Supplemental Digital Content is available in the text
Keywords: anabolic drugs, combination therapy, meta-analysis, osteoporosis
Abstract
Background:
According to the mechanisms of action, combination therapy of anabolic and antiresorptive agents may produce more effect for the treatment of osteoporosis. However, the combination therapy of anabolic agents and bisphosphonates reports no benefit and even reduced the anabolic effects of anabolic agents. This study aims to assess the effect of combination therapy of anabolic and nonbisphosphonates antiresorptive agents in adults with osteoporosis.
Methods:
Medline, EMBASE, and Cochrane Library were searched from January 1, 1980 to November 1, 2017 for randomized controlled trials (RCTs) of adults with osteoporosis treated in combination therapy of anabolic and nonbisphosphonates antiresorptive agents compared with monotherapy of either agent alone. The primary outcome was the incidence of fractures. The secondary outcomes were the bone mineral density (BMD) changes at lumbar spine and total hip. Continuous outcomes were expressed as standardized mean difference (SMD) and 95% confidence interval (CI), while dichotomous outcomes were expressed as risk ratio (RR) and 95% CI. The meta-analysis was performed using a random-effects model. I2 statistic (I2 > 50% as a threshold indicates significant heterogeneity) was used to assess the heterogeneity.
Results:
A total of 10 trials with a total of 1042 patients were included. The pooled results showed that the combination therapy demonstrated a significant advantage over a monotherapy in the BMD improvement at the lumbar spine (SMD 1.18; 95% CI, 0.63 to 1.72; I2 = 93%) and the total hip (SMD 0.89; 95% CI, 0.48 to 1.29; I2 = 88%) and further reduce the fracture risk (RR, 0.45; 95%CI, 0.21 to 0.94; I2 = 0%).
Conclusions:
Low-to-moderate-quality evidence shows that the combination therapy of anabolic and nonbisphosphonates antiresorptive agents is superior to monotherapy in improving the BMD and reducing the fracture risk. However, further high methodological quality studies are needed to determine the antifracture efficacy, cost-effectiveness and safety of this strategy of combination therapy.
1. Introduction
Osteoporosis is a common skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, causing an increase in bone fragility and susceptibility to fracture.[1] Fragility fractures are the major complication of osteoporosis, of various fragility fractures, vertebral and hip fractures are associated with pronounced morbidity and excess mortality.[2–4]
To date, a range of pharmacological interventions is available for the treatment of osteoporosis. Depending on their mechanism of action, antiosteoporosis medications can be classified into either antiresorptive agents or anabolic agents. Antiresorptive agents include bisphosphonates, hormone replacement therapy (HRT), raloxifene, denosumab, and calcitonin. Anabolic agents include the full-length molecule parathyroid hormone (PTH 1–84) and teriparatide (PTH 1–34). Despite various drugs, single use of antiresorptive or anabolic agents do not restore bone mineral density (BMD) to normal, current treatment approaches do not “cure” osteoporosis,[5] and options for those with severe osteoporosis remained limited.[6]
Owing to improve the treatment efficacy, combination strategy by using antiresorptive and anabolic agents simultaneously was proposed. Since bisphosphonates are the most commonly used antiresorptive agents for the treatment of osteoporosis, the combination therapy of anabolic agents and bisphosphonates was initially thought to be a promising approach. Unfortunately, this combination strategy reported no benefit and even reduced the anabolic effects of anabolic agents.[7,8] Conversely, a combination therapy of anabolic and other nonbisphosphonates antiresorptive agents (HRT, raloxifene, and denosumab) seemed to be a suitable combination strategy.[9] However, the conclusions among studies are still controversial.[10] And there is no evidence-based evaluation of this issue.
Thus, the objective of this meta-analysis of randomized controlled trials (RCTs) is to determine whether the combination therapy of anabolic and nonbisphosphonates antiresorptive agents produces more effects on BMD and reduce the incidence of fractures than monotherapy in adults with osteoporosis.
2. Methods
This meta-analysis protocol was reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.[11] A formal protocol was developed and registered on the PROSPERO international prospective register of systematic reviews (CRD42016038951). This study was not a human or animal experiment, so no ethical approval was required.
2.1. Information sources and search strategy
MEDLINE, EMBASE, and the Cochrane Library were searched from January 1, 1980 to November 1, 2017 without language restriction. The search strategy was developed using relevant text words as well as Medical Subject Headings that consisted of terms relevant to “osteoporosis,” “teriparatide,” “parathyroid hormone,” and “randomized control trial” (for the detailed search strategy, see the File S1 in the Supplement). Moreover, reference lists from retrieved trials, reports, conference abstracts, and reviews were manually scanned to further identify potentially eligible trials. Additionally, the clinicaltrials.gov website (www.clinicaltrials.gov) was searched for RCTs that were registered as completed but not yet published.
