Abstract
We investigated the relationships between body mass index change (ΔBMI) and prognoses and clinical effects of patients with advanced colorectal cancer (CRC).
From January 2008 to December 2012, 224patients with stage IV CRC were diagnosed in our hospital, and their clinical and pathological data were collected for this retrospective study. These patients were divided into lowΔ BMI group (ΔBMI ≤−0.45 kg/m2) and high ΔBMI (ΔBMI >−0.45 kg/m2) group.
After 2 cycles of chemotherapy, there were no significant differences between prediagnosis BMI, ΔBMI, and clinical effects (P = .196; P = .59).There was also no significant difference in median progression-free survival of the high ΔBMI and low ΔBMI groups (P = .530). The overall survival (OS) time of the high ΔBMI group was significantly longer than that of the low ΔBMI group (P = .002). Family history (P = .041), eastern cooperative oncology group performance status (ECOG PS) score (P = .001), ΔBMI (P = .023), and carcinoembryonic antigen, (P = 0.02) were independent predictive factors of OS rates in patients with CRC. The relative risk was 0.72-fold for patients with CRC patients with high ΔBMI levels, relative to those with lower ΔBMI levels.
Our results demonstrate that ΔBMI decreases predict poor prognoses for patients with advanced CRC, and elevated ΔBMI was a predictive factor for high survival rate. Thus, ΔBMI appears to be an independent predictive factor of CRC survival rates.
Keywords: advanced colorectal cancer, body mass index, chemotherapy, clinical effects, prognosis
1. Introduction
Colorectal cancer (CRC) is a very common malignant tumor with high recurrence rates and poor prognoses.[1] CRC accounts for about 8% of all newly diagnosed cancers and 8% to 9% of all new cancer-related mortalities.[2] Mortalities from CRC have greatly decreases, possibly because of new technologies that enable earlier diagnoses and better treatments.[3,4] However, the long-term survival rate is relatively low for patients with CRC,[1] wherein the 5-year relative survival rate ranges from >90% in patients with stage I CRC to slightly >10% in patients with stage IV of CRC.[5] In China, CRC ranks fifth in overall number of cancer deaths, and the CRC incidences are continually on the rise,[3] especially for patients with advanced, late stage. It has been reported that in 15% to 25% of patients with CRC, metastasis occurs before diagnosis. This has become the key obstacle in the effective treatment of colon cancer.
Accordingly, it is necessary to identify predictors related to CRC progression and invasion, which may help patients select the appropriate treatment and monitoring.[1] Body mass index (BMI), which is defined as the relation of one's weight in kilograms divided by the square of one's height in meters, is a useful tool in clinical practice for assessing adult weight and nutritional status. Higher BMIs are related to morbidity and prognoses[6,7] and can increase surgical complications, such as incision infections and laparoscopic operation difficulties.[8,9] A reverse association between weight change and cancer risk has been observed in locally advanced pancreatic cancer,[10] breast cancer,[11–13] gastric cancer,[14] CRC,[15] non-small cell lung cancer[16], and endometrial cancer.[17] Innominato et al[18] reported that weight loss during chemotherapy had a close relationship with poor overall survival (OS). However, few studies have investigated the associations between BMI change (ΔBMI) during chemotherapy, clinical effects, and CRC prognosis.
Therefore, we performed a retrospective cohort study to assess the relation of these factors to ΔBMI.
2. Materials and methods
2.1. Study population
From January 2008 to December 2012, a total of 224 patients with stage IV CRC were treated at our hospital and were included in this retrospective study. Among these patients, 132 (58.9%) were men, 92 (41.1%) were women, and the median age was (63.2 ± 12.2) years. Patients were diagnosed with colonoscopy in combination with histopathological examinations. Clinical pathological and survival data were collected. The factors of age, sex, type of pathology, differentiation, site of tumor and metastasis, lymph node metastasis, and survival were included in this study. This study was approved by the Ethics Committee of our hospital. All patients or their relatives gave informed consent.
