Abstract
Representative data on the prevalence of hypertension, a major non-infectious comorbidity in human immunodeficiency virus (HIV)-infected people, is lacking. We assessed the prevalence, awareness, treatment, and control, as well as determinants of hypertension in HIV-infected adults in South Africa.
A cross-sectional survey was conducted between March 2014 and February 2015 in a random sample of 827 adults (77.7% women), receiving care for HIV infection at 17 randomly selected public health facilities across the Western Cape Province, South Africa.
Participants’ mean age was 38.4 years overall, 41.1 years in men and 37.7 years in women (P < .001). The median diagnosed duration of HIV infection, similar in men and women, was 5 years, while the median CD4 count was 381 cell/mm3. Age-standardized prevalence, awareness, treatment, and control of hypertension was 38.6% (95% CI: 34.3–42.9), 46.3% (37.7–54.9), 76.4% (61.1–91.7), and 81.1% (62.9–99.3) in the overall sample; 40.0% (30.0–50.0), 36.3% (17.6–55.0), 84.8% (38.3–131.3), and 87.0% (38.2–135.8) in men; and 37.7% (32.9–42.5), 48.9% (38.9–58.9), 75.8% (59.1–92.5), and 81.3% (61.1–101.5) in women. Age and education were weakly associated with prevalent hypertension, while CD4 count and diagnosed duration of HIV infection were unrelated to prevalent hypertension.
Similar to reports in the general population in this and other countries in the region, hypertension is frequent in young South Africans receiving care for HIV infection, with similar diagnostic and treatment gaps. Integrating HIV and non-communicable disease (NCD) prevention and care will, at least in part, reduce missed opportunities for implementing NCD prevention in HIV-infected people in care.
Keywords: blood pressure, HIV infection, hypertension, South Africa
1. Introduction
According to the World Health Organization (WHO) Global Status Report, the worldwide prevalence of hypertension, a leading risk factor for cardiovascular disease (CVD), was 22% in 2014.[1] Notably, at 30%, the prevalence was highest in the Africa region, which is also experiencing a high infectious disease burden, particularly human immunodeficiency virus (HIV) infection. Africa is home to the largest number of HIV-infected individuals globally[2]; therefore, hypertension in HIV-infected populations will likely contribute substantially to the hypertension burden in the region. However, prevalence studies on hypertension in HIV-infected Africans have been conducted mainly in non-randomly selected small samples drawn from single healthcare facilities.[3] Therefore, this suggests that the prevalence of hypertension in HIV-infected populations has not been well-established. This is likely reflected in the wide hypertension prevalence range of 8.7% to 45.9% in low- and middle-income African countries as reported in a systematic review that included 13 African studies, of which 4 were South African.[3]
In view of the overall high burden of both hypertension and HIV infection in Africa, it is crucial to determine the hypertension prevalence in HIV-infected populations on the continent so as to optimize healthcare delivery to this vulnerable group. This is particularly pertinent today with the widespread implementation of antiretroviral therapy (ART), which has significantly prolonged the lifespans of HIV-infected individuals[4,5] to the extent that these are now comparable with the general population.[6,7] As a result, similar to the latter group, HIV-infected individuals are now exposed to diseases of ageing. These include CVDs and other non-communicable diseases (NCDs), and their risk factors such as hypertension.
With an estimated 6.3 million inhabitants infected with HIV in 2013, South Africa bears the greatest burden of this disease worldwide.[2] Approximately half of this HIV-infected population is receiving ART and has access to healthcare services. Consequently, these regular healthcare facility attenders are ideally positioned to receive optimal care for hypertension, a condition globally notorious for inadequate diagnosis, treatment, and control.[8] However, a parallel healthcare system exists in South Africa for the treatment of HIV infection and little is known about the overlap in management for infectious and NCDs, particularly hypertension, in those receiving ART. Such information is required to appropriately allocate resources, and develop cost-effective therapeutic strategies and programmes for combining the management of infectious and NCDs in those receiving ART. The current study aimed to determine the prevalence, awareness, treatment, and control of hypertension, and to identify the associations, if any, of HIV-related factors with hypertension in HIV-infected patients receiving treatment across multiple public healthcare facilities in the Western Cape Province of South Africa.
2. Methods
2.1. Study design and sampling
A cross-sectional survey was conducted from March 2014 to February 2015 in a random sample of HIV-infected adults, aged 18 years and older, receiving ongoing care at public healthcare facilities across the Western Cape Province in South Africa. The sample frame comprised healthcare facilities that provided ART to at least 325 HIV-infected patients per month to ensure the recruitment of an adequate number of participants within a reasonable time period. From the 62 public healthcare facilities providing ART in the Western Cape (42 in Cape Town and 20 in the surrounding rural municipalities), 17 sites were randomly selected for inclusion in this study. At each participating site, 15 to 60 patients were randomly sampled.
