Supplemental Digital Content is available in the text
Keywords: imaging findings, prostate, stromal sarcoma
Abstract
Rationale:
prostatic stromal sarcoma is a very rare malignant tumor that accounts for <0.1% of prostate malignancy.
Patient concerns:
we reported a 49-year-old man presented with dysuria and hematuria, whose computed tomography examination showed an enlarged prostate gland with an irregular shape.
Diagnoses:
the diagnosis was confirmed on the basis of imaging manifestations and histopathological findings which were proved as prostatic stromal sarcoma.
Interventions:
The patient underwent radical prostatectomy
Outcomes:
his postoperative condition was good.
1. Introduction
The prostate stromal tumor originates from mesenchymal components of the prostate. In 1998, it was first classified into 2 types by Gaudin et al[1] including prostatic stromal sarcoma (PSS) and stromal tumors of uncertain malignancy potential. It has been suggested that PSS was especially rare which only accounts for <0.1% of primary prostate malignancies in adults. Several scholars reported that the common symptom of PSS was urinary retention and the prostatic-specific antigen (PSA) in the blood often remains at a normal level.[2] However, with the low incidence, the clinical information was still unclear. Here we reported a case of PSS in a 49-year-old man presented with urinary retention and a large prostate mass, and discussed the imaging findings, treatment strategy and differential diagnosis.
2. Case report
2.1. Clinical data
A 49-year-old man was admitted to the local hospital 2 months ago due to repeated dysuria, he accepted catheterization there, and liquid yellow urine had been exported, then he was given antibiotic therapy until the catheter was removed 7 days later. The patient was admitted to our hospital with a complaint of hematuria that started 9 days before admission. The patient had no pain, fever, or abdominal distension when he was suffering from the urinary tract symptoms.
The physical examination was unremarkable. Blood analysis showed an increase in eosinophil, all other blood routine indexes were within the normal limits. PSA value was 0.64 ng/mL which was in normal range (<4.0 ng/mL), and other tumor markers including carcinoembryonic antigen and glucosaminidase were also within the normal limits. Urinalysis revealed the number of leukocyte was 15/HP (normal range is 0–2/HP) and positive urinary protein, bacterial count was 55.2/μL (reference range is 0–100/μL). Urine and blood cultures were not performed. Based on the details above, we gave the patient catheterization, as well as an oral antibiotic to fight the infection.
2.2. Imaging findings
The digital rectal examination confirmed a III ° enlarged pliable prostate with perceptible nodules and less clear border. Pelvic transabdominal ultrasound revealed a hypovascular enlarged prostate with a rough surface, the echo of the mass was heterogeneous. A pelvic computed tomography (CT) was performed on this patient, the unenhanced CT images showed an enlarged, partially ill-defined, and heterogeneous mass in the presacral space, which measured as 6.22 cm × 7.64 cm, there were seemingly some separations in it, and the average CT value of the tumor was 23 HU (Fig. 1A). After intravenous contrast medium injection, the mass slightly enhanced with CT value of 30 HU in arterial phase, 32 HU in venous phase, and 36 HU in delay phase; however, it should be noticed that the separations showed moderate enhancement and their CT values during arterial, venous, and delay phases were 51 HU (Fig. 1B), 65 HU (Fig. 1C), and 80 HU (Fig. 1D), respectively. Otherwise, the seminal vesicles and the angles between the bladder and seminal vesicles were normal. All symptoms, signs, and examination results led to a provisional diagnosis of prostate cancer, prostatic sarcoma was taken into consideration.
Figure 1.
