Abstract
In utero exposure to marijuana may cause various short- and long-term health problems, such as stillbirth, low birth weight and decreased cognitive function. Detection of in utero marijuana exposure with a relatively new specimen type, umbilical cord tissue, can be used to plan treatment and guide social management. In this study, a liquid chromatography–tandem mass spectrometry (LC–MS-MS) assay was developed for the simultaneous identification of four cannabinoids in umbilical cord tissue, including ∆9-tetrahydrocannabinol (THC), 11-nor-9-carboxy-∆9--THC (THC-COOH), 11-hydroxy-∆9-THC (11-OH-THC) and cannabinol (CBN). Within- and between-run imprecision, accuracy, linearity, sensitivity, carryover, recovery, matrix effects and specificity were evaluated using drug-free umbilical cord tissue spiked with non-deuterated and deuterated standards. Calibration curves were reproducible and linear (r > 0.995) for all four analytes in the range of 0.2 ng/g lower limit of quantitation (LLOQ) and 30 ng/g upper limit of quantitation (ULOQ). Total imprecisions (% coefficient of variation) were 7.8% (THC), 13.3% (THC-COOH), 11.8% (11-OH-THC) and 10.6% (CBN) at low QC (n = 15, 0.25 ng/g), and were 7.2% (THC), 10.0% (THC-COOH), 9.5% (11-OH-THC) and 5.8% (CBN) at high QC (n = 15, 4 ng/g), respectively. No interfering substances were identified, and no carryover was observed. The average accuracies (N = 25) were 94–95%. The average recoveries observed for THC, THC-COOH, 11-OH-THC and CBN were 74, 82, 58 and 86%, respectively. By analyzing authentic clinical specimens that had been previously tested for cannabinoids by enzyme-linked immunoassay, positive and negative result agreements were 100 and 53.8%. In summary, the presented method can be used for the assessment of in utero exposure to four common cannabinoids.
Introduction
Marijuana is the most commonly used illicit drug in the USA (1). Its use is widespread among young people. For example, in 2015, greater than 11,000 young Americans ages 18–25 admitted to use of marijuana in the past year. In the same survey, 4.7% of pregnant women admitted to use of marijuana in the past month (2). Published studies suggest that the adverse effects of in utero exposure to cannabinoids include growth restriction, reduced length of gestation, intelligence and decreased or diminished cognitive function (3–5). Drug testing has become a useful tool to identify in utero exposure to cannabinoids and test results can be used to develop a treatment plan as well as guide social management.
There are several specimen types that have been used for detection of drug exposure in newborns (6), such as urine, amniotic fluid, hair, meconium and umbilical cord tissue. Of the specimens routinely used for drug testing, urine testing results reflect recent drug uses and thus tend to underestimate the incidence rate. Amniotic fluid also reflects a recent time frame and the sampling procedure is complicated and invasive, which could lead to adverse consequences, e.g., pregnant loss. Meconium has been used to assess in utero drug exposure including cannabinoids for more than 20 years (7–10). Meconium is the first stool from the newborn, and its formation begins from the second trimester. Although drug testing with meconium could reflect drug use during the second and third trimesters, it is generally believed that meconium drug testing results primarily reflect exposure during the third trimester of a full-term birth (11–13). Drug detection windows for meconium vary, and are very much dependent on the specific drug of interest, maternal drug use patterns, and stability of drugs and metabolites in meconium (14). Moreover, meconium is not available in some cases (10–20%) due to early passing prior to birth; particularly, seen with stressed babies in the greatest need of testing. Hair testing is thought to represent drug exposures during the last trimester as well, but hair testing is often not feasible due to wide variation in the amount of hair available to collect at birth. Therefore, alternative specimen types are needed for detection of prenatal exposure to cannabinoids as well as other licit or illicit drugs. Umbilical cord tissue is a relatively new specimen type for detection of in utero drug exposure that has the distinct advantage over meconium, of universal availability at birth (15–17). Because, drug testing can be initiated with umbilical cord tissue immediately after birth, the time required to obtain results is reduced when compared with waiting to collect meconium. Moreover, ample cord specimens are typically available for testing whereas the quantity of meconium can be limited or not available. Although several studies have reported the agreement of drug screening results between cord and meconium among certain common drug classes (15, 18), one published study that reported overall agreement for cannabinoids was poor; as low as 76% (19).