2.2. Collection of data
A team of trained nurses and fieldworkers collected data by administering questionnaires and conducting clinical measurements. Data were captured electronically on personal digital assistants (PDAs) with built-in checks for quality control. These were encrypted at the point of collection and sent via mobile connection to a dedicated server, where it was further checked, downloaded, and stored for future use.
2.3. Interviews
The administered questionnaire was adapted from the WHO Stepwise approach to Surveillance (STEPS) tool. Data collected included socio-demographic details, medical history, duration of diagnosed HIV infection, and cluster of differentiation 4 (CD4) counts were obtained from clinical charts. Information pertaining to HIV treatment was obtained by capturing medications brought in by the participants.
2.4. Physical examination
Anthropometric parameters including height, weight and waist circumference (WC) were measured using standardized techniques. Height was measured to the nearest millimeter using a Leicester Height Scale (Seca, United Kingdom) with participants barefoot and in the upright position. Weight was measured to the nearest gram using A&D Personal Scale (Model UC-321, Japan) with participants in light clothes and without shoes. WC, recorded to the nearest millimeter, was taken at the level of umbilicus. After participants were seated in a resting position for at least 5 minutes, blood pressure (BP) was measured in mmHg on the right arm, using a digital automatic BP monitor (Omron, M6 Comfort, the Netherlands); 3 BP measurements were taken 3 minutes apart and average of the second and third BP measurements was used in the final analysis.
2.5. Definitions and calculations
Hypertension was defined as BP ≥140/90 mmHg or self-reported history of health professional diagnosed hypertension, irrespective of treatment status.[9] The proportion of participants aware of a hypertension diagnosis was determined among those with hypertension, while the proportion receiving treatment was calculated among aware hypertensive participants. Controlled hypertension was defined as BP <140/90 mmHg in those on treatment. Problematic alcohol intake was classified as the consumption of ≥5 standard alcoholic drinks for men and ≥4 standard alcoholic drinks for women in one sitting within the previous 30 days. A standard alcoholic drink is categorized as equivalent to 1 can (340 mL) of beer, 1 glass (125 mL) wine, or 1 shot (25 mL) of spirits.
2.6. Statistical analysis
The Statistical Package for Social Sciences (IBM SSPS Inc, Chicago, IL) V.23.0 software was used for the data analyses. Data are summarized as count and percentages for categorical variables and mean (standard deviation [SD]) for continuous variables. Age-standardized prevalence was based on the age distribution of people with HIV infection in the South African mid-year population estimates for 2015.[10] Direct standardization methods were applied.[11] Analysis of the variance (ANOVA), chi-square tests, and equivalents were used as appropriate for group comparisons. Logistic regression models were used to assess the association between selected characteristics and prevalent hypertension, and separately for the outcome of “any hypertension,” as well as “screen-detected hypertension” among participants with no prior history of diagnosed hypertension. A basic model was adjusted for age, sex, and recruitment center, while expanded multivariable models for the outcome of “screen-detected hypertension” further adjusted for significant predictors in basic models. A P-value <.05 was used to identify statistically significant results.
Permission to conduct the survey was obtained from the Health Research Office of the Western Cape Department of Health, and the relevant healthcare facilities. The study was approved by the South African Medical Research Council Ethics Committee and conducted in accordance with the principles of the Declaration of Helsinki.
