Abstract
Sanofi Pasteur has developed a chimeric yellow fever–dengue, live-attenuated, tetravalent dengue vaccine (CYD-TDV) that is currently approved for use in several countries. In clinical trials, CYD-TDV was efficacious at reducing laboratory-confirmed cases of dengue disease. Efficacy varied by dengue virus (DENV) serotype and prevaccination dengue immune status. We compared the properties of antibodies in naive and DENV-exposed individuals who received CYD-TDV. We depleted specific populations of DENV-reactive antibodies from immune serum samples to estimate the contribution of serotype-cross-reactive and type-specific antibodies to neutralization. Subjects with no preexisting immunity to DENV developed neutralizing antibodies to all 4 serotypes of DENV. Further analysis demonstrated that DENV4 was mainly neutralized by type-specific antibodies whereas DENV1, DENV2, and DENV3 were mainly neutralized by serotype cross-reactive antibodies. When subjects with preexisting immunity to DENV were vaccinated, they developed higher levels of neutralizing antibodies than naive subjects who were vaccinated. In preimmune subjects, CYD-TDV boosted cross-reactive neutralizing antibodies while maintaining type-specific neutralizing antibodies acquired before vaccination. Our results demonstrate that the quality of neutralizing antibodies induced by CYD-TDV varies depending on DENV serotype and previous immune status. We discuss the implications of these results for understanding vaccine efficacy.
Keywords: Dengue vaccine, flavivirus, neutralizing antibody, live attenuated vaccine.
Millions of persons living in tropical and subtropical parts of the world are infected by dengue viruses (DENVs) each year. Several hundred thousand of these infections, especially in children, progress to dengue hemorrhagic fever/dengue shock syndrome, a life-threatening disease [1]. DENVs belong to the family Flaviviridae, which includes other medically significant arboviruses, such as Japanese encephalitis, yellow fever, West Nile, tick-borne encephalitis, and Zika viruses. Vaccination is one of the most effective strategies for protecting the public from flavivirus infections, as demonstrated by the success of yellow fever, Japanese encephalitis, and tick-borne encephalitis vaccines.
In the case of dengue, vaccine development is complicated by the presence of 4 virus serotypes and the possibility of immune-enhanced dengue disease. Nevertheless, many dengue vaccines are currently at different stages of development. Sanofi Pasteur has developed a chimeric yellow fever–dengue, live-attenuated, tetravalent dengue vaccine (CYD-TDV) that is currently approved for use in the population aged 9–45 years in several countries. In 2 large-scale phase III efficacy trials, CYD-TDV was efficacious at reducing laboratory-confirmed cases of dengue disease, including severe cases and those requiring hospitalization [2, 3]. Efficacy varied by serotype, with higher efficacy rates against serotypes 3 and 4 than against serotypes 1 and 2 [2–4]. Furthermore, efficacy was higher in participants who had been previously exposed to dengue than in those who were dengue naive at baseline [2, 3, 5].
Although the results from live-attenuated dengue vaccine trials are encouraging, they also highlight the complexity of the human immune response to DENV vaccination, which is often not balanced across the 4 serotypes and also influenced by prior flavivirus immune status of vaccinees [6, 7]. The goal of the current study was to characterize serum from CYD-TDV recipients to better understand the specificity and functionality of DENV-specific vaccine induced antibodies and to generate hypotheses that may explain efficacy data.
DENV envelope (E) protein is the major target of neutralizing antibody [8]. The crystal structures of the E protein of dengue and other flaviviruses have been solved [9–12]. Individual subunits of E protein consist of 3 beta-barrel domains designated domains I, II, and III, with the native protein forming a head-to-tail homodimer. The hydrophobic fusion peptide is located at the tip of domain II and shielded by domain III of the adjacent subunit. A detailed picture of how E protein dimers are organized on the surface of the virion has been obtained by combining the crystal structures of E protein with cryo–electron microscopic reconstructions of the entire virion [13]. Each virion has 180 monomers of E protein organized into 90 dimers that lie flat on the surface of the virus.
DENVs have epitopes that are unique to each serotype and epitopes that are shared between serotypes. Persons who have recovered from primary DENV infections develop robust antibody responses that cross-react with the 4 serotypes [8, 14]. Despite the cross-reactivity, after a limited “grace” period of cross-protection, antibodies only prevent reinfection from the same serotype (homologous serotype), and individuals are susceptible to a second infection with a different serotype (heterologous serotype) [8, 15]. Serotype-specific, strongly neutralizing human antibodies have been mapped to different epitopes on E protein, including complex quaternary structure epitopes that span different E protein molecules [16–23]. Furthermore, recent studies indicate that DENV-immune individuals exposed to a new serotype develop neutralizing antibodies that bind to simple and quaternary epitopes that are conserved between serotypes [15, 19]. Thus, primary DENV infections mainly induce neutralizing antibodies that bind to epitopes that are unique to the infecting serotype, whereas repeated infections broaden this response and induce serotype cross-neutralizing antibodies. The cross-protective nature of immunity after repeated infections may account for the observation that third and fourth infections with new serotypes are not as clinically severe as first or second infections [24].