Traditional testing algorithms typically include screening and confirmation testing. Immunoassays are commonly utilized for screening due to ease of use and cost-effectiveness. Our laboratory employs an enzyme-linked immunoassay (ELISA) to screen for the presence of cannabinoids in cord (17). The antibodies used in this qualitative assay cross-react with five cannabinoids including ∆9-tetrahydrocannabinol (THC, 10%), 11-nor-9-carboxy-∆9-THC (THC-COOH, 125%), 11-hydroxy-∆9-THC (11-OH-THC, 33%), cannabinol (CBN, <0.25%) and cannabidiol (CBD, <0.25%). However, as with any immunoassay, there exists concern about the rates of false-positive or false negative results from the testing. In this study, we aimed to develop and validate a sensitive LC–MS-MS method for the simultaneous quantification of four cannabinoids, including THC, THC-COOH, CBN and 11-OH-THC, in umbilical cord tissue. In addition, 44 authentic clinical samples were analyzed by this LC–MS-MS method, and the results were compared with those obtained by ELISA.
Methods and Materials
Chemicals and reagents
Isopropanol, methanol and acetonitrile were HPLC grade and were purchased from VWR international (West Chester, PA, USA). Clinical laboratory reagent water was generated using a Barnstead Nanopure Infinity ultrapure water system (Thermo Fisher Scientific). The following reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA): formic acid, acetic acid, ammonium bicarbonate, ammonium hydroxide and sodium hydroxide. Stock standards were purchased from Cerilliant (Round Rock, Texas, USA).
Preparation of calibration standards and QC solutions
A primary stock solution containing THC, 11-OH-THC, THC-COOH and CBN at 1000 ng/mL was prepared in methanol. The stock solution was diluted to create 100 and 10 ng/mL calibrator solutions, respectively. A calibration curve was generated with the following calibrator concentrations 0.2, 0.5, 1, 5, 10, 15 and 30 ng/g in drug-free umbilical cord tissue. QC stock solutions were prepared with a different lot of each cerilliant standard except for THC-COOH; instead, THC-COOH-glucuronide was added as an indicator of hydrolysis. Blank cord samples were spiked with standard solutions at the concentrations of 0.25 and 4 ng/g, respectively. Primary stock solutions of THC-d3, THC-COOH-d3, 11-OH-THC-d3 and CBN-d3 were diluted in methanol to produce a mixed internal standard solution of 100 ng/mL. All stock and working standards were aliquoted and stored in −80°C in amber glass vials.
Drug-free sample preparation
Blank cord was evaluated by ELISA, as described previously (17). To ensure the absence of detectable cannabinoids, the ELISA absorbance of the selected residual cord samples was greater than that of negative control since the drug concentrations were indirectly proportional to the ELISA absorbance. Approximately 80 residual blank cord samples were de-identified and pooled to generate drug-free blank. Additionally, the presented LC–MS-MS method confirmed no detectable cannabinoids in the blank cord pool.
Sample extraction procedure
Each patient sample (1.0 ± 0.1 g) was sliced and weighted into a labeled 5 mL conical polypropylene tube with a screw top cap and 25 μL of deuterated internal standards were added. Next, 2.5 mL methanol and six stainless steel beads were added and each sample was homogenized using a Bead Rupter (Biotage, NC, USA) for 1.5 min. Homogenates were centrifuged for 10 min at 14,000 rpm and 4°C. Each supernatant was transferred to a 10 mL culture tube. Base hydrolysis was performed by adding 2.5 mL 0.5 M NaOH. Samples were incubated at ambient temperature (23–25°C) for 45 min. Following hydrolysis, solid phase extraction (SPE) was performed using Evolute AX SPE columns (60 mg/3 mL, Biotage), which were conditioned with 1 mL methanol and 1 mL water before sample loading. The specimens were washed with 1 mL 1% ammonium hydroxide in water and 1 mL methanol, respectively. Analytes were eluted into LC vials with 1.2 mL 2% acetic acid in methanol, and dried at 40°C under a gentle nitrogen stream with a Turbo Vap LV (Biotage, Charlotte, NC, USA) for ~15 min. Samples were reconstituted in 200 μL of water: methanol (40:60).
