Abstract
Background
Trends in CD4+ T-cell count at human immunodeficiency virus (HIV) infection diagnosis and antiretroviral therapy (ART) initiation can be characterized using laboratory tests from surveillance.
Methods
We used CD4+ T-cell counts and viral loads from New York City for persons who received a diagnosis of HIV infection during 2006–2012.
Results
From 2006 to 2012, the median CD4+ T-cell count increased from 325 to 379 cells/µL at diagnosis and from 178 to 360 cells/μL at ART initiation. CD4+ T-cell counts were consistently lower in women, blacks, Hispanics, persons who inject drugs, and heterosexuals.
Discussion
Increases in CD4+ T-cell count at diagnosis and ART initiation suggest that the time from HIV infection to ART initiation has been reduced substantially in New York City.
Keywords: HIV, CD4 cell count, antiretroviral therapy, trends
A major aim of the treatment-as-prevention approach is to minimize the time from human immunodeficiency virus (HIV) infection to sustained viral suppression [1]. Early initiation of antiretroviral therapy (ART) reduces HIV-associated morbidity and mortality and prevents onward transmission of HIV [2]. Prerequisites to early ART initiation include timely HIV infection diagnosis and linkage to care soon after HIV infection occurs. Many US jurisdictions, including New York City, support and promote immediate ART initiation among persons with HIV infection and have implemented geographically targeted HIV testing campaigns to facilitate earlier HIV infection diagnosis [3, 4].
Population-based trends in CD4+ T-cell count at HIV infection diagnosis and ART initiation can be used to assess and monitor efforts aimed at reducing the time from infection to diagnosis and ART initiation. However, population-based measures of immunodeficiency at the start of ART are largely missing in the United States because ART information is not routinely collected by HIV surveillance systems [5]. We used a novel surveillance-based measure of ART initiation to determine trends in CD4+ T-cell count at diagnosis and at ART initiation for all persons in New York City aged ≥13 years who received a diagnosis of HIV infection from 1 January 2006 to 31 December 2012.
METHODS
Data Source and Population
The primary data source for this analysis was the New York City Department of Health and Mental Hygiene HIV Surveillance Registry, which contains information on all diagnoses of HIV infection/AIDS, all HIV-related illness, and all CD4+ T-cell count, viral load, and genotype tests conducted for persons with HIV infection [6]. The population-based HIV Surveillance Registry is continuously updated with demographic, clinical, transmission risk, and other information for persons meeting the HIV surveillance case definitions, as established by the Centers for Disease Control and Prevention [7], and with results of HIV-related laboratory tests conducted in New York City.
This analysis included persons aged ≥13 years who received a diagnosis of HIV infection in New York City from 1 January 2006 to 31 December 2012. We excluded persons with an HIV load of < 400 copies/mL within 31 days of diagnosis, as diagnoses in these persons were not likely to be new and they were likely already taking ART. The final study population (Supplementary Figure 1) included all persons with newly diagnosed HIV infection and a CD4+ T-cell count obtained within 6 months of diagnosis (17 733 of 24 358 persons [73%] in whom HIV infection was newly diagnosed during the study period) and the subset of persons with a new diagnosis determined to have initiated ART with a CD4+ T-cell count reported at ART initiation (13 022 of 24 358 [54%]). All data were drawn from the HIV Surveillance Registry as of 31 March 2014.
Outcome Definitions
Definition of ART Initiation and Measure Validation
ART initiation in a person with newly diagnosed HIV infection was defined as the earlier occurrence of either (1) a ≥1-log drop in viral load over a 3-month period or (2) a detectable viral load followed by an undetectable viral load (defined as <400 copies/mL, to capture the range of viral load tests in use in New York City during the analytic period) [5]. The date of probable ART initiation was defined as the midpoint between the 2 viral loads bracketing the first occurrence of either (1) or (2) above.
