Abstract
The volatile alkylpyrazines methyl‐ and methoxypyrazines (MPs) present in the reflex bleeds of coccinellid beetles such as the harlequin ladybird beetle Harmonia axyridis are important semiochemicals that function in antipredatory defense behavior. Pyrazines have also been coadapted from a primarily defensive role into pheromones that function in intraspecific communication, attraction, and aggregation behavior. However, the biosynthesis of MPs in ladybird beetles is poorly understood. Here, we tested the hypothesis that MPs could be produced by microbial symbionts in H. axyridis, which generates four different MPs. The evaluation of tissue‐specific MP production showed that MP concentrations were highest in the gut tissue and hemolymph of the beetles rather than the fat body tissue as the presumed site of MP biosynthesis. Furthermore, manipulation of gut microbiota by antibiotic‐containing diets resulted in a lower MP content in adult beetles. The analysis of the bacterial community of the digestive tract revealed the presence of bacteria of the genera Serratia and Lactococcus which are reportedly able to produce MPs. In line with the known diet‐dependent production of MP in H. axyridis, we determined that the presence or relative abundance of some of the potential MP producers (Enterococcus and Staphylococcus) is also diet‐dependent. We hypothesize a potential role of the microbiota in MP production in H. axyridis as a possible example for outsourcing the synthesis of ecologically important semiochemicals to its gut bacteria.
Keywords: chemical defense, gut microbiota, Harmonia axyridis, Lactococcus lactis, methoxypyrazines (MPs), symbiosis
1. INTRODUCTION
The volatile alkylpyrazines, particularly methyl‐ and methoxypyrazines (MPs) are heterocyclic aromatic, nitrogen‐containing compounds, which produce odors of diverse biological significance. Apart from being important constituents of flavor in food, food products, several drinks and wine, numerous pyrazines also show antibacterial, and tuberculostatic properties, making them important agents in the food processing and the biomedical research industry (Bonde, Peepliwal, & Gaikwad, 2010; Maga, 1982). Pyrazines are also widespread in nature, with diverse roles ranging from plant defense and promotion of plant growth, to serving semiochemicals in plant–insect interactions, components of insect sex pheromones, and in inter‐specific defenses within fungi (von Beeren, Schulz, Hashim, & Witte, 2011; Murray, Shipton, & Whitfield, 1970; Murray & Whitfield, 1975).
In insects, pyrazines are present in diverse orders (Moore, Brown, & Rothschild, 1990; Supporting information Table S1). Beetles, particularly in the predacious subfamily Coccinellinae (Coleoptera: Coccinellidae) containing the two‐spotted ladybeetle Adalia bipunctata, the seven‐pointed ladybird Coccinella septempunctata, the convergent ladybird Hippodamia convergens as well as the harlequin ladybird Harmonia axyridis, have evolved to use pyrazine in several inter‐ and intraspecific interactions, as allomones or pheromones, which function as deterrents or attractants (Guilford, Nicol, Rothschild, & Moore, 1987, Rizzi, 1988, Woolfson & Rothschild, 1990, Rothschild & Moore, 1987, Moore et al., 1990, Rowe & Guilford, 1999, Siddall & Marples, 2008, Verheggen, Vogel, & Vilcinskas, 2017; Supporting information Table S2). H. axyridis like other members of Coccinellidae releases a noxious exudate from its tibio‐femoral joints through a defensive mechanism known as reflex bleeding to deter predators. The exudate, which can account for up to 20% of their body weight, functions to release several bitter, toxic compounds having a characteristic odor, which repel vertebrate and invertebrate predators (Daloze, Brackman, & Pasteels, 1994; Hemptinne & Dixon, 2000; King & Meinwald, 1996; Majerus & Majerus, 1997; Marples, 1993; Verheggen et al., 2017). The pyrazines form an important class of compounds responsible for this characteristic repellent. Several dull colored coccinellid beetles show little pyrazine content, whereas the aposematic species show relatively higher pyrazines content (Cai, Koziel, & O'Neal, 2007; Moore et al., 1990). Thus, there could be considerable selective pressure to use pyrazines as warning signals, given the substantial energetic costs involved in their synthesis and release (Holloway, Jong, Brakefield, & Vose, 1991). Semiochemicals in Coccinellidae tracks and feces also act as warning cues leading to avoidance behavior and reduction in plant colonization by prey animals such as aphids (Ninkovic, Al Abassi, & Pettersson, 2001; Youren, 2012). The odor of coccinellid feces also has an influence on feeding and oviposition activities of conspecific as well as heterospecific competing female coccinellids (Agarwala, Yasuda, & Kajita, 2003). The three main groups of MPs found in insects are 2‐isobutyl‐3‐methoxypyrazine (IBMP), 2‐isopropyl‐3‐methoxypyrazine (IPMP), and 2‐sec‐butyl‐3‐methoxypyrazine (SBMP). They are present in diverse species of Orthoptera, Hemiptera, Lepidoptera, and Coleoptera (Moore et al., 1990; Supporting information Table S1). Besides IBMP, IPMP, and SBMP, the coccinellid beetles H. axyridis and C. septempunctata exhibit a fourth distinct MP: 2,5‐dimethyl‐3‐methoxypyrazine (DMMP) which represents a further component of the coccinellid characteristic odor (Cai et al., 2007).
Although the importance of MPs in the ecology of Coccinellinae has received considerable attention, little is known about the site and mechanism of synthesis/acquisition in these beetles, leading to the question as to how the insects acquire these multi‐purpose semiochemicals. Several plants, including Pisum sativum, a host plant for aphids (on which some coccinellid beetles prey) produce MPs (Murray et al., 1970). A direct correlation between MP content of plants and those of carnivorous ladybeetles is however not yet known. Ladybirds may also gather semiochemicals with additional diets such as grapes in autumn (Dunlevy et al., 2010). Additionally, MPs could also be sequestered by feeding on pyrazine‐rich prey, such as aphids (Kögel, Eben, Hoffmann, & Gross, 2012).
Besides plants, more than 350 microbial volatiles are known to be released from bacteria with pyrazines as the most prevalent substances (Dickschat, Reichenbach, Wagner‐Döbler, & Schulz, 2005, reviewed in Schulz & Dickschat, 2007). The structural similarity of some bacterial volatiles to insect pheromones suggests the possibility of microbial involvement in pyrazine synthesis in the beetles (Davis, Crippen, Hofstetter, & Tomberlin, 2013; Dickschat et al., 2005; Supporting information Table S3). Since, (a) pyrazines are a major group of volatile compounds released by bacteria, and (b) H. axyridis MP concentration in adults and larvae is determined by their dietary components (Kögel et al., 2012), we hypothesize that a combination of diets and gut microbiota affects MP concentrations in the beetles. In this study, we test this hypothesis by characterizing the gut microbiota of H. axyridis fed on different diets and its effect on the concentration of MPs.
2. METHODS
2.1. Harmonia rearing
The experiments were conducted with a greenhouse strain of the Asian ladybeetle Harmonia axyridis collected from invasive wild populations in and around Giessen (Germany) and kept in groups of about 50 individuals in cages (60 × 60 × 30 cm) according to Gegner, Schmidtberg, Vogel, & Vilcinskas, 2018. Larvae and adults were reared on the pea aphid Acyrthosiphon pisum on bean plants (Vicia faber var. minor) and maintained under a photoperiod of 16:8 hours (L:D) at average temperature of 21°C. For testing the MP concentrations under diapause‐conditions, we collected adult beetles from aggregations sites in autumn and spring found in indoor overwintering sites and kept them in cages at 4–8°C in the dark.
2.2. Diets
Harmonia axyridis are polyphagous generalist insects. They are known to feed on diverse diets including aphids (along with other insects of the suborder Sternorrhyncha), immature stages or eggs of numerous invertebrate prey, honeydew, pollen, and fruits like grapes (Berkvens et al., 2008; Botezatu, Kotseridis, Inglis, & Pickering, 2013; Galvan, Koch, & Hutchison, 2008; Koch, 2003; Roy et al., 2016). They can also be reared on eggs of the Angoumois grain moth Sitotroga cereallella or the flour moth Ephesitia kuehniella (Gegner et al., 2018; Kögel et al., 2012; Laugier et al., 2013). To investigate the effect of diet on MP synthesis, we fed the beetles with five different plant‐based and insect‐based diets. We divided the beetles into five experimental groups based on the diets they received. The beetles were offered living aphids (group I, aphid), or bisected organic grapes (group II, grape). In group III, beetles were provided with 50 µl of 4:1 honey syrup (H) diluted with water. In group IV, beetles were fed with a diet containing 500 mg eggs of Sitotroga cerealella mixed with 1 ml honey syrup to increase palatability (group IV, HS). In group V, the beetles were fed antibiotics through a honey syrup‐egg mass containing 1:1 tetracycline/ampicillin mix (50 µg/ml, Roth, Germany) (group V, HSAB) to study the effect of manipulation of gut microbiota on MP concentrations (modified after Hurst, Majerus, & Walker, 1992, Noriyuki, Kameda, & Osawa, 2014). Diets were kept at the periphery of Petri plates that contained moistened filter paper and housed adults (females and males) and freshly hatched first instar (L1) larvae. The ratio of females to males was retained 1:1. For adults, ad libitum food was provided daily for 10 days, while larvae were fed until they reached L4 stage or the pupal stage. At the end of the experiment, adults and larvae were dissected to remove their entire alimentary canal, and the residual body was frozen in liquid nitrogen. Another group of L4 larvae was allowed to pupate, and emerging adults were frozen 0–24 hr after hatching. The samples were stored at −20°C for gas chromatography‐mass spectrometry (GC/MS) analysis and stored in 70% ethanol at −80°C for DNA extraction.