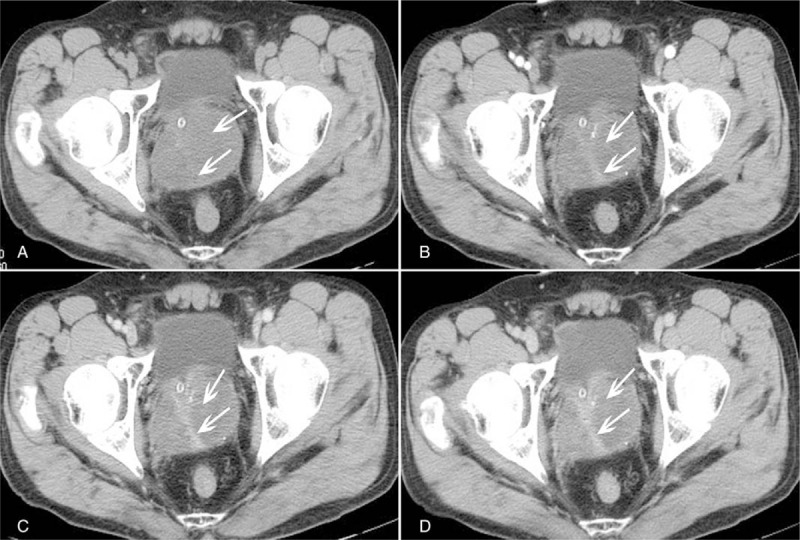
CT imaging findings of the PSS. Conventional CT scan reveals a multiloculated soft tissue mass in the prostate with slight separations in it (A). Contrast-enhanced CT scan shows a gradually enhancement in the separations in the arterial phase (B), venous phase (C) and delayed phase (D). CT = computed tomography, PSS = prostatic stromal sarcoma.
2.3. Surgical and histopathological findings
There was no other adjacent organ-invading or enlarged lymph node could be identified from CT examination. Since the patient had no surgical contraindication, he was advised to undergo radical prostatectomy in consideration of the inefficacy of conservative treatment. The incision was taken in the middle of the lower abdomen. Macroscopically, the prostate was enlarged and slightly squeezed the bladder, and an oval, soft tumor with smooth surface was visible in the prostate, there was no obvious synechia between the tumor and normal prostate tissue. Microscopically, the tumor was growing with the invasive potential, there were abundant heteromorphic interstitial cells with pleomorphic shapes and increased nucleocytoplasmic ratio (Fig. 2A). Combined with the immunohistochemical results which showed the vimentin was positive (Fig. 2B), PSA and CD34 were negative (Fig. 2C and D), the final diagnosis of prostate-specific stromal sarcoma was made definitely on the basis of the histopathological result.
Figure 2.
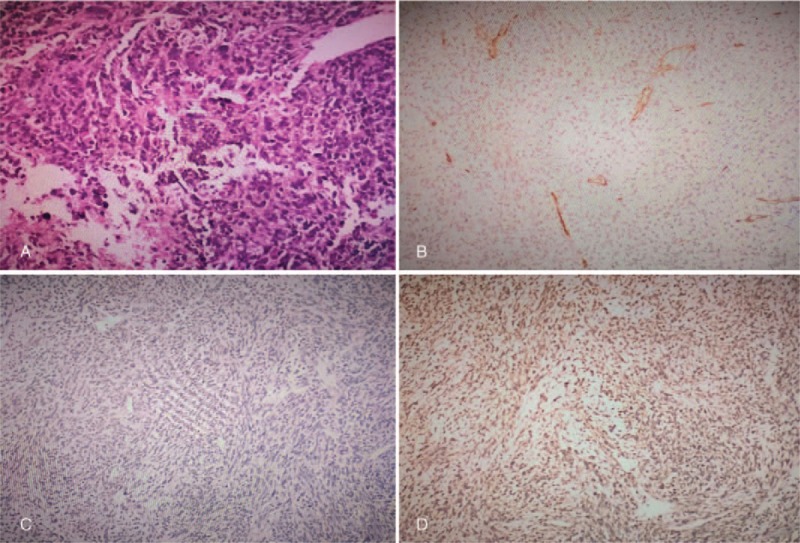
Histopathological presentation of the prostatic stromal sarcoma. There are abundant heteromorphic interstitial cells with pleomorphic shapes and increased nucleocytoplasmic ratio (A), (HE 4 × 200); immunohistochemical examination showed that the tumor was negative for CD34 (B) and PSA(C), positive for vimentin (D). PSA = prostate specific antigen.
