and ionomycin for 5 hours, with brefeldin A and monensin added for the last 4 hours. Expression of intracellular interferon (IFN) γ was analyzed in different subsets by flow cytometry. A, Frequency of total IFN-γ+ cells within CD4+CD3+ T cells. B, Frequency of IFN-γ+ expressing cells within the FOXP3+CD4+CD3+ and FOXP3− CD4+CD3+ T-cell populations. C, Frequency of the cell cycle protein Ki67+ cells within IFN-γ+ or IFN-γ− FOXP3 expressing splenic Tregs. Boxes represent 25th–75th percentiles; horizontal lines, medians; and whiskers, 5th and 95th percentile values (n = 5–6 per group); *P < .05 vs controls. In control animals, IFN-γ+FOXP3+ Tregs also tended to express more Ki67 than their IFN-γ− counterparts (P = .09; Mann–Whitney U test).
Intra-amniotic Injection of U. parvum Caused Uterine Inflammation
Although U. parvum was not detected with PCR in any of the maternal uteri at 3 days, it was present in 3 of 5 uteri at 7 days. Interestingly, in contrast to the very limited effects on fetal membranes, intra-amniotic U. parvum increased IL-1ß (3–4 fold), interleukin 6 (4–5 fold), and cyclooxygenase 2 (4–5 fold) mRNA expression in the uterus at both 3 and 7 days after exposure, compared with control animals (Figure 4A). Uterine TNF-α, MCP-1, and CXCL8 mRNA expression was also variably but not significantly increased. Similarly, the mRNA expression for prostaglandin F receptor (PTGFR), inducible nitric oxide synthase, matrix metalloproteinase 2, 8, and 9, and vascular endothelial growth factor A and C in the uterus did not change (Supplementary Table 3). There were modest but significant increases in neutrophil (MPO+), monocyte/macrophage (CD68+), and T cells (CD3+) in uterine tissue (Supplementary Figure 2). The inflammatory cells seemed to be located within the capillaries interspersed between smooth muscle cells rather than infiltrating the tissue. Connexin 43, a gap junction protein, is essential for parturition [25]. Compared with controls, intra-amniotic U. parvum increased the expression of connexin 43 at both 3 and 7 days, as detected by immunohistochemistry (Figure 4B and 4C). However, no change was seen in the expression of other genes associated with uterine contraction, namely, oxytocin receptor, myosin light chain kinase, RhoA, Rho-associated coiled-coil containing protein kinase 1 and 2, and finger E-Box binding homeobox 1 and 2 (Supplementary Table 3).
Figure 4.
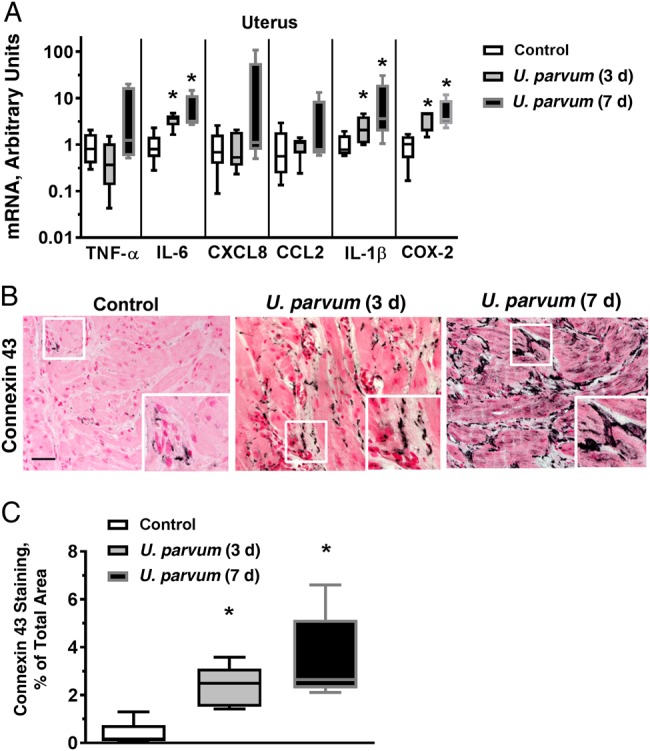
Intra-amniotic Ureaplasma parvum–induced modest inflammation and increased connexin 43 in the uterus. A, Messenger RNAs (mRNAs) were isolated from full-thickness uterine tissue including the adherent decidua. Quantitative PCR was performed using rhesus-specific Taqman probes. The values were first internally normalized to the endogenous 18S RNA, and the resultant values for the experimental animals were shown as fold increase compared with the mean control value. *P < .05 vs controls (Mann–Whitney U test). B, Immunostaining was performed for the contraction-associated gap junction protein connexin 43. Representative photomicrograph is shown for each study group; the inset in each panel shows a higher-magnification photomicrograph. Note more intensely stained areas of connexin 43 in both U. parvum groups compared with control (scale bar, 50 µm). C, Image quantitation for the connexin 43 immunostaining was performed and expressed as stained area relative to total area (mean of 5 random fields per animal was used as representative value for each animal). Boxes represents 25th–75th percentile; horizontal lines, medians; and whiskers, 5th and 95th percentile values (n = 5 per group). *P < .05 vs controls (Mann–Whitney U test). Abbreviations: IL-1β, interleukin 1β; IL-6, interleukin 6; TNF, tumor necrosis factor.