Building on results on the specificity and functionality of antibodies in persons exposed to natural DENV infections, in the current study we compared and contrasted the properties of DENV-specific antibodies in naive and dengue-exposed individuals who were vaccinated with 3 doses of CYD-TDV. Our results, which demonstrate differences in the quality of neutralizing antibodies depending on virus serotype and prevaccination immune status, provide a basis for better understanding the efficacy data from dengue vaccine trials.
MATERIAL AND METHODS
Clinical Trial Serum Samples
Serum samples from clinical trial subjects naive at baseline taken 28 days after the third vaccination of CYD-TDV in a 0/6/12-month vaccination schedule were used from a study conducted in a nonendemic area for dengue (www.clinicaltrials.gov NCT01134263) [25]. Serum samples from clinical trial subjects preimmune at baseline before initial vaccination and 28 days after the third vaccination of CYD-TDV in a 0/6/12-month vaccination schedule were used from a study conducted in a dengue-endemic area (NCT01187433) [26]. The serum samples tested from this trial were paired prevaccination and PD3 samples. Use of clinical trial serum samples for these analyses was performed in accordance with study protocols for NCT01134263 and NCT01187433.
Viruses and Cells
WHO reference strains, DENV1 (West Pac 74), DENV2 (S-16803), DENV3 (CH-53489) and DENV4 (TVP-376) were used in the present study unless otherwise noted. The strains were grown in C6/36 mosquito cells to generate infectious stocks and Vero-81 mammalian cells to generate purified antigen. DENVs from culture medium were purified by means of density gradient and ultracentrifugation, as described elsewhere [27]. DENV2 purified antigen was purchased from Microbix Biosystems.
Whole-Virus Depletion of DENV-Specific Antibodies From Vaccine Immune Serum Samples
Purified DENV was adsorbed onto 4.5-ÃÂÃÂm Polybead polystyrene microspheres (Polysciences; catalog No. 17135-5) at a bead (microliter) to ligand (microgram) ratio of 5:2. Beads were washed 3 times with 0.1 mol/L Borate buffer (pH 8.5), followed by an overnight incubation with purified DENV and 0.1 mol/L Borate buffer (8.5) at room temperature. Control beads were incubated with the equivalent amount of bovine serum albumin. The control and virus-coated beads were then blocked 3 times with a 10 mg/mL bovine serum albumin solution for 30 minutes at room temperature, followed by 4 washes with 0.1 mol/L borate buffer (pH 8.5) and then 4 washes with ×1 phosphate-buffered saline. DENV-specific antibodies were depleted from human serum samples by incubating virus-adsorbed beads with human serum samples diluted 1:10 in phosphate-buffered saline for 45 minutes at 37ÃÂÃÂC 3 times with end-over-end mixing. Successful depletion of DENV-specific antibodies was confirmed via enzyme-linked immunosorbent assay (ELISA), in which the plates are coated with the same DENV antigen used for depletion.
ELISA Methods
Initial titration ELISA is done on the serum samples, in which the ELISA plate is coated with the DENV antigen with which the serum will be depleted. The initial ELISA helps quantify the amount of DENV antigen needed to fully deplete the serum from the respective serotype. After depletion, confirmation ELISA was performed to confirm that the serum was fully depleted from all antibodies that could bind to the serotype of depletion.