2.2. Inclusion and exclusion criteria
Inclusion criteria were: complete clinicopathologic materials, pathologically established diagnosis pathologically, no previous radical resection, eastern cooperative oncology group performance status (ECOG PS) scores ≤2, distant metastases confirmed by image examinations, such as positron emission tomography-computed tomography (PET-CT), ECT, CT, and magnetic resonance imaging (MRI), undiagnosed multiple diseases, including coronary heart disease, cerebral infarction, diabetes mellitus, among others, completion of at least 2 cycles of XELOX or folinic acid (leucovorin)-fluorouracil-oxaliplatin (FOLFOX) regimens.
Exclusion criteria were: incomplete clinicopathologic materials, patients with stage I–III CRC, simple, localized liver or lung metastasis which were radically resected, relapse after radical resection, neoadjuvant chemotherapy, unwillingness to give informed consent or undergo follow-up.
2.3. How to calculate ΔBMI
Height and weight outcomes before chemotherapy and after 2 cycles of chemotherapy were collected. BMIs were calculated before and after 2 cycles of chemotherapy, based on BMI (weight [kg]/height squared [m2]). ΔBMI during chemotherapy was calculated as BMI (after 2 cycles of chemotherapy)–BMI (before chemotherapy).
2.4. Treatment
Patients were only treated with chemotherapy. The standard chemotherapy included the CapeOX scheme, which is a 3-hour intravenous infusion of oxaliplatin (130 mg/m2) on day 1, and on days 1 to 14, oral capecitabine (1000 mg/m2) was administered 2 times a day. Every 3 weeks, a total of 8 cycles were completed. The FOLFOX scheme included leucovorin (200 mg/m2), given as a 2-hour infusion, and oxaliplatin (85 mg/m2) as a 2-hour infusion, followed by a bolus infusion of 5-FU (400 mg/m2) and a continuous infusion of 5-FU (2400 mg/m2) for 46 hours. This regimen was repeated every 2 weeks for 12 cycles.[19] The bevacizumab dose was 7.5 mg/kg, and it was administered every 2 weeks, initially over 90 minutes. If the first infusion was well tolerated, the second was delivered over 60 minutes, and if the 60-minute infusion was well tolerated, all subsequent infusions were delivered over 30 minutes. Cetuximab was administered weekly (at an initial dose of 400 mg/m2, intravenously over 120 minutes, and subsequently at 250 mg/m2 over 60 minutes on day 1 of each cycle (CRC whose tumors do not harbor a KRAS mutation). Clinical effects were evaluated by imaging studies, after 2 cycles of chemotherapy.
2.5. Clinical effect
The Response Evaluation Criteria in Solid Tumors (RECIST) was proposed as a standard evaluation, the content of which was as follows: complete response (CR), partial response (PR), stable disease (SD),(progressive disease (PD).The disease control rate (DCR) was equal to (CR + PR + SD). The clinical efficacy was evaluated after two cycles of chemotherapy, and if disease progression was suspected, the evaluation was performed ahead of time.
2.6. Follow up
Follow-up studies were performed as follows: once every month for the first year, once every 3 months for years 2 to 3, and once every 6 months after year 3. The last follow-up date was December 1, 2015.The follow-up periods ranged from 3 to 78 months (median: 25 months). Progression-free survival (PFS) time was defined as the duration between pathological diagnosis and the development of local recurrence or distant metastases. OS time was defined as the duration between the date of pathological diagnosis to the date of death or final follow-up of deceased and surviving patients, respectively.
2.7. Statistical analysis
All statistical analyses were performed with the SPSS 20.0 (SPSS Inc, Chicago, IL). The Spearman rank correlation analysis was used to analyze ΔBMIs and clinical effects. Independent sample t tests were used to examine differences of normally distributed data.
The receiver-operating characteristics (ROC) curve identified −0.45 as the optimal cutoff value for ΔBMI, in terms of OS and PFS. Patients were then divided into ΔBMI high (ΔBMI≥ −0.45 kg/m2) and ΔBMI low (ΔBMI ≤−0.45 kg/m2) groups, according to the optimal cut-off.[20] The Spearman rank Pearson correlation tests were used to identify correlations among clinicopathological parameters and ΔBMI. Survival curves were constructed, and the Kaplan–Meier method was used to analyze OS and PFS in patients with CRC. Survival differences were analyzed by the log-rank test, which was used to compare the survival distributions of the variables. Noncategorical variables were transformed into 2 categorical variables, according to their respective mean values. The Cox proportional hazards model was used to analyze the influence of variables on OS time. All reported P values were 2-tailed, and those <.05 were considered statistically significant.