LC–MS-MS conditions
LC separation was performed on a Kinetex Evo C18 column (2.1 × 50 mm, 2.6 μm particle size) purchased from Phenomenex (Torrance, CA, USA). Solvent A (5 mM ammonium bicarbonate in nanopure water, pH 9.5) and solvent B (methanol) was used to develop a gradient: 60% B for 0.50 min, linear gradient from 60 B to 80% B between 0.50 and 1.25 min, hold at 80% B for 1 min, and a linear gradient from 80 B to 90% B between 2.25 and 3.25, and hold at 90% B for 0.5 min, and step from 90 B to 95% B, and hold at 95% for 0.8 min followed by re-equilibration to initial conditions (total run time 4.7 min). The LC eluent was diverted to waste for the first 0.5 min and the final 0.5 min. The LC flow rate was 0.5 mL/min. The injection volume was 50 μL. The column and autosampler were held at 28 and 4°C, respectively. MS-MS analysis were performed by AB SCIEX Triple QuadTM 5500 mass spectrometer interfaced with CTC PAL HTC-xt-DLW autosampler and Agilent 1260 infinity series binary pump, degasser and column oven. The instrument was in negative electrospray ionization mode. MS-MS parameters were optimized via direct infusion of a mixture of standards followed by a split tee injection with LC flow, shown in Table I. Data were collected in scheduled multiple reaction monitoring (MRM) mode. AB SCIEX Analyst software (version 1.6.1) was used for instrument control. Two transition ions were used to monitor each drug analyte, as shown in Table II. Ion ratios were calculated by dividing peak area of qualifier ion by peak area of quantifier ion. Quantitation was performed using MultiQuant (AB Sciex, Foster City, CA) with the MQ4 algorithm. Calibrators and controls were carried through the same processes as the specimens being tested. Calibration curves were constructed via linear least-squares regression with 1/× weighting factor.
Table I.
Mass spectrometry parameters
| MS-MS parameters | |
|---|---|
| Instrument mode | Negative |
| Curtain Gas (psi) | 30 |
| Collision Gas (psi) | 10 |
| IonSpray Voltage | −4000 |
| Temperature (°C) | 550 |
| Nebulizer Gas (psi) | 25 |
| Heater Gas (psi) | 30 |
Table II.
Drug testing components, cutoff concentrations, MRM transitions, RT and internal standards
| Non-deuterated analyte/deuterated internal standard | RT (min) | MRM transitions for non-deuterated analyte | MRM transitions for deuterated analyte | Cutoff (ng/g) |
|---|---|---|---|---|
| THC/ THC-d3 | 3.2 | 313.10 to 245.1 | 316.0 to 248.0 | 0.2 |
| 313.10 to 191.1 | 316.0 to 194.0 | |||
| THC-COOH/ THC-COOH-d3 | 1.4 | 343.1 to 244.9 | 346.0 to 248.2 | 0.2 |
| 343.1 to 191.0 | 346.0 to 194.0 | |||
| 11-OH-THC/11-OH-THC-d3 | 2.1 | 329.1 to 268.1 | 332.1 to 271.0 | 0.2 |
| 329.1 to 173.3 | 332.1 to 173.0 | |||
| CBN/CBN-d3 | 3.0 | 309.3 to 222.1 | 312.0 to 282.0 | 0.2 |
| 309.3 to 171.0 | 312.0 to 171.0 |
Method validation
Validation experiments were carried out as previously described (20). Within- and between-run precision, accuracy, linearity, sensitivity, carryover, analytical recovery, matrix effect, hydrolysis efficiency and on-board stability of extracts were evaluated using drug-free blank umbilical cord spiked with non-deuterated and deuterated standards.
“Within-run, between-run and total imprecisions” of the assay were evaluated by extracting samples along the analytical measurement range in triplicate in three different batches. The linearity of the assay was evaluated by a seven-point calibration curve for 5 days. Samples were prepared with drug-free umbilical cord spiked with an appropriate concentration of the analytes. “The limit of detection (LOD)” was determined by analyzing samples in decreasing concentrations in duplicate for two days until a signal to noise (S/N) ≥3 and acceptable quantitative results were observed. “The lower limit of quantitation (LLOQ)” was the lowest concentration that has S/N ≥ 10 as well as all qualitative parameters met acceptable criteria: acceptable chromatography, retention time (RT) within ± 2% of the target RT, all monitored ions present and all ion mass ratios (calculated by dividing the area of quantifier ions by the area of qualifier ions) within ± 30% of the mean ion mass ratios of calibrators, and at which all samples were accurate within 85–115% of the target concentrations. “The ULOQ” was the highest concentration at which all samples were accurate within 85–115% of the target concentrations.