CD4+ T-Cell Count Outcomes
The primary outcomes for the analysis were median CD4+ T-cell count at diagnosis and at ART initiation. The CD4+ T-cell count at diagnosis was a person's first CD4+ T-cell count within 6 months of diagnosis. The CD4+ T-cell count at ART initiation was the CD4+ T-cell count closest to and within the 3 months before the estimated date of ART initiation.
Statistical Analysis
We performed descriptive analysis of demographic characteristics and calculated the median CD4+ T-cell count at diagnosis and, among those who initiated ART, the median CD4+ T-cell count at ART initiation (with interquartile ranges). We plotted median CD4+ T-cell counts from 1 January 2006 to 31 December 2012 by year of diagnosis and ART initiation for trends.
To assess differences in CD4+ T-cell count trends by demographic and risk groups, we used quantile regression to model the median CD4+ T-cell count at diagnosis and ART initiation. All models included a third-order polynomial for time (year of diagnosis or year of ART initiation for CD4+ T-cell count at diagnosis and CD4+ T-cell count at ART initiation, respectively).
All analyses were conducted in SAS, version 9.3 (SAS Institute, Cary).
RESULTS
Analytic Population
A total of 24 346 persons had a new diagnosis of HIV infection from 1 January 2006 to 31 December 2012 in New York City (Supplementary Figure 1). Of these, 21 948 (90%) were ever linked to care after diagnosis. Of the 21 948 persons linked to care, CD4+ T-cell counts for 17 733 (81%) were obtained within 6 months of diagnosis. The remaining 4215 persons did not have a CD4+ T-cell count within 6 months of diagnosis. Among persons with a CD4+ T-cell count obtained within 6 months of diagnosis, 14 311 (81%) had evidence of probable ART initiation. Finally, 13 022 persons who initiated ART during the analytic period had a CD4+ T-cell count obtained within 3 months of ART initiation. Among this group, 10 192 persons (78%) initiated ART within 12 months of diagnosis.
Demographic Characteristics and CD4+ T-Cell Count Distribution
The majority of persons in the study population were male, aged 20–29 years, black or Hispanic, and men who have sex with men (MSM), similar to the overall profile of persons with a new diagnosis of HIV infection during this period (Table 1) [8].
Table 1.
Characteristics and Median CD4+ T-Cell Counts at Human Immunodeficiency Virus (HIV) Infection Diagnosis and Antiretroviral Therapy (ART) Initiation Among Persons With a CD4+ T-Cell Count at Diagnosis and Persons With a CD4+ T-Cell Count at ART Initiation, New York City (NYC), 2006–2012
| Characteristic | CD4+ T-Cell Count at
Diagnosisa |
CD4+ T-Cell Count at ART
Initiationa,b |
||
|---|---|---|---|---|
| Persons Evaluated, No. (%) | Cells/μL, Median (IQR) | Persons Evaluated, No. (%) | Cells/μL, Median (IQR) | |
| Overall | 17 733 (100.0) | 354 (367) | 13 022 (100.0) | 297 (305) |
| Age, y | ||||
| 13–19 | 784 (4.4) | 436 (247.5) | 567 (4.4) | 360 (237) |
| 20–29 | 5055 (28.5) | 416 (302) | 3692 (28.4) | 348 (258) |
| 30–39 | 4617 (26.0) | 363 (362) | 3427 (26.3) | 302 (304) |
