Abstract
Background. In Uganda, the tuberculosis vaccine BCG is administered on the first day of life. Infants delivered at home receive BCG vaccine at their first healthcare facility visit at 6 weeks of age. Our aim was to determine the effect of this delay in BCG vaccination on the induced immune response.
Methods. We assessed CD4+ and CD8+ T-cell responses with a 12-hour whole-blood intracellular cytokine/cytotoxic marker assay, and with a 6-day proliferation assay.
Results. We enrolled 92 infants: 50 had received BCG vaccine at birth and 42 at 6 weeks of age. Birth vaccination was associated with (1) greater induction of CD4+ and CD8+ T cells expressing either interferon γ (IFN-γ) alone or IFN-γ together with perforin and (2) induction of proliferating cells that had greater capacity to produce IFN-γ, tumor necrosis factor α (TNF-α), and interleukin 2 together, compared with delayed vaccination.
Conclusions. Distinct patterns of T-cell induction occurred when BCG vaccine was given at birth and at 6 weeks of age. We propose that this diversity might impact protection against tuberculosis. Our results differ from those of studies of delayed BCG vaccination in South Africa and the Gambia, suggesting that geographical and population heterogeneity may affect the BCG vaccine–induced T-cell response.
Keywords: Uganda, BCG, vaccination, birth, delayed, CD4+ and CD8+ T-cell responses
BCG vaccine is the only vaccine licensed for prevention of childhood tuberculosis [1]. BCG protects infants against severe forms of tuberculosis (meningitis and miliary tuberculosis) [2] and has a positive influence on overall infant morbidity and mortality [3]. Therefore, in settings where the Mycobacterium tuberculosis exposure risk is high, the World Health Organization (WHO) recommends BCG vaccination soon after birth [4].
In Uganda, tuberculosis is endemic [5], and BCG vaccine is routinely administered within 24 hours after birth among infants born in a healthcare facility. However, up to 50% of babies are born at home [6, 7]. These infants commonly receive BCG vaccine at the first contact with a healthcare facility, usually at 6 weeks of age, when other WHO Expanded Programme on Immunization–recommended vaccines are administered [8]. We aimed to assess the effect of this delay on the immune response induced by BCG vaccine.
We proposed to investigate CD4+ and CD8+ T-cell immunity, which is thought to be critical in the control of M. tuberculosis. BCG vaccine induces a T-helper type 1 (Th1) response, characterized by production of interferon γ (IFN-γ), tumor necrosis factor α (TNF-α), and interleukin 2 (IL-2) [9–12]. BCG vaccine also induces cytotoxic T cells [13], as well as interleukin 17 (IL-17; Th17)–producing T cells [14].
Compared with adults, neonates' innate immune cells produce less Th1-promoting interleukin 12 (IL-12) [15] and display diminished Toll-like receptor 4 (TLR) expression and signaling [16]. This potentially results in a suboptimal response to BCG vaccine. At 6 weeks of age, the infant immune system would start adapting to the ex utero environment [17], and enhanced priming of BCG-specific immune responses might occur. Multiple studies have investigated the effects of delaying BCG vaccination. Comparable purified protein derivative (PPD)–induced IFN-γ production was shown between infants vaccinated at birth or later [18–20]. In contrast, a South African study showed that delaying BCG vaccination from birth to 10 weeks of age induced a greater frequency of BCG-specific polyfunctional CD4+ T cells (ie, cells that express IFN-γ, TNF-α, and IL-2 together) [21]. Importantly, in all previous studies, infants were randomized to receive BCG vaccine at birth or later. In contrast, we addressed the effects in a setting where delayed vaccination occurred due to home births. We hypothesized that BCG vaccination at 6 weeks of age would result in an enhanced specific T-cell response, compared with administration at birth. We report a comprehensive immunological assessment of delayed BCG vaccination.
METHODS
Study Population and Sample Collection
Healthy 9-month-old infants were enrolled at the Child Health and Development Center in Mulago National Referral Hospital, Kampala, Uganda. A child health growth card was used to identify infants who received BCG vaccine at birth or at 6 weeks of age.
Infants were excluded if the mother had documented evidence of a positive HIV test result or had not participated in a program to prevent mother-to-child HIV transmission and if the infant lacked a BCG vaccination scar, was born before 37 weeks of gestation, had significant perinatal complications, had any acute or chronic disease symptoms at the time of enrollment or clinically apparent anemia, had a household contact with tuberculosis, or had an unexplained persistent cough or confirmed active tuberculosis.
The study was approved by the institutional review board of the School of Public Health, Makerere University College of Health Sciences, and the Uganda National Council for Science and Technology. Good clinical practice procedures were adhered to.
Blood Collection and Processing
From each study participant, a 4-mL whole-blood specimen was collected in a sodium-heparin tube and was transported to the laboratory for processing within 1 hour. One milliliter of heparinized whole blood was incubated with either BCG (Danish strain 1331; Statens Serum Institut; 1.2 × 106 colony-forming units [CFU]/mL) or phytohemagglutinin (PHA; Sigma-Aldrich; 5 µg/mL) or was left unstimulated, as previously described [22]. The costimulatory antibodies anti-CD28 and anti-CD49d (at 1 µg/mL each; BD Biosciences, San Jose, CA) were added to all assay conditions to enhance the responses [23]. Blood was incubated at 37°C for 7 hours, after which plasma was removed and stored at −80°C for later measurement of soluble cytokines. Thereafter, brefeldin A (Sigma-Aldrich; 10 µg/m) was added, and the blood was incubated for a further 5 hours. Cells were harvested, fixed in BD FACS Lysing Solution (BD Biosciences), and cryopreserved for later measurement of T-cell–associated cytokine expression.