Neutralization Assays
Neutralizing titers were initially measured through the standard DENV plaque reduction neutralization assay (PRNT) [28] In the present study, we used a flow cytometry–based assay that uses a human monocytic cell line (U937) expressing dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN), which generates neutralization titers qualitatively similar to those of PRNT [29]. Briefly, DENV depleted and control depleted serum samples are incubated with each of the 4 DENV serotypes separately for 45 minutes at 37ÃÂÃÂC. Cells are then added and incubated for 2 hours at 37ÃÂÃÂC. After 2 hours the cells are washed with medium and incubated for 24 hours at 37ÃÂÃÂC. After 24 hours the cells are fixed and permeabilized. The percentage of infection is measured by staining the cells with 2H2, a preMembrane (prM) protein specific mouse monoclonal antibody, conjugated to Alexa488 fluorophore using flow cytometry. Using the 50% neutralization antibody titers (Neut50), the percentage of cross-reactive (CR) and type-specific (TS) neutralizing antibodies against each serotype were calculated as follows:
and
RESULTS
Flavivirus-Naive Subjects Vaccinated With CYD-TDV
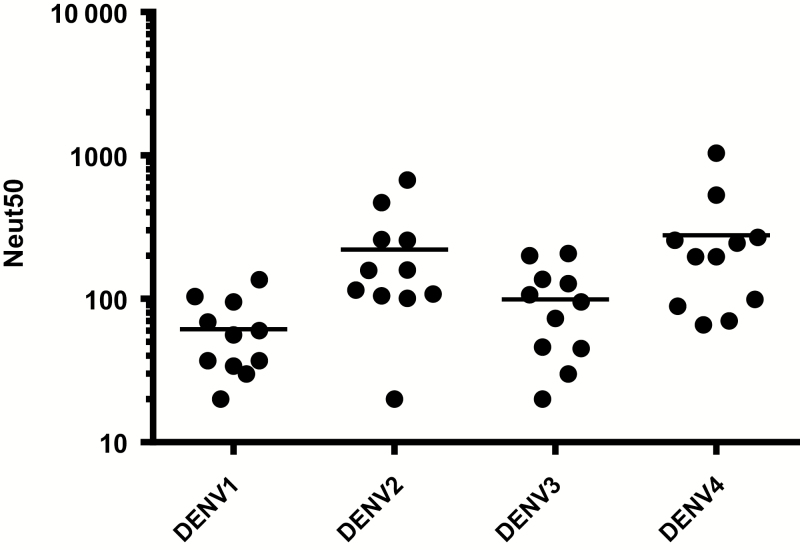
We first analyzed blood samples collected from 11 subjects who had no evidence of exposure to dengue before vaccination (Supplementary Table 1). To measure levels of DENV neutralizing antibodies induced by vaccination, we used a flow cytometry–based assay that uses a human monocytic cell line (U937) expressing DC-SIGN. Figure 1 and Supplementary Table 1 depicts the 50% neutralizing antibody titers against each of the 4 serotypes in these 11 subjects. Although there was considerable variability among subjects in the levels of neutralizing antibodies to the different serotypes, the mean levels to serotypes 2 and 4 were higher than those to serotypes 1 and 3.
Figure 1.
Neutralizing antibody levels in chimeric yellow fever–dengue, live-attenuated, tetravalent dengue vaccine (CYD-TDV) recipients who were dengue virus (DENV) naive before vaccination. Neutralizing antibodies were measured in samples collected from 11 subjects 1 month after the final vaccine dose. Each data point represents the 50% neutralization antibody titer (Neut50) for a single subject for the specified serotype; horizontal bars represent geometric mean titers.
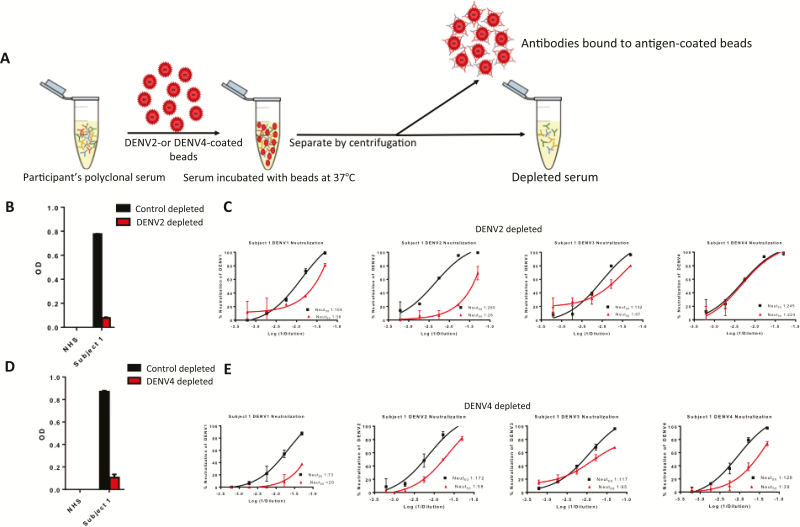
Persons exposed to primary DENV infections develop serotype-specific neutralizing antibodies [16]. After repeated DENV infections with different serotypes, individuals maintain both serotype-specific and cross-reactive neutralizing antibodies. We performed experiments to estimate levels of type-specific and cross-reactive neutralizing antibodies in naive subjects who had received 3 doses of CYD-TDV. Polystyrene beads coated with purified DENV antigen were used to selectively remove serum antibodies that bound to a particular serotype (Figure 2A). Antibody depletion was confirmed by ELISA before performing DENV neutralization assays with the 4 serotypes. A serotype-specific (homotypic) response was the fraction of neutralizing antibodies against a particular serotype that remained after depletion with antigen from heterologous serotypes. For example, as depicted in Figure 2B, when a sample from subject 1 was depleted with DENV2 antigen, ELISA confirmed loss of DENV2 binding antibodies. When the depleted sample was used in a neutralization assay with the 4 serotypes, we observed a large loss of DENV2, a partial of loss of DENV1 and DENV3, and no loss of DENV4 neutralization (Figure 2C).