3. Results
3.1. No significant relationships were identified between prediagnosis BMI, ΔBMI, and clinical effect
A total of 114 patients (50.9%) was in the high ΔBMI group and 110 (49.1%) were in the low ΔBMI group. Correlations between ΔBMI and clinical effects were studied by the Spearman rank correlation analysis. After 2 cycles of chemotherapy, the clinical effect parameters included CR (0.0%), PR (62.27.7%), SD (155.69.2%), PD (7.3.1%), and DCR (177.96.8%). There were no statistically significant differences between prediagnosis BMI, ΔBMI, and clinical effects (P = .196 and .59, respectively).
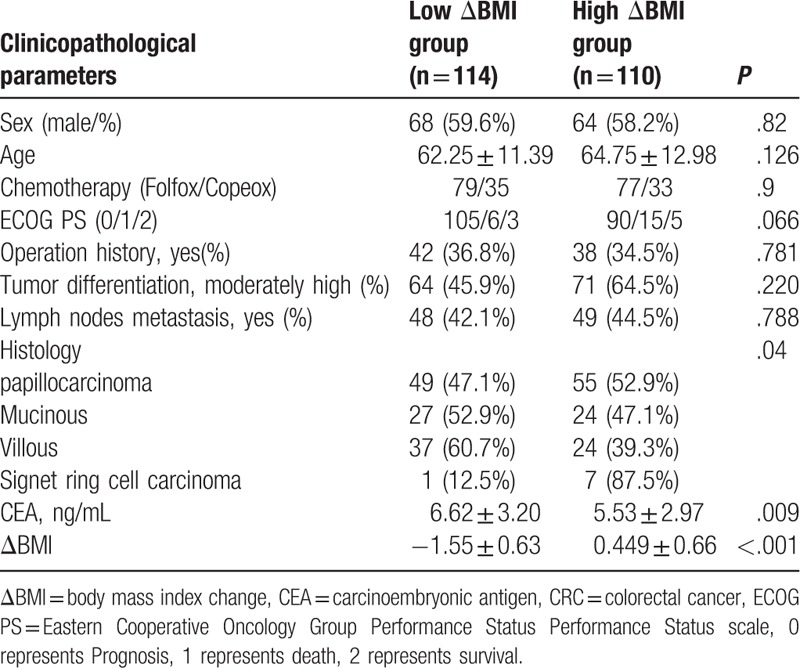
3.2. Significant correlation was found between ΔBMI and carcinoembryonic antigen
There were no significant differences between the high ΔBMI group and the low ΔBMI group in terms of sex, age, chemotherapy, ECOG PS, operation history, tumor differentiation, and lymph nodes metastasis (P = .82, .126,.90, .066, .781, .220, and .788,respectively). However, there were significant differences in histology (P = .04) and ΔBMI between the high ΔBMI and low ΔBMI groups (P < .001) (Table 1). The Pearson correlation test showed that ΔBMI and carcinoembryonicantigen (CEA) had a mild negative correlation, with a significant difference (R = −0.228, P = .001). The Spearman rank correlation results showed that ΔBMI and operation history also had a mild negative correlation, with a significant difference(R = −0.179, P = .007).
Table 1.
The basic clinicopathological parameters of 224 CRC patients.
3.3. Comparison between the high ΔBMI and low ΔBMI group survival rates
The independent sample t test results showed that there were no significant differences between the median PFS values of the high ΔBMI and low ΔBMI groups (P = .530) (Fig. 1). The median OS durations of the 2 groups were 27.0 ± 0.40 and 28.0 ± 0.29 months, respectively. The OS of the high ΔBMI group was significantly higher than that of the low ΔBMI group (Fig. 2, P = .002).