| 40–49 | 4293 (24.2) | 306 (414) | 3174 (24.4) | 252 (330) |
| 50–59 | 2151 (12.1) | 235 (361) | 1588 (12.2) | 205 (299) |
| ≥60 | 833 (4.7) | 187 (308) | 574 (4.4) | 182.5 (251) |
| Sex | ||||
| Male | 13 521 (76.3) | 358 (355) | 9979 (76.6) | 303 (303) |
| Female | 4212 (23.8) | 336 (397) | 3043 (23.4) | 277 (314) |
| Race/ethnicity | ||||
| Black | 8084 (45.6) | 334 (372) | 5751 (44.2) | 276 (309) |
| Hispanic | 5731 (32.3) | 350 (358) | 4322 (33.2) | 294 (294) |
| White | 3289 (18.6) | 415 (355) | 2471 (19.0) | 352 (291) |
| Asian/Pacific Islander | 552 (3.1) | 311.5 (316) | 427 (3.3) | 298 (263) |
| Native American | 54 (0.3) | 289 (427) | 38 (0.3) | 233 (274) |
| Other/unknown | 23 (0.1) | 411 (396) | 13 (0.1) | 418 (250) |
| Risk group | ||||
| MSM | 8530 (48.1) | 399.5 (315) | 6582 (50.6) | 338 (270) |
| Persons with IDU history | 984 (5.6) | 337 (397.5) | 663 (5.1) | 278 (323) |
| Heterosexuals | 4267 (24.1) | 320 (389) | 3144 (24.1) | 262 (309) |
| Unknown | 3952 (22.3) | 258 (386) | 2633 (20.2) | 219 (311) |
| Year of diagnosis | ||||
| 2006 | 2686 (15.2) | 325 (377) | 1981 (15.2) | 249 (286) |
| 2007 | 2712 (15.3) | 335 (375) | 1992 (15.3) | 265.5 (291) |
| 2008 | 2716 (15.3) | 341 (377.5) | 1944 (14.9) | 278 (295.5) |
| 2009 | 2578 (14.5) | 364 (354) | 1925 (14.8) | 310 (292) |
| 2010 | 2396 (13.5) | 360 (349) | 1766 (13.6) | 319.5 (287) |
| 2011 | 2392 (13.5) | 380.5 (353) | 1779 (13.7) | 342 (314) |
| 2012 | 2253 (12.7) | 379 (360.2) | 1635 (12.6) | 352 (330) |
| Year of ART initiation | ||||
| 2006 | … | … | 967 (7.4) | 178 (264) |
| 2007 | … | … | 1486 (11.4) | 227.5 (299) |
| 2008 | … | … | 1751 (13.5) | 256 (277) |
| 2009 | … | … | 1927 (14.8) | 287 (271) |
| 2010 | … | … | 2055 (15.8) | 317 (272) |
| 2011 | … | … | 1958 (15.0) | 335 (283) |
| 2012 | … | … | 2125 (16.3) | 360 (313) |
| 2013 | … | … | 753 (5.8) | 402 (333) |
| NYC borough at diagnosis | ||||
| Bronx | 3715 (21.0) | 344 (371) | 2745 (21.1) | 285 (304) |
| Brooklyn | 4869 (27.5) | 333 (378) | 3598 (27.6) | 278 (310) |
| Manhattan | 4987 (28.1) | 385 (355) | 3809 (29.3) | 322 (298) |
| Queens | 2789 (15.7) | 342 (348) | 2048 (15.7) | 291 (294.5) |
| Staten Island | 315 (1.8) | 314 (440) | 200 (1.5) | 273.5 (313.5) |
| Outside NYC | 1023 (5.8) | 376 (347) | 607 (4.7) | 321 (290) |
| Unknown | 35 (0.2) | 316 (487) | 15 (0.1) | 310 (480) |
Abbreviations: IDU, injection drug use; IQR, interquartile range; MSM, men who have sex with men.
a Inclusion criteria were as follows: received a diagnosis of HIV infection from 1 January 2006 to 31 December 2012, resident of NYC, and had a CD4+ T-cell count obtained within 182 days of diagnosis. Data exclude persons with an undetectable viral load within 31 days after the diagnosis date and persons with a detectable viral load >31 days before diagnosis date.
b Includes cases meeting the mixed log 1 ART definition (ie, ≥1-log drop or change in viral load from detectable to undetectable) and having a CD4+ T-cell count reported within 3 months before ART initiation. Excludes persons with a first viral load reported as undetectable.