2.3. Identification of gut microbiota
For analysis of the gut microbiome, we used male and female adult beetles fed on an aphid or grape diet. Beetles were washed in PBS (pH 7.2) before dissections. The gut samples were again washed in PBS and pooled in 70% ethanol. The residual bodies were also collected in 70% ethanol. This procedure was separately performed with females and males for two diets (aphids vs. grapes). Thus, we analyzed the following groups: I) guts from males fed on grapes (M‐gut‐gr), II) guts from males fed on aphids (M‐gut‐ap), III) guts from females fed on grapes (F‐gut‐gr), IV) guts from females fed on aphids (F‐gut‐ap), V) residual bodies from males fed on grapes (M‐body‐gr), VI) residual bodies from males fed on aphids (M‐body‐ap), VII) residual bodies from females fed on grapes (F‐body‐gr), and VIII) residual bodies from females fed on aphids (F‐body‐ap). DNA was extracted from pooled samples using the PowerSoil DNA extraction kit (MoBio Laboratories, USA) according to the manufacturer's recommended protocol.
To characterize the bacterial gut community, the V1–V3 region of the 16S rRNA gene was amplified from total genomic DNA using Gray28F and Gray519R primers (Sun, Wolcott, & Dowd, 2011) and sequenced using a Roche 454 FLX instrument with Titanium chemistry at an external facility (Molecular Research LP, Shallowater, TX). Sequences were quality filtered in QIIME (version 1.8.0; Caporaso, Kuczynski, et al., 2010b) using default parameters and denoised using Denoiser (Reeder & Knight, 2010). Sequences were quality filtered, chimeric sequences were removed, and high‐quality sequences were clustered using the USEARCH (Edgar, 2010) pipeline in QIIME. Taxonomy was assigned using RDP (Wang, Garrity, Tiedje, & Cole, 2007) at 80% confidence level using the with the Greengenes database version 13_8 (DeSantis et al., 2006 ) preclustered at 97% identity. Representative sequences were aligned using PyNAST (Caporaso, Bittinger, et al., 2010a), filtered, and a phylogenetic tree was constructed using FastTree (Price, Dehal, & Arkin, 2010). For downstream analyses, the OTU table was rarefied to 15,000 sequences per sample. Data were analyzed in R (Pinheiro, Bates, DebRoy, & Sarkar, 2014) and Qiime. Heatmaps were plotted using lattice (Sarkar, 2010) in R with OTUs summarized at the genus level, and those classified below family level, and with cumulative relative abundance <0.1% grouped into “Others.”.
2.4. GC/MS analysis of MPs
For MP analysis, at least five H. axyridis (larvae and adults), their tissues and the diets were individually measured for each experiment. They were washed in sterile PBS before they were frozen in liquid nitrogen and stored subsequently at −20°C until analysis. Whole gut tissue along with residual body tissue was also washed and frozen. Each specimen of the different diet groups was thawed, separately weighed using an electronic balance, and transferred into a vial. Beetles and larvae were macerated with a scoop, and guts were slightly pressed with the tip of a pipette. The headspace GC/MS analsyis was modified from Cudjoe, Wiedekher, and Brindle (2005). The collected headspace samples were analyzed on a GC‐MS system (model 5977B, Agilent, Santa Clara, CA, USA) equipped with a UNIS 500 split/splitless injector (JAS, Moers, Germany). Compounds were separated on a HP‐5MS Ultra Inert GC column (Agilent) with helium as carrier gas. The MS was operated in full scan mode (EI Energy: 70 eV, transfer line: 280°C, ion source: 230°C, quadrupole: 150°C) and spectra were recorded from m/z 50‐550. Signals were integrated in extracted ion chromatograms specific for the individual MP (IPMP: m/z 137, 11.1 min; IBMP: m/z 124, 12.5 min; SBMP: m/z 138, 12.4 min; IPEP (IS): m/z 166, 12.2 min). Concentrations were calculated based on calibration curves generated using the identical IS addition and NTD sampling protocol with MP standard dilutions and normalized on a ‘per fresh weight' or ‘per sample' basis.
2.5. Microscopy
For light microscopy, guts were dissected while submerged in ice‐cold PBS (pH 7.2). Prefixation was performed in 2.5% glutaraldehyde plus 2% paraformaldehyde in 0.1 M cacodylate buffer (pH 7.4) for 2 hr. After washing in cacodylate buffer, the samples were post‐fixed with 1% OsO4 in 0.1 M cacodylate buffer (pH 7.4) for 1.5 hr, dehydrated through a graded ethanol series and embedded in Araldite. Semi‐thin sections were prepared with a Reichert Om/U3 ultramicrotome stained with 0.5% toluidine blue in 0.5% sodium borate. The samples were observed under a Leica DM 5000 B microscope.
2.6. Statistical analysis
Statistical analysis for the different life stages and diets was carried out using SIGMAPLOT v. 12.0 (Systat Software Inc., San Jose, CA). Significant differences between groups of parametric data were determined by one‐way analysis of variance (ANOVA) with a subsequent Holm–Sidak test. Nonparametric data were analyzed by ANOVA on ranks with a subsequent Dunn's test. For the pairwise comparison of controls and antibiotic treatments, we used IBM SPSS Statistics v23 software (Armonk, USA). Statistical differences were calculated by Mann‐Whitney U test for nonparametric data and Student's t test for normally distributed data. Statistical significance was defined as a threshold of p < 0.05 (Appendix S1: Supplementary Material Part II—Statistical Analysis and Data Set).
3. RESULTS AND DISCUSSION
3.1. MP concentrations during life cycle of H. axyridis reared on aphid diet
The total MP contents in H. axyridis individuals were affected by their life stages. For groups that were exclusively fed with the aphid A. pisum, there was an overall increase in total MP concentration in their fresh weight. Whereas larval stages showed significantly lower concentrations (mean ± SE = 4.192 ± 0.908 pg/mg) than adults (24.982 ± 3.356 pg/mg), MP concentrations were comparable between newly hatched adults (22.798 ± 2.645 pg/mg), mature adults, and adults in diapause (23.364 ± 4.546 pg/mg; Figure 1a; Supporting information Table S4). However, total amount of MPs (per sample) was lower in diapause‐induced adults (235.943 ± 50.244 pg/mg) than in mature adults (403.410 ± 111.993 pg/mg). Generally, the egg stage showed relatively higher MP content per mg fresh weight (8.115 ± 0.908 pg/mg) than the L4 larvae (4.192 ± 0.5 pg/mg).
Figure 1.
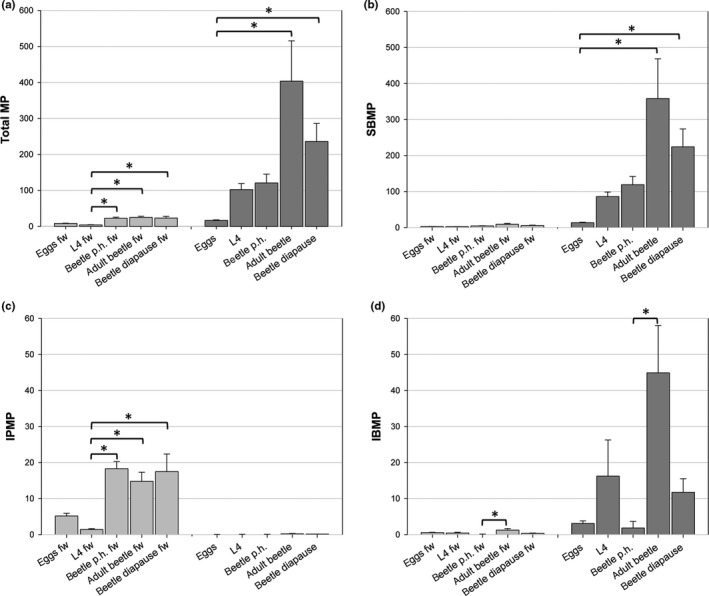
Methoxypyrazine (MP) content in life stages of the ladybird beetle H. axyridis. (a) Total MP content. (c) IPMP (2‐isopropyl‐3‐methoxypyrazine) content. (d) IBMP (2‐isobutyl‐3‐methoxypyrazine) content. Gray: data for MP pg/mg fresh weight (fw); dark gray: data for MP pg/sample. Life stages of H. axyridis: eggs, last larval instars (L4), beetles post hatching without food intake (beetle p.h.), mature beetles (adult beetle), beetles in diapause (beetle diapause). Statistical significant differences in values *p < 0.05
The different MPs tested (IPMP, IBMP, and SBMP) varied in their relative concentrations across beetle life stages that were exclusively fed with A. pisum (Figure 1b; Supporting information Table S4). The most abundant of the three tested MPs was SBMP, whose overall distribution over life stages was similar to that of the total MP content. In contrast, IPMP constituted a relatively smaller proportion of the total MP fraction (Figure 1c; Supporting information Table S4). It was the lowest in L4 larvae (1.414 ± 0.231) and significantly higher in beetles (newly hatched beetle 18.286 ± 1.985 pg/mg, mature beetle 14.769 ± 2.536 pg/mg, beetle in diapause 17.486 ± 4.870 pg/mg). IBMP concentrations also constituted a relatively smaller proportion of the total MP content. However, contrary to IPMP concentrations, IBMP levels were significantly higher in mature adult beetles (pg/per mg fresh weight 1.235 ± 0.428, per individual 44.906 ± 13.137) as compared to immature beetles. In the latter group, only one individual showed a detectable IBMP level. Thus, IBMP levels appeared more important in the larval stage and in mature adult beetles (Figure 1d; Supporting information Table S4). Our result that L4 larvae of H. axyridis show relatively lower MP production than their adult stages corroborates with the experiments by Ninkovic et al. (2001) and Moore et al. (1990) which show lower levels of MP volatiles in larvae which can produce lower avoidance behavior in aphids.