Figure 1.
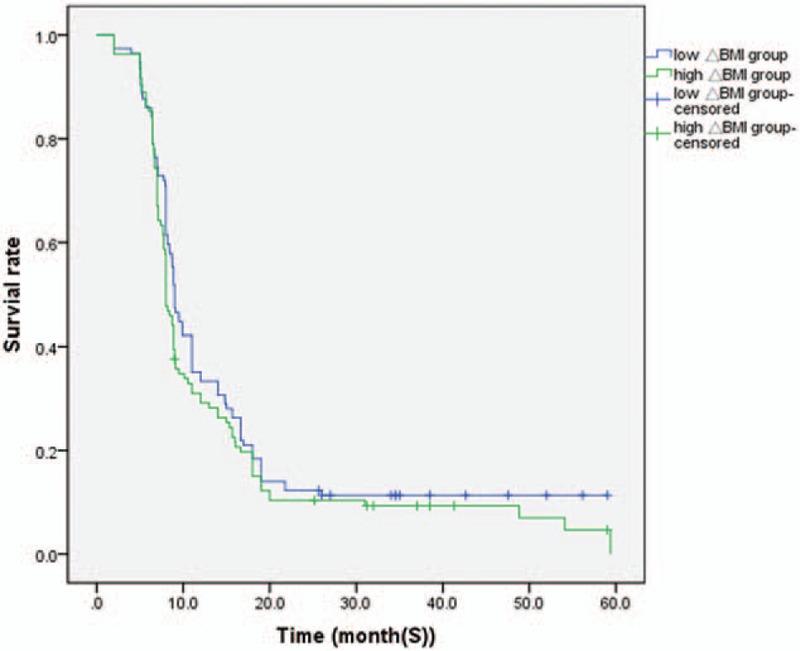
Kaplan-Meier survival analysis according to ΔBMI status (n = 224). The y-axis indicates the percentage of patients and x-axis depicts their survival in months. The green line represents the patients with low ΔBMI expression, who did not show a trend of worse survival than those with high ΔBMI, indicated by the blue line. The median progression-free survival of high ΔBMI group and low ΔBMI group were (8.0 ± 0.35), (9.0 ± 0.59) months, respectively (P > .05). ΔBMI = body mass index change.
Figure 2.
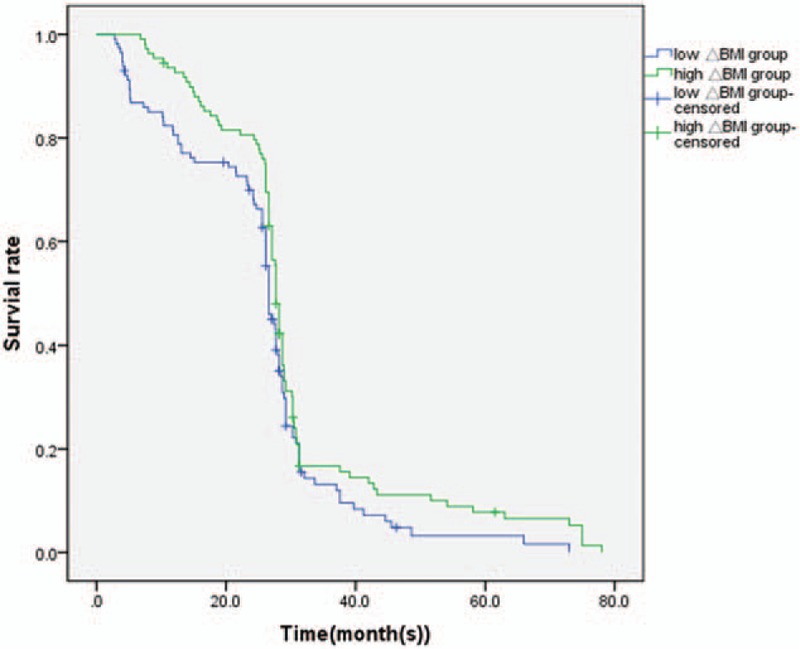
Kaplan-Meier survival analysis according to ΔBMI status (n = 224). The y-axis indicates the percentage of patients, and x-axis depicts their survival in months. The green line represents the patients with low ΔBMI expression, who showed a trend of worse survival than those with high ΔBMI, indicated by the blue line. The median OS of high ΔBMI group and low ΔBMI group were (28.0 ± 0.29), (27.0 ± 0.40) months, respectively (P < .05). ΔBMI = body mass index change.