A further 1 mL of whole blood was diluted in 9 mL of Roswell Park Memorial Institute medium and mixed in a sterile polypropylene tube. One milliliter of diluted blood was incubated with either BCG (Danish strain 1331; Statens Serum Institut; 1 × 105 CFU/mL) or PHA (Sigma-Aldrich; 1 µg/mL, added on the third day of incubation) or was left unstimulated. Incubation continued for 6 days at 37°C in 5% CO2 as previously described [24]. Four hours before the end of the cell culture, phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich; 20 ng/mL), ionomycin (Sigma-Aldrich; 2 µg/mL), and brefeldin A (Sigma-Aldrich; 10 µg/mL) were added to induce cytokine expression. Later, red cells were lysed with BD FACS Lysing Solution (BD Biosciences), and white blood cells were fixed and cryopreserved for measurement of the proliferation and cytokine-producing potential of proliferating cells.
Antibodies
The following monoclonal antibodies were used: anti-CD3 Pacific Blue (UCHT1), anti-CD8 PerCP-Cy5.5 (SK-1), anti-CD8 Horizon V500 (RPA-T8), anti-IFN-γ Alexa Fluor 700 (B27), anti-IL-2 FITC (5344.111), anti-Ki67 PE (B56), anti-CD45RA FITC (HI100), anti-CD27 APC (L128), and anti-CCR7 PE (150 503), from BD Biosciences (San Jose, CA); anti-CD4 QDot605 (S3.5) and anti-CD8 QDot565 (3B5), from Invitrogen (Eugene, OR); anti-TNF-αPE-Cy7 (Mab11) and anti-IL-17 Alexa Fluor 647 (eBio64CAP17), from eBiosciences (San Diego, CA); anti-CD69 PerCP-Cy5.5 (FN50), from Biolegend (San Diego, CA); and anti-Perforin PE (B-D48), from Diaclone (Besancon, France).
Intracellular Cytokine Staining (ICS) Assay
Fixed, cryopreserved white cells from the stimulated whole blood were thawed, washed, and permeabilized before staining, as previously described [22]. Stained cells were acquired on a LSRII flow cytometer (BD Biosciences). After acquisition, data were analyzed using FlowJo software (v9.4.11; Tree Star). Compensation was done with positive and negative anti-mouse immunoglobulin kappa beads (BD Biosciences) labeled with the respective fluorochrome-conjugated antibodies. Cytometer setting and tracking beads (BD Biosciences) were used for daily settings.
Flow cytometry data were exported to Pestle v1.7 (Mario Roederer, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health) and Spice (v5.1) for analysis [25].
Multiplex Soluble Cytokine Assay
Plasma collected after 7 hours of incubation during the short-term whole-blood assay was used to measure levels of IFN-γ, IL-2, interleukin 4 (IL-4), interleukin 5 (IL-5), interleukin 10 (IL-10), and interleukin 13 (IL-13) with the Milliplex MAP assay (Millipore, Billerica, MA), in accordance with the manufacturer's instructions. Fluorescence was detected using a Luminex 100 IS machine (xMAP technology; Luminex). Data were acquired using the Bio-Plex Manager Software. The standards for each assay ranged from 3.2 to 10 000 pg/mL.
Data Analysis
The response detected in the negative controls (unstimulated) was subtracted from the response detected in the BCG-stimulated samples. For short-term ICS, proliferation, and multiplex analyses, samples were excluded from the final analysis if the response detected in the positive control (PHA) was lower than the median plus 3 times the median absolute deviations of the negative control samples for all infants.
For memory phenotype analysis, in addition to the above criteria, data were excluded if (1) the frequency of BCG-specific cells was <0.01%, (2) the ratio of BCG to unstimulated frequencies was <2, and (3) there were <20 positive events in the BCG-stimulated sample minus the number of events in the unstimulated sample.
For multiplex analysis, data were excluded if (1) the positive control response was less than that of the negative control and (2) the positive control response was <3.2 pg/mL.
The Mann–Whitney U test was used to compare immunological outcomes between the 2 groups. A P value of <.05 was considered statistically significant. Spearman rank correlation was used to test for associations between frequencies of the specific T cells and the levels of soluble cytokines. Prism v5.0 (GraphPad Software) was used for statistical analyses. The influence of vaccination group, sex, household income, and weight on the frequency of cytokine-expressing T cells was determined by linear regression analysis.
RESULTS
Participants
We enrolled 92 infants at 9 months of age between October 2008 and February 2009 in Uganda. Fifty of these infants received BCG vaccine at birth and 42 received the vaccine at 6 weeks of age. Six infants who had received BCG vaccine at birth and 2 infants who had received BCG vaccine at 6 weeks of age were excluded because of inadequate blood volumes. The body weight and sex distribution between the 2 groups were not different at recruitment (Table 1). The birth weight for home-born infants was not available. Infants who received BCG vaccine at birth were more likely to be from a household with higher income than infants vaccinated at 6 weeks of age (Table 1).
Table 1.
Demographic Characteristics of the Study Participants at 9 Months of Age
| Variable | Vaccinated at Birth (n = 44) | Vaccinated at 6 Weeks of Age (n = 40) | P |
|---|---|---|---|
| Sex | |||
| Female | 18 (41) | 20 (53) | .29a |
| Male | 26 (59) | 18 (47) | |
| Income | |||
| Less than $125 | 12 (27) | 25 (64) | .001a |
| Greater than $125 | 32 (73) | 14 (36) | |
| Season of BCG vaccination | |||
| Dry | 28 (64) | 24 (60) | .73a |
| Rainy | 16 (36) | 16 (40) | |
| Weight at 9 mo of age, kg | 9.0 (8–9.55) | 8.5 (8–9.45) | .26b |
| Weight-for-age z score at 9 mo of age | 0.27 (−0.70 to 1.08) | 0.04 (−0.42 to 0.89) | .53b |
Data are no. (%) of infants or median (interquartile range).
a By χ2 analysis.
b By the Mann–Whitney U test.