DISCUSSION
Although Ureaplasma species are the most common organisms associated with intrauterine infection [3, 4], their contribution to perinatal pathology is still not well understood [26, 27]. Part of the problem is that in humans, naturally occurring Ureaplasma infections are commonly polymicrobial [3, 28]. We used a preterm rhesus macaque model and a well characterized U. parvum serovar to explore these relationships, and our results provide a nuanced picture of immune changes and inflammation in different fetal-maternal compartments with intrauterine infection with U. parvum.
The lack of chorioamnionitis was surprising given prior reports by Novy et al [15, 29] demonstrating severe chorioamnionitis in rhesus macaques at about the same gestational age. We used the same strain, dose, and source of U. parvum and the organism efficiently grew in the amniotic fluid, but unlike those studied by Novy et al, the rhesus macaques in our study were not instrumented. The lack of infiltration of inflammatory cells in the decidua after U. parvum is in sharp contrast to a robust deciduitis induced by intra-amniotic IL-1ß [21]. Our results in the rhesus are thus more reminiscent of modest inflammatory response in the maternal compartments in fetal sheep [10, 30] and consistent with a finding that ≥25% of amniocenteses or placentas with Ureaplasma species in humans did not have histologic chorioamnionitis or amniotic inflammation [4, 31]. We also found no significant growth of U. parvum in the rhesus fetal membranes. These observations raise a question regarding the ability of U. parvum to directly attach to and signal intact amniotic epithelium in vivo, although U. parvum was reported to signal dissociated amniotic epithelial cells in vitro [32]. The presence of other microorganisms along with U. parvum in the amniotic fluid or placenta may modulate its virulence [4, 28].
Consistent with fetal airways being in contact with contents of the amniotic fluid, we demonstrated fetal lung colonization with U. parvum in all the animals. However, the fetal inflammation was modest, similar to our data in the fetal sheep [30]. Surfactant protein mRNAs were also not increased after intra-amniotic U. parvum infection. U. parvum–induced lung maturational changes were measured after chronic exposure (45 days) to intra-amniotic U. parvum in sheep, but not after shorter exposures (3–14 days) [30, 33]. Similarly, although dissemination of U. parvum in the fetal central nervous system was described clinically [9], we could not detect invasion of U. parvum in the fetal brain, nor could we detect inflammation or injury responses in the brain. In contrast, intra-amniotic LPS causes brain inflammation, microglia activation, and impaired myelination in the fetal rhesus macaque [34], and fetal sheep [35]. Novy et al also infrequently detected U. parvum in fetal brains of rhesus macaques, with culture or PCR [15].