3. Results
3.1. General characteristics of the study sample
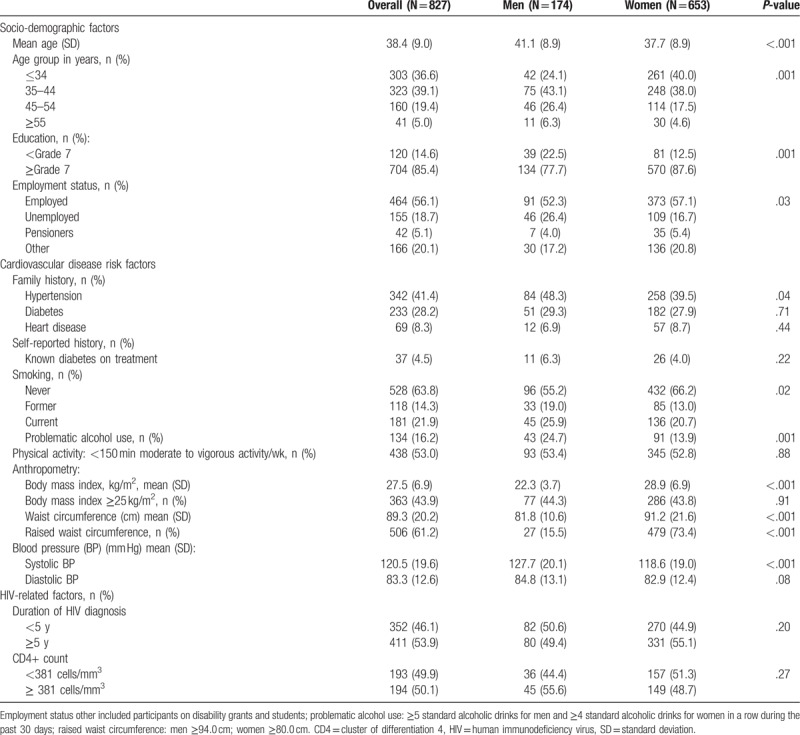
Of the 833 randomly selected HIV positive patients, participants whose questionnaires were incomplete and those with missing data on BP levels were excluded. Therefore, the final analytic sample comprised 827 participants (77.7% women). The general characteristics of the participants are presented in Table 1. The mean age (standard deviation [SD]) was 38.4 (9.0) years overall, 41.1 (8.9) years in men and 37.7 (8.9) years in women; P < .001. The majority of participants (85.4%) had ≥7 years of education and over half were employed (56.1%) with significant sex differences (both P ≤ .03). Men compared with women were more likely to be current smokers (P = .02), problematic alcohol users (P = .001), and have a family history of hypertension (P = .04). Levels of physical activity did not differ between men and women (P = .88), and neither did family history of diabetes and heart diseases (both P ≥ .44). Mean body mass index and WC were significantly higher in women (28.9 kg/m2 [SD 93.7] and 91.3 cm [SD 21.7]) than in men (22.3 kg/m2 [SD 6.9] and 81.8 cm [SD 10.6]); both P < .001. Overall, the proportion of overweight/obese participants was 43.9%, with no difference between men and women (44.3% vs 43.8%, P = .91).
Table 1.
Socio-demographic, cardiovascular disease risk, and HIV-related factors presented by sex.
3.2. Profile of HIV infection
The median duration of diagnosed HIV infection was 5 years overall, with no significant difference in the distribution of men and women above and below this median (P = .20). Likewise, the median CD4 count was 381 cells/mm3, again, with no significant sex difference in the distribution of participants above and below this median (P = .27), Table 1.
3.3. Blood pressure profile
Mean (SD) systolic BP was 120.5 mmHg (19.6) in the overall sample and significantly higher in men than in women at 127.7 mmHg (20.1) versus 118.7 mmHg (19.0) (P < .001). Mean diastolic BP was 83.3 mmHg (13.6) overall and similar in men (84.8 mmHg [13.01]) and women (82.9 mmHg [12.4]), (P = .08) (Table 1). In the overall sample, mean systolic BP increased progressively with age while mean diastolic BP, in contrast, initially increased with age but plateaued between 35 to 44 years and 45 to 54 years of age, and declined slightly thereafter (Fig. 1).
Figure 1.
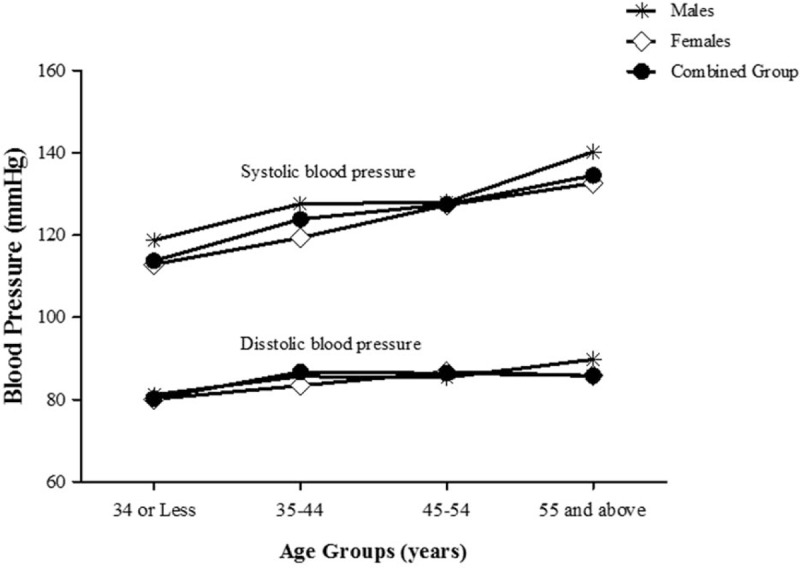
Mean systolic and diastolic blood pressures presented by age group and sex.