Greater Frequencies of BCG-Specific CD4+ and CD8+ T Cells Expressing IFN-γ, With or Without Perforin, in Infants Vaccinated at Birth, Compared With Infants Vaccinated at 6 Weeks of Age
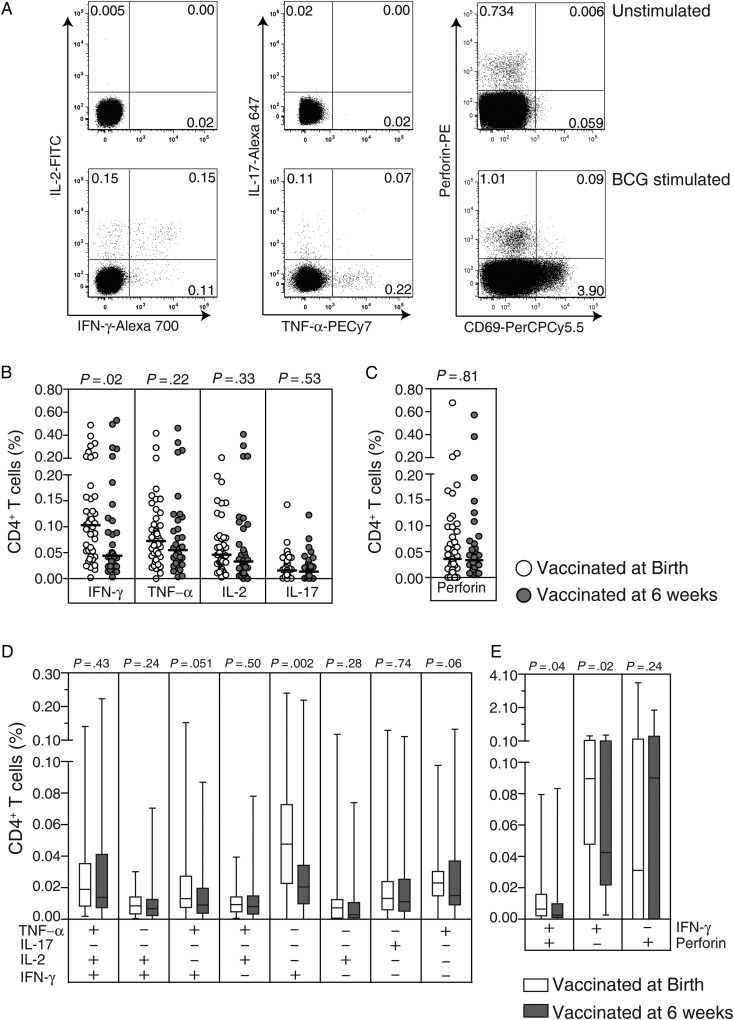
We compared the frequency of BCG-specific IL-2–, IL-17–, IFN-γ–, TNF-α–, and perforin-expressing CD4+ T cells in infants who received BCG vaccine at birth or at 6 weeks of age, using a short-term WB-ICS assay (Figure 1A and Supplementary Figure 1). The great majority of infants vaccinated at either time point had a detectable specific IL-2, IL-17, IFN-γ, TNF-α, and perforin CD4+ T-cell response (Figure 1B and 1C). The frequencies of BCG-specific CD4+ T cells expressing IL-2, IL-17, TNF-α, or perforin were comparable in the 2 vaccination groups (Figure 1B and 1C). However, infants who received BCG vaccine at birth had greater frequencies of IFN-γ–expressing CD4+ T cells, compared with infants vaccinated at 6 weeks of age (Figure 1B).
Figure 1.
Specific CD4+ T-cell cytokine and perforin responses measured in the short-term whole-blood assay. Representative flow cytometry data of cytokine and perforin expression in a negative control (unstimulated) sample (top row) or BCG-stimulated sample (bottom row) from a 9-month-old infant who received BCG vaccine at birth (A). Scatterplots depict frequencies of total: interferon γ (IFN-γ)–, tumor necrosis factor α (TNF-α)–, interleukin 2 (IL-2)–, and interleukin 17 (IL-17)–expressing CD4+ T cells (B) and perforin-expressing CD69+CD4+ T cells (C). In the scatterplots, the horizontal lines represent the median frequencies. Box and whisker plots show the frequencies of distinct subsets of specific CD4+ T cells based on combinations of expression of IFN-γ, TNF-α, IL-2, and IL-17 (D) and the frequencies of CD69+CD4+ T cells expressing IFN-γ and perforin singly or in combination (E). For box and whiskers plots, the horizontal line represents the median, the boxes represent the interquartile range, and the whiskers represent the 10th and 90th percentiles. Values shown are corrected for background responses in the negative control condition. The Mann–Whitney U test was used to assess differences in frequencies of cytokine- or perforin-expressing CD4+ T cells between infants vaccinated at birth (open dots/bars) and 6 weeks of age (closed dots/bars).
Next, we compared the profile of BCG-specific CD4+ T cells expressing IL-2, IL-17, IFN-γ, or TNF-α alone or in different combinations between the 2 groups of infants. We did not observe coexpression of IL-17 with any of the Th1 cytokines (Figure 1D and data not shown), whereas perforin was coexpressed with IFN-γ only (Figure 1E and data not shown). Frequencies of BCG-specific polyfunctional (IL-2+IFN-γ+TNF-α+), double positive (IL-2+IFN-γ+, IL-2+TNF-α+, or IFN-γ+TNF-α+), and single-positive (IL-2+, IL-17+, TNF-α+, or perforin+) CD4+ T-cell subsets were not different between the 2 groups (Figure 1D and 1E). However, the group vaccinated at birth had greater frequencies of BCG-specific IFN-γ single-positive and IFN-γ+perforin+ double-positive CD4+ T cells, compared with those vaccinated at 6 weeks (Figure 1D and 1E).
Next, we analyzed specific CD8+ T cells. In both groups, BCG-specific CD8+ T-cell responses were dominated by IFN-γ and perforin expression (Figure 2B and 2C). As observed for CD4+ T cells, frequencies of BCG-specific IL-2–, IL-17–, TNF-α–, and perforin-expressing CD8+ T cells in both groups were not different, whereas infants who received BCG vaccine at birth had greater frequencies of IFN-γ–expressing CD8+ T cells, compared with infants vaccinated at 6 weeks of age (Figure 2B). Also, similar to CD4+ T cells, the group vaccinated at birth had greater frequencies of specific CD8+ T cells expressing IFN-γ alone (Figure 2D) or in combination with perforin (Figure 2E).
Figure 2.