An important finding of our study is that U. parvum decreased the CD4+FOXP3+ Treg population, in both the thymus and the periphery. Wolfs et al [36] reported that the proportion of CD4+FOXP3+ cells decreased in the gut of U. parvum–exposed fetal sheep at 3 and 7 days after infection, but this study did not characterize fetal immune responses in depth. In our current study, the effect on Tregs was surprising because the levels of inflammatory cytokines measured in the amniotic fluid and fetal plasma were unchanged or modestly increased after U. parvum infection, although we might have missed an early increase (before 3 days) in amniotic fluid cytokines, as previously reported [15]. Furthermore, U. parvum–induced Treg changes (mainly at 3 days) preceded higher amniotic fluid colonization rates (colony-forming unit counts were higher on day 7 than on day 3). Similarly to U. parvum infection, both intra-amniotic IL-1ß and LPS diminished the proportion and absolute number of fetal Tregs [23, 24]. However, U. parvum induced a IFN-γ response in Tregs (with approximately 25% of fetal spleen Tregs adopting a bifunctional phenotype), whereas intra-amniotic LPS induced a predominant IL-17 response [24].
This finding is new and provocative, because neonatal T-helper (Th) 1 responses have long been considered impaired, mostly because neonatal antigen-presenting-cells seem deficient at producing the major pro-Th1 cytokine, interleukin 12 (reviewed in [37]). However, this concept has been challenged recently, because IFN-γ expression was detected in a population of activated neonatal T cells in the absence [38] or presence [39] of exposure to maternal inflammation. These results thus suggest the existence of a subset of cells that can develop a Th1 phenotype early in life, and this response may be increased in the presence of inflammation. Future studies will be needed to better understand the mechanisms that dictate the response of fetal T cells to in utero inflammation, particularly the emergence and functional characteristics of these “inflammatory” Tregs.
The effects of intra-amniotic U. parvum infection on the uterus is a novel observation. There were small but significant increases in proinflammatory cytokine mRNA expression, increases in leukocytes, mostly in the capillaries, and increased expression of connexin 43 in the uterus after exposure to U. parvum. These findings are consistent with intrauterine U. parvum inducing preterm labor in rhesus monkey [15, 29]. However, we did not detect overt preterm labor in any of the intra-amniotic U. parvum groups. Because uterine inflammation was detected in the absence of U. parvum demonstrable by PCR at 3 days, the results suggest that indirect signaling to the uterus via the amniotic compartment may play a role in pathogenesis.
In conclusion, U. parvum efficiently colonized rhesus macaque amniotic fluid, causing maternal uterine inflammation but no chorioamnionitis. Despite the modest fetal inflammation, Treg populations decreased in the fetal thymus and peripheral lymphoid organs, adopting an “inflammatory” phenotype. These results mirror the moderate pathogenicity of Ureaplasma in human pregnancy. However, our results also suggest that infections with indolent microorganisms such as U. parvum can affect the developing fetal immune system. Their influence might be underestimated owing to the absence of overt chorioamnionitis at birth.
Supplementary Material
Notes
Acknowledgments. We thank the California National Primate Research Center staff for its outstanding technical support, especially Sarah Davis, Jennifer Vandevegte, and Sona Santos for their invaluable help in animal management and autopsy. We also thank Manuel Alvarez for excellent technical support and Donna Crabb and Amy Ratliff, University of Alabama at Birmingham Diagnostic Mycoplasma Laboratory, for technical assistance in performing Ureaplasma cultures and PCR assays.
Financial support. This work was supported by Burroughs-Wellcome Fund, Preterm Initiative (C. A. C.), March of Dimes Innovation (catalyst award to S. G. K.), Cincinnati Children's Hospital Medical Center (Arnold W. Strauss fellow research award to A. F. S.), and National Primate Research Centers Program (base grant P51OD011107 to L. A. M.).
