Abstract
Patients with mild cognitive impairment (MCI) are at high risk of dementia, but early identification and active intervention can reduce its morbidity and the incidence of dementia. There is currently no suitable neuropsychological assessment scale to effectively identify MCI in neurological outpatient departments in China. The Mini-Mental State Examination (MMSE) is often used to screen for MCI in outpatient departments in China.
To compare the value of Mini-Cog and MMSE in screening patients for MCI in a neurological outpatient department, and determine differences in the value of Mini-Cog for different ages and educational levels.
This was a retrospective study of 229 patients with suspected MCI who visited the Cangzhou Central Hospital between March 2012 and April 2016. The MCI group included 119 patients diagnosed with MCI and 110 cases without MCI (non-MCI group). The MCI patients were subgrouped as 40 to 60 years of age, 61 to 80 years, and >80 years; and as without education, ≤6 years education, and >6 years education. All subjects were assessed using the Mini-Cog and MMSE.
There were significant differences in Mini-Cog (P < .05) and MMSE (P < .05) between the MCI and non-MCI groups. The sensitivity, specificity, positive predictive value, negative predictive value, and Youden index (85.71%, 79.41%, 0.8108, 0.8438, and 0.6550) of Mini-Cog were all higher than those of MMSE (64.76%, 71.57%, 0.7010, 0.6364, and 0.3370) in identifying MCI, but there was no significant difference in specificity (P > .05). Mini-Cog was better than MMSE (P < .05) for MCI patients with different ages and education levels.
These results showed that the Mini-Cog was superior to MMSE in identifying MCI patients. Mini-Cog was less affected by age and education level than MMSE. The Mini-Cog assessment was short (3–4 minutes) and easily accepted by the patients. Mini-Cog could be more suitable for application in outpatient department in primary hospitals.
Keywords: cognitive dysfunction, diagnostic tests and procedures, Mini cognitive scale, Mini-Mental State Examination
1. Introduction
Mild cognitive impairment (MCI) refers to patients who have memory or cognitive impairment but without obvious influence on their daily activities.[1] Therefore, MCI can be considered as an intermediate state between normal aging and dementia.[2] Patients with MCI are at high risk of developing dementia, with a risk 10 times greater than that of non-MCI elderly people.[3] MCI also represents an early stage of dementia in some patients.[4] MCI (particularly amnestic MCI) was found to develop easily into Alzheimer disease (AD).[2]
Alzheimer's Disease International (ADI) reported in 2015 that there were up to 46 million people with dementia in the world, and that 58% of dementia patients were living in low- and middle-income countries. This number is predicted to increase to more than 130 million by 2050, and in 2015 alone, there were about 9.9 million new cases of dementia. Therefore, this predicts that there would be one more dementia patient every three seconds in the world. With the increase in social aging, the incidence of AD is increasing year by year in China. In 2014, the incidence of dementia in people over the age of 65 years old was 5.14% and AD accounted for 3.21%.[5]
Although there are no evidence-based medications that can be recommended for patients with MCI to prevent the development of dementia,[6] physicians should be actively looking for the causes of MCI and then provide targeted therapy or attempt to delay disease progression if any causes are considered treatable.[7] Therefore, neuropsychological assessment is important for diagnosis and further research is needed into therapies for MCI.[8] The early identification of patients with cognitive impairment is mainly focused on first-line physicians who work with outpatients. Therefore, it is vitally important that these physicians use a short, sensitive, and accurate screening scale.[9] Nevertheless, currently in Chinese neurological outpatient departments there is no suitable neuropsychological assessment scale for physicians to effectively identify MCI.
The Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA) are the most commonly used cognitive function screening scales. MMSE has a short assessment time (5–10 minutes) and is simple and easy to use, but MMSE is not sensitive to MCI.[10] MoCA has a higher sensitivity to MCI,[11] but takes longer (10–15 minutes), and is not suitable for use by physicians for outpatients. The Mini cognitive scale (Mini-Cog) only takes 3 minutes, and is slightly influenced by language and education level. It is easily accepted by patients and outpatient physicians,[9] and the sensitivity and specificity of screening patients with dementia are higher than those of MMSE.[12] Nevertheless, while Mini-Cog is now mainly used in screening patients with dementia, little research has been undertaken on whether it performs better than MMSE for screening patients at the MCI stage.[13]
Therefore, the aim of this study was to compare the value of Mini-Cog with MMSE in screening patients with MCI in a neurological outpatient department in China. In addition, we investigated differences in the value of the scales in assessing patients of different ages and educational levels. The results of this study will provide the basis for the early diagnosis of MCI, the early prevention and treatment of dementia, and the selection of a simple and reliable screening tool.
2. Methods
2.1. Subjects
This was a retrospective study of 229 patients with suspected MCI who visited the Cangzhou Central Hospital between March 2012 and April 2016 and consulted a first-line physician at the outpatient department.
The inclusion criteria were patients who were assessed in the neurological outpatient department of Cangzhou City Central Hospital between March 2012 and April 2016 for MCI. The exclusion criteria were patients with psychiatric history or congenital mental retardation; patients with long-term use of antipsychotic drugs; patients with depression or anxiety neurosis; patients with visual, hearing, or severe limb dysfunction, and could not communicate with language, and patients with mental diseases.
This study was approved by the ethics committee of the author's hospital. The need for individual consent was waived by the committee because of the retrospective nature of the study.
2.2. Study design
The patients were divided into the MCI (n = 119) and non-MCI (n = 110) groups according to the final evaluation by a neurologist. The diagnosis of MCI met the diagnostic standards of the Petersen's criteria,[14,15] including the following 5 criteria: memory problems; objective memory disorder; absence of any other cognitive disorder or impact on daily life; normal general cognitive function; and no dementia. The above criteria are the general criteria of MCI and they are usually supplemented by objective indexes such as clinical dementia rating (CDR) (scoring 0.5 point), Mini-Mental State Examination (MMSE) (scoring >24 points).[16]
Patients in the MCI group were also investigated in terms of the assessment value according to their ages and education level, by subgrouping the patients into those aged 40 to 60 years, aged from 61 to 80 years, and >81 years; and according to without education, ≤6 years education, and >6 years education.
2.3. Clinical data collection
MMSE, Mini-Cog, Hamilton's Depression Scale (HAMD), Hamilton's Anxiety Scale (HAMA), CDR, and Hachinski Ischemic Rating Scale were used to assess all research subjects to exclude the patients with cognitive impairment and dementia due to causes of anxiety, depression, and cerebrovascular diseases. There were 4 physicians involved in this study, and they were all trained by neuropsychology and qualified deputy directors and physicians in the Department of Neurology. The final diagnosis of MCI was made based on the Petersen's criteria.[14,15] A Chinese version of the MMSE was used.[17] The Mini-Cog was composed of the Three Objects Recall and Clock Drawing Test (CDT) from the Cognitive Abilities Screening Instrument (CASI).[18] In the Three Objects Recall, 3 points were counted for the instantaneous recall, and 3 points for the short-term delayed recall. In CDT, spontaneously drawing of a circular clock (time of 11:10) was used. A 3-point method was used for scoring: 1 point for drawing a circle, 1 point for drawing correct clock digits, and 1 point for drawing correct clock time. Normal CDT was considered when all time scales were correct and the hand position was consistent with the specified time. In this study, the total score of the Mini-Cog was 9 points. Mini-Cog was evaluated according to the scoring rules published in 2000 by Borson et al[18] (Fig. 1). Scoring standards: 1 point for correctly recalling each word after the CDT test; 0 point suggested cognitive impairment; 3 points suggested no cognitive impairment; and patients with 1 or 2 points were classified according to CDT (normal CDT suggested no cognitive impairment, while abnormal CDT suggested cognitive impairment).
Figure 1.