Figure 2.
Dengue virus (DENV) antibody depletion assay to estimate proportions of serotype-specific and cross-reactive neutralizing antibodies in serum samples from chimeric yellow fever–dengue, live-attenuated, tetravalent dengue vaccine (CYD-TDV) recipients. A, Schematic of the antibody depletion method. Serum is incubated with polystyrene beads coated with purified DENV2 or DENV4 virions. A negative control depletion is performed using beads coated with bovine serum albumin. The virus-coated beads (or control beads) were pelleted, and the supernatant was collected for further analysis. BÃÂÃÂÃÂE, Antibody depletion and DENV neutralization results for CYD-TDV subject 1. Abbreviations: NHS, normal human serum; OD, optical density. B, D, Enzyme-linked immunosorbent assay was used to confirm removal of DENV2 or DENV4 binding antibodies. C, E, DENV neutralization assays were performed with the DENV2 or DENV4 antibody-depleted samples to estimate proportions of type-specific and cross-reactive neutralizing antibodies against each of the 4 DENV serotypes.
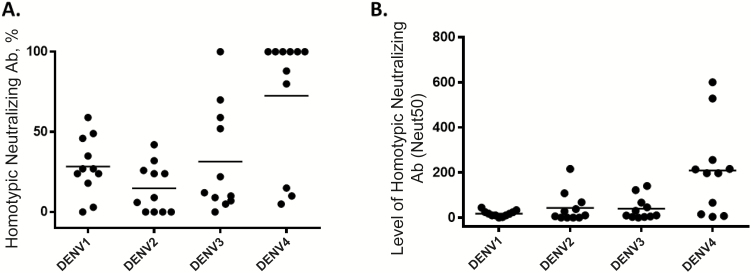
These results indicate that DENV4 is mainly neutralized by type-specific antibodies, whereas a mixture of type-specific and cross-reactive antibodies neutralize DENV1 and 3. The data are inconclusive about the properties of DENV2 neutralizing antibodies, because both DENV2 type-specific and cross-reactive antibodies have been depleted from the sample. To determine the quality of DENV2 neutralizing antibodies, the sample was separately depleted using beads coated with DENV4 antigen (Figure 2D and 2E). The DENV4 depletion resulted in a large loss of DENV2 neutralization, demonstrating that cross-reactive antibodies dominated the DENV2 response. Using this approach, we estimated levels of homotypic neutralizing antibodies in all 11 naive subjects who were vaccinated. As depicted in Figure 3, the DENV4 response was dominated by type-specific neutralizing antibodies, whereas DENV1, DENV2, and DENV3 responses had lower levels of type-specific neutralizing antibody. This pattern of type-specific antibodies dominating the DENV4 response was observed when we analyzed an additional 14 flavivirus-naive subjects who had received 3 doses of CYD-TDV (Supplementary Figure 1).
Figure 3.
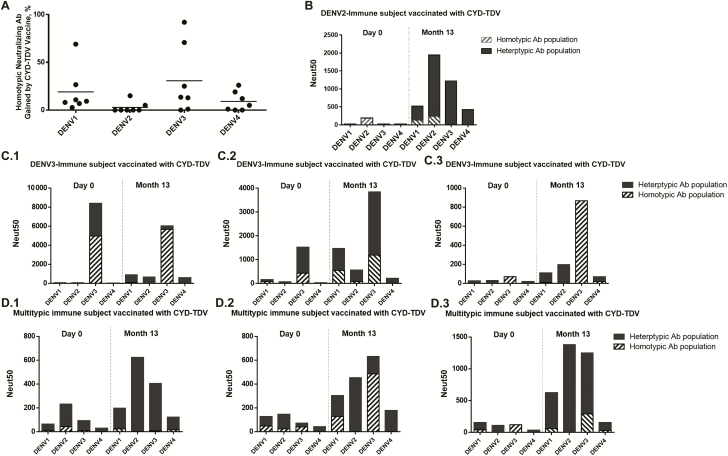
Proportion and level of homotypic neutralizing antibodies to each serotype in dengue virus (DENV)–naive subjects who received the chimeric yellow fever–dengue, live-attenuated, tetravalent dengue vaccine (CYD-TDV). The antibody depletion assay was used to measure proportions of DENV type-specific (homotypic) neutralizing antibodies induced by CYD-TDV in DENV-naive subjects. A, Each data point represents the percentage of the total neutralizing antibody response to the specified serotype attributed to type-specific antibodies; horizontal bars represent geometric mean titers. Two-way analysis of variance (ANOVA) was used to calculate P values (DENV4 vs DENV1, P = .003; DENV4 vs DENV2, P < .001; DENV4 vs DENV3, P = .005). B, Each data point represents the absolute level of homotypic neutralizing antibody to the specified serotype (50% neutralization antibody titer [Neut50]); horizontal bars represent geometric mean titers. Two-way ANOVA was used to calculate P values (DENV4 vs DENV1, P = .001; DENV4 vs DENV2, P = .006; DENV4 vs DENV3, P = .004).