2.2. Study eligibility
Titles, abstracts, and full-text articles were screened independently by 2 authors (SHL and LFW) for eligibility, with discrepancies discussed with a third author (YSW). We used the following inclusion criteria. Participants were adults with osteoporosis. Diagnosis criterion of osteoporosis was as follow: (1) T scores ≤ −2.5 at the spine, hip or femoral neck; (2) T scores ≤–2.0 with at least 1 BMD-independent risk factor; or (3) T scores ≤–1.0 with a history of fragility fracture. The intervention was a combination therapy of anabolic agents and nonbisphosphonates antiresorptive agents. The comparator was a monotherapy with either anabolic agents or nonbisphosphonates agents alone (or monotherapy plus placebo). The outcomes included the incidence of fractures and the BMD variation. All relevant RCTs written in any language were included.
2.3. Data extraction
Data were extracted by 2 independent reviewers (SHL and LFW) using a standardized data collection form. Discrepancies were resolved through discussion with a third reviewer (YSW). Extracted data included the following major categories: (1) study characteristics; (2) participant characteristics; and (3) outcome characteristics. For continuous outcomes, the sample size, mean, and SD were extracted for each experimental group and control group. For dichotomous outcomes, we extracted the original data regarding the events and the total number in both the experimental group and the control group. We attempted to contact study authors for additional information when necessary.
2.4. Outcomes
The primary outcome is the incidence of fractures (confirmed by x-ray radiography). Both vertebral and nonvertebral fractures were included. Nonvertebral fractures were documented as wrist, humerus, clavicle, pelvis, vertebrae, hip, ankle, metatarsal, or other. The secondary outcomes are BMD changes (measured by dual-energy x-ray absorptiometry, DXA) at the lumbar spine and the total hip.
2.5. Risk of bias assessment
Two authors (SHL and LFW) independently assessed the risk of bias using the Cochrane risk-of-bias tool.[12] Any disagreements were resolved through discussion, and sometimes with another reviewer (YSW) if necessary. Bias was assessed across the following 7 domains: (1) random-sequence generation (selection bias); (2) allocation concealment (selection bias); (3) blinding of participants and personnel (performance bias); (4) blinding of outcome assessment (detection bias); (5) incomplete outcome data (attrition bias); (6) selective reporting (reporting bias); (7) other bias. Each aspect could further be classified as a low, high or unclear risk. For the study design, we assessed random-sequence generation, allocation concealment, blinding of the participants and outcome reporting. For each outcome, we assessed blinding of the outcome assessors and loss to follow-up.
2.6. Data synthesis and analysis
The continuous outcomes are expressed as the standardized mean differences (SMD) and the 95% confidence interval (CI), using the generic inverse variance methods. The dichotomous outcomes are expressed as the risk ratios (RR) and the 95% CI, using the Mantel–Haenszel method.
The meta-analysis was performed using a random-effects model, which provided more conservative estimated effects. To assess heterogeneity in results of individual studies, we used Cochrane's Q statistic and the I2 statistic (I2 >50% as a threshold indicates significant heterogeneity).[13] Publication bias was assessed using the Egger regression test for funnel asymmetry in addition to visual inspection of the funnel plots.[14] All analyses were conducted in Review Manager (version 5.3) and Comprehensive Meta-Analysis (version 2.0).
When there was a significant heterogeneity, sensitivity analyses were conducted using sequential omission of a single study from the total studies to evaluate the influence of each study on the pooled effect estimates. To further explore possible sources of heterogeneity, preplanned subgroup analyses were performed based on the different mechanisms of antiosteoporosis medications (anabolic agents versus nonbisphosphonates antiresorptive agents). And meta-regression analyses were used to evaluate the relationship between the duration of therapy, the age, and the outcomes. A 2-sided P value of less than or equal to .05 was deemed statistically significant.
The quality of the evidence was assessed according to using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) guidelines, which uses the domains of risk of bias, inconsistency, indirectness, imprecision, and publication bias in in results.[15]
3. Results
3.1. Search results
A total of 1346 articles were obtained through electronic and hand searches. After 307 duplicates were removed, the titles and abstracts of 1039 records were reviewed, 1024records were excluded for not meeting the inclusion criteria, and thus the remaining 15 articles were retrieved, all written in English, for further assessment. Five trials were excluded due to reports of repeated data[16–19] and nonosteoporotic patients.[20] Ten trials[6,21–29] fulfilled our inclusion criteria and were included in our meta-analysis (Fig. 1).
Figure 1.