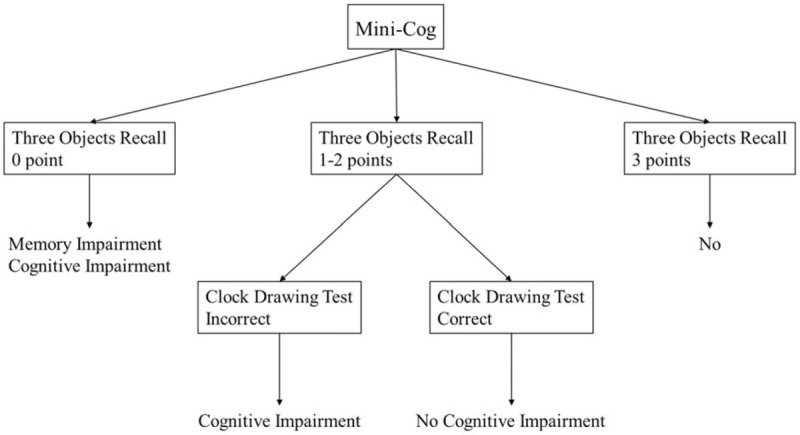
Scoring rule of Mini-Cog.
2.4. Statistical methods
SPSS17.0 (IBM, Armonk, NY) was used to analyze the data. The continuous data were shown as means ± standard deviation (χ ± s) and categorical data were shown as n (%). The 2 independent samples t test was used for the comparison between the 2 groups. The Chi-square test was used for the comparison of categorical data. P < .05 was considered as a statistically significant difference. The intraobserver and interobserver reliability of Mini-Cog were evaluated by calculation of the intraclass coefficient (ICC).
3. Results
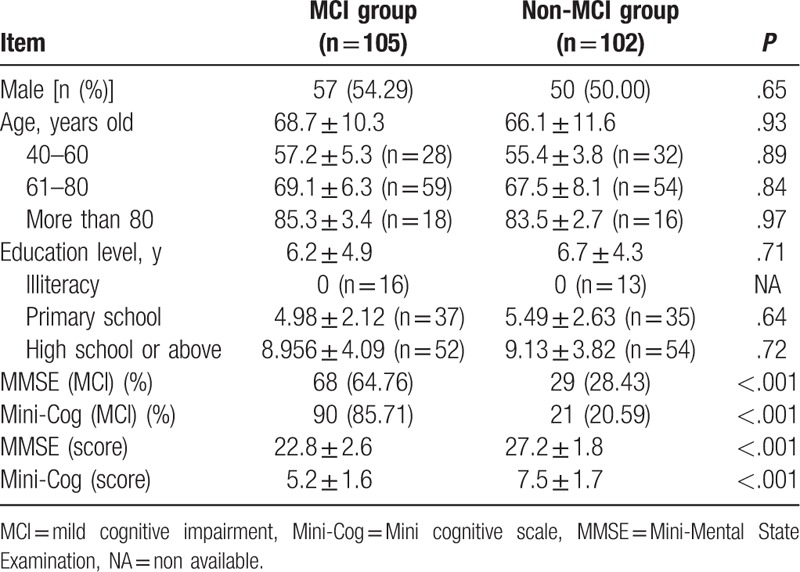
During the study period, 229 patients with suspected MCI visited the outpatient clinic and were included in the study. Initially, there were 119 patients in the MCI group and 110 patients in the non-MCI group, but 105 patients in the MCI group and 102 patients in the non-MCI group were included in the final analysis, because patients with incomplete clinical data were excluded. In the MCI group, there were 57 males and 48 females with a mean age of 67.4 ± 10.3 years and a mean of 12.2 ± 7.3 years of education. In the non-MCI group, there were 50 males and 52 females with a mean age of 73.1 ± 8.3 years and a mean of 12.2 ± 7.3 years of education. There were no significant differences in age, gender, and education level between the 2 groups (P > .05, Table 1).
Table 1.
Comparisons of baseline data between the 2 groups.
3.1. Comparisons of the Mini-Cog and MMSE scores in 2 groups
The Mini-Cog scores in the MCI group and non-MCI group were 5.2 ± 1.6 points and 7.5 ± 1.7 points, respectively, and the difference was statistically significant (P < .01). MMSE scores were 22.8 ± 2.6 and 27.2 ± 1.8, respectively, and the difference was statistically significant (P < .05, Table 1).