Flavivirus-Preimmune Subjects Vaccinated With CYD-TDV
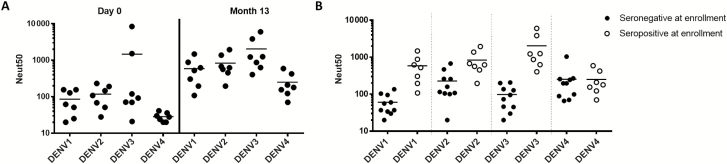
To evaluate the antibody response among dengue-exposed individuals who were vaccinated with CYD-TDV, we analyzed blood from 7 subjects who were recruited in a dengue-endemic region. Blood samples collected 1 month after the final vaccine dose were analyzed using the same approaches described for dengue-naive CYD-TDV recipients. Before vaccination, these subjects had varying levels of neutralizing antibodies to DENV serotypes consistent with prior exposure to primary or repeated DENV infections (Figure 4A and Supplementary Table 2). The overall levels of neutralizing antibodies to all 4 serotypes increased when preimmune subjects were vaccinated with CYD-TDV (Figure 4A and Supplementary Table 2). Moreover, preimmune subjects attained higher levels of neutralizing antibodies than naive subjects who were vaccinated, with the exception of DENV4, for which both groups had similar titers after vaccination (Figure 4B).
Figure 4.
Neutralizing antibody levels in chimeric yellow fever–dengue, live-attenuated, tetravalent dengue vaccine (CYD-TDV) recipients who were dengue virus (DENV) preimmune when vaccinated. A, DENV neutralizing antibody levels in DENV-preimmune subjects vaccinated with CYD-TDV. Serum samples from 7 DENV-preimmune subjects at base line were tested at day 0 before CYD-TDV administration. Samples from the same subjects were tested 1 month after the final dose. Each data point represents the 50% neutralization antibody titer (Neut50) for a single subject for the specified serotype; horizontal bars represent geometric mean titers. B, Comparison of DENV neutralizing antibody levels induced by CYD-TDV in naive and preimmune subjects. Each data point represents the Neut50 for a single subject for the specified serotype; horizontal bars depict geometric mean titers.
We next performed antibody depletions to determine whether CYD-TDV induced type-specific or cross-reactive neutralizing antibodies in preimmune subjects. As depicted in Figure 5A, the vaccine mainly induced cross-reactive neutralizing antibodies in preimmune individuals. This pattern is clear when persons with primary DENV2, DENV3, or multitypic responses are analyzed as separate groups (Figure 5B–5D). The single subject categorized as having had a past DENV2 primary infection had high levels of DENV2-specific neutralizing antibodies at enrollment (Figure 5B). After vaccination, this subject developed high levels of neutralizing antibodies to all 4 serotypes, which were mainly cross-reactive antibodies. A similar pattern of cross-reactive antibody boosting was also observed for subjects who had evidence of past primary DENV3 infection (Figure 5C) or multitypic immunity (Figure 5D). It is also important to note that in nearly all cases preexisting type-specific neutralizing antibodies were maintained after vaccination, and in some cases the vaccine actually boosted preexisting type-specific responses to DENV3.
Figure 5.
Levels of homotypic and heterotypic dengue virus (DENV) neutralizing antibodies (Abs) induced by chimeric yellow fever–dengue, live-attenuated, tetravalent dengue vaccine (CYD-TDV) in preimmune subjects. A, Proportions of DENV homotypic neutralizing antibodies induced by vaccine. The antibody depletion assay was used to measure proportions of DENV type-specific (homotypic) neutralizing antibodies induced by CYD-TDV in DENV-preimmune subjects. Each data point represents the percentage of the total neutralizing antibody response to a serotype attributed to type-specific antibodies; horizontal bars represent geometric mean titers. B–D, Absolute levels of type-specific DENV neutralizing antibodies before and after vaccination with CYD-TDV. The antibody depletion assay was used to measure absolute levels of DENV type-specific (homotypic) and cross-reactive (heterotypic) neutralizing antibodies induced by CYD-TDV. Homotypic antibody is shown in solid black; heterotypic antibody, in stripes. Bars represents the total 50% neutralization antibody titer (Neut50) for each serotype. Neutralizing antibody levels in subjects with evidence of prevaccination DENV2 exposure (B), DENV3 exposure (C, left to right), and secondary exposure (D, left to right) are depicted as separate graphs.