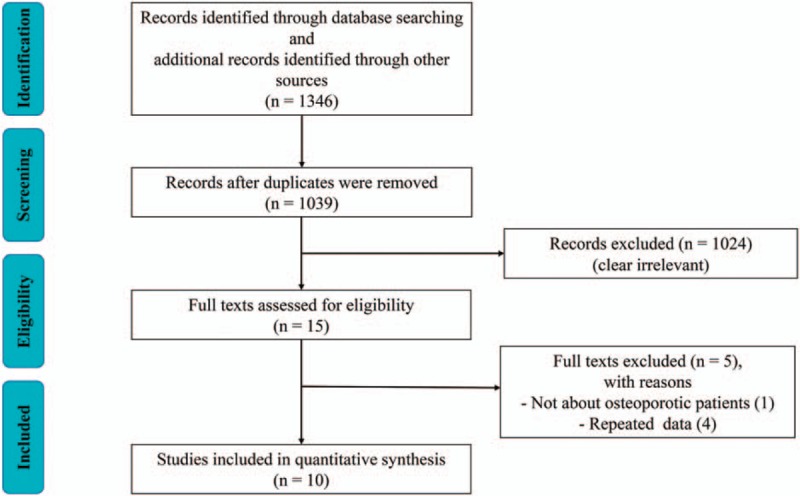
Flow diagram shows the process of literature selection.
3.2. Study characteristics
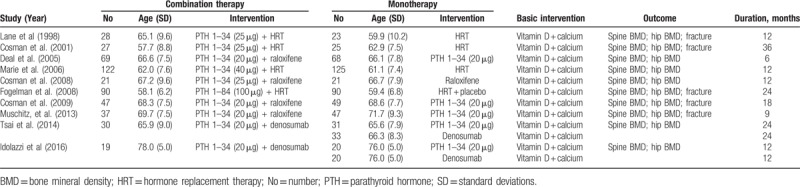
The main characteristics of the included trials are summarized in Table 1. These trials were published from 1998 to 2016 and involved totally 1042 patients, with the sample sizes ranging from 42 to 247. Anabolic agents included PTH 1–34 (20, 25 or 40 μg) and PTH 1–84 (100 μg); nonbisphosphonates antiresorptive agents included HRT, raloxifene and denosumab. The duration of treatment lasted from 6 to 36 months. All patients received oral calcium and vitamin D supplements daily.
Table 1.
Characteristics of included randomized controlled trials.
3.3. Risk of bias assessment
Figure 2 summarizes the details of the risk of bias. Random sequence generation was reported in 5 trials[6,21–23,28] and was not described in the remaining trials. Allocation concealment was adequately reported in 2 trials.[6,22] Four trials[6,22–24] were open-label design, which might lead to a potential performance bias. However, whether or not the participants and investigators were blind has limited impact on the changes in BMD. Blinding of outcome assessment was adequately reported in all the 10 trials except for 2.[21,29] One trial[24] has a high risk of attrition bias because of a high loss to follow-up (over 20%). There was a low risk of reporting bias, and other biases in all the included trials.