3.2. Comparisons of sensitivity and specificity of the Mini-Cog and MMSE in the diagnosis of MCI
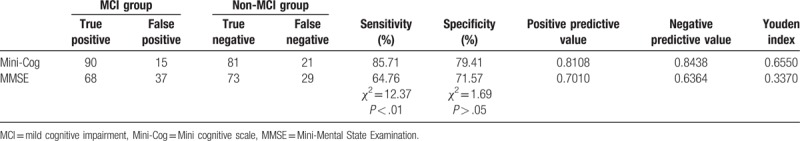
The sensitivity of Mini-Cog in the diagnosis of MCI (85.71%) was higher than that of MMSE (64.76%), and the difference was statistically significant (χ2 = 12.37, P < .05). The specificity of Mini-Cog (79.41%) was higher than that of MMSE (71.57%), but the difference was not statistically significant (χ2 = 1.69, P > .05). Please see Table 2.
Table 2.
Comparisons of the sensitivity and specificity of Mini-Cog and MMSE for the identification of patients with MCI (n = 207).
3.3. Effect of age on sensitivity
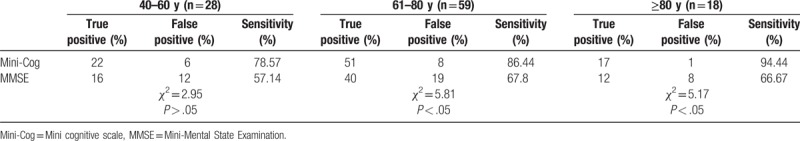
For the MCI group, the sensitivity of the Mini-Cog in each age group was higher than that of MMSE. In patients aged 40 to 60 years, there was no significant difference in the sensitivity between the 2 scales (χ2 = 2.95, P > .05). In those aged 61 to 80 years and those above 81 years, the sensitivity of the 2 scales was significantly different (61–80: χ2 = 5.81, P < .05, and ≥81: χ2 = 5.17, P < .05). Please see Table 3.
Table 3.
Comparison of the sensitivity of the Mini-Cog and MMSE in MCI patients with different ages (n = 105).
3.4. Effect of the education level on sensitivity
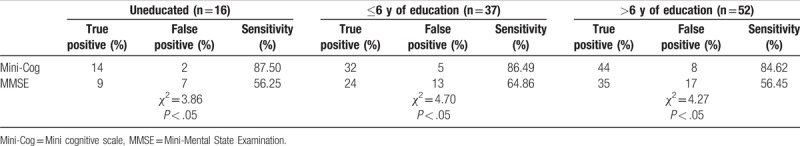
For MCI patients, the sensitivity of the Mini-Cog in each education group was higher than that of MMSE, and there were significant differences in the sensitivity of the 2 scales in each subgroup (uneducated: χ2 = 3.86, P < .05; ≤6 years of education: χ2 = 4.70, P < .05; >6 years of education: χ2 = 4.27, P < .05). Please see Table 4.
Table 4.
Comparisons of the sensitivity of Mini-Cog and MMSE in MCI patients with different education levels (n = 105).
4. Discussion
The aim of this study was to compare the value of Mini-Cog and MMSE in screening patients for MCI in a Chinese neurological outpatient department, and determine whether the Mini-Cog test was influenced by age and educational level. The results show that the Mini-Cog was better than MMSE for sensitivity, specificity, positive predictive value, negative predictive value, and Youden index in identifying MCI, but the difference in specificity was not significant. Overall, investigation of subgroups based on age and education level suggested that the Mini-Cog was better than MMSE for screening MCI. Therefore, we suggest that the Mini-Cog could be superior to MMSE in identifying MCI patients.