Both non‐diapausing and diapausing beetles produced MPs (Figure 1), indicating that the potential aggregating pheromone of reflex bleeds are also produced under nonwintering conditions (Durieux et al., 2015; Jeanson & Deneubourg, 2009). However, these data also indicate the relative proportion of SBMP, IPMP, and IBMP differed between non‐diapause and diapause, such that a change in the relative proportion within the MP cocktail could enable the beetles to use the same set of volatile compounds to display different signals under different conditions (Figure 1b–d). Diapausing and non‐diapausing A. bipunctata produce the same three methoxypyrazines (IBMP, IBMP, and SBMP) regardless of physiological state, but IBMP (alone or combined with IPMP) can result in aggregation and causing diapause (Susset et al., 2013). Since adult beetles can secrete MPs through reflex bleeding throughout their lifetimes, including during autumnal aggregation, MPs form part of a multi‐modal display strategy in H. axyridis. In conjunction with other traits such as coloration, gregarious behavior, and reflex bleeding that are primarily defensive, MPs have likely been co‐opted as aggregation pheromones (Wheeler & Cardé, 2013). MPs are also associated with inducing aggregation; for example, A. bipunctata spend more time in the vicinity of an MP source when an extract containing cuticular hydrocarbons from diapausing individuals was present (Durieux et al., 2015; Susset et al., 2013). In H. convergens, IBMP has the strongest aggregative effect, whereas beetles aggregate only in specific doses of SBMP, while IPMP is repellent. IBMP is released only by adults that are on target for their overwintering locations attaining the diapause (McCord, 2015; Wheeler & Cardé, 2013). In our experiments, the adult beetles of H. axyridis showed the highest IBMP content (pg per mg fresh weight 1.235 ± 0.428, per individual 44.906 ± 13.137), whereas diapausing beetles had comparatively low amounts (per mg fresh weight 0.307 ± 0.102, per individual 11.728 ± 3.779) (Figure 1d; Supporting information Table S4). If IBMP also functions in inducing aggregation in H. axyridis, these data suggest that the release of IBMP could occur prior to beetles entering diapause, when it would function to induce aggregation among diapausing adults.
Male H. axyridis beetle guts showed higher MP concentrations than females quantified in both fresh weight (males 11.956 ± 3.424 pg/mg, females 5.783 ± 1.622 pg/mg) and per individual (males 30.640 ± 9.382 pg/mg, females 20.013 ± 4.448 pg/mg) (Figure 2a; Supporting information Table S4). The magnitude of the differences was even stronger in residual body samples (males 426.478 ± 70.175 pg/mg, females 205.908 ± 34.576 pg/mg) that were analyzed after removal of the gut, indicating that beetles carry substantial MP content in extra‐intestinal tissue.
Figure 2.
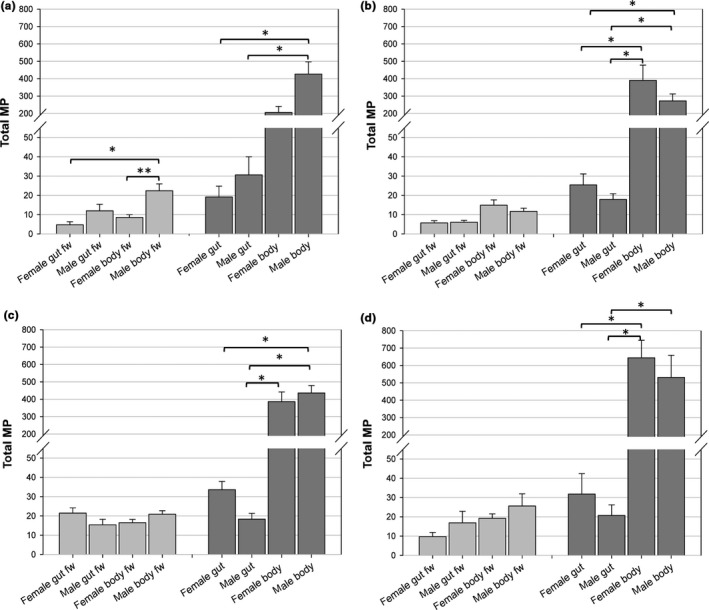
Influence of diets on total MP content in mature beetles. Quantification of MP content in gut and residual body (body) of male and female H. axyrids. Gray: data for MP pg/mg fresh weight (fw); dark gray: data for MP pg/sample. The following feeding assays were performed for 10 days: (a) Aphid diet on Petri dishes. (b) Grape diet. (c) Honey syrup diet. (d) Honey syrup/S. cerealella eggs diet. Statistical significant differences in values *p < 0.05; **p < 0.01.
3.2. Source of MPs in ladybird beetles
To investigate the source of MPs in H. axyridis, we explored two scenarios through which the beetles could acquire or generate MPs: external acquisition through diets, or through autogenous synthesis. Potential “external” sources include sequestration of compounds from their host plants or host prey (such as aphids), or acquisition through their microbiota (von Beeren et al., 2011; Dettner, 1987; Rothschild, Euw, & Reichste, 1973; Witte, Ehmke, & Hartmann, 1990).
To test the influence of diet on the MP content of H. axyridis, we provided the beetles with plant‐based and insect (prey)‐based diets: A. pisum, grapes, honey syrup, and S. cerealella eggs mixed with honey (Figure 2). MPs were below the detection level and likely absent in all these diets (Appendix S1: Supplementary Material Part II—Statistical Analysis and Data Set), and thus, we ruled out these diets as a direct source of sequestering pyrazines in beetles. Despite this, all three MPs were detected in beetle life stages, and these concentrations were influenced on the diets on which they fed, the life stage of the beetles and the sex of the adults. Residual body tissue showed greater than tenfold increase in total MP content in mg/sample in all four diets. Aphid‐fed males (Figure 2a; Supporting information Table S4) showed higher MP levels than the females, significantly in the residual body (males 22.386 ± 3.552 pg/mg, females 8.514 ± 1.437 pg/mg). Generally, aphid‐fed males had higher MP concentrations in mg fresh weight (gut 11.956 ± 3.424 pg/mg, residual body 22.386 ± 3.552 pg/mg) than grape‐fed male beetles (gut 7.253 ± 1.411 pg/mg, residual body 11.717 ± 1.641 pg/mg) (Figure 2b; Supporting information Table S4). On the grape diet, the MP contents of females were generally higher than in males. Both sexes of beetles with honey syrup diet show higher MP amounts in mg/fresh weight particularly in the gut tissue (females 21.487 ± 2.692 pg/mg, males 15.384 ± 2.832 pg/mg) in comparison to the grape (females 5.741 ± 1.075 pg/mg, males 7.235 ± 1.411 pg/mg) and aphid diet (females 5.783 ± 1.622 pg/mg, males 11.956 ± 3.424 pg/mg) (Figure 2c; Supporting information Table S4). The addition of S. cerealella eggs to honey syrup led to the highest measured MP levels in the residual body of H. axyridis (pg per mg fresh weight 19.251 ± 2.259, per individual 643.689 ± 101.086; Figure 2d; Supporting information Table S4).
To further investigate the site of MP synthesis in H. axyridis beetles, we used different tissues from dissected beetles that were fed on an aphid diet. We isolated the beetles' hemolymph, fat body, muscle, and the alimentary canal to analyze their MP content. We found no detectable levels of total MPs in the muscle or the fat body (Appendix S1: Supplementary Material Part II—Statistical Analysis and Data Set). However, the hemolymph showed the highest concentration of MP content (37.65 ± 2.486 pg/mg fresh weight), which was approximately four times greater than MP concentration in the alimentary canal (9.07 ± 2.375 pg/mg fresh weight; Appendix S1: Supplementary Material Part II—Statistical Analysis and Data Set). As a reflex bleeder, it is likely that pyrazines are secreted from the hemolymph, facilitating a spontaneous as well as voluminous exudate during warning displays. In conclusion, these results indicated that MPs were not present in the diets themselves, but were detected in gut of the beetles that feed on these diets, with a high prevalence in their hemolymph. We therefore hypothesized that the alimentary canal is the site of synthesis of the MPs from which they are released into the hemolymph, which act as a storage tissue for release during reflex bleeding.
We thus ruled out the sequestration of MPs in H. axyridis either through plant sources (grapes, honey syrup) or through insect prey (aphids and Sitotroga eggs). An MP uptake via other food sources could also be excluded, because H. axyridis individuals were exclusively fed on their assigned diets throughout these experiments. Although Moore et al. (1990) examined several insect host plants for the presence of alkylpyrazines and found IPMP, IBMP, and SBMPs in Asclepiadaceae, Aristolochiaceae, Passifloraceae, Asteraceae, and Papaveraceae families, we show here that MP levels were not detected in grapes that were fed to our experimental beetles. Thus, we infer that since beetles could not acquire MPs from their diets, they must synthesize them within their body. This could be achieved either by the host themselves, or through the metabolic activity of microbial symbionts.
3.3. The bacteria community of the gut of H. axyridis
To investigate the effect of diet on the H. axyridis microbiome, we characterized the bacterial community of gut and residual body samples of adult beetles that were fed on either aphids or grapes. We used two approaches to characterize the effect of diet on the beetle microbiome. First, we identified a core bacterial community separately for gut tissue and residual body tissue by identifying OTUs (summarized at the genus level) that were present in 100% sample replicates for the two categories (irrespective of the diet). Secondly, we identified which OTUs were differentially abundant as an effect of the diets in the two tissue types.
A total of 14 OTUs were identified as the core bacterial community for the male and female gut tissue. These included the genera Lactococcus, Serratia, and unclassified Enterobacteriaceae and Enterococcaceae. The core community for the residual body tissue for adults consisted of 35 OTUs consisting Lactococcus, Staphylococcus, Serratia, Corynebacterium, Delftia, Pseudomonas, Stenotrophomonas, Acinetobacter, Providencia, Methylobacterium, Flavobacterium, Comamonas, and several unclassified families. Proteobacteria and Firmicutes were the two most dominant bacterial phyla in male and female samples (Figure 3). At the species level, some Lactococcus OTUs were assigned to L. garvieae, while the most abundant OTU was identified as L. lactis (BLAST against the nr database, 98% sequence identity). The Serratia OTUs were identified as S. marcescens (BLAST against the nr database, 98% sequence identity), and the Enterobacteriaceae were identified as Enterobacter hormaechei (BLAST against the nr database, 98% sequence identity; Table 1).