Figure 2.
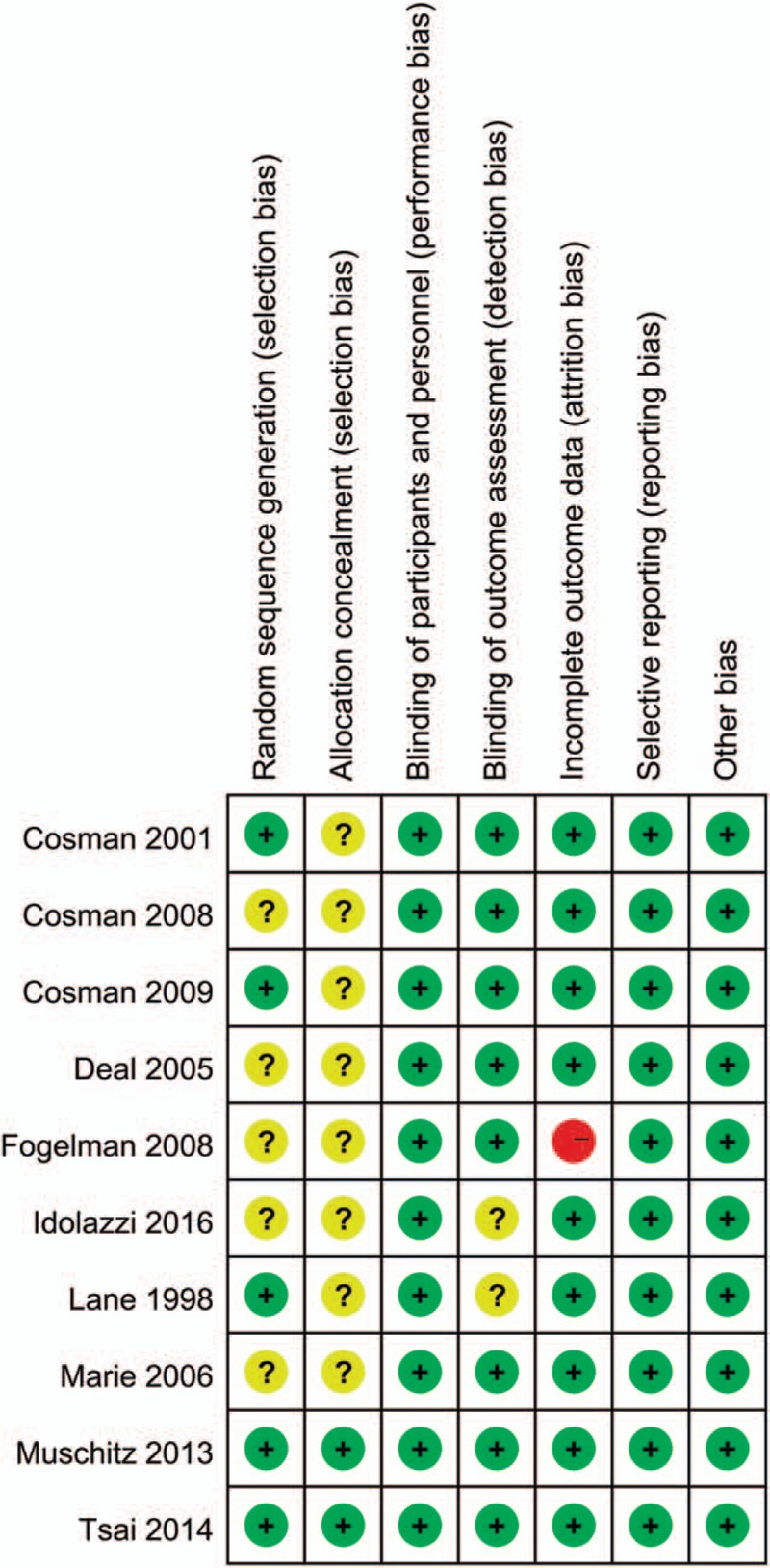
The methodological quality of the RCTs. Risk of bias summary. “+” means low risk; “?” means unclear risk; “-” means high risk. RCT = randomized controlled trial.
3.4. Incidence of fractures
Five trials[21–24,28] provided the available data about the incidence of fractures. Compared with monotherapy, the combination therapy could achieve a greater reduction of fracture incidence (RR, 0.45; 95%CI, 0.21 to 0.94; I2 = 0%) (Fig. 3). Subgroup analyses showed that when compared with nonbisphosphonates agents alone, the combination therapy could reduce the risk of fractures (RR, 0.31; 95%CI, 0.12 to 0.81; I2 = 0%). However, the currently available evidence is insufficient to support the combination therapy is superior to monotherapy with anabolic agents for the prevention of fractures (RR, 0.83; 95%CI, 0.21 to 3.33; I2 = 21%). The meta-regression analyses did not show any statistically significant differences in association by treatment duration (P = .11 for slope, data not shown) or by the age (P = .17 for slope, data not shown). There was no evidence of publication bias in the overall pooled result, with Egger's test P value of .18 (Figure S1).
Figure 3.
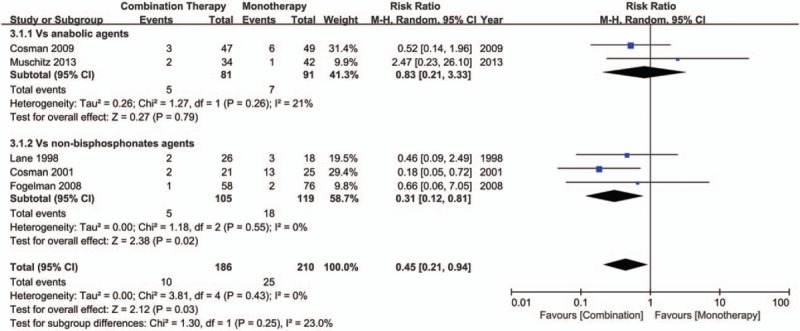
Forest plot for the incidence of fractures.