At present, both MMSE and Mini-Cog are widely used as screening tools for cognitive function by departments of neurology, departments of geriatrics, and general practitioners in a number of countries.[19,20] In China, MMSE remains the most widely used cognitive screening scale and the Mini-Cog is less popular. Nevertheless, this preference for MMSE may be mistaken as it takes a relatively long time, about 10 minutes, and is affected by language and education level, and the patients need to have relatively good hearing and vision. It also covers fewer cognitive areas than the Mini-Cog (5 cognitive components such as directional ability, immediate memory, computational ability, short-term memory, and language ability). The questions are too simple for MCI patients and lack the evaluation of some features such as executive function and abstract thinking, which can easily lead to the “ceiling effect.” Its sensitivity in diagnosing MCI is low, and a meta-analysis found that the sensitivity and specificity in distinguishing between elderly without MCI and those with MCI were 63.4% and 65.4%, respectively.[10] Therefore, it is not very suitable for use in neurology outpatient departments not only because of the long testing time but also the sensitivity and specificity in screening MCI patients. The Montreal Cognitive Assessment (MoCA) is also used for screening MCI patients[21] and it covers cognitive areas of attention, executive function, memory, language, visuospatial skills, abstract thinking, calculation, and orientation. International studies found that when that using 26 points as the boundary value, the sensitivity and specificity of identifying MCI patients were 90% and 87%, respectively.[11] Nevertheless, the MoCA takes a long time, about 15 minutes, which is suitable for specialized cognitive screening outpatient, but is difficult for first-line neurology physicians to apply in primary hospitals.
The Mini-Cog was proposed by Borson et al in 2000.[18] It is a simple and convenient tool for identifying the existence of cognitive impairment in elderly people. Its sensitivity is ∼76% to 99% and specificity is ∼89% to 93%.[18,19,22] It has strong predictive value in various clinical situations. The scale consists of 2 parts, including short term memory (delayed recall of three words based on the 3-word recall in ACSI) and the CDT. It seems simple, but involves a variety of cognitive functions such as immediate memory, short-term memory, comprehension, structural concept, visual spatial ability, executive function, abstract thinking, and attention. It takes a short time, about 3 minutes, and is not affected by education, culture, and language.[23] It has recently been suggested as an effective tool for use in screening for dementia in Chinese communities in comparison to MMSE, CDT, and AD8.[24] Simple short-term training can help physicians to accurately use the Mini-Cog. Compared with other complex cognitive assessment methods, the pressure of using Mini-Cog for assessment on patients is small. The accuracy in heterogeneous populations could improve the detection rate of cognitive impairment, and it is especially suitable for populations with pluralistic language, culture and education level.[9,18] Both validity and sensitivity of the Mini-Cog in elderly people were found to be high,[25] and it was able to identify MCI at an early stage.[26]
We found that the Mini-Cog took a short time and was easily accepted by patients in our outpatient department. None of the patients rejected the test, but 5 patients in the non-MCI group and three in the MCI group indicated that the MMSE test was tedious and were not interested in taking the test, and even refused to cooperate. The results of this study showed that the predictive value of screening for MCI with the Mini-Cog was significantly different from that of MMSE. Both sensitivity and specificity of the Mini-Cog in identifying MCI patients were higher than with MMSE, but there was no statistical difference in the specificity. Mini-Cog was less affected by education level and age than MMSE. Our research results were consistent with those of Borson et al,[27] and the research results showed that the Mini-Cog was able to find subjects with MCI who were difficult to identify. Milian et al[28] showed that the severity of depression did not affect the specificity of the Mini-Cog and CDT, but it did for MMSE. Shulman[29] showed that the individual application of CDT had the best sensitivity and specificity when using 3 points as the boundary value. Compared with the Mini-Cog, CDT had similar specificity, but less sensitivity, which was only 72.28% (Mini-Cog: 82.04%). Mini-Cog was able to quickly and easily evaluate cognition in a variety of medical conditions.[29]
Nevertheless, it is worth noting that in our opinion the cognitive areas contained in the Mini-Cog were not comprehensive enough for a fully sensitive test. Therefore, the Mini-Cog could not be used alone in assessing cognitive outpatients. Another study has used the Mini-Cog in combination with a functional activities questionnaire with good results.[30] Therefore, there may be some added value in combining testing methods for the most effective screening.