Figure 3.
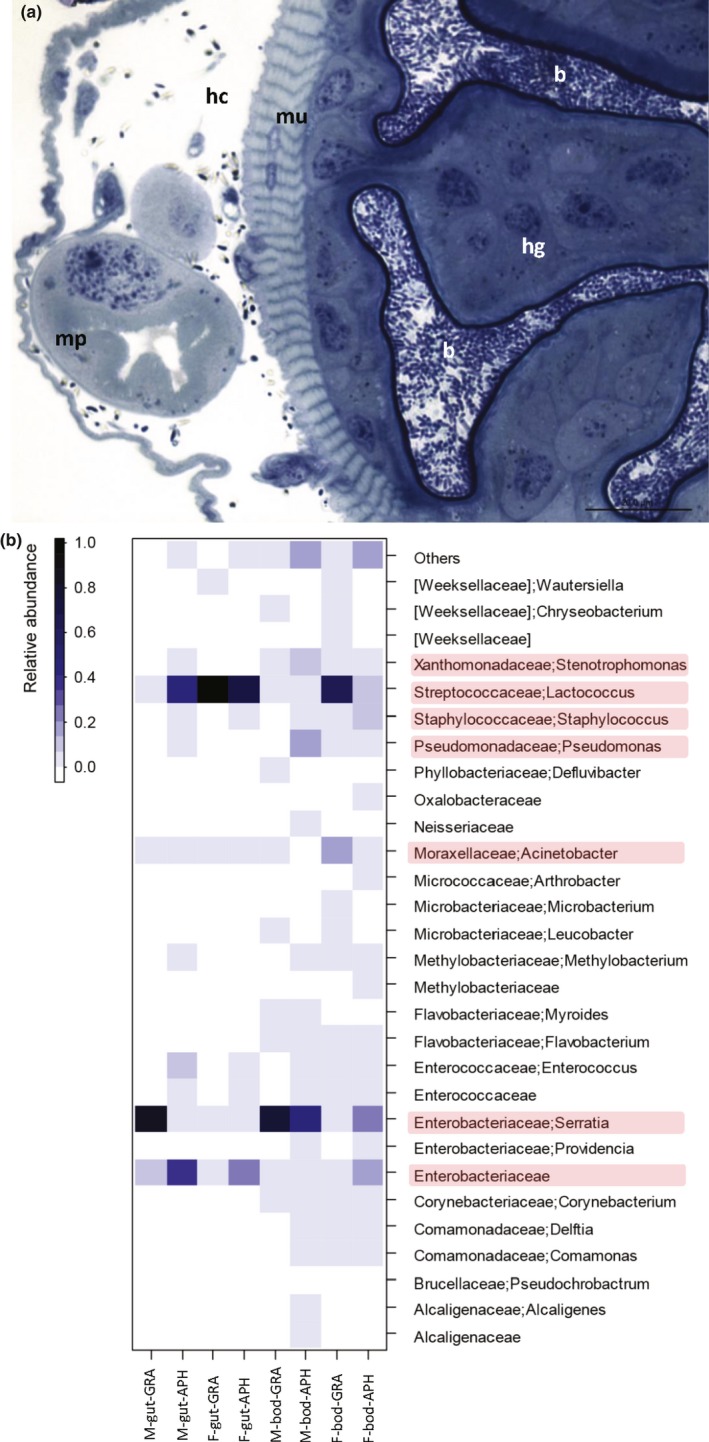
Microbiome analysis of gut of Harmonia axyridis. (a) Micrograph of bacteria in the lumen of the hindgut of a female H. axyridis. b: bacteria; hc: hemocoel, hg: hindgut; mu: muscle layer; mp: Malpighian tubules. (b) Heatmap showing relative proportion of bacteria summarized at genus level for male and female samples (gut tissue and residual body tissue) for adult beetles fed on aphid and grape diets. ap: aphid diet; bod: residual body tissue; M: males; F: females; gr: grape diet. Bacteria highlighted in red are presumed to produce MPs
Table 1.
Bacterial community of the gut of H. axyridis
| Bacteria in gut of H. axyridis | MP production verified in strains or closely related strains | |||
|---|---|---|---|---|
| Phylum | Order | Family | Genus/species | |
| Firmicutes | Bacillales | Staphylococcaceae | Staphylococcus kloosii | Staphylococcus sciuri , S. aureus (Robacker, 2007; Robacker et al., 1991, 1998) |
| Staphylococcus succinus | ||||
| Staphylococcus sciuri | ||||
| Lactobacillales | Enterococcaceae | Unclassified Enterococcaceae | No information | |
| Enterococcus | ||||
| Enterococcus mundtii | ||||
| Streptococcaceae | Lactococcus | Lactococcus lactis subsp. lactis biovar. diacetilactis (Heon & Lee, 1991; Lee, DeMilo, Moreno, & Martinez, 1995) | ||
| Lactococcus lactis subsp.lactis | ||||
| Lactococcus garvieae | ||||
| Bacillales | See Table 2 | |||
| γ‐Proteobacteria | Enterobacteriales | Enterobacteriaceae | Unclassified Enterobacteriaceae | Enterobacter agglomerans (Robacker et al., 1998, Robacker & Lauzon, 2002, Robacker et al. 2007) |
| Enterobacter hormaechei | ||||
| Serratia sp. | Serratia marcescens , S. ficaria, S. rubidea, S. odorifera (Gallois & Grimont, 1985) | |||
| Serratiamarcescens | ||||
| Aeromonodales | Aeromonadaceae | Aeromonas sp. | no information | |
| Pseudomonaldales | Pseudomonadaceae | Pseudomonas | Pseudomonas perolens, P. taetroleus, P. aeruginosa (Morgan et al., 1972, Miller et al., 1973, Dumont et al., 1983, Gallois et al., 1988, Cheng et al., 1991, Cheng & Reineccius 1991) | |
| Moxarellaceae | Acinetobacter | Acinetobacter baumannii (Gao et al., 2016) | ||
| Xanthomonadales | Xanthomonadaceae | Stenotrophomonas | Stenotrophomonas maltophilia (Zou et al., 2007) | |
| α‐Proteobacteria | Rhizobiales | Methylobacteriaceae | Methylobacterium | Methylobacterium sp.: diverse pyrazines (Balachandran, Duraipandiyan, & Ignacimuthu, 2012) |
| Bacteroidetes | Flavobacteriales | Flavobacteriaceae | Wautersiella | no information |
Bold: species with already described MP production and the respective references; underlined: core bacteria of the gut of H. axyridis.
Several OTUs showed differential relative abundances based on the tissue sampled and the diets. Although, OTUs assigned to the genus Lactococcus were present in all samples, they showed higher relative abundances in female and male gut tissue with aphid diets and female gut tissue with grape diet. Serratia showed highest relative abundance in male body and male gut tissue fed on grapes. Corynebacterium was exclusively found in residual body tissue in males and females. Based on the Bray‐Curtis dissimilarity measure, the female gut tissues across diets clustered together, indicating greater similarity in gut microbiomes in females despite feeding differences.
The bacteria community composition described here shows overlap with the community composition of native H. axyridis (from Korea), which consisted of α‐ and γ‐Proteobacteria, Actinobacteria, Firmicutes, and Deinococcus–Thermus (Kim, Han, Moon, Yu, & Whang, 2011; Moon et al., 2011). In contrast, the beetles used in this study showed Enterococcus and Lactococcus strains which were not identified in samples of native H. axyridis. However, the microbiome of invasive H. axyridis from Poland encompassed Lactococcus, but not Enterococcus (Dudek, Humińska, Wojciechowicz, & Tryjanowski, 2017). However, it remains uncertain whether the differences in these taxa were an effect of diet.
Some of the bacteria we detected in the gut of H. axyridis are known producers of methoxypyrazines (Table 1): viz. Firmicutes with the genus Lactococcus, and particularly the species Lactococcus lactis subsp. lactis and the species Staphylococcus sciuri; γ‐Proteobacteria with the genera Serratia, Pseudomonas, Acinetobacter, Stenotrophomonas, and those belonging to the family Enterobacteriaceae. For example, Lactococcus lactis subsp. lactis, which was highly abundant in male and female beetles in this study, produces tetramethylpyrazines (Heon & Lee, 1991). Several Serratia species are also known to generate MPs (Dalamaga & Vrioni, 2011; Gallois & Grimont, 1985; Tables 1 and 2). In the current study, Serratia was relatively more abundant in males. It is presumed that Serratia strains in the insect digestive tract probably originate from plants (Grimont & Grimont, 2006), and it is known that several Serratia strains generate potato‐like odors with a combination of different pyrazine compounds such as 3‐isopropyl‐2‐methoxy‐5‐methylpyrazine, 3‐sec‐butyl‐2‐methoxy‐5(6)‐methylpyrazine, and 3‐isobutyl‐2‐methoxy‐6‐methylpyrazine (Gallois & Grimont, 1985) (Table 2). Staphylococcus was detected in male and female tissue of beetles feeding on aphids and is also known to produce methoxypyrazines (Robacker, 2007; Robacker, DeMilo, & Voaden, 1991; Robacker, Martinez, Garcia, & Bartelt, 1998). In aphids, S. sciuri is known to produce volatile semiochemicals that attract natural enemies. Bacteria of the genus Pseudomonas are known to produce IPMP and SBMP (Chen & Reineccius, 1991; Cheng, Reineccius, Bjorklund, & Leete, 1991; Dumont, Mourgues, & Adda, 1983; Filipiak et al., 2012; Gallois, Kergomard, & Adda, 1988; Miller, Scanlan, Lee, Libbey, & Morgan, 1973; Morgan, Libbey, & Scanlan, 1972) and Kim et al. (2011) and Moon et al. (2011) also described two Pseudomonas strains in native H. axyridis. Bacteria associated with Acinetobacter and Stenotrophomonas that were detected in the gut tissue of adults feeding on aphids are reported to synthesize pyrazines (Gao et al., 2016; Zou, Mo, Gu, Zhou, & Zhang, 2007).