3.5. BMD changes at the lumbar spine
Ten trials[6,21–29] reported the BMD changes at the lumbar spine. Compared with monotherapy, the combination therapy could significantly improve BMD (SMD 1.18; 95% CI, 0.63 to 1.72; I2 = 93%) (Fig. 4). Sensitivity analyses were performed to examine the robustness of our analysis by omitting each study in turn, and the pooled SMD was significantly affected by the study of Cosman[28] (SMD 0.82; 95% CI, 0.42–1.22; I2 = 87.5%, after removed) (Figure S2). Subgroup analyses showed that whether compared with anabolic agents (SMD 0.23; 95% CI, 0.03–0.44; I2 = 0%) or nonbisphosphonates antiresorptive agents (SMD 1.91; 95% CI, 1.16–2.67; I2 = 92%), the BMD did increase significantly (Fig. 3). The tests for the subgroup differences indicated that the differences were statistically significantly between each other (P < .05 for interaction). Meta-regression analyses suggested that the results have a significant relationship with the treatment duration (P < .05 for slope, data not shown), but not with the age (P = .056 for slope, data not shown). Both funnel plot and Egger's test suggested no presence of publication bias for the BMD changes at the lumbar spine (Egger's test P = .17) (Figure S3).
Figure 4.
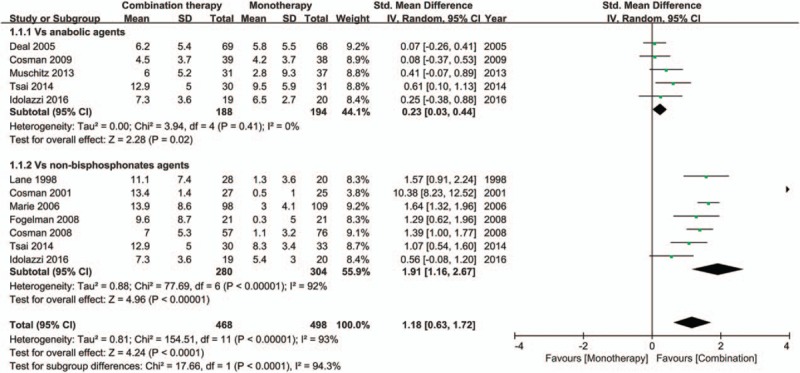
Forest plot for the BMD changes at the lumbar spine. BMD = bone mineral density.
3.6. BMD changes at the total hip
Ten trials[6,21–29] provided total hip BMD data and were included in the analysis. Combination therapy demonstrated a significant advantage over monotherapy in total hip BMD improvement (SMD 0.89; 95% CI, 0.48–1.29; I2 = 88%) (Fig. 5). Sensitivity analyses showed that the trail of Cosman[28] greatly affected the pooled SMD (SMD 0.68; 95% CI, 0.37 to 1.00; I2 = 79.7%, after removed) (Figure S4). Subgroup analyses showed that compared with monotherapy with either anabolic agents (SMD 0.81; 95% CI, 0.21–1.41; I2 = 87%) or nonbisphosphonates antiresorptive agents (SMD 0.97; 95% CI, 0.36–1.57; I2 = 90%), the BMD did increase significantly and the differences were not statistically significantly (P = .71 for interaction) (Fig. 4). The meta-regression analyses showed statistically significant differences in association by treatment duration (P < .05 for slope, data not shown), but not by the age (P = .45 for slope, data not shown). There was an evidence of publication bias, with the Egger's test P value of .04 and the asymmetric funnel plot (Figure S5).
Figure 5.
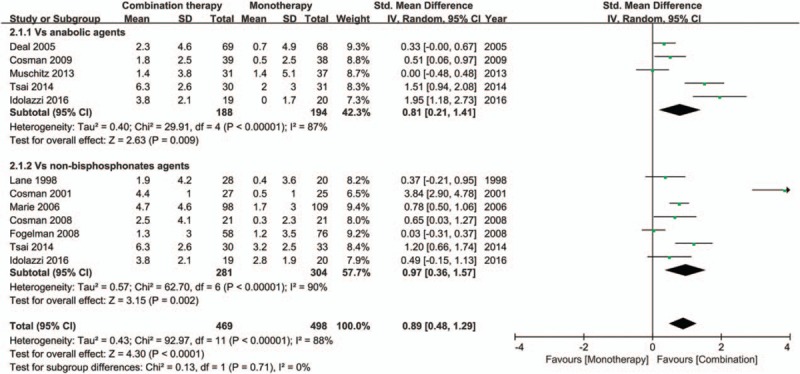
Forest plot for the BMD changes at the total hip. BMD = bone mineral density.