3.4. Prevalence, awareness, treatment, and control of hypertension
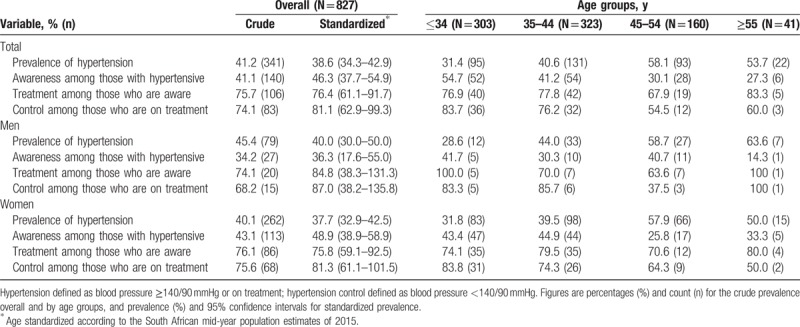
The prevalence, awareness, treatment, and control of hypertension are summarized in Table 2. The crude prevalence of hypertension was 41.2% overall, 45.4% in men, and 40.1% in women (P = .21). The age-standardized prevalence (95% confidence interval [CI]) was 38.6% (95% CI: 34.3–42.9) overall, 40.0% (95% CI: 30.0–50.0) in men and 37.7% (95%CI: 32.9–42.5) in women. The crude prevalence of hypertension increased with older age from 31.4% in participants ≤34 years old to 53.5% in those ≥55 years of age in the overall sample. Among participants with hypertension, 41.1% overall, 34.2% of men and 43.1% of women (P = .16) were aware of their hypertensive status. The age-standardized awareness rate was 46.3% overall, 36.3% in men and 48.9% in women. Of the patients who were aware of their hypertensive status, 75.7% overall, 74.1% in men and 76.1% in women, were on BP lowering medications. Among those receiving anti-hypertensive treatment, 74.1% overall (68.2% of men and 75.6% of women) were at target BP levels, Table 2.
Table 2.
Hypertension prevalence, awareness, treatment, and control presented by age and sex categories.
3.5. Prevalence of hypertension by HIV specific characteristics
The prevalence of hypertension in participants below the median CD4 count (381 cells/mm3) was 34.2% versus 43.3% in those at or above the median (P = .07). The prevalence of hypertension in participants with median duration of diagnosed HIV infection of less compared with >5 years of infection was 41.2% versus 40.9%; P = .80.
3.6. Comparative profile of participants by hypertension status and determinants of hypertension
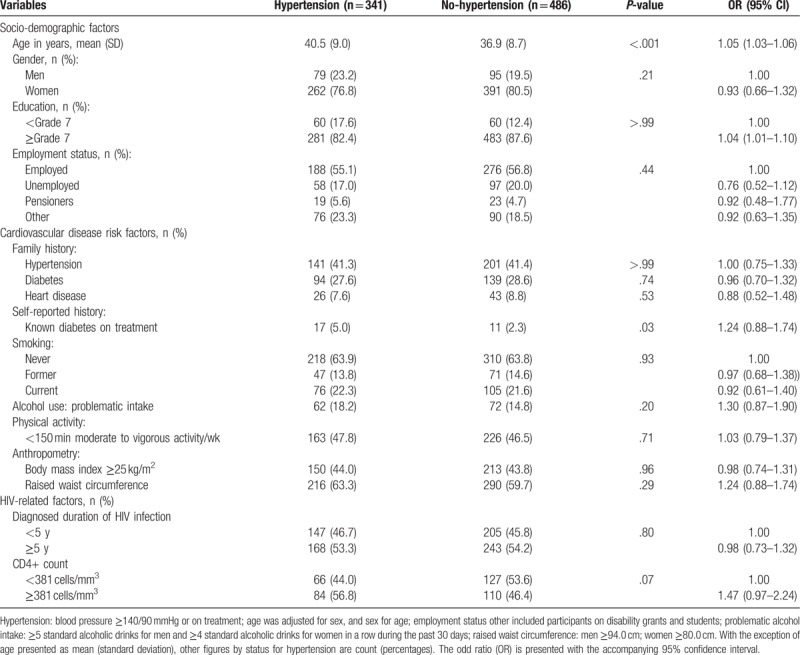
The profile of participants with and without hypertension is shown in Table 3. Participants with hypertension were older (P < .001), more likely to be known diabetics (P = .03), and to have higher CD4 count (P = .07). None of the other characteristics considered varied significantly between hypertensive and non-hypertensive participants. In an age, sex, and center adjusted logistic regression models a significant association was apparent between increasing age and any hypertension (odds ratio [OR] per year older age 1.05 [95% CI: 1.03–1.06]), and between higher education and prevalent hypertension (1.04; 1.01–1.10).