DISCUSSION
As exemplified by the yellow fever 17D vaccine developed >75 years ago, live virus vaccines can be very effective against flaviviruses. Several live DENV vaccines are in clinical trials and the leading product developed by Sanofi (CYD-TDV) has been licensed in several countries. In clinical trials, CYD-TDV efficacy varied by serotype and efficacy was higher in participants who had been previously exposed to dengue than in those who were dengue naive when vaccinated [2, 3, 5]. In CYD-TDV efficacy trials, while the mere presence of DENV neutralizing antibodies was not correlated with protection [4], vaccine efficacy curve models have now identified a correlation between PRNT50 titers and efficacy [30]. However, although high titers were a remarkably consistent signature of high vaccine efficacy, other factors besides Neut50 titers may play a role when considering lower titers. Therefore, beyond quantitative levels, it is important to explore additional qualitative aspects of the immune response.
The goal of the current study was then to characterize serum from CYD-TDV recipients to clarify the specificity and functionality of DENV-specific vaccine-induced antibodies and to generate hypotheses that may explain vaccine efficacy data. DENV-naive subjects who received 3 doses of CYD-TDV developed neutralizing antibodies to each of the 4 DENV serotypes. DENV serotypes 1, 2, and 3 were mainly neutralized by cross-reactive antibodies, whereas serotype-specific antibodies were mainly responsible for DENV4 neutralization.
In naive subjects, why did CYD-TDV mainly induce type-specific neutralizing antibodies to DENV4 but not to DENV1, DENV2, and DENV3? Previous studies have demonstrated that the DENV4 component in CYD-TDV replicates to higher titers in animals and in persons compared with the other 3 serotypes [6]. The superior replication of DENV4 is most likely responsible for the type-specific responses to this serotype, whereas the cross-reactive neutralizing antibodies against the other 3 serotypes could result to a significant degree from unbalanced vaccine virus replication. However, some persons did develop substantial levels of type-specific responses against DENV1, DENV2, and DENV3, suggesting that these vaccine components replicated well in some individuals. This exemplifies expected variability from individual to individual, also seen in the level of neutralization achieved across a given population [2].
CYD-TDV efficacy was lower in naive subjects even though the vaccine induced neutralizing antibodies to all 4 serotypes in the PRNT50 assay, albeit at different levels [4, 30]. In fact early clinical studies with CYD-TDV demonstrated lower replication and antibody responses to DENV1 and 2 components compared with DENV4 [31, 32]. In persons exposed to natural primary DENV infections, serotype-specific antibodies have been linked to long-protective immunity [15]. Tertiary quaternary structure antibody epitopes displayed on the surface of intact dengue virions are the main targets of neutralizing antibodies in persons exposed to natural infections [16, 33]. Induction of these antibodies are likely to be dependent on active viral replication and stimulation of naive B cells by intact virus particles. Although natural primary infections can also stimulate cross-reactive antibodies with measurable neutralizing activity in cell culture, these antibodies do not seem to provide durable cross-protection from other serotypes. We propose that in dengue-naive subjects who are vaccinated, the levels of type-specific antibodies that target complex epitopes displayed on the viral surface are likely to contribute significantly to vaccine efficacy. This hypothesis is testable using data and samples from the recently completed CYD-TDV 14 and 15 efficacy trials.
CYD-TDV performed particularly well in persons with preexisting immunity to DENV. Our data demonstrate that in dengue-immune subjects, CYD-TDV induced high levels of cross-reactive antibodies that broadly cross-neutralized DENV serotypes. Persons exposed to natural second DENV infections also develop broadly cross-reactive neutralizing antibodies. In fact, the cross-protective nature of second infection–induced antibodies may explain why third DENV infections are mild or clinically unapparent [24]. We propose that the superior efficacy of CYD-TDV in DENV-preimmune individuals is mediated by the induction of cross-reactive and cross-protective antibodies that are similar to antibodies induced after natural second DENV infections. Recent studies have identified complex E protein interdimer epitopes on the viral surface targeted by serotype cross-neutralizing antibodies produced by plasmablasts in persons exposed to repeated DENV infections [18]. Studies are ongoing to determine whether the cross-reactive neutralizing antibodies induced by the CYD-TDV in preimmune subjects target E protein interdimer or related epitopes. Our results are consistent with the hypothesis proposed by Guy and Jackson [34] that CYD-TDV may act as a primary infection in those who are seronegative and a secondary infection in those who are seropositive–with different impacts on vaccine efficacy.