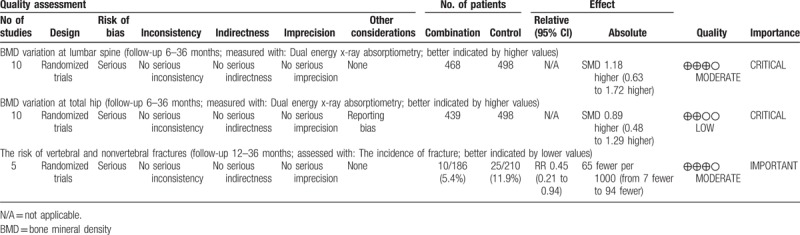
3.7. The quality of evidence
The GRADE evidence profiles for each outcome are shown in Table 2. All the included trials were RCTs and had no serious inconsistency, indirectness, or imprecision. Risk of bias existed in each outcome, and the most common causes of the decreased level of evidence were the unclear random sequence generation and the unclear allocation concealment. Reporting bias existed in the outcome of BMD changes at the total hip. Although the included RCTs were considered as high-quality evidence, the quality was lowered because of the above limitations. The strength of inference was therefore limited and the available evidence of each outcome was moderate to low.
Table 2.
The GRADE evidence quality for each outcome.
4. Discussion
Our meta-analysis comprehensively and systematically reviews the current available literature and provides low-to-moderate-quality evidence that the combination therapy of anabolic nonbisphosphonates antiresorptive agents is superior to monotherapy in improving the BMD at the lumbar spine and total hip. This study also provides moderate-quality evidence that this combination therapy has an advantage than monotherapy in reducing the fracture incidence.
Meanwhile, our meta-regression analyses suggested that the treatment duration had a significant relationship with the results, which might be the main reason for the significant heterogeneity. Among the included trials, the treatment duration lasted from 6 to 36 months. It was suggested that owing to the subsequent resistance to anabolic agents suggested that to increase BMD, anabolic agents might best be used for periods of 6 to 12 months or less.[30] Since the effect of anabolic agents was affected by the treatment duration, the effect of combination therapy should be affected as well. In addition, it was suggested that the effect of combination therapy seemed to be affected by the potency of antiresorptive agents.[10] Since there were 3 different kinds of antiresorptive agents (HRT, raloxifene, and denosumab) included in our study, and the potency of them was different, which was another explanation for the significant heterogeneity. Although the diverse settings brought some heterogeneity, the heterogeneity has been well explained, and the diverse settings considerably improved the generalizability and usefulness of our meta-analysis.[31]
Monotherapy was the current standard treatment for osteoporosis. Antiresorptive agents could reduce bone resorption, were the first line drugs. Anabolic agents could increase bone formation, were the second line drugs. However, due to the coupling of bone resorption and formation, antiresorptive agents not only inhibit bone resorption but also inhibit bone formation, thereby mitigating the potential benefit of the antiresorptive effect, similarly, antiresorptive agents increase bone formation also increase bone resorption, which affecting the anabolic ability.[32] Theoretically, if bone resorption is inhibited by an antiresorptive agent while bone formation is being stimulated by an anabolic agent, a combination therapy of anabolic and antiresorptive agents could ‘uncouple’ bone resorption and bone formation, produced greater increases in BMD, greater improvement of bone strength, and perhaps greater antifracture efficacy compared to monotherapy.[33] Our study showed that combining anabolic agents and nonbisphosphonates antiresorptive agents produced a synergistic effect on BMD and greater efficacy against fracture in comparison to monotherapy, which confirmed this hypothesis. Based on our results, although current evidence does not yet support a change in clinical practice, combination therapy may be appropriate for certain patients with osteoporosis.[5,34] For example, (1) for patients previously treated with antiresorptive agents who yet continue to lose significant bone density; (2) for patients who need rapidly bone density increases while receiving monotherapy.
Moreover, since anabolic agents were approved for a limited period (18–24 months), a sequential therapy was required due to the short duration. According to current evidence, when using sequential therapy with monotherapy, for patients previously treated with anabolic agents alone, sequential therapy with an antiresorptive agent is recommended to maintain and further increase the BMD.[35,36] However, for patients previously treated with antiresorptive agents, the BMD response to anabolic agents would be blunted.[37,38] When using the combination therapy as a part of the sequential therapy, switching from monotherapy (anabolic or antiresorptive agents) to the combination therapy might be more appropriate.[22,23,35,39,40] For patients previously treated with antiresorptive agents, the full period (18–24 months) of the combination therapy produced greater BMD increases compared with teriparatide monotherapy.[23] For patients previous treated with anabolic agents, stopping anabolic agents at the 12th month, then starting the combination therapy was appropriate,[30] the combination therapy could extend the “anabolic window” and further enhance the anabolic effects of anabolic agents.[22,41]
This study also has limitations. (1) There were some methodological limitations in the included trials, such as the unclear random method, the inadequate concealment of treatment allocation, and the high loss of follow-up. (2) The possibility of publication bias existed for the limited number of included trials. (4) Owing to the methodological limitations and the potential publication bias of the included trials, the quality of evidence was only moderate or low. (5) There was significant heterogeneity in some outcomes. (6) Since all the patients in this study were osteoporotic women, whether the results presented in this meta-analysis are applicable to osteoporotic men need to be further studied. Given these limitations, results of this meta-analysis should be interpreted cautiously.