3.4. ΔBMI was an independent prognostic determinant of OS in patients with CRC
The log-rank showed that there were no significant differences in sex (P = .644), distant metastasis (P = .879), tumor differentiation (P = .134), diseases location (P = .898), chemotherapy (P = .553), lymph nodes metastasis (P = .925), T stage (P = .644), histology (P = .86), and the survival rates of patients with CRC. However, there were significant differences in CEA (P < .001), family history (P = .008), ΔBMI (P < .001), ECOG PS (P < .001), age at diagnosis (P < .001), operation history (P = .011), prechemotherapy BMI (P < .001), and OS.
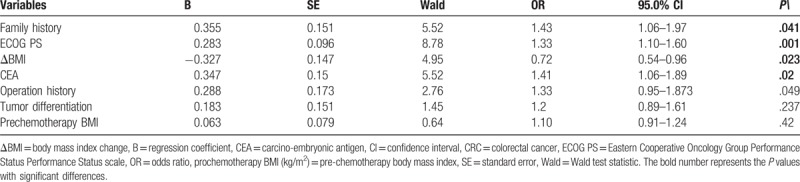
The cox regression analysis confirmed that family history (P = .041), ECOG PS (P = .001), ΔBMI (P = .023), and CEA (P = .02) were independent factors in the prediction of OS rates in patients with CRC. The above data indicate that decreased ΔBMI can predict OS rates in patients with CRC. Operation history (P = .10), tumor differentiation (P = .23), and prochemotherapy BMI (P = .42) cannot predict OS rates in patients with CRC (Table 2).
Table 2.
Cox regression analysis of 224 CRC patients for OS.
4. Discussion
Worldwide, CRC is the second most commonly diagnosed cancer in women and the third in men. There have been an estimated 1,360,000 new cases of CRC, and nearly 700,000 deaths, in 2012.[21–23] Numerous previous studies have shown that BMI not only relates to CRC morbidity, but also mortality.[24] Prediagnostic BMI has been associated with survival of patients with CRC and follows a U-shape pattern. Both obesity [25–27] (BMI ≥30 kg/m2) and underweight (BMI <18.5 kg/m2) [28] are associated with high mortality[29] and are strong risk factors for the long-term prognosis of patients with CRC. Furthermore, Laake et al[27] also found that weight gain was associated with higher CRC-specific mortality in women with CRC. However, these results are controversial. Junzhong et al[30] reported that weight loss during preoperative chemoradio therapy was an independent prognostic factor for OS. Other research has shown that severe weight loss directly contributes to up to one-fifth of cancer deaths and has a significant effect on patient quality of life.[31] Hoffmeister et al[32] suggested that overweight and obesity maybe associated with increased risk, in female patients with microsatellite-instable-high CRC. Croft et al doubted whether BMI could be used to measure the value of obesity in the prognosis of local CRC.[33] However, in a recent study, Shiao et al[34] reported that BMI is a predictor of CRC. Whether BMI changes influence the survival of patients with CRC has been unclear.
In this study, there were no significant differences between prediagnosis BMI, ΔBMI, and clinical effects. These results indicate that prediagnosis BMI and ΔBMI could not predicate clinical effects or provide reliable references for making or changing chemotherapy decisions. Furthermore, other studies have reported similar results.[35]
In our study, there were significant differences between ΔBMI and histology and CEA. There were no significant differences between ΔBMI and sex, chemotherapy, ECOG PS, operation history, tumor differentiation, age of diagnosis, and lymph node metastases. Innominato et al[18] reported that weight loss during chemotherapy was closely related to poor prognosis. Daniel et al[36] observed that weight change did not correlate with PFS, which is in agreement with our results; however, Lee et al[37] found that weight gain during therapy was associated with unfavorable survivor rates. These results differ from ours, and in our study, ΔBMI could be used to predict OS rates in patients with CRC.