Specific CD8+ T-cell cytokine and perforin responses measured in the short-term whole-blood assay. Representative flow cytometry data of cytokine and perforin expression in a control (unstimulated) sample (top row) or a BCG-stimulated sample (bottom row) from a 9-month-old infant who received BCG vaccine at birth (A). Scatterplots depict frequencies of total: interferon γ (IFN-γ)–, tumor necrosis factor α (TNF-α)–, interleukin 2 (IL-2)–, and interleukin 17 (IL-17)–expressing CD8+ T cells (B) and perforin-expressing CD69+CD8+ T cells (C). On the scatterplots, the horizontal lines represent the median frequencies. Box and whisker plots show the frequencies of distinct subsets of specific CD8+ T cells based on combinations of cytokine expression of IFN-γ TNF-α IL-2, and IL-17 (D) and the frequencies of CD69+CD8+ T cells expressing IFN-γ and perforin singly or in combination (E). For box and whisker plots, the horizontal line represents the median, the boxes represent the interquartile range, and the whiskers represent the 10th and 90th percentiles. Values shown are corrected for background responses in the negative control condition. The Mann–Whitney U test was used to assess the differences in frequencies of cytokine- or perforin-expressing CD8+ T cells between infants vaccinated at birth (open dots/bars) and 6 weeks of age (closed dots/bars).
No Difference in Proportions of BCG-Specific CD4+ and CD8+ T-Cell Memory Phenotypes Between the 2 Groups
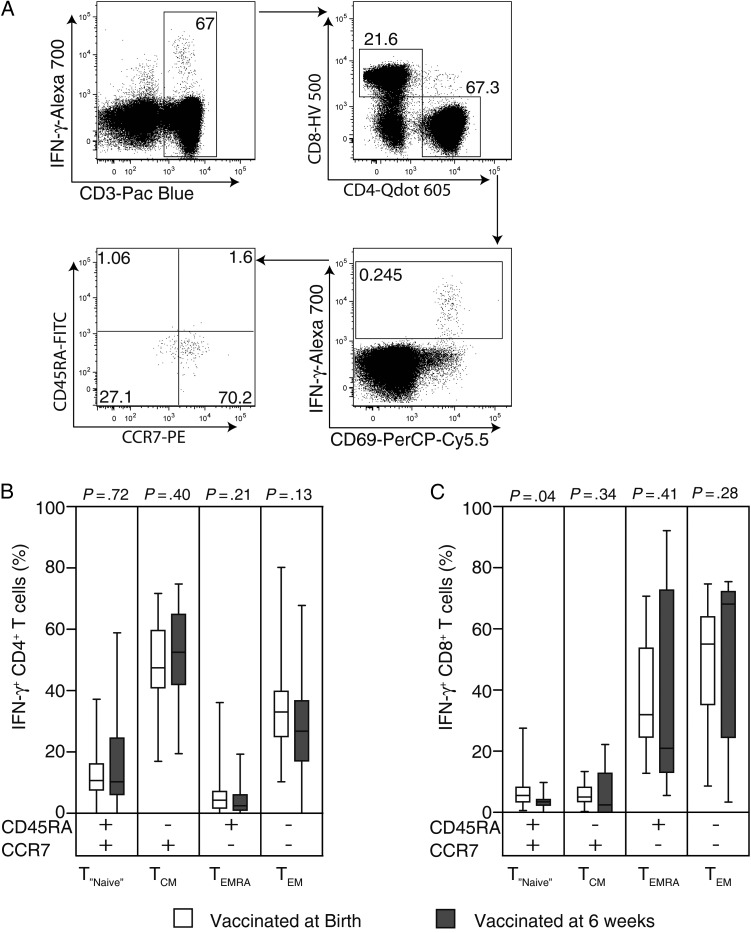
Next, we evaluated whether the observed differences in frequencies of BCG-specific IFN-γ–expressing CD4+ and CD8+ T cells could be associated with differential T-cell memory phenotypes, as defined by CCR7 and CD45RA expression (Figure 3A) [26]. We hypothesized that birth vaccinated infants would show a greater proportion of BCG-specific effector memory (TEM) cells, compared with those vaccinated at 6 weeks of age. Phenotypic markers were measured following stimulation of whole blood for 12 hours.
Figure 3.
Memory phenotype of BCG-specific CD4+ and CD8+ T cells identified in the short-term whole blood intracellular cytokine assay. For the gating strategy to analyze the expression of CD45RA and CCR7 memory markers on BCG-specific CD4+ T cells, CD3+ cells were separated into CD4+ and CD8+ T cells. Then, cells expressing interferon γ (IFN-γ) or CD69, both considered specific to BCG stimulation, were selected from the CD4+ T cells. Finally, CD45RA and CCR7 expression were assessed from these specific cells (A). A similar analysis strategy was used for CD8+ T cells. Box and whiskers plots show the frequencies of BCG-specific IFN-γ+ CD4+ T cells (B) and IFN-γ+ CD8+ T cells (C) expressing the CD45RA and CCR7 memory markers singly or in combination. The open and closed bars represent the infants vaccinated at birth and 6 weeks of age, respectively. The horizontal line represents the median, the boxes represent the interquartile range, and the whiskers represent the 10th and 90th percentiles. The Mann–Whitney U test was used to assess the differences in proportions of memory phenotypes of specific CD4+ and CD8+ T cells between the 2 groups.
The majority of BCG-specific IFN-γ–expressing CD4+ T cells showed a central memory (TCM) phenotype, in both groups of infants (Figure 3B). Proportions of BCG-specific IFN-γ–expressing CD4+ T cells showing a naive-like (TNaive), TCM, TEM, and effector memory RA (TEMRA) phenotype were not different in the infant groups (Figure 3B). We also measured memory phenotypes of specific CD8+ T cells. BCG-specific IFN-γ–expressing CD8+ T cells mainly showed TEM and TEMRA phenotypes in both groups of infants, and no differences were observed between the 2 vaccination groups (Figure 3C).