“The extraction recovery” was evaluated by analyzing the same samples with standards added before and after SPE extraction. The recovery was calculated based on the difference in the area counts between the pre-extracted and post-extracted samples. “Hydrolysis efficiency” was calculated for THC-COOH-glucuronide using a control spiked at 1.51 times the target concentration due to mass differences between glucuronidated and free drugs. “Mass spectrometry ion suppression” was evaluated by injecting negative cord sample extract while infusing a solution containing each analyte of interest at 100 ng/g. “Carryover studies” were performed to assess (i) contamination carryover due to a contaminated syringe from a previous high specimen to a following specimen and (ii) septum/injection port/column carryover from a high specimen to a following specimen by extracting a low and a high concentration spike. High concentration spikes outside of the analytical measurement range (2 × ULOQ) were prepared to ensure carryover adequately assessed. The low spikes used were near the low end of the AMR. Internal standard counts within a range of 50–200% of average of the internal standards across the run. “On-board stability” of the extracted analytes was evaluated by re-analysis of a control set every 24 h for 7 days, and the concentration and area counts at each time point was compared to the initial concentration. The ratios of the results were calculated. EP Evaluator software, Multiquant, and Excel were used for data analysis.
Authentic clinical specimens
Forty-four residual umbilical cord tissue specimens were selected for analysis by LC–MS-MS based on results obtained by the ELISA (Immunalysis, Pomona, CA, USA) routinely used in our laboratory (17). The cutoff for the ELISA is based on signal generated with a 1 ng/g THC-COOH calibrator. Quality control (QC) samples prepared at 50 and 150% of the calibrator are also analyzed with patient samples. The residual specimens were classified into three groups based on ELISA results: high positive (HP, ≥150% calibrator, n = 9), positive (P, 100–150% calibrator, n = 9), negative (Neg, ≤100% calibrator, n = 26). The concentrations of four cannabinoids in authentic specimens were analyzed by the LC–MS-MS method described above. The data were analyzed in MultiQuantTM 3.0.2 (AB Sciex, Foster City, CA) and imported into Excel Spreadsheets (Microsoft) and EP Evaluator (Data Innovations, Burlington, USA) to perform analysis.
Results and Discussion
Umbilical cord is a relatively new specimen type for in utero drug exposure. Currently, very few commercial laboratories are able to offer a definitive/quantitative assay for detection of in utero exposure to cannabinoids. In this study, we presented a method for the simultaneous quantitation of THC, THC-COOH, 11-OH-THC and CBN in umbilical cord tissue. Compared to a previously published study that presented methods for identification of one cannabinoid (i.e., THC-COOH) in cord, the presented method was able to identify three additional cannabinoids in cord tissue. Since umbilical cord tissue is a relatively new specimen type, the cannabinoid deposition patterns in cord have not been well-characterized. Although, THC-COOH has been reported to be the predominant metabolite in meconium, we do not know whether it would be the same case in umbilical cord tissue. We believe the additional drug testing information could improve the detection sensitivity of in utero exposure to cannabinoids as well as help us to understand their deposition patterns.
Table III detailed the results of accuracy, within- and between-run imprecisions, and extraction recoveries of THC, 11-OH-THC, THC-COOH and CBN. Within-run (n = 15) and between-run (n = 15) imprecisions at low control (n = 15, 0.25 ng/g) ranged from 7 to 10% and 4 to 9%, respectively, while, at high control (n = 15, 4 ng/g), within- and between-run precisions ranged from 4–6% and 1–5%, respectively. The average recoveries observed for THC, THC-COOH, 11-OH-THC and CBN were 74, 82, 58 and 86%, respectively. The presented method was able to achieve higher recoveries (58–86%) compared to the previously published study that reported 34–73% recoveries for cannabinoids in urine (21), while our results were found to be similar to the recoveries for cannabinoids in oral fluid (75.9–86.1%) reported by Huestis et al. (22) as well as those in plasma (61.3–91.6%) reported by Andrenyak et al. (20). The sample loss during the extraction process typically results from the complexity of specimen extraction procedure, and hydrophobicity of analytes; particularly, hydrophobic analytes tend to adhere to collagen of cord tissue as well as HPLC vials during the sample handling process. Nevertheless, the presented method was optimized to achieve good recoveries for all four analytes and thus allowed us to achieve LLOQs for cannabinoid analytes. Using THC-COOH-glucuronide as a control, base hydrolysis efficiency was found to be 100%. Short-term on-board stability of THC, 11-OH-THC, THC-COOH and CBN were evaluated using three concentrations (i.e., 0.2, 0.5 and 1 ng/g). No loss was found in the samples maintained at 4°C in the autosampler for 72 h.
Table III.