This difference could be attributed to the enrollment of patients with stage II-III CRC. Most patients with early-stage CRC need operative treatment, and patients with a high ΔBMI, especially those who are obese, should undergo operations with caution because of their increased postoperative morbidity risk.[25,38] High ΔBMI might cause metabolic abnormalities, low immunogenicities, and intestinal inflammation, which leads to poor prognoses of patients with CRC. Advanced stages of cancer are often accompanied by malnutrition, and such patients cannot tolerate operations. Therefore, high ΔBMI provides as a marker for nutritional assessments[39] that can be utilized to improve poor nutritional statuses, which could ultimately enhance chemotherapy tolerance. Therefore, ΔBMI has a direct connection with favorable prognosis.
CEA, family history, ΔBMI, ECOG PS, tumor differentiation, operation history, and prochemotherapy BMI were significant risk factors for OS. The results of the cox analysis confirmed that family history, ECOG PS, ΔBMI, and CEA were independent predictors of OS rates in patients with CRC. The above results indicate that downregulated ΔBMI is predictive of poor prognoses in patients with CRC. Operation history, tumor differentiation, and prochemotherapy BMI were not independent predictors of OS. The relative risk is 0.72-fold for patients with CRC with high ΔBMI levels, compared to those with those with lower ΔBMI levels. ΔBMI is predictive factor of CRC survival rates.
In our study, patients with stage IV CRC who could not accept operations were enrolled. Lower ΔBMI levels often suggest poor nutritional statuses, which might compromise prognoses of patients with types of cancers, before and during anti-tumor treatments.[30] ΔBMI decreases owing to cachexia is a common symptom for patients with advanced cancer,[40] and decreased ΔBMI is also a risk factor for CRC-specific mortality.[34] However, because of our small sample size and inclusion of patients with higher TNM stages, we should further study ΔBMI levels across larger patient cohorts. Lee et al[37] reported that weight gain was a poor prognosis factor, especially for patients who are overweight or obese. The prognostic differences might also be associated with different TNM stages and treatments.
5. Conclusions
We demonstrated that ΔBMI decrease predicts a poor prognosis for patients with advanced CRC, and high ΔBMI is a predictive factor for survival. ΔBMI is an independent predictive factor of CRC survival rates.
Author contributions
Data curation: Zhao Cong.
Funding acquisition: Delin Wang.
Investigation: Zhao Cong, Delin Wang, Yujuan Cao.
Resources: Yujuan Cao.
Software: Zhao Cong, Delin Wang, Yujuan Cao.
Supervision: Yujuan Cao.
Writing – original draft: Zhao Cong.
Writing – review & editing: Delin Wang.
Footnotes
Abbreviations: ΔBMI = body mass index change, CEA = carcino-embryonic antigen, CEA = carcinoembryonicantigen, CR = complete response, CRC = colorectal cancer, DCR = disease control rate, ECOG PS = Eastern Cooperative Oncology Group Performance Status, FOLFOX = folinic acid(leucovorin)– fluorouracil- oxaliplatin, LV = leucovorin, MRI = Magnetic Resonance Imaging, S = overall survival, PD = progressive disease, PET-CT = positron emission tomography-computed tomography, PFS = progression-free survival, PR = partial response, SD = stable disease.
This study was funded by capital clinical characteristics applied research and attainment expanding (Grant No. Z161100000616003); the risk factors of malignancy, based on prospective cohort study is certified and analyzed (Grant No2016YPC1302605).
The authors report no conflicts of interest.