Several gaps remain regarding the combination therapy. First, both meta-regression analyses and sensitivity analyses determined that there is a close association between treatment duration and outcomes. Future research should aim to identify the long-term (24 months) effect of combination therapy. Second, further studies are required to establish the cost-effectiveness and safety of combination therapy. Third, there are no adequately powered fracture outcome studies, additional studies are needed to evaluate the antifracture efficacy of combination therapy. Finally, trials are needed to establish an optimal strategy of combination therapy, considering active controls to investigate the comparative effectiveness of different combination strategies.
5. Conclusion
Low-to-moderate-quality evidence shows that the combination therapy of anabolic and nonbisphosphonates antiresorptive agents is superior to monotherapy in in improving the BMD and reducing the fracture risk. However, there is still a need for further high methodological quality studies to determine the antifracture efficacy, cost-effectiveness and safety of this strategy of combination therapy.
Supplementary Material
Footnotes
Abbreviations: BMD = bone mineral density, CI = confidence interval, GRADE = Grading of Recommendations Assessment, Development and Evaluation, HRT = hormone replacement therapy, PTH = parathyroid hormone, RCT = randomized controlled trial, RR = risk ratio, SDC = supplemental digital content, SMD = standard mean difference.
SL and LW contributed equally to this work.
Author contributions: YW conceived and designed the experiments and reviewed the draft. SL and LW performed the experiments, analyzed the data, wrote the paper, prepared the figures and tables and reviewed the draft. YW, YJ and JL contributed to the design of the search strategy, prepared the figures and tables and reviewed the draft.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001;285:785–95. [DOI] [PubMed] [Google Scholar]
- [2].Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet 2002;359:1761–7. [DOI] [PubMed] [Google Scholar]
- [3].Johnell O, Kanis JA. An estimate of the worldwide prevalence, mortality and disability associated with hip fracture. Osteoporos Int 2004;15:897–902. [DOI] [PubMed] [Google Scholar]
- [4].Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006;17:1726–33. [DOI] [PubMed] [Google Scholar]
- [5].Cosman F. Combination therapy for osteoporosis: a reappraisal. Bonekey Rep 2014;3:518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Leder BZ, Tsai JN, Uihlein AV, et al. Two years of Denosumab and teriparatide administration in postmenopausal women with osteoporosis (The DATA Extension Study): a randomized controlled trial. J Clin Endocrinol Metab 2014;99:1694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Li W, Chen W, Lin Y. The efficacy of parathyroid hormone analogues in combination with bisphosphonates for the treatment of osteoporosis: a meta-analysis of randomized controlled trials. Medicine (Baltimore) 2015;94:e1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhang Q, Qian J, Zhu Y. Parathyroid hormone plus alendronate in osteoporosis: a meta-analysis of randomized controlled trials. Int J Clin Exp Med 2015;8:3338–48. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [9].McClung MR. Using osteoporosis therapies in combination. Curr Osteoporos Rep 2017;15:343–52. [DOI] [PubMed] [Google Scholar]
- [10].Shen Y, Gray DL, Martinez DS. Combined pharmacologic therapy in postmenopausal osteoporosis. Endocrinol Metab Clin North Am 2017;46:193–206. [DOI] [PubMed] [Google Scholar]
- [11].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Egger M, Davey SG, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lindsay R, Nieves J, Formica C, et al. Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet 1997;350:550–5. [DOI] [PubMed] [Google Scholar]
- [17].Tsai JN, Uihlein AV, Lee H, et al. Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial. Lancet 2013;382:50–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tsai JN, Burnett-Bowie SM, Lee H, et al. Relationship between bone turnover and density with teriparatide, denosumab or both in women in the DATA study. Bone 2017;95:20–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tsai JN, Jiang LA, Lee H, et al. Effects of teriparatide, denosumab, or both on spine trabecular microarchitecture in DATA-switch: a randomized controlled trial. J Clin Densitom 2017;20:507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Finkelstein JS, Klibanski A, Arnold AL, et al. Prevention of estrogen deficiency-related bone loss with human parathyroid hormone-(1–34): a randomized controlled trial. JAMA 1998;280:1067–73. [DOI] [PubMed] [Google Scholar]