It is important to note that in preimmune individuals, CYD-TDV induced broadly cross-reactive neutralizing antibodies, while maintaining, and in some cases enhancing, preexisting type-specific responses. In our small subset of DENV-primed individuals, several subjects had type-specific responses to DENV2 or DENV3 indicative of previous exposure to these serotypes. These type-specific responses were maintained even after receipt of CYD-TDV and in the case of DENV3, the vaccine enhanced DENV3 type-specific responses. We propose that CYD-TDV can stimulate both cross-reactive and type-specific memory B cells, depending on the replication of individual vaccine components in preimmune subjects.
The main limitation of our study is that we were able to analyze only a relatively small number of samples because of the complex and laborious nature of antibody depletion studies. For DENV-naive subjects, we first analyzed 11 subjects and then an additional 14 subjects and observed a clear trend wherein the DENV serotype 4 neutralizing antibody response was type specific, whereas the responses for serotypes 1, 2, and 3 were mainly due to cross-reactive antibodies. For the preimmune population, we analyzed 7 subjects and observed a large increase in serotype cross-reactive neutralizing antibodies. Although CYD-TDV continues to be evaluated in short- and long-term clinical trials, 2 other companies have also recently launched large phase III trials with different live dengue vaccines. The results we report here provide a foundation for analyzing vaccine data and samples and establishing correlates and mechanisms of protective immunity.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank all the parents and participants who agreed to take part in the clinical trials. We also thank Fernando Noriega, Alain Bouckenooghe, Anh Wartel-Tram, Diane Van der Vliet, Betzana Zambrano, and Jean Lang, and we thank members of the laboratories at the University of North Carolina for their assistance with these studies.
Financial support. This work was supported by Sanofi Pasteur, Lyon, France (A. M. d. S.) and the National Institute of Allergy and Infectious Diseases (grant 1-R01-AI125198-01 to A. M. d. S.).
Potential conflicts of interest. A. M. B., J. M. M., S. F. S., M. B., N. J., and B. G. are employees of Sanofi Pasteur, France. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med 2004; 10:S98–109. [DOI] [PubMed] [Google Scholar]
- 2. Capeding MR, Tran NH, Hadinegoro SR, et al. ; CYD14 Study Group Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet 2014; 384:1358–65. [DOI] [PubMed] [Google Scholar]
- 3. Villar L, Dayan GH, Arredondo-GarcÃÂÃÂa JL, et al. ; CYD15 Study Group Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med 2015; 372:113–23. [DOI] [PubMed] [Google Scholar]
- 4. Sabchareon A, Wallace D, Sirivichayakul C, et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet 2012; 380:1559–67. [DOI] [PubMed] [Google Scholar]
- 5. Hadinegoro SR, Arredondo-GarcÃÂÃÂa JL, Capeding MR, et al. ; CYD-TDV Dengue Vaccine Working Group Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med 2015; 373:1195–206. [DOI] [PubMed] [Google Scholar]
- 6. Guy B, Briand O, Lang J, Saville M, Jackson N. Development of the Sanofi Pasteur tetravalent dengue vaccine: one more step forward. Vaccine 2015; 33:7100–11. [DOI] [PubMed] [Google Scholar]
- 7. Thomas SJ. Developing a dengue vaccine: progress and future challenges. Ann N Y Acad Sci 2014; 1323:140–59. [DOI] [PubMed] [Google Scholar]
- 8. Roehrig JT. Antigenic structure of flavivirus proteins. Adv Virus Res 2003; 59:141–75. [DOI] [PubMed] [Google Scholar]
- 9. Modis Y, Ogata S, Clements D, Harrison SC. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc Natl Acad Sci U S A 2003; 100:6986–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Modis Y, Ogata S, Clements D, Harrison SC. Variable surface epitopes in the crystal structure of dengue virus type 3 envelope glycoprotein. J Virol 2005; 79:1223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nybakken GE, Nelson CA, Chen BR, Diamond MS, Fremont DH. Crystal structure of the West Nile virus envelope glycoprotein. J Virol 2006; 80:11467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rey FA, Heinz FX, Mandl C, Kunz C, Harrison SC. The envelope glycoprotein from tick-borne encephalitis virus at 2 A resolution. Nature 1995; 375:291–8. [DOI] [PubMed] [Google Scholar]
- 13. Kuhn RJ, Zhang W, Rossmann MG, et al. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell 2002; 108:717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rothman AL. Immunology and immunopathogenesis of dengue disease. Adv Virus Res 2003; 60:397–419. [DOI] [PubMed] [Google Scholar]