References
- [1].Zong Z, Zhou T, Rao L, et al. Musashi2 as a novel predictive biomarker for liver metastasis and poor prognosis in colorectal cancer. Cancer Med 2016;5:623–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11–30. [DOI] [PubMed] [Google Scholar]
- [3].Zhang X, Yu J, Li M, et al. The association of HMGB1 expression with clinicopathological significance and prognosis in Asian patients with colorectal carcinoma: a meta-analysis and literature review. Onco Targets Ther 2016;9:4901–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zeng YJ, Lai W, Wu H, et al. Neuroendocrine-like cells -derived CXCL10 and CXCL11 induce the infiltration of tumor-associated macrophage leading to the poor prognosis of colorectal cancer. Oncotarget 2016;7:27394–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet 2014;383:1490–502. [DOI] [PubMed] [Google Scholar]
- [6].Han J, Wang Z, Wei G, et al. [Risk factors associated with incisional surgical site infection in colorectal cancer surgery with primary anastomosis]. Zhonghua Wai Ke Za Zhi 2014;52:415–9. [PubMed] [Google Scholar]
- [7].Tao W, Konings P, Hull MA, et al. Colorectal cancer prognosis following obesity surgery in a population-based cohort study. Obes Surg 2017;27:1233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yin H, Xu L, Porter NA. Free radical lipid peroxidation: mechanisms and analysis. Chem Rev 2011;111:5944–72. [DOI] [PubMed] [Google Scholar]
- [9].Murphy TK, Calle EE, Rodriguez C, et al. Body mass index and colon cancer mortality in a large prospective study. Am J Epidemiol 2000;152:847–54. [DOI] [PubMed] [Google Scholar]
- [10].Yildirim BA, Ozdemir Y, Colakoglu T, et al. Impact of presence and degree of pretreatment weight loss in locally-advanced pancreatic cancer patients treated with definitive concurrent chemoradiotherapy. Pancreatology 2016;16:599–604. [DOI] [PubMed] [Google Scholar]
- [11].Warner ET, Ballman KV, Strand C, et al. Impact of race, ethnicity, and BMI on achievement of pathologic complete response following neoadjuvant chemotherapy for breast cancer: a pooled analysis of four prospective Alliance clinical trials (A151426). Breast Cancer Res Treat 2016;159:109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Valle CG, Deal AM, Tate DF. Preventing weight gain in African American breast cancer survivors using smart scales and activity trackers: a randomized controlled pilot study. J Cancer Surviv 2017;11:133–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Vargas-Meza A, Chavez-Tostado M, Cortes-Flores AO, et al. Body weight changes after adjuvant chemotherapy of patients with breast cancer: results of a Mexican cohort study. Eur J Cancer Care (Engl) 2016;26: [DOI] [PubMed] [Google Scholar]
- [14].Ock CY, Oh DY, Lee J, et al. Weight loss at the first month of palliative chemotherapy predicts survival outcomes in patients with advanced gastric cancer. Gastric Cancer 2016;19:597–606. [DOI] [PubMed] [Google Scholar]
- [15].Vergidis J, Gresham G, Lim HJ, et al. Impact of weight changes after the diagnosis of stage III colon cancer on survival outcomes. Clin Colorectal Cancer 2016;15:16–23. [DOI] [PubMed] [Google Scholar]
- [16].Topkan E. Weight gain as a surrogate marker of longer survival in advanced non-small cell lung cancer patients. Ann Transl Med 2016;4:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Welti LM, Beavers DP, Caan BJ, et al. Weight fluctuation and cancer risk in postmenopausal women: the women's health initiative. Cancer Epidemiol Biomarkers Prev 2017;26:779–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Innominato PF, Giacchetti S, Moreau T, et al. Fatigue and weight loss predict survival on circadian chemotherapy for metastatic colorectal cancer. Cancer 2013;119:2564–73. [DOI] [PubMed] [Google Scholar]
- [19].Takata K, Fujita KI, Kubota Y, et al. Cost-minimization analysis of adjuvant chemotherapy regimens given to patients with colorectal cancer in Japan. J Pharm Health Care Sci 2016;2:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhang Q, Ma X, Xu Q, et al. Nomograms incorporated serum direct bilirubin level for predicting prognosis in stages II and III colorectal cancer after radical resection. Oncotarget 2016;8:71138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rezaei-Tavirani M, Safaei A, Zali MR. The association between polymorphismsin insulin and obesity related genes and risk of colorectal cancer. Iran J Cancer Prev 2013;6:179–85. [PMC free article] [PubMed] [Google Scholar]