Table 2.
“Harmonia” MPs and others produced by bacteria and fungi
| Origin—bacteria/fungi | Mainly produced pyrazines | Reference |
|---|---|---|
|
Enterobacter agglomerans
from mouthparts of Anastrepha ludens and Rhagoletis pomonella |
2,5‐dimethylpyrazine, trimethylpyrazine | Robacker et al. (1998), Robacker, Lauzon, and He (2004), Robacker and Lauzon (2002) |
| Cedecea davisae | SBMP, 3‐isopropyl‐2‐methoxypyrazine, IBMP | Dalamaga and Vrioni (2011), Gallois and Grimont (1985) |
| Serratia ficaria | IPMP, 3‐s‐butyl‐2‐methoxy‐5(6)‐methylpyrazine | |
| S. marcescens | 3‐isopropyl‐2‐methoxy‐5‐methylpyrazine, 2,3,5‐trimethylpyrazine | |
| S. odorifera | 3‐isopropyl‐2‐methoxy‐5‐methylpyrazine, 3‐isobutyl‐2‐methoxy‐6‐methylpyrazine | |
| S. rubidaea | 3‐isopropyl‐2‐methoxy‐5‐methylpyrazine, 3‐s‐butyl‐2‐methoxy‐5(6)‐methylpyrazine, 2,3,5‐trimethylpyrazine, 2‐ethyl‐6‐methylpyrazine | |
| Citrobacter freundii | 2,5‐dimethylpyrazine | DeMilo, Lee, Moreno, and Martinet (1996), Robacker and Bartelt (1997), Robacker (2007) |
| Klebsiella pneumoniae | 2,5‐dimethylpyrazine, 2‐isopropyl‐5‐methylpyrazine | Martinez, Robacker, Garcia, and Esau (1994), Lee et al. (1995), Robacker et al. (2004), Robacker (2007), Schulz and Dickschat (2007) |
| Pseudomonas perolens | IPMP, SBMP | Morgan et al. (1972), Miller et al. (1973), Dumont et al. (1983), Cheng et al. (1991), Cheng & Reineccius (1991) |
| Pseudomonas taetrolens | IPMP | Gallois et al. (1988) |
| Acinetobacter baumannii | 2,5‐dimethylpyrazine | Gao et al. (2016) |
| Stenotrophomonas maltophilia | 2,3,5‐trimethylpyrazine, 2,5‐dimethylpyrazine | Zou et al. (2007) |
|
Staphylococcus aureus
isolated from A. ludens |
2,5‐ dimethylpyrazine | Rohbacker and Flath (1995), Robacker et al. (1998), Robacker (2007) |
| Lactococcus lactis subsp lactis biovar. diacetilactis | tetramethylpyrazine | Heon and Lee (1991), Lee et al. ( 1995) |
| Bacillus subtilis | 2,5‐dimethylpyrazine, 2,3,5,6‐tetramethylpyrazine | Besson, Creuly, Gros, and Larroche (1997), Larroche, Besson, and Gros (1999), Xiao, Xie, Liu, Hua, and Xu (2006), Zou et al. (2007), Zhu, Xu, and Fan (2009) |
| Bacillus licheniformes | 2,3 dimethylpyrazine, trimethylpyrazine, tetramethylpyrazine | Zhang, Wu, and Xu (2013) |
| Bacillus thuringiensis | 2,5‐ dimethylpyrazine | Robacker et al. (1991), Robacker et al. (1998), Robacker (2007) |
| Bacillus sphaericus | 2,5‐ dimethylpyrazine | |
| Bacillus megaterium | 2,5‐ dimethylpyrazine | |
| Bacillus popilliae | 2,5‐ dimethylpyrazine | |
| Paenibacillus polymyxa | tetramethylpyrazine, methylethylpyrazine, 2,5‐di(propan‐2‐yl)pyrazine, 2,5diisopropylpyrazine | Beck, Hansen, and Lauritsen (2003), Schulz and Dickschat (2007) |
|
Alicyclobacillus acidoterrestris
isolated from apple juice |
IBMP | Siegmund and Pöllinger‐Zierler (2006) |
| Micrococcus luteus | 2,5‐ dimethylpyrazine | Robacker et al. (1991), Robacker et al. (1998), Robacker (2007) |
|
Actinomycetes sp isolated from apple juice |
IBMP | Siegmund and Pöllinger‐Zierler (2006) |
| Corynebacterium glutamicum | tetramethylpyrazine | Dickschat et al. (2010) |
| Chondromyces crocatus | 2,5‐dialkylpyrazine, 3‐methoxy‐2,5‐dialkylpyrazine (side‐chains: methyl, isopropyl, isobutyl, or sec‐butyl) | Dickschat et al. (2005) |
| unclassified on fruit surface | 2,5‐diisopropylpyrazine | Zilkowski, Bartelt, Blumberg, James, and Weaver (1999) |
| unclassified | 3,5‐dimethyl‐2‐methoxypyrazine | Mottram and Patterson (1984) |
| unclassified | 2,5‐diisopropylpyrazine | Zilkowski et al. (1999) |
| Aspergillus parasiticus | IPMP, 2‐hydroxy‐3,6‐di‐sec‐butylpyrazine | Buchanan and Houston (1982) |
| Septoria nodorum | IPMP | Devys, Bousquet, Kollmann, and Barbier (1978) |
IBMP: 2‐isobutyl‐3‐methoxypyrazine; IPMP: 2‐isopropyl‐3‐methoxypyrazine); SBMP: 2‐sec‐butyl‐3‐methoxypyrazine.
3.4. Role of gut bacteria on the MP production
To investigate the role of gut bacteria in MP production in H. axyridis, we fed adult beetles with S. cerealella eggs (containing honey) or a HS diet supplemented with ampicillin and tetracycline (HSAB) for 10 days (Figure 4). The HSAB‐fed beetles were then dissected, and the total MP content was analyzed in their gut tissue and residual body tissue. Antibiotic‐fed beetles showed lower MP concentrations (per mg fresh weight) compared to the control beetles, both in residual body tissues as well as in the gut tissues (Figure 4a; Supporting information Table S4). There was reduction in MP levels in the residual body tissue of antibiotic‐fed males and females, but no difference in the gut tissues when MP concentrations were analyzed per sample (Figure 4b; Supporting information Table S4).
Figure 4.
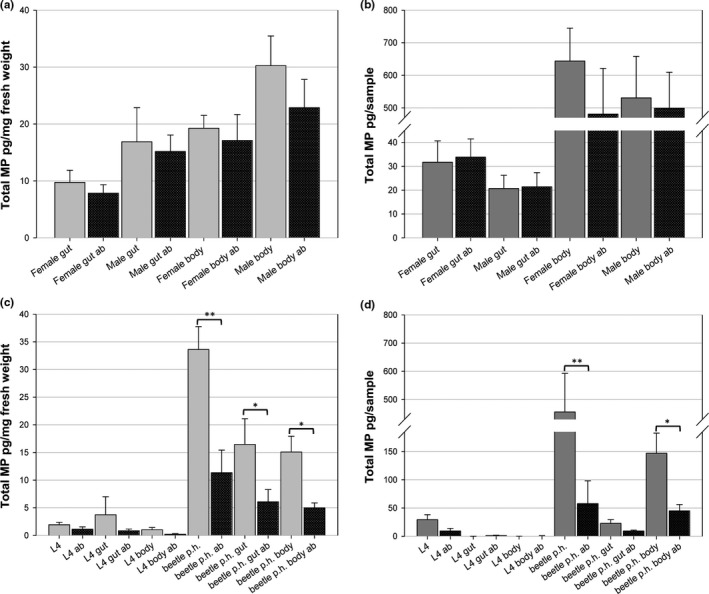
Influence of antibiotics on total MP content of H. axyridis. A Total MP concentration (pg/mg) fresh weight of guts and residual bodies (body) of female and male H. axyridis fed for 10 days with Sitotroga eggs (in honey) (gray) and Sitotroga eggs mixed with honey and antibiotics (dark spotted). (b) Total MP contents in pg/sample after same procedure as shown in 4A. (c) Total MP (pg/mg) fresh weight of whole individuals, dissected guts (gut) and residual bodies (body) from L4 larval instars and newly hatched beetles (beetle p.h.) under Sitotroga eggs (gray) and Sitotroga eggs‐antibiotic mix (dark spotted). (d) Total MP pg/sample of experiment presented in C. Statistical significant differences in values *p < 0.05; **p < 0.01
Diets can have considerable impact on MP levels at larval stages (Kögel et al., 2012); hence, we analyzed MP concentrations in larval stages and newly eclosed beetles fed on the HSAB diet. HSAB‐fed larvae had lower MP concentration than the controls, both in the gut as well as the residual body tissue. A stronger trend was also observed in newly hatched beetles, where HSAB‐fed beetles showed significantly reduced MP concentrations (control beetles 33.622 ± 4.134 pg/mg, HSAB‐fed beetles 11.329 ± 4.099 pg/mg), also in the gut (control 16.438 ± 4.674 pg/mg, HSAB 6.077 ± 2.219 pg/mg) and in the residual body tissue (control 15.092 ± 2.842 pg/mg, HSAB 4.997 ± 0.872 pg/mg) (Figure 4c; Supporting information Table S4). Thus, these data indicate that the gut microbiota seems important for MP synthesis, especially in early life stages of the beetles. We further show, that dysbiosis (the imbalance of the microbial gut community) at larval stages, can influence MP levels at adult stages, indicating that larval gut community could have lifelong influences on the beetles physiology and display signaling, if the native gut microbiota is disrupted.