Median CD4+ T-cell count among persons who received a diagnosis from 1 January 2006 to 31 December 2012 in New York City was 354 cells/µL (Table 1). Median CD4+ T-cell count at diagnosis decreased with increasing age at diagnosis. Men had a higher median CD4+ T-cell count at diagnosis than women, and whites had a higher median CD4+ T-cell count at diagnosis than blacks, Hispanics, Asian/Pacific Islanders, and Native Americans. By transmission risk group, MSM had the highest CD4+ T-cell count at diagnosis. Over the entire analytic period, 59% of persons (14 311 of 24 358) who received a new diagnosis of HIV infection in New York City and had a CD4+ T-cell count reported within 6 months of diagnosis initiated ART; median CD4+ T-cell count at ART initiation among persons who received a new diagnosis during this period was 297 cells/µL. Differences in median CD4+ T-cell count at ART initiation across demographic subgroups were similar to subgroup differences in median CD4+ T-cell count at diagnosis.
Trends in Median CD4+ T-cell Count at Diagnosis and at ART Initiation
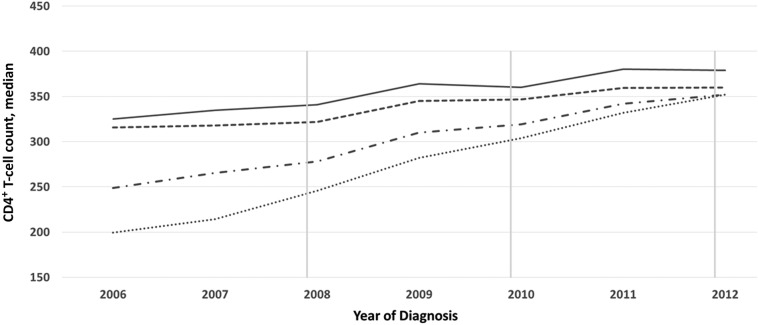
Among all diagnoses in New York City (n = 24 358), the proportion of persons with a CD4+ T-cell count within 6 months of diagnosis increased from 66.7% to 80.1% during 1 January 2006 to 31 December 2012, and the proportion initiating ART within 12 months in each annual diagnostic cohort increased from 32.4% to 55.5%. The median CD4+ T-cell count at diagnosis increased from 325 cells/μL in 2006 to 379 cells/μL in 2012 (mean increase, 7.7 cells/µL per year; Figure 1). The median CD4+ T-cell count at diagnosis among those who initiated ART was consistently lower than the median CD4+ T-cell count at diagnosis among all persons with a new diagnosis.
Figure 1.
Trends in CD4+ T-cell count at human immunodeficiency virus (HIV) infection diagnosis and antiretroviral therapy (ART) initiation, New York City, 2006–2012. Data denote the CD4+ T-cell count at diagnosis, defined as a person's first CD4+ T-cell count within 6 months of diagnosis, overall (solid line; n = 17 773) and among ART initiators (dashed line; n = 14 311); and the CD4+ T-cell count at ART initiation, defined as the CD4+ T-cell count closest to and within the 3 months before the estimated date of ART initiation, (dash/dot line; n = 13 022) and among individuals who initiated ART within 12 month of diagnosis (dotted line; n = 10 192). The US Department of Health and Human Services panel on ART guidelines recommended that the CD4+ T-cell count threshold for treatment initiation increase from <200 cells/μL to <350 cells/μL in December 2007 and then to <500 cells/μL in December 2009. As of December 2011, the New York City Department of Health and Mental Hygiene recommended that all persons should initiate treatment, regardless of CD4+ T-cell count.
During this period, the median CD4+ T-cell count at ART initiation, by year of diagnosis, increased from 249 cells/μL in 2006 to 352 cells/μL in 2012 (mean increase, 14.7 cells/µL per year; Figure 1). Among persons who initiated ART within 12 months of diagnosis, the median CD4+ T-cell count at ART initiation by year of diagnosis increased more dramatically, from 199.5 cells/μL in 2006 to 352 cells/μL in 2012 (mean, 21.8 cells/µL per year).