This study has some limitations. The sample size was quite small and from a single center. A larger study that includes more hospital outpatient departments would add more evidence to these results. The data was analyzed retrospectively after the patients had been diagnosed, so there may have been some unintentional bias being introduced. Therefore, further studies are needed to fully evaluate the clinical value of the Mini-Cog in Chinese outpatient departments.
Our study suggests that the Mini-Cog was more suitable for use in a Neurology Outpatient Department in a primary Chinese hospital than MMSE. As a MCI scale, the Mini-Cog was simple, rapid, economical, and easily accepted by the patients. Nevertheless, the cognitive areas contained in the Mini-Cog are not comprehensive enough for a fully sensitive test. Therefore, the Mini-Cog could not be used alone in assessing MCI outpatients.
Author contributions
Xueyan Li and Jie Dai carried out the studies, participated in collecting data, and drafted the manuscript. Shasha Zhao, Wangen Liu, and Haimei Li performed the statistical analysis and participated in its design. Xueyan Li and Jie Dai helped to draft the manuscript. All authors read and approved the final manuscript.
Resources: Shasha Zhao.
Validation: Wangen Liu.
Visualization: Haimei Li.
Writing – original draft: Xueyan Li.
Writing – review & editing: Jie Dai.
Footnotes
Abbreviations: ADI = Alzheimer's Disease International, CASI = Cognitive Abilities Screening Instrument, CDR = clinical dementia rating, CDT = Clock Drawing Test, HAMD = Hamilton's Depression Scale, ICC = intraclass coefficient, MCI = mild cognitive impairment, MMSE = Mini-Mental State Examination, MoCA = Montreal Cognitive Assessment.
Data availability: Data are available upon demand by contacting the corresponding author.
The authors have no conflicts of interest to disclose.
References
- [1].Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA 2014;312:2551–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 2004;256:240–6. [DOI] [PubMed] [Google Scholar]
- [3].Bozoki A, Giordani B, Heidebrink JL, et al. Mild cognitive impairments predict dementia in nondemented elderly patients with memory loss. Arch Neurol 2001;58:411–6. [DOI] [PubMed] [Google Scholar]
- [4].Eshkoor SA, Hamid TA, Mun CY, et al. Mild cognitive impairment and its management in older people. Clin Interv Aging 2015;10:687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jia J, Wang F, Wei C, et al. The prevalence of dementia in urban and rural areas of China. Alzheimers Dement 2014;10:1–9. [DOI] [PubMed] [Google Scholar]
- [6].Cooper C, Li R, Lyketsos C, et al. Treatment for mild cognitive impairment: systematic review. Br J Psychiatry 2013;203:255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Writing Group of the Dementia and Cognitive Society of Neurology Committee of Chinese Medical Association; Alzheimer's Disease Chinese. [Guidelines for dementia and cognitive impairment in China: the diagnosis and treatment of mild cognitive impairment]. Zhonghua Yi Xue Za Zhi 2010;90:2887–93. [PubMed] [Google Scholar]
- [8].Vega JN, Newhouse PA. Mild cognitive impairment: diagnosis, longitudinal course, and emerging treatments. Curr Psychiatry Rep 2014;16:490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Borson S, Scanlan JM, Watanabe J, et al. Simplifying detection of cognitive impairment: comparison of the Mini-Cog and Mini-Mental State Examination in a multiethnic sample. J Am Geriatr Soc 2005;53:871–4. [DOI] [PubMed] [Google Scholar]
- [10].Mitchell AJ. A meta-analysis of the accuracy of the mini-mental state examination in the detection of dementia and mild cognitive impairment. J Psychiatr Res 2009;43:411–31. [DOI] [PubMed] [Google Scholar]
- [11].Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–9. [DOI] [PubMed] [Google Scholar]