4. CONCLUSIONS
The secretion of repellents containing methoxypyrazines through reflex bleeds is an important ecological adaptation in several insects, especially in the family Coccinellidae. The invasive ladybird beetle H. axyridis is known to coadapt multiple pyrazines in its reflex bleeds, which function in defense, aggregation and as warning signals. In this study, we quantified MPs in H. axyridis and found that MP concentrations vary across beetle life stages, sexes, and physiological status. We rule out their plant‐based and insect (prey)‐based diets as a potential source of these pyrazines, but show several fold higher accumulation in adult stages, indicating that beetles generate pyrazines endogenously or through their microbiota. Further, adults showed relatively high MP concentrations in the gut and the hemolymph, but not in the fat body or other tissue. Manipulating the gut microbiota of larvae with antibiotics significantly reduced MP concentrations. Thus, we hypothesize that the gut is the site for MP synthesis possibly involving the gut microbiota, from where it is transported to the hemolymph for release through reflex bleeds. The abundance of bacteria particularly of the genera Lactobacillus and Serratia which are known MP producers supports this hypothesis.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
HS performed developmental and diet‐dependent studies, microscopic analysis, data analysis, and wrote the first draft of the paper. RH designed, organized, and performed the GC/MS analysis. SPS designed, performed and described the microbiome analysis. AV and HV conceived and designed the work, and revised and edited the manuscript.
Supporting information
ACKNOWLEDGMENTS
This research was in part funded by the ERA‐Net BiodivERsA, with the national funders ANR (France), DFG (Germany) and BELSPO (Belgium), as part of the 2012–2013 BiodivERsA call for research proposals (VI 219/71, VO 841/71). AV acknowledges funding provided by the Hessian State Ministry of Higher Education, Research and the Arts (HMWK), including a generous grant for the LOEWE Center for Insect Biotechnology and Bioresources. The authors want to thank Sabrina Stiehler for technical assistance, Marisa Skaljac for statistic advices, Martin Hardt for providing the ultramicrotome equipment, and Richard M. Twyman for editing of the manuscript.
Schmidtberg H, Shukla SP, Halitschke R, Vogel H, Vilcinskas A. Symbiont‐mediated chemical defense in the invasive ladybird Harmonia axyridis . Ecol Evol. 2019;9:1715–1729. 10.1002/ece3.4840
DATA ACCESSIBILITY
Microbiome 16S amplicon sequences and data descriptions, methoxypyrazine GC‐MS data and supplemental figures are deposited in the Open Access Data Repository EDMOND and can be directly accessed at the following https://doi.org/10.17617/3.1c.
REFERENCES
- Agarwala, B. K. , Yasuda, H. , & Kajita, Y. (2003). Effect of conspecific and heterospecific feces on foraging and oviposition of two predatory ladybirds: Role of fecal cues in predation avoidance. Journal of Chemical Ecology, 29, 357–376. [DOI] [PubMed] [Google Scholar]
- Balachandran, C. , Duraipandiyan, V. , & Ignacimuthu, S. (2012). Cytotoxic (A549) and antimicrobial effects of Methylobacterium sp. Isolate (ERI‐135) from Nilgrils forest soil, India. Asian Pacific Journal of Tropical Biomedicine, 2(9), 712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, H. B. , Hansen, A. M. , & Lauritsen, F. R. (2003). Novel pyrazine metabolites found in polymyxin biosynthesis by Paenibacillus polymyxa . FEMS Microbiology Letters, 220, 67–73. [DOI] [PubMed] [Google Scholar]
- Berkvens, N. , Bonte, J. , Berkvens, D. , Deforce, K. , Tirry, L. , & Clercoq, P. (2008). Pollen as an alternative food for Harmonia axyridis In Roy H. E., & Wajnberg E. (Eds.), From biological control to invasion: The ladybird Harmonia axyridis as a model species (pp. 201–210). The Netherlands: Springer. [Google Scholar]
- Besson, I. , Creuly, C. , Gros, J. B. , & Larroche, C. (1997). Pyrazine production by Bacillus subtilis in solid‐state fermentation on soybeans. Applied Microbiology Biotechnology, 47, 489–495. 10.1007/s002530050961 [DOI] [Google Scholar]
- Bonde, C. G. , Peepliwal, A. , & Gaikwad, N. J. (2010). Synthesis and antimycobacterial activity of azetidine‐, quinazoline‐ and triazolo‐thiadiazole‐containing pyrazines. Archiv Der Pharmazie – Chemistry in Life Sciences, 343(4), 228–236. 10.1002/ardp.200900165 [DOI] [PubMed] [Google Scholar]
- Botezatu, A. , Kotseridis, Y. , Inglis, D. , & Pickering, G. (2013). Occurrence and contribution of alkylmethoxyprazines in wine tainted by Harmonia axyridis and Coccinella septempunctata . Journal of the Science of Food and Agriculture 93, 803–810. [DOI] [PubMed] [Google Scholar]
- Buchanan, R. L. , & Houston, W. M. (1982). Production of blue fluorescent pyrazines by Aspergillus parasiticus . Journal of Food Science 47, 779‐782. [Google Scholar]
- Cai, L. S. , Koziel, J. A. , & O'Neal, M. E. (2007). Determination of characteristic odorants from Harmonia axyridis beetles using in vivo solid‐phase microextraction and multidimensional gas chromatography‐mass spectrometry‐olfactometry. Journal of Chromatography A, 1147, 66–78. 10.1016/j.chroma.2007.02.044 [DOI] [PubMed] [Google Scholar]
- Caporaso, J. G. , Bittinger, K. , Bushman, F. D. , DeSantism, T. Z. , Andersenm, G. L. , & Knightm, R. (2010a). PyNAST: A flexible tool for aligning sequences to a template alignment. Bioinformatics, 26, 266–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso, J. G. , Kuczynski, J. , Stombaugh, J. , Bittinger, K. , Bushman, F. D. , Costello, E. K. , … Knight, R. (2010b). QIIME allows analysis of high‐throughput community sequencing data. Nature Methods, 7, 335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, T.‐B. , & Reineccius, G. A. (1991). A study factors influencing 2‐methoxy‐3‐isopropylpyrazine production by Pseudomonas perolens using acid trap and UV spectroscopy. Applied Microbiology and Biotechnology, 36, 304–308. [Google Scholar]
- Cheng, T. B. , Reineccius, G. A. , Bjorklund, J. A. , & Leete, E. (1991). Biosynthesis of 2‐methoxy‐3‐isopropylpyrazine in Pseudomonas perolens . Journal of Agricultural and Food Chemistry, 39, 1009–1012. 10.1021/jf00005a042 [DOI] [Google Scholar]
- Cudjoe, E. , Wiederkehr, T. B. , & Brindle, I. D. (2005). Headspace gas chromatography‐mass spectrometry: A fast approach to the identification and determination of 2‐alkyl‐3‐methoxypyrazine pheromones in ladybugs. Analyst, 130, 152–155. 10.1039/b410659h [DOI] [PubMed] [Google Scholar]
- Dalamaga, M. , & Vrioni, G. (2011). Cedecea In Liu D. (Ed.), Molecular detection of human bacterial pathogens (pp. 817–826). London, UK: CRC Press. [Google Scholar]
- Daloze, D. , Brackman, J. C. , & Pasteels, J. M. (1994). Ladybird defense alkaloids: Structural, chemotaxonomic and biosynthetic aspects (Col.: Coccinellidae). Chemoecology, 5(6), 173–183. [Google Scholar]
- Davis, T. S. , Crippen, T. L. , Hofstetter, R. W. , & Tomberlin, J. K. (2013). Microbial volatile emissions as insect semiochemicals. Journal of Chemical Ecology, 39(7), 840–859. 10.1007/s10886-013-0306-z [DOI] [PubMed] [Google Scholar]
- DeMilo, A. B. , Lee, C. J. , Moreno, D. S. , & Martinet, A. J. (1996). Identification of volatiles derived from Citrobacter freundii fermentation of a trypticase soy broth. Journal of Agriculture and Food Chemistry, 44, 607–612. [Google Scholar]
- DeSantis, T. Z. , Hugenholtz, P. , Larsen, N. , Rojas, M. , Brodie, E. L. , Keller, K. , … Andersen, G. L. (2006). Greengenes, a chimera‐checked 16S rRNA gene database and workbench compatible with ARB. Applied and Environmental Microbiology, 72, 5069–5072. 10.1128/AEM.03006-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettner, K. (1987). Chemosystematics and evolution of beetle chemical defenses. Annual Review of Entomology, 32, 17–48. 10.1146/annurev.en.32.010187.000313 [DOI] [Google Scholar]
- Devys, M. , Bousquet, F. , Kollmann, A. , & Barbier, M. (1978). La septorine, nouvelle pyrazine substituée, isolée du milieu de culture de Septoria nodorum Berk champignon phytophatogéne. Comptes Rendues L'académie Des Sciences, 286, 457–458. [Google Scholar]
- Dickschat, J. S. , Reichenbach, H. , Wagner‐Döbler, I. , & Schulz, S. (2005). Novel pyrazines from myxobacterium Chondromyces crocatus and marine bacteria. European Journal of Organic Chemistry, 10.1002/ejoc.200500280 [DOI] [Google Scholar]
- Dickschat, J. S. , Wickel, S. , Bolten, C. J. , Nawrath, T. , Schulz, S. , & Wittmann, C. (2010). Pyrazine synthesis in Corynebacterium glutamicum . European Journal of Organic Chemistry, 14, 2687–2695. [Google Scholar]