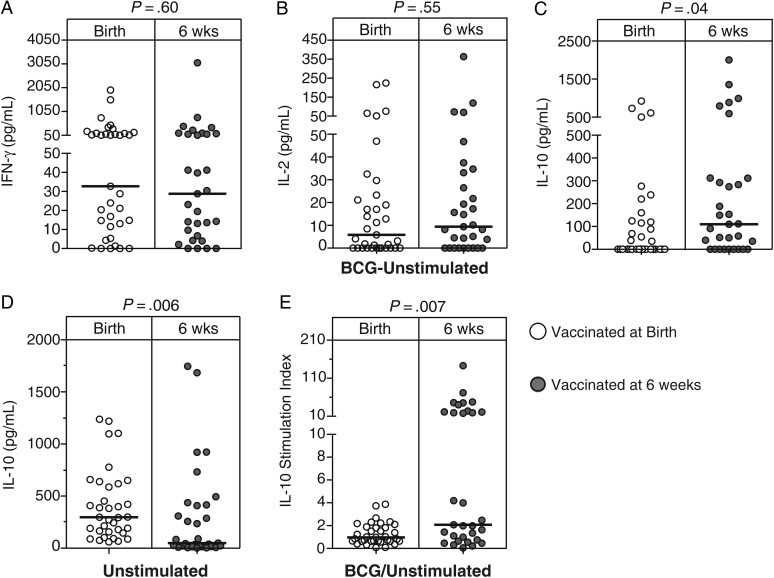
Higher IL-10 Levels in Infants Vaccinated at 6 Weeks of Age, Compared With Those Vaccinated at Birth
We also evaluated levels of Th1 and Th2 cytokines and IL-10 in plasma. We hypothesized that the cytokine milieu would impact the BCG-primed T-cell response (eg, the presence of high levels of Th2 cytokines [IL-4, IL-5, and IL-13], as well as IL-10, might attenuate Th1 immunity) [27, 28]. Levels of BCG-induced IL-10 were higher in the infants vaccinated at 6 weeks of age, compared with the group vaccinated at birth (Figure 4A–C and 4E). However, infants vaccinated at birth had higher levels of IL-10 in the unstimulated controls, compared with infants vaccinated at 6 weeks of age (Figure 4D). We therefore calculated a stimulation index and could confirm higher specific induction of IL-10 when vaccinated later (Figure 4E); IL-10 was not measured with the WB-ICS, because preliminary experiments showed that T-cell–specific expression was too low for valid analysis. Levels of all other cytokines were not different between the 2 groups of infants (Figure 4A–C and 4E).
Figure 4.
BCG-specific cytokine levels in plasma. The scatterplots show levels of 3 different cytokines measured by multiplex bead array in plasma collected after whole blood was incubated with BCG for 7 hours. The following cytokines are shown: interferon γ (IFN-γ; A), interferon 2 (IL-2; B), and interleukin 10 (IL-10; C). All values are corrected for unstimulated levels (BCG-unstimulated) implies the response detected in the unstimulated sample was subtracted from that of the stimulated sample. IL-10 levels of the unstimulated samples and the ratio of stimulated divided by unstimulated IL-10 levels are shown in panels D and E, respectively. The horizontal lines represent the median frequencies. The Mann–Whitney U test was used to assess for differences between the cytokine levels in the 2 groups.
Greater Capacity of Specific Proliferating CD4+ T Cells to Coexpress IL-2, IFN-γ, and TNF-α in Infants Vaccinated at Birth Than at 6 Weeks of Age
The ability of T cells to proliferate in response to secondary antigen encounter is an important feature of memory responses [29]. We measured this ability of the T cells in a 6-day assay by measuring upregulation of Ki67, a nuclear protein expressed during the active phases of cell division [30], as a marker for proliferation (Figure 5A and 5B) [24, 31]. The proliferative capacity of CD4+ and CD8+ T cells were similar between the 2 groups (Figure 5C and 5D).
Figure 5.
Capacity of BCG-specific CD4+ and CD8+ T cells to proliferate and produce cytokines. Representative flow cytometry data of cytokine expression by proliferating CD8− (herein referred to as CD4+; A) and CD8+ (B) T cells in a control (unstimulated) sample (top row) or BCG-stimulated sample (bottom row) from a 9-month-old infant who received BCG vaccine at birth. Scatterplots depict frequencies of proliferating CD4+ (C) and CD8+ (D) T cells. The capacity of the proliferating CD4+ T cells to express interleukin 2 (IL-2), interleukin 17 (IL-17), interferon γ (IFN-γ), and tumor necrosis factor α (TNF-α) alone or in combination is shown in (E). In the scatterplots, the horizontal lines represent the median frequencies. Finally, proportions of distinct subsets assessed by the cytokine coexpression of the proliferating CD4+ T cells were analyzed (D). The open and closed bars or dots represent the infants vaccinated at birth and 6 weeks of age, respectively. The Mann–Whitney U test was used to assess for differences between the 2 groups.
We also assessed the cytokine-producing capacity of specific proliferating CD4+ T cells after stimulating the cells on day 6 with PMA/ionomycin. Although the capacity of the specific cells (ie, Ki67+ cells) to produce any of the cytokines did not differ (data not shown), we observed that cells from infants vaccinated at birth had a greater capacity to coexpress IL-2, IFN-γ, and TNF-α, compared with those vaccinated at 6 weeks of age. However, the proportion of these cells expressing TNF-α only was lower in the group vaccinated at birth, compared with those vaccinated at 6 weeks of age (Figure 5E). Cytokine production capacity among specific CD8+ T cells did not differ between the groups (data not shown).
DISCUSSION
We compared BCG vaccine–induced immunity in Ugandan infants either vaccinated at birth or at 6 weeks of age. We showed that age of vaccination impacted specific immune response measured at 9 months of age. Infants vaccinated at birth had higher frequencies of BCG-specific CD4+ and CD8+ T cells producing IFN-γ alone or coexpressing IFN-γ and perforin. Furthermore, although the T-cell proliferative potential was similar in the 2 groups, a higher proportion of proliferating BCG-specific CD4+ T cells coexpressed IL-2, IFN-γ, and TNF-α in birth vaccinated infants. These infants also had lower levels of specific IL-10, compared with those vaccinated at 6 weeks of age.