Table 3.
Socio-demographic, cardiovascular risk, and HIV-related factors presented by hypertension status and adjusted for age and sex.
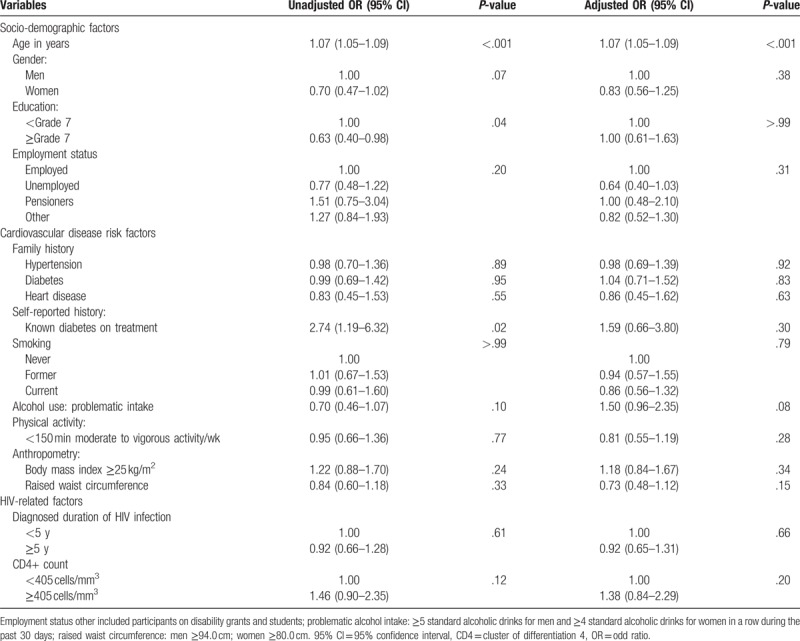
In similar logistic regression models in the subsample of participants short of those previously diagnosed with hypertension a significant association was apparent between age and screen-detected hypertension, with an odd ratio (OR) of 1.07 (95% CI: 1.05–1.09) per year older age, Table 4. No other characteristic was associated with screen-detected hypertension in this subsample.
Table 4.
Odd ratios (OR) and 95% confidence intervals (95% CI) for predictors of unknown hypertension.
4. Discussion
This study is to first attempt to provide generalizable data on the burden of hypertension in HIV-infected South Africans in care for HIV infection, through a random selection of participants across multiple public HIV care facilities. Our study broadly extends to the young population of HIV-infected people, observations already made on BP levels, and hypertension in the general population across Africa and beyond. For instance, average BP levels increased with older age up to the age of 45 to 55 years where the trajectory of systolic blood pressure (SBP) became steeper while that for diastolic BP rather flattened between age 35 and 45 years with a slight decline thereafter. Nearly 2 in 5 participants had prevalent hypertension (similar in men and women), with less than half of them being aware of their condition at the time of the survey. The majority of those previously diagnosed with hypertension were on treatment, with about 2 in 3 treated patients being at target control BP levels. The profile of hypertension was unaffected by HIV predictive characteristics such as diagnosed duration of the infection and ART use, while it was correlated with traditional risk factors for hypertension such as increasing age. Altogether, our findings highlight the missed opportunity of optimally coaddressing comorbid hypertension in this population already in regular contact with the health system.
A systematic review of published studies on prevalent hypertension in people with HIV infection reported prevalence rates ranging from 8% to 45.9% across 13 studies in Africa, and 19.1% to 40.9% across 3 South African studies.[3] But these studies provided essentially crude prevalence rates from participants non-randomly selected and generally from a single health institution. Findings and shortcomings of African studies were generally shared with studies from other regions around the world,[3] as well as subsequently published studies.[12,13] The age-standardized prevalence rate of hypertension of 31.4% in our study is within the range of the pooled estimates from a recent meta-analysis of hypertension prevalence in sub-Saharan Africa.[14] The similarity of BP profile across age in people with HIV infection and the general population,[15] as well as the non-apparent effect of HIV-predictive characteristics on hypertension prevalence in our sample, would support factors operating in the general population as the main drivers of hypertension risk in people with HIV infection.