- [22].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [23].Sato H, Kotake K, Sugihara K, et al. Clinicopathological factors associated with recurrence and prognosis after R0 resection for stage IV colorectal cancer with peritoneal metastasis. Dig Surg 2016;33:382–91. [DOI] [PubMed] [Google Scholar]
- [24].Guo L, Li N, Wang G, et al. [Body mass index and cancer incidence:a prospective cohort study in northern China]. Zhonghua Liu Xing Bing Xue Za Zhi 2014;35:231–6. [PubMed] [Google Scholar]
- [25].Walter V, Jansen L, Hoffmeister M, et al. Prognostic relevance of prediagnostic weight loss and overweight at diagnosis in patients with colorectal cancer. Am J Clin Nutr 2016;104:1110–20. [DOI] [PubMed] [Google Scholar]
- [26].Kocarnik JM, Chan AT, Slattery ML, et al. Relationship of prediagnostic body mass index with survival after colorectal cancer: Stage-specific associations. Int J Cancer 2016;139:1065–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Laake I, Larsen IK, Selmer R, et al. Pre-diagnostic body mass index and weight change in relation to colorectal cancer survival among incident cases from a population-based cohort study. BMC Cancer 2016;16:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kaneko M, Sasaki S, Ozaki K, et al. Underweight status predicts a poor prognosis in elderly patients with colorectal cancer. Mol Clin Oncol 2016;5:289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wang N, Khankari NK, Cai H, et al. Prediagnosis body mass index and waist-hip circumference ratio in association with colorectal cancer survival. Int J Cancer 2017;140:292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lin J, Peng J, Qdaisat A, et al. Severe weight loss during preoperative chemoradiotherapy compromises survival outcome for patients with locally advanced rectal cancer. J Cancer Res Clin Oncol 2016;142:2551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Helfenstein SF, Uster A, Ruhlin M, et al. Are four simple questions able to predict weight loss in outpatients with metastatic cancer? A prospective cohort study assessing the simplified nutritional appetite questionnaire. Nutr Cancer 2016;68:743–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hoffmeister M, Bläker H, Kloor M, et al. Body mass index and microsatellite instability in colorectal cancer: a population-based study. Cancer Epidemiol Biomarkers Prev 2013;22:2303–11. [DOI] [PubMed] [Google Scholar]
- [33].Croft B, Reed M, Patrick C, et al. Diabetes, obesity, and the metabolic syndrome as prognostic factors in stages I to III colorectal cancer patients. J Gastrointest Cancer 2018. [DOI] [PubMed] [Google Scholar]
- [34].Shiao SPK, Grayson J, Yu CH, et al. Gene environment interactions and predictors of colorectal cancer in family-based, multi-ethnic groups. J Pers Med 2018;81:pii:E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Renfro LA, Loupakis F, Adams RA, et al. Body mass index is prognostic in metastatic colorectal cancer: pooled analysis of patients from first-line clinical trials in the ARCAD database. J Clin Oncol 2016;34:144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Daniel CR, Shu X, Ye Y, et al. Severe obesity prior to diagnosis limits survival in colorectal cancer patients evaluated at a large cancer centre. Br J Cancer 2016;114:103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lee DW, Han SW, Cha Y, et al. Prognostic influence of body mass index and body weight gain during adjuvant FOLFOX chemotherapy in Korean colorectal cancer patients. BMC Cancer 2015;15:690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Langella S, Russolillo N, Forchino F, et al. Impact of obesity on postoperative outcome of hepatic resection for colorectal metastases. Surgery 2015;158:1521–9. [DOI] [PubMed] [Google Scholar]
- [39].Moghadamyeghaneh Z, Hanna MH, Hwang G, et al. Outcome of preoperative weight loss in colorectal surgery. Am J Surg 2015;210:291–7. [DOI] [PubMed] [Google Scholar]
- [40].Escamilla DM, Jarrett P. The impact of weight loss on patients with cancer. Nurs Times 2016;112:20–2. [PubMed] [Google Scholar]