We measured specific Th1 cytokine– and perforin-producing cells based on the proposed roles for these molecules in control of mycobacteria. For example, humans with mutations in the IL-12/IFN-γ pathway show an increased risk of mycobacterial disease [32, 33]. The role of perforin in mediating immunity against M. tuberculosis involves perforation of the cell membrane of an infected cell to permit entry of cytolytic granzymes that may directly kill M. tuberculosis or infected cells [34]. Rahman et al showed that, following vaccination of nonhuman primates with a recombinant BCG vaccine expressing a pore-forming toxin and the M. tuberculosis antigens Ag85A, Ag85B, and TB10.4, which was then boosted with an adenovirus 35 (rAd35) vaccine vector encoding the same M. tuberculosis antigens, better protection against M. tuberculosis challenge correlated with greater frequencies of vaccination-induced perforin-expressing T cells [35]. In our study, greater frequencies of BCG-specific CD4+ and CD8+ T cells expressing either IFN-γ and coexpressing IFN-γ and perforin in the group vaccinated at birth, compared with the group vaccinated at 6 weeks of age, could be a reflection of more effective vaccine-take in the former group. However, we cannot speculate about clinical relevance of this observation in human infants in terms of protection against tuberculosis [14]; BCG-induced correlates of protection are not known.
In chronic viral infections, persistence of antigen is associated with induction of effector memory cells; these cells may be functionally defined by their ability to produce IFN-γ [36]. The current study and previous studies from our laboratory have shown BCG-specific responses in infants are dominated by IFN-γ–producing CD4+ and CD8+ T cells [12, 21]. On the basis of this definition, it would appear as if vaccination at birth is more likely to induce an effector phenotype, compared with delayed vaccination. However, we have shown here and previously [37] that most BCG-induced IFN-γ–expressing cells have a TCM surface phenotype—the cells express the surface molecules required by classical TCM to home to lymph nodes [38]. The functional implication of the apparent discrepancy between functional and phenotypic definitions of T-cell populations induced by BCG is the focus of ongoing research; this may be important, given that BCG is likely to remain a prime vaccine for quite some time.
We evaluated levels of BCG-induced IL-10 on the basis of studies showing that this cytokine may attenuate Th1 responses. For example, in M. tuberculosis–susceptible CBA/J mice, antibody blockade of IL-10R during BCG vaccination resulted in an enhanced BCG-specific IFN-γ response and better protection against subsequent M. tuberculosis challenge [39]. We have shown that infants who received BCG vaccine at 6 weeks of age had greater levels of BCG-specific IL-10 production, compared with infants vaccinated at birth. However, we showed no strong negative correlation between IL-10 levels and CD4+ T-cell IFN-γ (Data not shown). The greater BCG-induced IL-10 levels observed in the infants vaccinated at 6 weeks of age may potentially result in attenuation of mycobacteria-specific Th1 immune responses during infection with M. tuberculosis in infants vaccinated at 6 weeks of age, compared with those vaccinated at birth.
We expanded the specific memory T cells in a long-term proliferation assay and measured the capacity of the specific proliferating cells to secrete cytokines. Proliferating CD4+ T cells from the infants vaccinated at birth showed greater capacity to coexpress IL-2, IFN-γ, and TNF-α. This was surprising, given our findings from the short-term assay, which indicated that BCG vaccination at birth was more likely to induce cells able to produce IFN-γ alone. It is possible that greater IL-10 levels observed in the infants vaccinated at 6 weeks of age may have attenuated the expansion of polyfunctional CD4+ T cells. Polyfunctional CD4+ T cells are thought to be important in tuberculosis immunity [40] and are therefore routinely measured in clinical trials assessing the immunogenicity of novel tuberculosis vaccines [41–43]. However, a clinical study from our laboratory reported no association between greater proportions of BCG-specific polyfunctional T cells and the risk of developing tuberculosis [14].
What are the possible explanations for the differences observed in the BCG-induced T-cell immunity between the 2 groups of infants? First, we observed that a higher proportion of infants vaccinated at 6 weeks of age were from families of lower social economic status, compared with infants vaccinated at birth (Table 1). Lower social economic status may negatively impact health-seeking behavior, nutrition, helminth exposure, and other factors, possibly leading to altered immune responses [44]. We showed no difference in the frequencies of BCG-specific IFN-γ–expressing CD4+ and CD8+ T-cell responses in infants from families with a higher income, compared with those from families in the lower income categories (Supplementary Figure 2) A and B. Nevertheless, we observed a significant association between the frequency of IFN-γ–expressing CD4+ T cells and household income (Supplementary Table 1). Second, we speculate that the lower Th1 responses observed in infants vaccinated at 6 weeks of age may be due to coadministration of BCG vaccine with alum, which is present in other vaccines [45]. Alum induces predominantly Th2 immune responses [46], which might have attenuated the BCG-induced Th1 responses [47].
We assessed the immune responses at 9 months of age. We do not know whether other differences in BCG-induced immunity may exist at earlier time points after vaccination. Our study setting limited us from accessing the participants at the peak of BCG-induced T-cell immune response [37]. However, in a clinical study of delayed BCG vaccination, Kagina et al showed that the greatest difference in BCG-induced T-cell immunity was at 1 year of age and not at the peak time point after the vaccination [21]. Differences between our study findings and those previously reported may be explained by many factors. First, we used a cross-sectional design, whereas previous studies were randomized controlled trials [18, 19, 21]. Second, diverse assays were used to measure outcomes in previous studies. For example, we used BCG as antigen, as did Burl et al [18], while others used PPD [19]. Third, the duration of incubation and choice of T-cell outcomes differed [18, 19, 21]. Finally, environmental and genetic variation may impact the mycobacterial immune response, as recently demonstrated by diverse patterns of antigen recognition and cytokine production in M. tuberculosis–infected persons from the Gambia, Uganda, and South Africa [48]. Environmental factors may include exposure to helminths [49], other infections, and diverse nutritional practices [44]. Genetic variation across Africa is well documented in descriptions of single-nucleotide polymorphisms [50], which may affect the immune response.
In summary, our findings appear to support WHO recommendations that infants in areas of high endemicity, such as Uganda, be vaccinated as soon as possible after birth. In this setting, delaying vaccination does not appear to hold vaccine-induced immunological advantages.