Potential conflicts of interest. All authors: No potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Liu L, Johnson HL, Cousens S et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 2012; 379:2151–61. [DOI] [PubMed] [Google Scholar]
- 2. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008; 371:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. DiGiulio DB. Diversity of microbes in amniotic fluid. Semin Fetal Neonatal Med 2012; 17:2–11. [DOI] [PubMed] [Google Scholar]
- 4. Combs CA, Gravett M, Garite TJ et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol 2014; 210:125.e1–15. [DOI] [PubMed] [Google Scholar]
- 5. DiGiulio DB, Callahan BJ, McMurdie PJ et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc Natl Acad Sci U S A 2015; 112:11060–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kacerovsky M, Vrbacky F, Kutova R et al. Cervical microbiota in women with preterm prelabor rupture of membranes. PLoS One 2015; 10:e0126884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lowe J, Watkins WJ, Edwards MO et al. Association between pulmonary Ureaplasma colonization and bronchopulmonary dysplasia in preterm infants: updated systematic review and meta-analysis. Pediatr Infect Dis J 2014; 33:697–702. [DOI] [PubMed] [Google Scholar]
- 8. Okogbule-Wonodi AC, Gross GW, Sun CC et al. Necrotizing enterocolitis is associated with Ureaplasma colonization in preterm infants. Pediatr Res 2011; 69:442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Viscardi RM, Hashmi N, Gross GW, Sun CC, Rodriguez A, Fairchild KD. Incidence of invasive Ureaplasma in VLBW infants: relationship to severe intraventricular hemorrhage. J Perinatol 2008; 28:759–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Knox CL, Dando SJ, Nitsos I et al. The severity of chorioamnionitis in pregnant sheep is associated with in vivo variation of the surface-exposed multiple-banded antigen/gene of Ureaplasma parvum. Biol Reprod 2010; 83:415–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dando SJ, Nitsos I, Kallapur SG et al. The role of the multiple banded antigen of Ureaplasma parvum in intra-amniotic infection: major virulence factor or decoy? PLoS One 2012; 7:e29856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gravett MG, Haluska GJ, Cook MJ, Novy MJ. Fetal and maternal endocrine responses to experimental intrauterine infection in rhesus monkeys. Am J Obstet Gynecol 1996; 174:1725–33. [DOI] [PubMed] [Google Scholar]
- 13. Golos TG, Bondarenko GI, Dambaeva SV, Breburda EE, Durning M. On the role of placental major histocompatibility complex and decidual leukocytes in implantation and pregnancy success using non-human primate models. Int J Dev Biol 2010; 54:431–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dambaeva SV, Breburda EE, Durning M, Garthwaite MA, Golos TG. Characterization of decidual leukocyte populations in cynomolgus and vervet monkeys. J Reprod Immunol 2009; 80:57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Novy MJ, Duffy L, Axthelm MK et al. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod Sci 2009; 16:56–70. [DOI] [PubMed] [Google Scholar]
- 16. Waites KB, Duffy LB, Schwartz S, Talkington DF. Mycoplasma and Ureaplasma. In: Garcia L, ed. Clinical microbiology procedure handbook. 3rd ed Washington, DC: American Society for Microbiology Press, 2010:3.15.1–3.7. [Google Scholar]
- 17. Xiao L, Glass J, Paralanov V et al. Detection and characterization of human Ureaplasma species and serovars by real-time PCR. J Clin Microbiol 2010; 48:2715–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mulder WM, Koenen H, van de Muysenberg AJ, Bloemena E, Wagstaff J, Scheper RJ. Reduced expression of distinct T-cell CD molecules by collagenase/DNase treatment. Cancer Immunol Immunother 1994; 38:253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van Damme N, Baeten D, De Vos M et al. Chemical agents and enzymes used for the extraction of gut lymphocytes influence flow cytometric detection of T cell surface markers. J Immunol Methods 2000; 236:27–35. [DOI] [PubMed] [Google Scholar]
- 20. Abuzakouk M, Feighery C, O'Farrelly C. Collagenase and dispase enzymes disrupt lymphocyte surface molecules. J Immunol Methods 1996; 194:211–6. [DOI] [PubMed] [Google Scholar]