Validation results for THC, THC-COOH, 11-OH-THC and CBN in umbilical cord by LC–MS-MS
| Analyte | Average Accuracy (N = 25) (%) | Imprecision (%CV) | Recovery | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low (N = 15) 0.25 ng/g | High (N = 15) 4.0 ng/g | Low (N = 9) 0.25 ng/g (%) | High (N = 9) 4.0 ng/g (%) | Average (%) | ||||||
| Total (%) | Between run (%) | Within-run (%) | Total (%) | Between run (%) | Within-run (%) | |||||
| THC | 94.1 | 7.8 | 8.1 | 7.2 | 7.2 | 0.9 | 5.7 | 73 | 74 | 74 |
| THC-COOH | 94.5 | 13.3 | 5.6 | 8.7 | 10.0 | 3.6 | 6.3 | 83 | 80 | 82 |
| 11-OH-THC | 94.5 | 11.8 | 9.3 | 10.0 | 9.5 | 5.1 | 3.8 | 59 | 57 | 58 |
| CBN | 95.2 | 10.6 | 3.8 | 7.8 | 5.8 | 3.8 | 4.6 | 83 | 89 | 86 |
Linear ranges were 0.2–5 ng/g for THC, 11-OH-THC, THC-COOH and CBN (r > 0.995). The LODs (LLOQs) were established at 0.1 ng/g (0.2 ng/g) for THC, 11-OH-THC, THC-COOH and CBN. No interfering substances were identified, and no carryover was observed immediately after a sample containing 60 ng/g of each analyte.
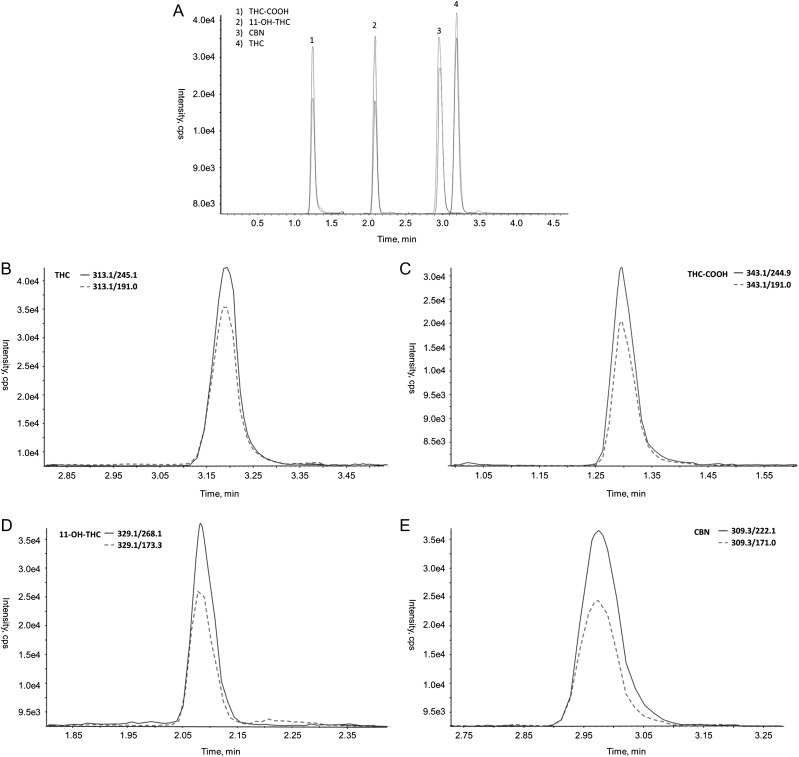
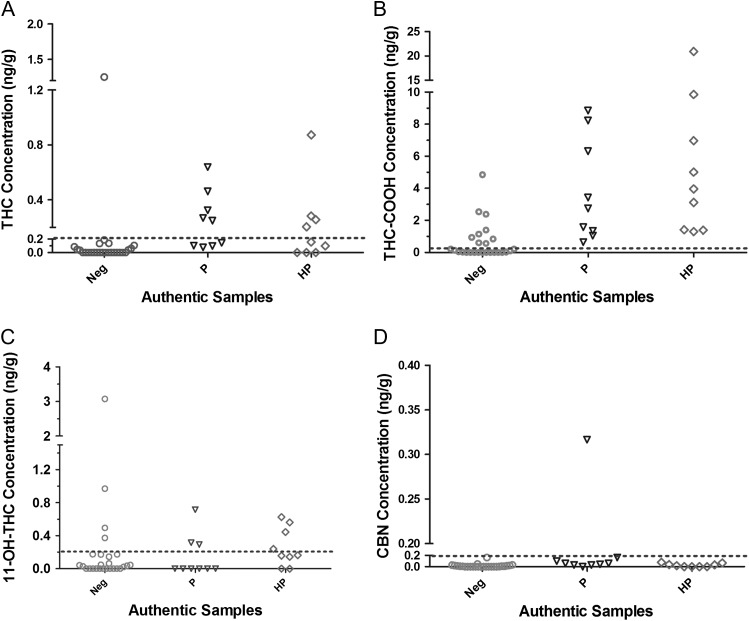
Figure 1 showed representative extracted ion chromatograms (EIC) of the four analytes. Figure 2 showed the concentration distribution of four analytes quantified by LC–MS-MS method in each group of authentic clinical samples (i.e., HP, positive and negative). The results were summarized in the Supplementary Table 1. EP evaluator identified that the positive result agreement was 100% and the negative agreement was 53.8% (shown in Table IV). About 18 ELISA positive specimens were found to be positive by LC–MS-MS. Within the HP group, THC-COOH exhibited the highest concentrations ranging from 1.3 to 20.9 ng/g. For positive (P) specimens (ELISA results between 100 and 150% calibrators), one specimen was positive for all four analytes. THC-COOH showed the highest concentrations ranging from 0.6 to 8.9 ng/g. Among negative specimens identified by ELISA (<100% calibrator), we found that the concentrations of at least one cannabinoid (s) in 12 out of 26 specimens (46.2%) were greater than the LC–MS-MS cutoffs (0.2 ng/g). THC-COOH concentrations in 4 out of 26 ELISA negative specimens were found greater than 1 ng/g by LC–MS-MS; 11-OH-THC in 4 out of 26 ELISA negative specimens were