The drivers of hypertension in the context of HIV in general, are still to be fully elucidated, with current suggestion that hypertension in HIV reflect the combined effect of risk factors operating in the general population, and of other factors that are specific to the HIV environment.[16] The latter factors in untreated HIV include immune deficiency, immune activation, and chronic inflammation, which may persist event after commencement of ART; while in treatment HIV, components of ART can have direct effect on blood pressure levels or indirect effects through ART-related changes in body composition.[3] In people with comorbid hypertension and HIV, there is a likelihood of interaction between some ART and blood pressure control medications.[16] Plasma levels of some calcium channel blockers (CCB), beta-blockers (BB), diuretics and angiotensin receptor blockers (ARAII) have been reported to decrease or increase when administered together with non-nucleoside reverse transcriptase inhibitors (NNRTIs), protease inhibitors, or elvitegravir/cobicistat. Coadministration of diuretics and indinavir can increase the risk of kidney stones, while certain PIs taken together with certain CCB or BB can cause prolonged PR-interval.[3,16]
Previous reports on the awareness of hypertension in people with HIV are very scanty, and provided awareness rates in African studies tend to be lower than the crude 41.1% or the 46.3% age standardized rates found in our study.[3] Awareness of hypertension in community based studies in the general population across Africa is around 27%,[14] mostly reflecting the lack of periodic access to prepared health systems where screening should be conducted to detect this mostly asymptomatic condition. The low awareness among HIV-infected people who are already in frequent contact with the health system likely reflect the failure to actively coscreen for comorbid NCDs including hypertension during routine visits, or the failure to recognize the diagnosis in those who are screened, since BP measurement is part of routine general medical practice. This failure to coscreen and/or recognize major NCD risk factors in people with HIV infection in care is a direct consequence of the verticality of HIV care programmes. Indeed, improved access to HIV care across Africa has been achieved through dedicated parallel programmes to specifically address HIV and related infectious comorbidities.[17] This approach has disconnected HIV care from the general health stream where opportunities are likely available for the screening and management of non-infectious comorbidities such as hypertension. To rectify the situation, the integration of HIV and common NCD care is increasingly advocated including in South Africa. However, how this integration should be operationalized is still variably interpreted across health systems and settings.[18]
A key finding of our study was the high treatment rates among those with previously diagnosed hypertension, around 75%. Existing studies have reported very low treatment rate of hypertension among African people with HIV infection, while studies from the West have reported treatment rates ranging from 42% to 75%.[3] Population-based studies on hypertension across Africa are generally in support of higher treatment rates among previously diagnosed South Africans compared with other countries in the region. This rate was around 50% across South African studies in a recent systematic review, while the average for Africa was 18%, with South Africa being outperformed only by Seychelles.[14] At the global level, treatment rate of hypertension among aware hypertensive individuals has been estimated at 56%.[19] The driver of the high treatment rate in our study is likely the free access to BP control medications in the public health system in South Africa, which is not the case in most other countries in the region.
Over 2/3rd of the treated participants were at target BP control level. This rate is higher than the global average of 13.8% in the general population,[19] the African average of 7%,[14] but overlaps with levels in the general population in South Africa.[14,20] Our findings therefore support a non-optimal treatment target in people with higher-than-optimal BP across the board in South Africa. Optimal treatment of hypertension particularly in African people regularly requires combination of several BP control agents. Concerns about pill burden can affect the implementation of polypharmacy required to achieve optimal BP control in people with HIV infection, both from the patient and health care providers’ perspectives.[21,22] A combination pill has already been positioned as an appropriate strategy for simplifying the prescriptions of and adherence to ART among people with HIV.[23] A similar strategy has been advocated to overcome inertia, improve adherence to medication, and reduce the cost of preventive cardiovascular prescriptions,[24] resulting in the ongoing race to develop, test, and promote a polypill for CVD prevention.[25] It has relevance to explore the opportunity of using a polypill for CVD prevention to improve the uptake of CVD risk reducing therapy in people with HIV infection, without negatively affecting the acceptable care they are already receiving for HIV and related infectious comorbidities.
Our investigation of the risk factors for hypertension was mostly disappointing, with most traditional risk factors as well as HIV-specific characteristics failing to show an association with prevalent hypertension. Indeed, only some weak associations were apparent for age, in relation to prevalent hypertension. Our study population was on average overweight, which will lead to a more homogenous population and mask any effect of adiposity on the risk of hypertension. Elsewhere, our statistical power was likely low to carefully explore the determinants of screen-detected hypertension, while associations with any hypertension (combination of known and screen-detected hypertension) tend to be diluted by the effects of treatments following diagnosis. The lack of associations of HIV specific characteristics with prevalent hypertension, unlike suggestions from the literature,[26] would tend to support that hypertension in people with HIV infection is mostly driven by the same factors operating in the general population.