- 15. Wahala WMPB, de Silva AM. The human antibody response to dengue virus infection. Viruses 2011; 3:2374–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Alwis R, Smith SA, Olivarez NP, et al. Identification of human neutralizing antibodies that bind to complex epitopes on dengue virions. Proc Natl Acad Sci U S A 2012; 109:7439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fibriansah G, Ibarra KD, Ng TS, et al. Dengue virus: cryo-EM structure of an antibody that neutralizes dengue virus type 2 by locking E protein dimers. Science 2015; 349:88–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rouvinski A, Guardado-Calvo P, Barba-Spaeth G, et al. Recognition determinants of broadly neutralizing human antibodies against dengue viruses. Nature 2015; 520:109–13. [DOI] [PubMed] [Google Scholar]
- 19. Dejnirattisai W, Wongwiwat W, Supasa S, et al. A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus. Nat Immunol 2015; 16:170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fibriansah G, Tan JL, Smith SA, et al. A potent anti-dengue human antibody preferentially recognizes the conformation of E protein monomers assembled on the virus surface. EMBO Mol Med 2014; 6:358–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fibriansah G, Tan JL, Smith SA, et al. A highly potent human antibody neutralizes dengue virus serotype 3 by binding across three surface proteins. Nat Commun 2015; 6:6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaufmann B, Vogt MR, Goudsmit J, et al. Neutralization of West Nile virus by cross-linking of its surface proteins with Fab fragments of the human monoclonal antibody CR4354. Proc Natl Acad Sci U S A 2010; 107:18950–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Teoh EP, Kukkaro P, Teo EW, et al. The structural basis for serotype-specific neutralization of dengue virus by a human antibody. Sci Transl Med 2012; 4:139ra83. [DOI] [PubMed] [Google Scholar]
- 24. Olkowski S, Forshey BM, Morrison AC, et al. Reduced risk of disease during postsecondary dengue virus infections. J Infect Dis 2013; 208:1026–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Torresi J, Heron LG, Qiao M, et al. Lot-to-lot consistency of a tetravalent dengue vaccine in healthy adults in Australia: a randomised study. Vaccine 2015; 33:5127–34. [DOI] [PubMed] [Google Scholar]
- 26. Dayan GH, Garbes P, Noriega F, et al. Immunogenicity and safety of a recombinant tetravalent dengue vaccine in children and adolescents ages 9-16 years in Brazil. Am J Trop Med Hyg 2013; 89:1058–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wahala WM, Kraus AA, Haymore LB, Accavitti-Loper MA, de Silva AM. Dengue virus neutralization by human immune sera: role of envelope protein domain III-reactive antibody. Virology 2009; 392:103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Timiryasova TM, Bonaparte MI, Luo P, Zedar R, Hu BT, Hildreth SW. Optimization and validation of a plaque reduction neutralization test for the detection of neutralizing antibodies to four serotypes of dengue virus used in support of dengue vaccine development. Am J Trop Med Hyg 2013; 88:962–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kraus AA, Messer W, Haymore LB, de Silva AM. Comparison of plaque- and flow cytometry-based methods for measuring dengue virus neutralization. J Clin Microbiol 2007; 45:3777–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jackson N, Boaz M, Hu BT, Langevin E, Byers A. Investigations of the observed efficacy of the CYD tetravalent dengue vaccine in the phase 2b trial in Ratchaburi, Thailand. Am J Trop Med Hyg 2014; 91:149. [Google Scholar]
- 31. Morrison D, Legg TJ, Billings CW, Forrat R, Yoksan S, Lang J. A novel tetravalent dengue vaccine is well tolerated and immunogenic against all 4 serotypes in flavivirus-naive adults. J Infect Dis 2010; 201:370–7. [DOI] [PubMed] [Google Scholar]
- 32. Poo J, Galan F, Forrat R, Zambrano B, Lang J, Dayan G. Live-attenuated tetravalent dengue vaccine in dengue-naÃÂÃÂve children, adolescents, and adults in Mexico City: randomized controlled phase 1 trial of safety and immunogenicity. Pediatr Infect Dis J 2011; 30:e9–17. [DOI] [PubMed] [Google Scholar]
- 33. Gallichotte EN, Widman DG, Yount BL, et al. A new quaternary structure epitope on dengue virus serotype 2 is the target of durable type-specific neutralizing antibodies. mBio 2015; 2015; 6:e01461–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guy B, Jackson N. Dengue vaccine: hypotheses to understand CYD-TDV-induced protection. Nat Rev Microbiol 2016; 14:45–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.