found above the LC–MS-MS cutoff, and THC concentration in one sample was 1.25 ng/g. After lowering the ELISA cutoff to 0.5 ng/g, the negative result agreement was found to increase to 78% while the positive result agreement still was 100%. The discrepancies between these two methods might be attributed to the different sample handling processes, i.e., hydrolysis used for the presented method but not ELISA, as well as different cutoffs used (1 ng/g vs.0.2 ng/g). Of note, because antibodies used in the ELISA cross-react with five cannabinoids, a higher cutoff typically is used for the ELISA than that for LC–MS-MS method as LC–MS-MS can detect individual analytes. Additionally, using the ELISA on hydrolyzed specimens might provide some knowledge on the causes of discrepancies between two results. But this is out of the range of this study. In summary, the presented LC–MS-MS method increases positivity rates compared to ELISA.
Figure 1.
(A) Representative LC separation and extracted ion chromatograms of (B) THC, (C) THC-COOH, (D) 11-OH-THC and (E) CBN at 4 ng/g.
Figure 2.
Concentration distribution of (A) THC, (B) THC-COOH, (C) 11-OH-THC and (D) CBN in authentic samples. Neg, negative tests by ELISA; P, positive tests by ELISA; HP, high positive tests by ELISA. ‘---’ indicates cutoffs used in the LC–MS-MS method.
Table IV.
Comparison of LC–MS-MS and ELISA methods
| ELISA (cutoff 1 ng/g) | ||||
|---|---|---|---|---|
| Negative | Positive | Total | ||
| (LC–MS-MS) (cutoff 0.2 ng/g) | Negative | 14 | 0 | 14 |
| Positive | 12 | 18 | 30 | |
| Total | 26 | 18 | 44 | |
| Agreement | 53.8% | 100% | ||
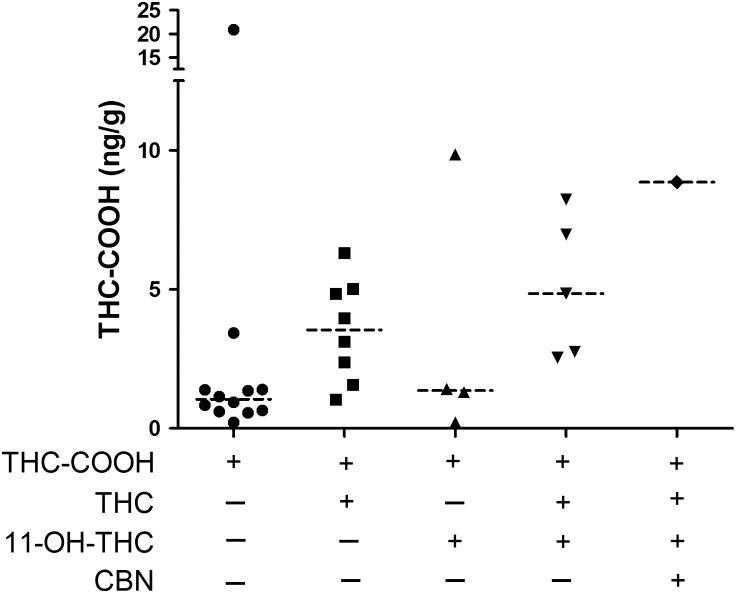
Figure 3 showed the concentration distribution and medians of THC-COOH observed in cord by LC–MS-MS. Of the note, 30 out of 44 authentic samples were found to be positive with at least one analyte above LC–MS-MS cutoff. Among the 30 positive samples, 46% of samples (n = 14) were positive for THC-COOH only with concentrations ranging from 0.21 to 20.9 ng/g, and 27% of samples (n = 6) were positive for THC-COOH and THC with concentration ranging from 1.5 to 6.3 ng/g and 0.2 to 1.25 ng/g, respectively; 16.7% of samples (n = 5) were positive for THC-COOH and 11-OH-THC with concentrations ranging from 0.2 to 9.9 ng/g and 0.2 to 1.0 ng/g, respectively; We