By year of ART initiation, the median CD4+ T-cell count at ART initiation increased from 178 cells/μL in 2006 to 402 cells/μL in 2013 (mean, 28 cells/µL per year; Table 1). Over half (56%) of persons who initiated ART in 2012 received a diagnosis that same year, compared with 48% in 2006 (data not shown).
All demographic and risk subgroups experienced increases in the median CD4+ T-cell count at diagnosis and ART initiation during 1 January 2006 to 31 December 2013 (Supplementary Figure 2). However, the median CD4+ T-cell counts were consistently lower for women, blacks, Hispanics, persons with a history of injection drug use, and heterosexuals, and there was a downward trend in recent years for persons with a history of injection drug use. Black or Hispanic MSM had lower median CD4+ T-cell counts at diagnosis and ART initiation compared with white MSM.
DISCUSSION
From 1 January 2006 to 31 December 2013, the CD4+ T-cell count at ART initiation among persons with HIV infection in New York City increased rapidly and more rapidly than did the CD4+ T-cell count at HIV infection diagnosis. These findings provide evidence that local and national recommendations for early initiation of ART after diagnosis are increasingly being implemented in New York City among persons who enter HIV care [4, 9]. At the current average increase of 28 cells/µL per year among persons with HIV infection in our sample, the median CD4+ T-cell count at ART initiation should reach 500 cells/µL by 2017. Modeling studies have shown that the estimated time for the median CD4+ T-cell count to reach 500 cells/µL in a person with untreated HIV infection is 2 years (95% confidence interval, 1.5–2.7 years) following HIV infection [10].
Reducing HIV-related health disparities remains a key goal of the US National HIV/AIDS Strategy [11]. Despite overall population-level and within-subgroup gains in New York City during this period vis-à-vis earlier identification of undiagnosed HIV infections and earlier initiation of treatment after HIV diagnosis, our analysis revealed persistent disparities across subgroups. Our results suggest that persons of color, including MSM of color, women, and persons with non-MSM risk receive a diagnosis and initiate ART later than their counterparts.
Recently published results of the START trial provide the first evidence from a randomized controlled trial of the clinical benefits of early ART initiation after HIV diagnosis, including major reductions in serious AIDS-related events, serious non–AIDS-related events, and death [2]. All patients had CD4+ T-cell counts of >500 cells/µL at the time of enrollment in the study. Patients in the intervention arm of the START trial all initiated ART at CD4+ T-cell counts of >500 cells/µL, and those in the control arm started ART when the CD4+ T-cell count dropped below 350 cells/µL [2]. However, while our data suggest improvements in earlier diagnosis, the median CD4+ T-cell count among all persons with a new diagnosis in 2012 in our sample was still only 379 cells/µL. Further, our analyses suggest that the median CD4+ T-cell count at diagnosis is increasing at a rate of only 7.7 cells/µL per year, meaning that it will be almost 16 years (2028) before the median CD4+ T-cell count at diagnosis would reach 500 cells/µL, when only half of persons with newly diagnosed HIV infection in New York City would be in a position to initiate ART at the 500 cells/µL threshold in the START trial. Therefore, continued and redoubled efforts aimed at earlier identification of undiagnosed HIV infections and prompt linkage to medical care in New York City, both prerequisites to starting treatment earlier, are greatly needed.
This analysis has several strengths and limitations. A major strength of this analysis is its use of population-based, longitudinal surveillance data that allow for analysis of trends over time and a large enough sample to examine trends by subgroup. The analysis also has limitations. First, our analysis excluded persons without a CD4+ T-cell count within 6 months of diagnosis. Second, persons with a more recent diagnosis of HIV infection, in 2012, may have been excluded from the group of ART initiators in our study, owing to a shorter postdiagnosis observation period. However, disparities and patterns in CD4+ T-cell count at ART initiation across subgroups were similar. Third, the CD4+ T-cell count can drop precipitously during the acute phase of HIV infection as the body mounts an immune response to the new pathogen [12]. Our study included persons with acute HIV infection at diagnosis, which might have artificially lowered our estimate of the median CD4+ T-cell count at diagnosis for the group. Over the entire analytic period, there were 887 persons with a diagnosis of acute HIV infection (3.2% of all HIV diagnoses) in New York City [5].