- 21. Presicce P, Senthamaraikannan P, Alvarez M et al. Neutrophil recruitment and activation in decidua with intra-amniotic IL-1beta in the preterm rhesus macaque. Biol Reprod 2015; 92:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol 2003; 6:435–48. [DOI] [PubMed] [Google Scholar]
- 23. Kallapur SG, Presicce P, Senthamaraikannan P et al. Intra-amniotic IL-1beta induces fetal inflammation in rhesus monkeys and alters the regulatory T cell/IL-17 balance. J Immunol 2013; 191:1102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rueda CM, Presicce P, Jackson CM et al. Lipopolysaccharide-induced chorioamnionitis promotes IL-1-dependent inflammatory FOXP3+ CD4+ T cells in the fetal rhesus macaque. J Immunol 2016; 196:3706–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tong D, Lu X, Wang HX et al. A dominant loss-of-function GJA1 (Cx43) mutant impairs parturition in the mouse. Biol Reprod 2009; 80:1099–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Viscardi RM, Kallapur SG. Role of Ureaplasma respiratory tract colonization in bronchopulmonary dysplasia pathogenesis: current concepts and update. Clin Perinatol 2015; 42:719–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kallapur SG, Kramer BW, Jobe AH. Ureaplasma and BPD. Semin Perinatol 2013; 37:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Onderdonk AB, Delaney ML, DuBois AM, Allred EN, Leviton A. Detection of bacteria in placental tissues obtained from extremely low gestational age neonates. Am J Obstet Gynecol 2008; 198:110.e1–7. [DOI] [PubMed] [Google Scholar]
- 29. Grigsby PL, Novy MJ, Sadowsky DW et al. Maternal azithromycin therapy for Ureaplasma intra-amniotic infection delays preterm delivery and reduces fetal lung injury in a primate model. Am J Obstet Gynecol 2012; 207:475.e1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Collins JJ, Kallapur SG, Knox CL et al. Inflammation in fetal sheep from intra-amniotic injection of Ureaplasma parvum. Am J Physiol Lung Cell Mol Physiol 2010; 299:L852–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sweeney EL, Kallapur SG, Gisslen T et al. Placental infection with Ureaplasma species is associated with histologic chorioamnionitis and adverse outcomes in moderately preterm and late-preterm infants. J Infect Dis 2016; 213:1340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Triantafilou M, De Glanville B, Aboklaish AF, Spiller OB, Kotecha S, Triantafilou K. Synergic activation of toll-like receptor (TLR) 2/6 and 9 in response to Ureaplasma parvum & urealyticum in human amniotic epithelial cells. PLoS One 2013; 8:e61199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moss TJ, Knox CL, Kallapur SG et al. Experimental amniotic fluid infection in sheep: effects of Ureaplasma parvum serovars 3 and 6 on preterm or term fetal sheep. Am J Obstet Gynecol 2008; 198:122.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schmidt AF, Kannan PS, Chougnet CA et al. Intraamniotic LPS causes acute neuro-inflammation in preterm rhesus macaques. J Neuroinflammation 2016; 13:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kuypers E, Jellema RK, Ophelders DR et al. Effects of intra-amniotic lipopolysaccharide and maternal betamethasone on brain inflammation in fetal sheep. PLoS One 2013; 8:e81644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wolfs TG, Kallapur SG, Knox CL et al. Antenatal Ureaplasma infection impairs development of the fetal ovine gut in an IL-1-dependent manner. Mucosal Immunol 2013; 6:547–56. [DOI] [PubMed] [Google Scholar]
- 37. Velilla PA, Rugeles MT, Chougnet CA. Defective antigen-presenting cell function in human neonates. Clin Immunol 2006; 121:251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang X, Mozeleski B, Lemoine S et al. CD4 T cells with effector memory phenotype and function develop in the sterile environment of the fetus. Sci Transl Med 2014; 6:238ra72. [DOI] [PubMed] [Google Scholar]
- 39. Matsuoka T, Matsubara T, Katayama K, Takeda K, Koga M, Furukawa S. Increase of cord blood cytokine-producing T cells in intrauterine infection. Pediatr Int 2001; 43:453–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