found 4 out of 30 samples were positive for THC, THC-COOH and 11-OH-THC together, and the concentrations of THC, THC-COOH and 11-OH-THC ranged from 0.3 to 1.3 ng/g, 2.8 to 8.2 ng/g and 0.3 to 3.1 ng/g, respectively; We only identified one sample that was positive for all four analytes. Our results showed that THC-COOH is the predominant metabolite in cord.
Figure 3.
The cannabinoid patterns in cord detected by LC–MS-MS. ‘---‘ indicates median.
There are limitations in this study. First, the maternal patterns of drug use and specific products used were unknown, nor were the outcomes of the associated children. As such, the cutoff concentration required to detect newborns at-risk of drug-related adverse outcomes is not known. Second, we were not able to detect CBD in umbilical cord tissues using the presented method. We found that the methods described were not suitable for the determination of CBD in the umbilical cord tissue since the recovery for CBD was low (<10%). Therefore, a separate method might be necessary for the analysis of CBD since CBD is a major cannabinoid in medical marijuana preparation and is likely present in umbilical cord.
Conclusion
In this study, we developed and validated a sensitive LC–MS-MS method for simultaneous quantification of THC, 11-OH-THC, THC-COOH and CBN in umbilical cord tissues. The presented method was acceptable for the assessment of in utero exposure to cannabinoids providing quantitative results for four cannabinoids.
Supplementary Material
Supplementary data
Supplementary data are available at Journal of Analytical Toxicology online.
Funding
This work was supported by the ARUP Institute for Clinical and Experimental Pathology. Dr Metz was supported by the National Institute on Child Health and Human Development under Award Number 5K12HD001271-18. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1. Huestis M.A., Choo R.E. (2002) Drug abuse’s smallest victims: In utero drug exposure. Forensic Science International, 128, 20–30. [DOI] [PubMed] [Google Scholar]
- 2.Substance abusecenter for behavioral health statistics and quality. Results from the 2015 national survey on drug use and health: Detailed tables SAMHSA https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf Published September 8, 2016. (accessed Jan 18, 2017).