- [21].Lane NE, Sanchez S, Modin GW. Parathyroid hormone treatment can reverse corticosteroid-induced osteoporosis. Results of a randomized controlled clinical trial. J Clin Invest 1998;102:1627–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Muschitz C, Kocijan R, Fahrleitner-Pammer A, et al. Antiresorptives overlapping ongoing teriparatide treatment result in additional increases in bone mineral density. J Bone Miner Res 2013;28:196–205. [DOI] [PubMed] [Google Scholar]
- [23].Cosman F, Wermers RA, Recknor C, et al. Effects of teriparatide in postmenopausal women with osteoporosis on prior alendronate or raloxifene: differences between stopping and continuing the antiresorptive agent. J Clin Endocrinol Metab 2009;94:3772–80. [DOI] [PubMed] [Google Scholar]
- [24].Fogelman I, Fordham JN, Fraser WD, et al. Parathyroid hormone (1–84) treatment of postmenopausal women with low bone mass receiving hormone replacement therapy. Calcif Tissue Int 2008;83:85–92. [DOI] [PubMed] [Google Scholar]
- [25].Cosman F, Nieves JW, Zion M, et al. Effect of prior and ongoing raloxifene therapy on response to PTH and maintenance of BMD after PTH therapy. Osteoporos Int 2008;19:529–35. [DOI] [PubMed] [Google Scholar]
- [26].Ste-Marie LG, Schwartz SL, Hossain A, et al. Effect of teriparatide [rhPTH(1–34)] on BMD when given to postmenopausal women receiving hormone replacement therapy. J Bone Miner Res 2006;21:283–91. [DOI] [PubMed] [Google Scholar]
- [27].Deal C, Omizo M, Schwartz EN, et al. Combination teriparatide and raloxifene therapy for postmenopausal osteoporosis: results from a 6-month double-blind placebo-controlled trial. J Bone Miner Res 2005;20:1905–11. [DOI] [PubMed] [Google Scholar]
- [28].Cosman F, Nieves J, Woelfert L, et al. Parathyroid hormone added to established hormone therapy: effects on vertebral fracture and maintenance of bone mass after parathyroid hormone withdrawal. J Bone Miner Res 2001;16:925–31. [DOI] [PubMed] [Google Scholar]
- [29].Idolazzi L, Rossini M, Viapiana O, et al. Teriparatide and denosumab combination therapy and skeletal metabolism. Osteoporos Int 2016;27:3301–7. [DOI] [PubMed] [Google Scholar]
- [30].Cosman F, Nieves J, Zion M, et al. Daily and cyclic parathyroid hormone in women receiving alendronate. N Engl J Med 2005;353:566–75. [DOI] [PubMed] [Google Scholar]
- [31].Gotzsche PC. Why we need a broad perspective on meta-analysis. It may be crucially important for patients. BMJ 2000;321:585–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lewiecki EM. Combination therapy: the Holy Grail for the treatment of postmenopausal osteoporosis. Curr Med Res Opin 2011;27:1493–7. [DOI] [PubMed] [Google Scholar]
- [33].Cosman F. Anabolic and antiresorptive therapy for osteoporosis: combination and sequential approaches. Curr Osteoporos Rep 2014;12:385–95. [DOI] [PubMed] [Google Scholar]
- [34].Compston J. The use of combination therapy in the treatment of postmenopausal osteoporosis. Endocrine 2012;41:11–8. [DOI] [PubMed] [Google Scholar]
- [35].Lou S, Lv H, Wang G, et al. The effect of sequential therapy for postmenopausal women with osteoporosis: a PRISMA-compliant meta-analysis of randomized controlled trials. Medicine (Baltimore) 2016;95:e5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Palacios S, Mejía A. Antiresorptives and anabolic therapy in sequence or combination for postmenopausal osteoporosis. Climacteric 2015;18:453–5. [DOI] [PubMed] [Google Scholar]
- [37].Obermayer-Pietsch BM, Marin F, McCloskey EV, et al. Effects of two years of daily teriparatide treatment on BMD in postmenopausal women with severe osteoporosis with and without prior antiresorptive treatment. J Bone Miner Res 2008;23:1591–600. [DOI] [PubMed] [Google Scholar]
- [38].Ettinger B, San MJ, Crans G, et al. Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res 2004;19:745–51. [DOI] [PubMed] [Google Scholar]
- [39].Black DJKSLTTJCJ. One year of alendronate after one year of parathyroid hormone (1–84) for osteoporosis. N Engl J Med 2005;353:555–65. [DOI] [PubMed] [Google Scholar]
- [40].Leder BZ, Tsai JN, Uihlein AV, et al. Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-Switch study): extension of a randomised controlled trial. Lancet 2015;386:1147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bilezikian JP. Combination anabolic and antiresorptive therapy for osteoporosis: opening the anabolic window. Curr Osteoporos Rep 2008;6:24–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