The current study has some limitations. The scope of HIV specific characteristics was limited to diagnosed duration of the infection and ART use, which were available only in a subsample of participants. The proportion of men in our sample was relatively small, leading to unstable estimates in some sex specific analyses, and precluding reliable men versus women comparisons. This, however, reflects a general pattern in South Africa and across Africa where participation of women in population-based research tends to be higher than that for men. The study was conducted only in the province of Western Cape, where health care standards tend to be better than in other provinces in South Africa. It is therefore likely that some of our findings reflect the best case scenario and not the typical national average. Lastly, our study conducted exclusively in HIV clinic did not include a comparative group of HIV-uninfected people. Published comparative studies have reported variables findings ranging from no difference in prevalence of hypertension by HIV status, to differences favoring either people with HIV or those without.[3] A major strength of our study is the random selection of participants across many randomly selected urban and rural public health facilities, therefore increasing the generalizability of our findings. By providing age-standardized prevalence estimates, we also increase the opportunity for external comparison of our findings with those of other investigators.
In conclusion, our study in a representative sample of HIV-infected adults receiving care across public health facilities in the Western Cape Province (South Africa), provide findings to extend to people with HIV infection, together with evidence from the general population in South Africa and Africa on the high prevalence of hypertension, low detection and non-optimal treatment and control rates. However, because people with HIV infection are already in regular contact with the health care systems, our findings highlight the missed opportunities of coscreening and addressing comorbid hypertension and other major CVD risk factors in this population. That hypertension in HIV-infected people seems to be largely driven by factors operating in the general population, support the need to integrate into the routine care of people with HIV infection strategies already in application in the general population to screen and mitigate the risk of hypertension and NCDs at large.
Acknowledgment
The authors are grateful to Deborah Jonathan and Erica April from the SAMRC's NCD Research unit, and their team, for the huge effort to collect data for this study.
Author contributions
APK, EJM, and BM conceived the study and acquired the funding. ADV operationalized and supervised data collection in collaboration with TEM, MM, NP analyzed the data and drafted the manuscript. All co-authors substantially revised the manuscript and approved the submission. APK (Epidemiologist) is the guarantor.
Conceptualization: Barbara Mukasa, Edward J. Mills, Andre-Pascal Kengne.
Formal analysis: Muyunda Mutemwa, Andre-Pascal Kengne.
Funding acquisition: Edward J. Mills, Andre-Pascal Kengne.
Investigation: Andre-Pascal Kengne.
Project administration: Anniza de Villiers.
Supervision: Nasheeta Peer, Andre-Pascal Kengne.
Validation: Tandi E. Matsha.
Writing – original draft: Muyunda Mutemwa.
Writing – review & editing: Nasheeta Peer, Anniza de Villiers, Barbara Mukasa, Tandi E. Matsha, Edward J. Mills, Andre-Pascal Kengne.
Footnotes
Abbreviations: ANOVA = analysis of the variance, ART = antiretroviral therapy, BP = blood pressure, CD4 = cluster of differentiation 4, CI = confidence interval, CVD = cardiovascular disease, HIV = human immunodeficiency virus, NCD = non-communicable disease, NNRTIs = non-nucleoside reverse transcriptase inhibitors, OR = odds ratio, PDA = personal digital assistant, SBP = systolic blood pressure, SD = standard deviation, STEPs = stepwise approach to surveillance, WC = waist circumference, WHO = World Health Organization.
Study supported by Grand Challenge Canada, through the Global Alliance on Chronic Diseases initiative; and the South African Medical Research Council. The funders had no role in the conduct of the study, data analysis, and reporting, as well as the decision to submit this work for publication.
No competing interest for all co-authors.