Supplementary Material
Notes
Financial support. This work was supported by the European and Developing Countries Clinical Trials Partnership (EDCTP CT grant 2005.32090.003).
Potential conflict of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Fine PE, Carneiro IAM, Milstien JB. Issues relating to the use of BCG immunisation programmes. Geneva: World Health Organization; 1999. WHO/V&B/99.23. [Google Scholar]
- 2.Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367:1173–80. doi: 10.1016/S0140-6736(06)68507-3. [DOI] [PubMed] [Google Scholar]
- 3.Aaby P, Roth A, Ravn H, et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J Infect Dis. 2011;204:245–52. doi: 10.1093/infdis/jir240. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. BCG vaccine. WHO position paper. Wkly Epidemiol Rec. 2004;79:27–38. [PubMed] [Google Scholar]
- 5.World Health Organization. WHO global tuberculosis control; 2011. [Google Scholar]
- 6.Fadnes LT, Nankabirwa V, Sommerfelt H, Tylleskar T, Tumwine JK, Engebretsen IM. Is vaccination coverage a good indicator of age-appropriate vaccination? A prospective study from Uganda. Vaccine. 2011;29:3564–70. doi: 10.1016/j.vaccine.2011.02.093. [DOI] [PubMed] [Google Scholar]
- 7.Nankabirwa V, Tumwine JK, Tylleskar T, Nankunda J, Sommerfelt H. Perinatal mortality in eastern Uganda: a community based prospective cohort study. PLoS One. 2011;6:e19674. doi: 10.1371/journal.pone.0019674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ministry of Health, Uganda. Promotion of Immunisation in Uganda: Booklet for Leaders. Vaccine Resource Library 2002. http:\\www.path.org/vaccineresources/files/Uganda_immunisation_advocacy.pdf . Accessed 11 November 2013. [Google Scholar]
- 9.Hoft DF, Blazevic A, Stanley J, et al. A recombinant adenovirus expressing immunodominant TB antigens can significantly enhance BCG-induced human immunity. Vaccine. 2012;30:2098–108. doi: 10.1016/j.vaccine.2012.01.048. [DOI] [PubMed] [Google Scholar]
- 10.Murray RA, Mansoor N, Harbacheuski R, et al. Bacillus Calmette Guerin vaccination of human newborns induces a specific, functional CD8+ T cell response. J Immunol. 2006;177:5647–51. doi: 10.4049/jimmunol.177.8.5647. [DOI] [PubMed] [Google Scholar]
- 11.Smith SM, Malin AS, Pauline T, et al. Characterization of human Mycobacterium bovis bacille Calmette-Guerin-reactive CD8+ T cells. Infect Immun. 1999;67:5223–30. doi: 10.1128/iai.67.10.5223-5230.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soares AP, Scriba TJ, Joseph S, et al. Bacillus Calmette-Guerin vaccination of human newborns induces T cells with complex cytokine and phenotypic profiles. J Immunol. 2008;180:3569–77. doi: 10.4049/jimmunol.180.5.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Semple PL, Watkins M, Davids V, et al. Induction of granulysin and perforin cytolytic mediator expression in 10-week-old infants vaccinated with BCG at birth. Clin Dev Immunol. 2011;2011:438463. doi: 10.1155/2011/438463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kagina BM, Abel B, Scriba TJ, et al. Specific T cell frequency and cytokine expression profile do not correlate with protection against tuberculosis after bacillus Calmette-Guerin vaccination of newborns. Am J Respir Crit Care Med. 2010;182:1073–9. doi: 10.1164/rccm.201003-0334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corbett NP, Blimkie D, Ho KC, et al. Ontogeny of Toll-like receptor mediated cytokine responses of human blood mononuclear cells. PLoS One. 2010;5:e15041. doi: 10.1371/journal.pone.0015041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sadeghi K, Berger A, Langgartner M, et al. Immaturity of infection control in preterm and term newborns is associated with impaired toll-like receptor signaling. J Infect Dis. 2007;195:296–302. doi: 10.1086/509892. [DOI] [PubMed] [Google Scholar]
- 17.Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–4. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 18.Burl S, Adetifa UJ, Cox M, et al. Delaying bacillus Calmette-Guerin vaccination from birth to 4 1/2 months of age reduces postvaccination Th1 and IL-17 responses but leads to comparable mycobacterial responses at 9 months of age. J Immunol. 2010;185:2620–8. doi: 10.4049/jimmunol.1000552. [DOI] [PubMed] [Google Scholar]
- 19.Hussey GD, Watkins ML, Goddard EA, et al. Neonatal mycobacterial specific cytotoxic T-lymphocyte and cytokine profiles in response to distinct BCG vaccination strategies. Immunology. 2002;105:314–24. doi: 10.1046/j.1365-2567.2002.01366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marchant A, Goetghebuer T, Ota MO, et al. Newborns develop a Th1-type immune response to Mycobacterium bovis bacillus Calmette-Guerin vaccination. J Immunol. 1999;163:2249–55. [PubMed] [Google Scholar]
- 21.Kagina BM, Abel B, Bowmaker M, et al. Delaying BCG vaccination from birth to 10 weeks of age may result in an enhanced memory CD4 T cell response. Vaccine. 2009;27:5488–95. doi: 10.1016/j.vaccine.2009.06.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanekom WA, Hughes J, Mavinkurve M, et al. Novel application of a whole blood intracellular cytokine detection assay to quantitate specific T-cell frequency in field studies. J Immunol Methods. 2004;291:185–95. doi: 10.1016/j.jim.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Waldrop SL, Pitcher CJ, Peterson DM, Maino VC, Picker LJ. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry: evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. J Clin Invest. 1997;99:1739–50. doi: 10.1172/JCI119338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soares A, Govender L, Hughes J, et al. Novel application of Ki67 to quantify antigen-specific in vitro lymphoproliferation. J Immunol Methods. 2010;362:43–50. doi: 10.1016/j.jim.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roederer M, Nozzi JL, Nason MX. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A. 2011;79A:167–74. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 27.Rook GA. Th2 cytokines in susceptibility to tuberculosis. Curr Mol Med. 2007;7:327–37. doi: 10.2174/156652407780598557. [DOI] [PubMed] [Google Scholar]