- 3. Sithisarn T., Granger D.T., Bada H.S. (2012) Consequences of prenatal substance use. International Journal of Adolescent Medicine and Health, 24, 105–112. [DOI] [PubMed] [Google Scholar]
- 4. Jutras-Aswad D., DiNieri J.A., Harkany T., Hurd Y.L. (2009) Neurobiological consequences of maternal cannabis on human fetal development and its neuropsychiatric outcome. European Archives of Psychiatry and Clinical Neuroscience, 259, 395–412. [DOI] [PubMed] [Google Scholar]
- 5. Behnke M., Smith V.C.. Committee on Substance, A., Committee on, F. and Newborn. (2013) Prenatal substance abuse: Short- and long-term effects on the exposed fetus. Pediatrics, 131, e1009–e1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Narkowicz S., Plotka J., Polkowska Z., Biziuk M., Namiesnik J. (2013) Prenatal exposure to substance of abuse: A worldwide problem. Environment International, 54, 141–163. [DOI] [PubMed] [Google Scholar]
- 7. McMillin G.A., Wood K.E., Strathmann F.G., Krasowski M.D. (2015) Patterns of drugs and drug metabolites observed in meconium: What do they mean? Therapeutic Drug Monitoring, 37, 568–580. [DOI] [PubMed] [Google Scholar]
- 8. Tynon M., Porto M., Logan B.K. (2015) Simplified analysis of 11-hydroxy-delta-9-tetrahydrocannabinol and 11-carboxy-delta-9-tetrahydrocannabinol in human meconium: Method development and validation. Journal of Analytical Toxicology, 39, 35–40. [DOI] [PubMed] [Google Scholar]
- 9. ElSohly M.A., Feng S. (1998) Delta 9-thc metabolites in meconium: Identification of 11-oh-delta 9-thc, 8 beta,11-dioh-delta 9-thc, and 11-nor-delta 9-thc-9-cooh as major metabolites of delta 9-thc. Journal of Analytical Toxicology, 22, 329–335. [DOI] [PubMed] [Google Scholar]
- 10. Ostrea E.M. Jr., Brady M., Gause S., Raymundo A.L., Stevens M. (1992) Drug screening of newborns by meconium analysis: A large-scale, prospective, epidemiologic study. Pediatrics, 89, 107–113. [PubMed] [Google Scholar]
- 11. Moore C., Negrusz A., Lewis D. (1998) Determination of drugs of abuse in meconium. Journal of Chromatography. B, Biomedical Sciences and Applications, 713, 137–146. [DOI] [PubMed] [Google Scholar]
- 12. Moriya F., Chan K.M., Noguchi T.T., Wu P.Y. (1994) Testing for drugs of abuse in meconium of newborn infants. Journal of Analytical Toxicology, 18, 41–45. [DOI] [PubMed] [Google Scholar]
- 13. Ostrea E.M., Jr. (2001) Understanding drug testing in the neonate and the role of meconium analysis. The Journal of Perinatal & Neonatal Nursing, 14, 61–82. quiz 105-106. [DOI] [PubMed] [Google Scholar]
- 14. Wu F., Marin S.J., McMillin G.A. (2017) Stability of 21 cocaine, opioid and benzodiazepine drug analytes in spiked meconium at three temperatures. Journal of Analytical Toxicology, 41, 196–204. [DOI] [PubMed] [Google Scholar]
- 15. Concheiro M., Jones H.E., Johnson R.E., Choo R., Shakleya D.M., Huestis M.A. (2010) Umbilical cord monitoring of in utero drug exposure to buprenorphine and correlation with maternal dose and neonatal outcomes. Journal of Analytical Toxicology, 34, 498–505. [DOI] [PubMed] [Google Scholar]
- 16. Haglock-Adler C.J., McMillin G.A., Strathmann F.G. (2016) Development of a liquid chromatography-tandem mass spectrometry method to address the increased utilization of umbilical cord in the assessment of in utero drug exposure. Clinical Bochemistry, 49, 1092–1095. [DOI] [PubMed] [Google Scholar]
- 17. Chittamma A., Marin S.J., Williams J.A., Clark C., McMillin G.A. (2013) Detection of in utero marijuana exposure by gc-ms, ultra-sensitive elisa and lc-tof-ms using umbilical cord tissue. Journal of Analytical Toxicology, 37, 391–394. [DOI] [PubMed] [Google Scholar]
- 18. de Castro A., Jones H.E., Johnson R.E., Gray T.R., Shakleya D.M., Huestis M.A. (2011) Methadone, cocaine, opiates, and metabolite disposition in umbilical cord and correlations to maternal methadone dose and neonatal outcomes. Therapeutic Drug Monitoring, 33, 443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Colby J.M. (2017) Comparison of umbilical cord tissue and meconium for the confirmation of in utero drug exposure. Clinical biochemistry. 10.1016/j.clinbiochem.2017.03.006.. [DOI] [PubMed] [Google Scholar]
- 20. Andrenyak D.M., Moody D.E., Slawson M.H., O’Leary D.S., Haney M. (2017) Determination of -9-tetrahydrocannabinol (thc), 11-hydroxy-thc, 11-nor-9-carboxy-thc and cannabidiol in human plasma using gas chromatography-tandem mass spectrometry. Journal of Analytical Toxicology, 41, 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Scheidweiler K.B., Desrosiers N.A., Huestis M.A. (2012) Simultaneous quantification of free and glucuronidated cannabinoids in human urine by liquid chromatography tandem mass spectrometry. Clinica Chimica Acta; International Journal of Clinical Chemistry, 413, 1839–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Desrosiers N.A., Scheidweiler K.B., Huestis M.A. (2015) Quantification of six cannabinoids and metabolites in oral fluid by liquid chromatography-tandem mass spectrometry. Drug Testing and Analysis, 7, 684–694. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.