- Dudek, K. , Humińska, K. , Wojciechowicz, J. , & Tryjanowski, P. (2017). Metagenomic survey of bacteria associated with the invasive ladybird Harmonia axyridis (Coleoptera: Coccinellidae). European Journal of Entomology, 114, 312–316. 10.14411/eje.2017.038 [DOI] [Google Scholar]
- Dumont, J. P. , Mourgues, R. , & Adda, J. (1983). Potato‐like off flavor in smear coated cheese: A defect induced by bacteria In Williams A. R., & Atkin R. K. (Eds.), Sensory quality in foods and beverages: Definition, measurement and control (pp. 424–428). Chichester, UK: Ellis Horwood Publisher. [Google Scholar]
- Dunlevy, J. D. , Soole, K. L. , Perkins, M. V. , Dennis, E. G. , Keyzers, R. A. , Kalua, C. M. , & Boss, P. K. (2010). Two O‐methyltransferases involved in the biosynthesis of methoxypyrazines: Grape‐derived aroma compounds important to wine flavor. Plant Molecular Biology, 74(1–2), 77–89. [DOI] [PubMed] [Google Scholar]
- Durieux, D. , Fassotte, B. , Deneubourg, J.‐L. , Brostaux, Y. , Vandereycken, A. , Joie, E. , … Verheggen, F. J. (2015). Aggregation behavior of Harmonia axyridis under non‐wintering conditions. Insect Science, 22, 670–678. [DOI] [PubMed] [Google Scholar]
- Edgar, R. C. (2010). Search and clustering orders of magnitude faster than BLAST. Bioinformatics, 26, 2460–2461. 10.1093/bioinformatics/btq461 [DOI] [PubMed] [Google Scholar]
- Filipiak, W. , Sponring, A. , Baur, M. M. , Filipiak, A. , Ager, C. , Wiesenhofer, H. , … Amann, A. (2012). Molecular analysis of volatile metabolites released specifically by Staphylococcus aureus and Pseudomonas aeruginosa . BMC Microbiology, 12, 113–129. 10.1186/1471-2180-12-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallois, A. , & Grimont, P. A. D. (1985). Pyrazines responsible for the potato‐like odor produced by some Serratia and Cedecea strains. Applied and Environmental Microbiology, 50(4), 1048–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallois, A. , Kergomard, A. , & Adda, J. (1988). Study of the biosynthesis of 3‐isopropyl‐2‐methoxypyrazine produced by Pseudomonas taetrolens . Food Chemistry, 28, 299–309. 10.1016/0308-8146(88)90105-7 [DOI] [Google Scholar]
- Galvan, T. L. , Koch, R. , & Hutchison, W. D. (2008). Impact of fruit feeding on overwintering survival of the multicolored Asian lady beetle, and the ability of this insect and paper wasps to injure wine grape berries. Entomologia Experimentalis Et Applicata, 128(3), 429–436. 10.1111/j.1570-7458.2008.00731.x [DOI] [Google Scholar]
- Gao, J. , Zou, Y. , Wang, Y. , Wang, F. , Lang, L. , Wang, P. , … Ying, K. (2016). Breath analysis for noninvasively differentiating Acinetobacter baumannii ventilator‐associated pneumonia from its respiratory tract colonization of ventilated patients. Journal of Breath Research, 10, 027102. [DOI] [PubMed] [Google Scholar]
- Gegner, T. , Schmidtberg, H. , Vogel, H. , & Vilcinskas, A. (2018). Population‐specific expression of antimicrobial peptides conferring pathogen resistance in the invasive ladybird Harmonia axyridis . Scientific Reports, 8, 3600 10.1038/s41598-018-21781-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimont, F. , & Grimont, P. A. D. (2006). The Genus Serratia. Prokaryontes, 6, 219–244. [Google Scholar]
- Guilford, T. , Nicol, C. , Rothschild, M. , & Moore, B. P. (1987). The biological roles of pyrazines – evidence for warning odor function. Biological Journal of the Linnean Society, 31, 113–128. [Google Scholar]
- Hemptinne, J.‐L. , & Dixon, F. G. (2000). Defence, oviposition and sex: Semiochemical parsimony in two species of ladybird beetles (Coleoptera, Coccinellidae). A short review. European Journal of Entomology, 97, 443–447. 10.14411/eje.2000.068 [DOI] [Google Scholar]
- Heon, K. K. , & Lee, H. J. (1991). Optimum conditions for the fermentation of acetoin as a precursor of tetramethylpyrazine during the citrate fermentation by Lactococcus lactis subsp. lactis biovar. diacetilacitis FC1. Journal of Microbiology and Biotechnology, 1(3), 202–203. [Google Scholar]
- Holloway, G. J. , de Jong, P. W. , Brakefield, P. M. , & de Vose, H. (1991). Chemical defense in ladybird beetles (Coccinellidae). I. Distribution of coccineline and individual variation in defence in 7‐spot ladybirds (Coccinella septempunctata). Chemoecology, 2, 7–14. [Google Scholar]
- Hurst, G. D. D. , Majerus, M. E. N. , & Walker, L. E. (1992). Cytoplasmic male killing elements in Adalia bipunctata (Linnaeus) (Coleoptera: Coccinellidae). Heredity, 69, 84–91. 10.1038/hdy.1992.97 [DOI] [Google Scholar]
- Jeanson, R. , & Deneubourg, J. L. (2009). Positive feedback, convergent collective patterns and social transitions in Arthropods In Gadau J., & Fewell J. (Eds.), Organization of insect societies: from genome to sociocomplexity (pp. 460–482). Cambridge, MA: Harvard University Press. [Google Scholar]
- Kim, K. K. , Han, S. I. , Moon, C. W. , Yu, Y. M. , & Whang, K. S. (2011). Biodiversity and isolation of gut microbes from digestive organs of Harmonia axyridis . Korean Journal of Microbiology, 47(1), 66–73. [Google Scholar]
- King, G. K. , & Meinwald, J. (1996). Review of the defensive chemistry of coccinellids. Chemical Reviews, 96, 1105–1122. 10.1021/cr950242v [DOI] [PubMed] [Google Scholar]
- Koch, R. L. (2003). The multicolored Asian Lady beetle, Harmonia axyridis: A review of its biology, uses in biological control, and non‐target impacts. Journal of Insect Science, 3, 32–47. 10.1093/jis/3.1.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kögel, S. , Eben, A. , Hoffmann, C. , & Gross, J. (2012). Influence of diet on fecundity, immune defense and content of 2‐Isopropyl‐3‐Methoxypyrazine in Harmonia axyridis Pallas. Journal of Chemical Ecology, 38(7), 854–864. 10.1007/s10886-012-0139-1 [DOI] [PubMed] [Google Scholar]
- Larroche, C. , Besson, I. , & Gros, J. B. (1999). High pyrazine production by Bacillus subtilis in solid substrate fermentation on ground soybeans. Process Biochemistry, 34, 667–674. 10.1016/S0032-9592(98)00141-1 [DOI] [Google Scholar]
- Laugier, G. J. M. , Le Moguédec, G. , Tayeh, A. , Loiseau, A. , Osawa, N. , Estoup, A. , & Facon, B. (2013). Increase in male reproductive success and female reproductive investment in invasive populations of the harlequin ladybird Harmonia axyridis . PLoS ONE, 8(10), e77083 10.1371/journal.pone.0077083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, C. J. , DeMilo, A. B. , Moreno, D. S. , & Martinez, A. J. (1995). Analyses of the volatile components of a bacterial fermentation that is attractive to the Mexican fruit fly, Anastrepha ludens . Journal of Agriculture and Food Chemistry, 43, 1348–1351. [Google Scholar]
- Maga, J. A. (1982). Pyrazines in food: An update. Critical Reviews in Food Science and Nutrition, 20, 1–48. [DOI] [PubMed] [Google Scholar]
- Majerus, M. E. N. , & Majerus, T. M. O. (1997). Predation of ladybirds by birds in the wild. The Entomologist's Monthly Magazine, 133, 1592–1595. [Google Scholar]
- Marples, N. M. (1993). Is the alkaloid in 2spotted ladybirds (Adalia bipunctata) a defence against ant predation? Chemoecology, 4(1), 29–32. [Google Scholar]
- Martinez, A. J. , Robacker, D. C. , Garcia, J. A. , & Esau, K. L. (1994). Laboratory and field olfactory attraction of the Mexican fruit fly (Diptera: Tephritidae) to metabolites of bacterial species. Florida Entomologist, 77, 117–126. 10.2307/3495878 [DOI] [Google Scholar]
- McCord, J. S. (2015). A comparative study of eastern and western North American populations of Hippodamia convergens (Coleoptera: Coccinellidae). 22. http://uknowledge.uky.edu/entomology_etds/22
- Miller, A. , Scanlan, R. A. , Lee, J. S. , Libbey, L. M. , & Morgan, M. E. (1973). Volatile compounds produced in sterile fish muscle (Sebastes melanops) by Pseudomonas perolens . Journal of Applied Microbiology, 25, 257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon, C. W. , Kim, K. K. , Whang, K. S. , Seo, M. J. , Youn, Y. N. , & Yu, Y. M. (2011). Characteristics of Enterobacteria from Harmonia axyridis and effects of Staphylococcus spp. on development of H. axyridis . Korean Journal of Applied Entomology, 50(2), 157–165. 10.5656/KSAE.2011.06.0.30 [DOI] [Google Scholar]
- Moore, B. P. , Brown, W. V. , & Rothschild, M. (1990). Methylalkylpyrazines in aposematic insects, their hostplants and mimics. Chemoecology, 1, 43–51. 10.1007/BF01325227 [DOI] [Google Scholar]
- Morgan, M. E. , Libbey, L. M. , & Scanlan, R. A. (1972). Identity of the musty‐potato aroma compound in milk cultures of Pseudomonas taetrolens . Journal of Dairy Science, 55, 666. [Google Scholar]
- Mottram, D. S. , & Patterson, L. S. (1984). 2,6‐Dimethyl‐3‐methoxypyrazine: A microbiologically‐produced compound with obnoxious musty odour. Chemistry & Industry, 12, 448–449. [Google Scholar]