Population-based surveillance data indicate that the time from HIV infection to ART initiation has been reduced substantially among persons with newly diagnosed HIV infection accessing HIV care after diagnosis in New York City, possibly because of a combination of local HIV testing campaigns [3] and changes in clinical practice related to initiation of treatment in response to guideline changes [4, 9]. The median CD4+ T-cell count at diagnosis among New York City persons with HIV infection is increasing but more slowly than the CD4+ T-cell count at ART initiation. Additional efforts are needed to achieve earlier HIV diagnosis and linkage to care if the full benefits of treatment as prevention are to be realized.
This work has implications beyond New York City for analyses of HIV cascade outcomes at both the local and national levels, particularly among persons with a new diagnosis of HIV infection. The median CD4+ T-cell count at diagnosis and our measure of ART initiation can be used by other jurisdictions to improve and deepen measurement of HIV care continuum measures related to ART and viral suppression and inform treatment-as-prevention efforts.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Supplementary Material
Notes
Acknowledgments. We thank staff from the HIV Epidemiology and Field Services Program, for their contributions to the New York City HIV surveillance system, the data source for this analysis.
Financial support. This work was supported by the Centers for Disease Control and Prevention (grant 5U62PS003993-04).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet 2009; 373:48–57. [DOI] [PubMed] [Google Scholar]
- 2. INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Myers JE, Braunstein SL, Shepard CW et al. . Assessing the impact of a community-wide HIV testing scale-up initiative in a major urban epidemic. J Acquir Immune Defic Syndr 2012; 61:23–31. [DOI] [PubMed] [Google Scholar]
- 4. New York City Department of Health and Mental Hygiene. Recommendation to expand antiretroviral therapy to all persons living with HIV frequently asked questions (FAQ) for healthcare providers—December 1, 2011. http://www.nyc.gov/html/doh/downloads/pdf/ah/nyc-hivart-faq-provider.pdf Accessed 20 July 2015.
- 5. Braunstein SL, Robertson MM, Myers J, Nash D. Using HIV Viral Load from Surveillance to Estimate the Timing of Antiretroviral Therapy Initiation. J Acquir Immune Defic Syndr 2016; 73:222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. State of New York. HIV Testing and Counseling. Amendment to New York State Public Health Law Article 21, Amendment of Part 63 of Title 10, Codes, Rules and Regulations of the State of New York (HIV/AIDS Testing, Reporting and Confidentiality of HIV-Related Information). Chapter 308. Albany, NY: State of New York Laws, 2010. [Google Scholar]
- 7. Selik RM, Mokotoff ED, Branson B, Owen SM, Whitmore S, Hall HI. Revised surveillance case definition for HIV infection - United States, 2014. MMWR Recomm Rep 2014; 63:1–10. [PubMed] [Google Scholar]
- 8. HIV Epidemiology and Field Services Program. HIV surveillance annual report, 2013. New York, NY: New York City Department of Health and Mental Hygiene, December 2014. [Google Scholar]
- 9. Department of Health and Human Services Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf Accessed 16 August 2016.
- 10. Jansson J, Kerr CC, Mallitt KA, Wu J, Gray RT, Wilson DP. Inferring HIV incidence from case surveillance with CD4+ cell counts. AIDS 2015; 29:1517–25. [DOI] [PubMed] [Google Scholar]
- 11. The White House Office of National AIDS Policy. National HIV/AIDS strategy for the United States. Washington, DC: The White House, 2015. [Google Scholar]
- 12. CASCADE Collaboration. Differences in CD4 cell counts at seroconversion and decline among 5739 HIV-1-infected individuals with well-estimated dates of seroconversion. J Acquir Immune Defic Syndr 2003; 34:76–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.