- 28.Schreiber T, Ehlers S, Heitmann L, et al. Autocrine IL-10 induces hallmarks of alternative activation in macrophages and suppresses antituberculosis effector mechanisms without compromising T cell immunity. J Immunol. 2009;183:1301–12. doi: 10.4049/jimmunol.0803567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Combadiere B, Boissonnas A, Carcelain G, et al. Distinct time effects of vaccination on long-term proliferative and IFN-gamma-producing T cell memory to smallpox in humans. J Exp Med. 2004;199:1585–93. doi: 10.1084/jem.20032083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–5. [PubMed] [Google Scholar]
- 31.Cellerai C, Harari A, Vallelian F, Boyman O, Pantaleo G. Functional and phenotypic characterization of tetanus toxoid-specific human CD4+ T cells following re-immunization. Eur J Immunol. 2007;37:1129–38. doi: 10.1002/eji.200636885. [DOI] [PubMed] [Google Scholar]
- 32.Bogunovic D, Byun M, Durfee LA, et al. Mycobacterial disease and impaired IFN-gamma immunity in humans with inherited ISG15 deficiency. Science. 2012;337:1684–8. doi: 10.1126/science.1224026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ottenhoff TH, de Boer T, Verhagen CE, Verreck FA, van Dissel JT. Human deficiencies in type 1 cytokine receptors reveal the essential role of type 1 cytokines in immunity to intracellular bacteria. Microbes Infect. 2000;2:1559–66. doi: 10.1016/s1286-4579(00)01312-5. [DOI] [PubMed] [Google Scholar]
- 34.Thiery J, Keefe D, Boulant S, et al. Perforin pores in the endosomal membrane trigger the release of endocytosed granzyme B into the cytosol of target cells. Nat Immunol. 2011;12:770–7. doi: 10.1038/ni.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rahman S, Magalhaes I, Rahman J, et al. Prime-boost vaccination with rBCG/rAd35 enhances CD8(+) cytolytic T-cell responses in lesions from Mycobacterium tuberculosis-infected primates. Mol Med. 2012;18:647–58. doi: 10.2119/molmed.2011.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harari A, Vallelian F, Pantaleo G. Phenotypic heterogeneity of antigen-specific CD4 T cells under different conditions of antigen persistence and antigen load. Eur J Immunol. 2004;34:3525–33. doi: 10.1002/eji.200425324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soares AP, Kwong Chung CK, Choice T, et al. Longitudinal changes in CD4(+) T-cell memory responses induced by BCG vaccination of newborns. J Infect Dis. 2013;207:1084–94. doi: 10.1093/infdis/jis941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campbell JJ, Butcher EC. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr Opin Immunol. 2000;12:336–41. doi: 10.1016/s0952-7915(00)00096-0. [DOI] [PubMed] [Google Scholar]
- 39.Pitt JM, Stavropoulos E, Redford PS, et al. Blockade of IL-10 signaling during bacillus Calmette-Guerin vaccination enhances and sustains Th1, Th17, and innate lymphoid IFN-gamma and IL-17 responses and increases protection to Mycobacterium tuberculosis infection. J Immunol. 2012;189:4079–87. doi: 10.4049/jimmunol.1201061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forbes EK, Sander C, Ronan EO, et al. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J Immunol. 2008;181:4955–64. doi: 10.4049/jimmunol.181.7.4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abel B, Tameris M, Mansoor N, et al. The novel tuberculosis vaccine, AERAS-402, induces robust and polyfunctional CD4+ and CD8+ T cells in adults. Am J Respir Crit Care Med. 2010;181:1407–17. doi: 10.1164/rccm.200910-1484OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sander CR, Pathan AA, Beveridge NE, et al. Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in Mycobacterium tuberculosis-infected individuals. Am J Respir Crit Care Med. 2009;179:724–33. doi: 10.1164/rccm.200809-1486OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scriba TJ, Tameris M, Smit E, et al. A phase IIa trial of the new tuberculosis vaccine, MVA85A, in HIV- and/or Mycobacterium tuberculosis-infected adults. Am J Respir Crit Care Med. 2012;185:769–78. doi: 10.1164/rccm.201108-1548OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodriguez L, Gonzalez C, Flores L, Jimenez-Zamudio L, Graniel J, Ortiz R. Assessment by flow cytometry of cytokine production in malnourished children. Clin Diagn Lab Immunol. 2005;12:502–7. doi: 10.1128/CDLI.12.4.502-507.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ota MO, Odutola AA, Owiafe PK, et al. Immunogenicity of the tuberculosis vaccine MVA85A is reduced by coadministration with EPI vaccines in a randomized controlled trial in Gambian infants. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3002461. 88ra56. [DOI] [PubMed] [Google Scholar]
- 46.Bungener L, Geeraedts F, Ter Veer W, Medema J, Wilschut J, Huckriede A. Alum boosts TH2-type antibody responses to whole-inactivated virus influenza vaccine in mice but does not confer superior protection. Vaccine. 2008;26:2350–9. doi: 10.1016/j.vaccine.2008.02.063. [DOI] [PubMed] [Google Scholar]
- 47.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–93. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 48.Black GF, Thiel BA, Ota MO, et al. Immunogenicity of novel DosR regulon-encoded candidate antigens of Mycobacterium tuberculosis in three high-burden populations in Africa. Clin Vaccine Immunol. 2009;16:1203–12. doi: 10.1128/CVI.00111-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elias D, Wolday D, Akuffo H, Petros B, Bronner U, Britton S. Effect of deworming on human T cell responses to mycobacterial antigens in helminth-exposed individuals before and after bacille Calmette-Guerin (BCG) vaccination. Clin Exp Immunol. 2001;123:219–25. doi: 10.1046/j.1365-2249.2001.01446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shey MS, Randhawa AK, Bowmaker M, et al. Single nucleotide polymorphisms in toll-like receptor 6 are associated with altered lipopeptide- and mycobacteria-induced interleukin-6 secretion. Genes Immun. 2010;11:561–72. doi: 10.1038/gene.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.