- Murray, K. E. , Shipton, J. , & Whitfield, F. B. (1970). 2‐Methoxypyrazines and flavour of green peas (Pisum sativum). Chemistry and Industry, 27, 897–898. [PubMed] [Google Scholar]
- Murray, K. E. , & Whitfield, F. B. (1975). Occurrence of 3‐alkyl‐2‐methoxypyrazines in raw vegetables. Journal of the Science of Food and Agriculture, 26, 973–986. [Google Scholar]
- Ninkovic, V. , Al Abassi, S. , & Pettersson, J. (2001). The influence of aphid‐induced plant volatiles on ladybird beetle searching behavior. Biological Control, 21, 191–195. 10.1006/bcon.2001.0935 [DOI] [Google Scholar]
- Noriyuki, S. , Kameda, Y. , & Osawa, N. (2014). Prevalence of male‐killer in a sympatric population of two sibling ladybird species, Harmonia yedoensis and Harmonia axyridis (Coleoptera: Coccinellidae). European Journal of Entomology, 111(2), 307–311. 10.14411/eje.2014.029 [DOI] [Google Scholar]
- Pinheiro, J. , Bates, D. , DebRoy, S. , & Sarkar, D. (2014). R Core Team (2014) nlme: linear and nonlinear mixed effects models. R package version 3.1‐117. http://CRAN.R-project.org/package=nlme
- Price, M. N. , Dehal, P. S. , & Arkin, A. P. (2010). FastTree 2–approximately maximum‐likelihood trees for large alignments. PLoS ONE, 5, e9490 10.1371/journal.pone.0009490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeder, J. , & Knight, R. (2010). Rapid denoising of pyrosequencing amplicon data: Exploiting the rank‐abundance distribution. NatureMethods, 7, 668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzi, G. P. (1988). The biogenesis of food related pyrazines. Food Reviews International, 4(3), 375–400. 10.1080/87559128809540839 [DOI] [Google Scholar]
- Robacker, D. C. (2007). Chemical ecology of bacterial relationships with fruit flies. IOBC/WPRS Bulletin, 30(9), 9–22. [Google Scholar]
- Robacker, D. C. , & Flath, R. A. (1995). Attractants from Staphylococcus aureus cultures for Mexican fruitfly, Anastrepha ludens . Journal of Chemical Ecology, 21, 1861–1874. [DOI] [PubMed] [Google Scholar]
- Robacker, D. C. , & Bartelt, R. J. (1997). Chemicals attractive to Mexican fruit fly from Klebsiella pneumoniae and Citrobacter freundii cultures sampled by solid‐phase microextraction. Journal of Chemical Ecology, 23, 2897–2915. 10.1023/A:1022579414233 [DOI] [Google Scholar]
- Robacker, D. C. , DeMilo, A. B. , & Voaden, D. (1991). Mexican fruit fly attractants: Effects of 1‐pyrroline and other amines on attractiveness of a mixture of ammonia, methylamine, and putrescine. Journal of Chemical Ecolology, 23, 1263–1280. 10.1023/B:JOEC.0000006463.93138.2a [DOI] [Google Scholar]
- Robacker, D. C. , & Lauzon, C. R. (2002). Purine metabolizing capability of Enterobacter agglomerans affects volatiles production and attractiveness to Mexican fruit fly. Journal of Chemical Ecology, 28, 1549–1563. [DOI] [PubMed] [Google Scholar]
- Robacker, D. C. , Lauzon, C. R. , & He, X. (2004). Volatiles production and attractiveness to the Mexican fruit fly of Enterobacter agglomerans isolated from apple maggot and Mexican fruit flies. Journal of Chemical Ecology, 30, 1329–1347. 10.1023/B:JOEC.0000037743.98703.43 [DOI] [PubMed] [Google Scholar]
- Robacker, D. C. , Martinez, A. J. , Garcia, J. A. , & Bartelt, R. J. (1998). Volatiles attractive to the Mexican fruit fly (Diptera: Tephritidae) from eleven bacteria taxa. Florida Entomologist, 81, 497–508. 10.2307/3495948 [DOI] [Google Scholar]
- Rothschild, M. , Euw, J. V. , & Reichste, T. (1973). Cardiac‐glycosides in a scale insect (Aspidiotus), a ladybird (Coccinella) and lacewing (Chrysopa). Journal of Entomology A, 48, 89–90. [Google Scholar]
- Rothschild, M. , & Moore, B. (1987). Pyrazines as alerting signals in toxic plants and insects In Labeyrie V., Fabres G., & Lachaise D. (Eds.), Proceedings of the 16th International Symposium on Insect‐Plant Relationships (pp. 97–101). Dordrecht, The Netherlands: Junk W. [Google Scholar]
- Rowe, C. , & Guilford, T. (1999). The evolution of multimodal warning displays. Evolutionary Ecology, 13, 655–671. 10.1023/A:1011021630244 [DOI] [Google Scholar]
- Roy, H. , Brown, P. M. J. , Adriaens, T. , Berkvens, N. , Borges, I. , Clusella‐Trullas, S. , … Zhao, Z. (2016). The harlequin ladybird, Harmonia axyridis: Global perspectives on invasionhistory and ecology. Biological Invasions, 18, 997–1044. 10.1007/s10530-016-1077-6 [DOI] [Google Scholar]
- Sarkar, D. (2010). lattice: lattice graphics. R package version 0.18‐3. http://CRAN.R-project.org/package=lattice
- Schulz, S. , & Dickschat, J. S. (2007). Bacterial volatiles: The smell of small organisms. Natural Product Reports, 24, 814–842. 10.1039/b507392h [DOI] [PubMed] [Google Scholar]
- Siddall, E. C. , & Marples, N. M. (2008). Better to be bimodal: The interaction of color and odor on learning and memory. Behavioral Ecology, 19, 425–432. 10.1093/beheco/arm155 [DOI] [Google Scholar]
- Siegmund, B. , & Pöllinger‐Zierler, B. (2006). Odor thresholds of microbially induced off‐flavor compounds in apple juice. Journal of Agriculture and Food Chemistry, 54(16), 5984–5989. 10.1021/jf060602n [DOI] [PubMed] [Google Scholar]
- Sun, Y. , Wolcott, R. D. , & Dowd, S. E. (2011). Tag‐encoded FLX amplicon pyrosequencing for the elucidation of microbial and functional gene diversity in any environment In Kwon Y. M., & Ricke S. C. (Eds.), High‐throughput next generation sequencing: Methods and applications (pp. 129–141). New York, NY: Humana Press. [DOI] [PubMed] [Google Scholar]
- Susset, E. C. , Ramon‐Portugal, F. , Hemptinne, J. L. , Dewhirst, S. Y. , Birkett, M. A. , & Magro, A. (2013). The role of semiochemicals in short‐range location of aggregation sites in Adalia bipunctata (Coleoptera, Coccinellidae). Journal of Chemical Ecology, 39, 591–601. 10.1007/s10886-013-0285-0 [DOI] [PubMed] [Google Scholar]
- Verheggen, F. J. , Vogel, H. , & Vilcinskas, A. (2017). Behavioral and Immunological Features Promoting the Invasive Performance of the Harlequin Ladybird Harmonia axyridis . Frontier Ecology and Evolution, 5, 156. [Google Scholar]
- Von Beeren, C. , Schulz, S. , Hashim, R. , & Witte, V. (2011). Acquisition of chemical recognition cues facilities integration into ant societies. BMC Ecology, 11, 30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q. , Garrity, G. M. , Tiedje, J. M. , & Cole, J. R. (2007). Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and Environmental Microbiology, 73, 5261–5267. 10.1128/AEM.00062-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler, C. A. , & Cardé, R. T. (2013). Defensive allomones function as aggregation pheromones in diapausing ladybird beetles, Hippodamia convergens . Journal of Chemical Ecology, 39, 723–732. 10.1007/s10886-013-0293-0 [DOI] [PubMed] [Google Scholar]
- Witte, L. , Ehmke, A. , & Hartmann, T. (1990). Interspecific flow of pyrrolizidine alkaloids from plants via aphids to ladybirds. Naturwissenschaften, 77, 540–543. 10.1007/BF01139268 [DOI] [Google Scholar]
- Woolfson, A. , & Rothschild, M. (1990). Speculating about pyrazines. Proceedings of the Royal Society B, 242(1304), 113–119. 10.1098/rspb.1990.0113. [DOI] [PubMed] [Google Scholar]
- Xiao, Z. J. , Xie, N. Z. , Liu, P. H. , Hua, D. L. , & Xu, P. (2006). Tetramethylpyrazine production from glucose by a newly isolated Bacillus mutant. Applied Microbiology Biotechnology, 73, 512–518. 10.1007/s00253-006-0491-6 [DOI] [PubMed] [Google Scholar]
- Youren, F. (2012). Responses of aphids to semiochemicals released from ladybirds. Master thesis. (pp. 1–25). Uppsala, Sweden: Department of Ecology. [Google Scholar]
- Zhang, R. , Wu, Q. , & Xu, Y. (2013). Aroma characterisitcs of Moutai‐flavour liquor produced with Bacillus licheniformis by solid‐state fermentation. Letters in Applies Microbiology, 57, 11–18. [DOI] [PubMed] [Google Scholar]
- Zhu, B. , Xu, Y. , & Fan, W. (2009). High‐yield fermentative preparation of tetramethylpyrazine by Bacillus sp. using an endogenous precursor approach. Journal of Industrial Microbiology & Biotechnology, 37, 179–186. [DOI] [PubMed] [Google Scholar]
- Zilkowski, B. W. , Bartelt, R. J. , Blumberg, D. , James, D. G. , & Weaver, D. K. (1999). Identification of host‐related volatiles attractive to pineapple beetle Carpophilus humeralis . Journal of Chemical Ecolology, 25(1), 229–252. [Google Scholar]
- Zou, C.‐S. , Mo, M.‐H. , Gu, Y.‐Q. , Zhou, J.‐P. , & Zhang, K.‐Q. (2007). Possible contributions of volatile‐producing bacteria to soil fungistasis. Soil Biology and Biochemistry, 39(9), 2371–2379. 10.1016/j.soilbio.2007.04.009 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Microbiome 16S amplicon sequences and data descriptions, methoxypyrazine GC‐MS data and supplemental figures are deposited in the Open Access Data Repository EDMOND and can be directly accessed at the following https://doi.org/10.17617/3.1c.