References
- [1].World Health Organisation. Global status report on noncommunicable diseases 2014. In: WHO, ed. Geneva; 2014. Available at: http://apps.who.int/iris/bitstream/10665/148114/1/9789241564854_eng.pdf?ua=1. Accessed January 16, 2018. [Google Scholar]
- [2].Joint United Nations Programme on HIV/AIDS. The gap report. Geneva: UNAIDS; 2014. Available at: http://files.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2014/UNAIDS_Gap_report_en.pdf. Accessed March 23, 2016. [Google Scholar]
- [3].Nguyen KA, Peer N, Mills EJ, et al. Burden, determinants, and pharmacological management of hypertension in HIV-positive patients and populations: a systematic narrative review. AIDS Rev 2015;17:83–95. [PubMed] [Google Scholar]
- [4].Moore RD, Chaisson RE. Natural history of HIV infection in the era of combination antiretroviral therapy. AIDS 1999;13:1933–42. [DOI] [PubMed] [Google Scholar]
- [5].Palella FJ, Jr, Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr 2006;43:27–34. [DOI] [PubMed] [Google Scholar]
- [6].Johnson LF, Mossong J, Dorrington RE, et al. Life expectancies of South African adults starting antiretroviral treatment: collaborative analysis of cohort studies. PLoS Med 2013;10:e1001418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nakagawa F, May M, Phillips A. Life expectancy living with HIV: recent estimates and future implications. Curr Opin Infect Dis 2013;26:17–25. [DOI] [PubMed] [Google Scholar]
- [8].Kayima J, Wanyenze RK, Katamba A, et al. Hypertension awareness, treatment and control in Africa: a systematic review. BMC Cardiovasc Disord 2013;13:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289:2560–72. [DOI] [PubMed] [Google Scholar]
- [10].Haffner SM, Stern MP, Hazuda HP, et al. Cardiovascular risk factors in confirmed prediabetic individuals. Does the clock for coronary heart disease start ticking before the onset of clinical diabetes? JAMA 1990;263:2893–8. [DOI] [PubMed] [Google Scholar]
- [11].Woodward M. Epidemiology: Study Design and Data Analysis. Third Edition.Boca Raton, Florida, USA: Chapman & Hall/CRC Texts in Statistical Sciences, CRC Press, Taylor & Francis Group; 2013. [Google Scholar]
- [12].Dimala CA, Atashili J, Mbuagbaw JC, et al. Prevalence of hypertension in HIV/AIDS patients on highly active antiretroviral therapy (HAART) compared with HAART-Naive Patients at the Limbe Regional Hospital, Cameroon. PLoS One 2016;11:e0148100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mashinya F, Alberts M, Van Geertruyden JP, et al. Assessment of cardiovascular risk factors in people with HIV infection treated with ART in rural South Africa: a cross sectional study. AIDS Res Ther 2015;12:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ataklte F, Erqou S, Kaptoge S, et al. Burden of undiagnosed hypertension in sub-saharan Africa: a systematic review and meta-analysis. Hypertension 2015;65:291–8. [DOI] [PubMed] [Google Scholar]
- [15].Dzudie A, Kengne AP, Muna WF, et al. Prevalence, awareness, treatment and control of hypertension in a self-selected sub-Saharan African urban population: a cross-sectional study. BMJ Open 2012;2:pii: e001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].van Zoest RA, van den Born BH, Reiss P. Hypertension in people living with HIV. Curr Opin HIV AIDS 2017;12:513–22. [DOI] [PubMed] [Google Scholar]
- [17].Kawonga M, Blaauw D, Fonn S. Aligning vertical interventions to health systems: a case study of the HIV monitoring and evaluation system in South Africa. Health Res Policy Syst 2012;10:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kawonga M, Blaauw D, Fonn S. The influence of health system organizational structure and culture on integration of health services: the example of HIV service monitoring in South Africa. Health Policy Plan 2016;31:1270–80. [DOI] [PubMed] [Google Scholar]
- [19].Mills KT, Bundy JD, Kelly TN, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation 2016;134:441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Peer N, Steyn K, Lombard C, et al. A high burden of hypertension in the urban black population of Cape Town: the cardiovascular risk in Black South Africans (CRIBSA) study. PLoS One 2013;8:e78567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Langness J, Cook PF, Gill J, et al. Comparison of adherence rates for antiretroviral, blood pressure, or mental health medications for HIV-positive patients at an academic medical center outpatient pharmacy. J Manag Care Spec Pharm 2014;20:809–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Libby AM, Fish DN, Hosokawa PW, et al. Patient-level medication regimen complexity across populations with chronic disease. Clin Ther 2013;35:385.e1–98.e1. [DOI] [PubMed] [Google Scholar]
- [23].Badowski ME, Perez SE, Biagi M, et al. New antiretroviral treatment for HIV. Infect Dis Ther 2016;5:329–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wald NJ, Law MR. A strategy to reduce cardiovascular disease by more than 80%. BMJ 2003;326:1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lafeber M, Spiering W, Visseren FL, et al. Multifactorial prevention of cardiovascular disease in patients with hypertension: the cardiovascular polypill. Curr Hypertens Rep 2016;18:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nduka CU, Stranges S, Bloomfield GS, et al. A plausible causal link between antiretroviral therapy and increased blood pressure in a sub-Saharan African setting: a propensity score-matched analysis. Int J Cardiol 2016;220:400–7. [DOI] [PubMed] [Google Scholar]