- [12].Milian M, Leiherr AM, Straten G, et al. The Mini-Cog versus the Mini-Mental State Examination and the Clock Drawing Test in daily clinical practice: screening value in a German Memory Clinic. Int Psychogeriatr 2012;24:766–74. [DOI] [PubMed] [Google Scholar]
- [13].Fage BA, Chan CC, Gill SS, et al. Mini-Cog for the diagnosis of Alzheimer's disease dementia and other dementias within a community setting. Cochrane Database Syst Rev 2015;Cd010860. [DOI] [PubMed] [Google Scholar]
- [14].Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol 2001;58:1985–92. [DOI] [PubMed] [Google Scholar]
- [15].Portet F, Ousset PJ, Visser PJ, et al. Mild cognitive impairment (MCI) in medical practice: a critical review of the concept and new diagnostic procedure. Report of the MCI Working Group of the European Consortium on Alzheimer's Disease. J Neurol Neurosurg Psychiatry 2006;77:714–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med 2005;352:2379–88. [DOI] [PubMed] [Google Scholar]
- [17].Chen L, Yu C, Fu X, et al. Using the Montreal Cognitive Assessment Scale to screen for dementia in Chinese patients with Parkinson's disease. Shanghai Arch Psychiatry 2013;25:296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Borson S, Scanlan J, Brush M, et al. The mini-cog: a cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry 2000;15:1021–7. [DOI] [PubMed] [Google Scholar]
- [19].Shulman KI, Herrmann N, Brodaty H, et al. IPA survey of brief cognitive screening instruments. Int Psychogeriatr 2006;18:281–94. [DOI] [PubMed] [Google Scholar]
- [20].Milne A, Culverwell A, Guss R, et al. Screening for dementia in primary care: a review of the use, efficacy and quality of measures. Int Psychogeriatr 2008;20:911–26. [DOI] [PubMed] [Google Scholar]
- [21].Trzepacz PT, Hochstetler H, Wang S, et al. Relationship between the Montreal Cognitive Assessment and Mini-mental State Examination for assessment of mild cognitive impairment in older adults. BMC Geriatr 2015;15:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Borson S, Scanlan JM, Chen P, et al. The Mini-Cog as a screen for dementia: validation in a population-based sample. J Am Geriatr Soc 2003;51:1451–4. [DOI] [PubMed] [Google Scholar]
- [23].Brodaty H, Low LF, Gibson L, et al. What is the best dementia screening instrument for general practitioners to use? Am J Geriatr Psychiatry 2006;14:391–400. [DOI] [PubMed] [Google Scholar]
- [24].Yang L, Yan J, Jin X, et al. Screening for dementia in older adults: comparison of Mini-Mental State Examination, Mini-Cog, Clock Drawing Test and AD8. PLoS ONE 2016;11:e0168949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].McCarten JR, Anderson P, Kuskowski MA, et al. Screening for cognitive impairment in an elderly veteran population: acceptability and results using different versions of the Mini-Cog. J Am Geriatr Soc 2011;59:309–13. [DOI] [PubMed] [Google Scholar]
- [26].Carolan Doerflinger DM. How to try this: the mini-cog. Am J Nurs 2007;107:62–71. quiz 71–72. [DOI] [PubMed] [Google Scholar]
- [27].Borson S, Scanlan JM, Watanabe J, et al. Improving identification of cognitive impairment in primary care. Int J Geriatr Psychiatry 2006;21:349–55. [DOI] [PubMed] [Google Scholar]
- [28].Milian M, Leiherr AM, Straten G, et al. The Mini-Cog, Clock Drawing Test, and the Mini-Mental State Examination in a German memory clinic: specificity of separation dementia from depression. Int Psychogeriatr 2013;25:96–104. [DOI] [PubMed] [Google Scholar]
- [29].Shulman KI. Clock-drawing: is it the ideal cognitive screening test? Int J Geriatr Psychiatry 2000;15:548–61. [DOI] [PubMed] [Google Scholar]
- [30].Steenland NK, Auman CM, Patel PM, et al. Development of a rapid screening instrument for mild cognitive impairment and undiagnosed dementia. J Alzheimers Dis 2008;15:419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]