Hemostasis, anti-inflammation, fluid infusion, and incision dressing change were given to the patient for postoperative management. The retropubic drainage tube was removed 3 days after the surgery and the urinary catheter 7 days after the surgery. The patient had no abdominal pain or distention when he was discharged from the hospital, the incision showed no exudation or swelling as well. The importance of follow-up has been emphasized in case of recurrence and lymph node metastasis, while unfortunately there were no further follow-ups in our hospital.
3. Discussion
Primary prostate sarcoma is a rare malignancy of the prostate with poor prognosis.[3,4] It originated from the mesoderm in the reproductive tract, and its risk factors may be related to prostatitis, perineal trauma, previous prostate biopsy, and radiation induced.[5] The subtypes of prostate sarcoma include leiomyosarcoma, rhabdomyosarcoma, malignant fibrous histiocytoma, and unclassified sarcoma. Among the above mentioned, rhabdomyosarcoma, mostly with high malignancy, is the most common histological subtype that accounts for about 40% of prostate sarcoma in children. The secondary subtype is leimyosarcoma which accounts for nearly 25% and mostly occurs in the elderly with a slightly lower degree of malignancy. Unclassified sarcoma can be further divided into PSS and stromal tumors of uncertain malignancy potential on the basis of the invasive area of mesenchyme and mitosis, as well as the extent of mesenchyme overgrowth.[1,6–8]
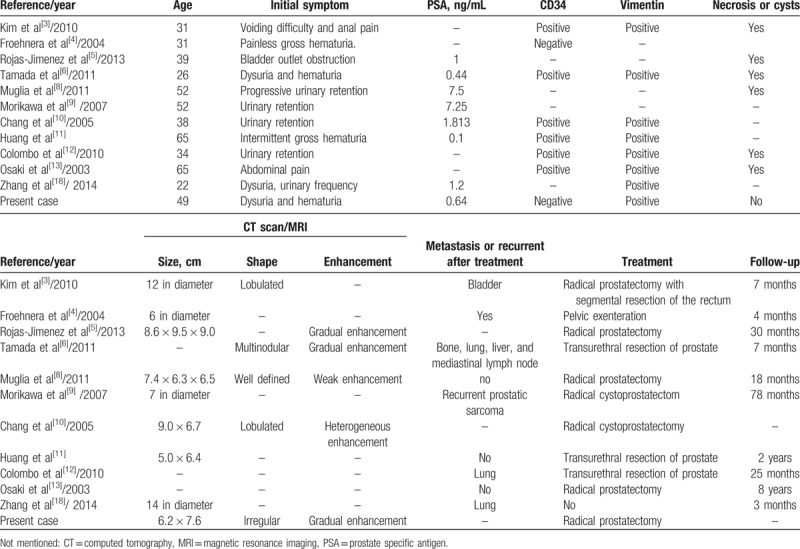
To the best of our knowledge, since the first case of PSS was reported in 1998, there are about 30 reports up to now.[3,9–13] We made a conclusion about some previous cases with detailed information, and our present case was also included (Table 1). Among the twelve cases of PSS, patients’ age ranged from 22 to 65 years old, the median age was 38.5 years old. The vast majority of initial symptoms were urinary retention and hematuria, except only 1 patient who had the first complaint of anal pain and another patient of abdominal pain.[3,13] Except for 2 cases and one of which is about recurrent prostatic sarcoma, PSA levels of all other patients in the Table 1 were within the normal limits. In immunohistochemical studies, PSS are typically positive for vimentin and CD34; however, 2 cases that include the present one showed negative CD34,[4,5] which means that immunohistochemistry gives an important reference significance for diagnosing PSS, but it cannot be exclusive diagnostic criteria.
Table 1.
Examples of prostate sarcoma cases reported in present literatures.
There are no specific presentations of PSS, patients normally complain about dysuria, urinary retention, hematuria at the late stage but have no symptom during early stage.[4] Due to the lack of typical clinical symptoms, the tumor is easily overlooked or misdiagnosed as benign prostatic hyperplasia, therefore, in many cases the prostate was significantly enlarged when the tumor was discovered, for example, in the present case the size of the tumor was 6.22 cm × 7.64 cm, occupying the majority of the prostate, which indicates that the patient was in a serious condition, therefore early detection is vital to the patients with PSS.
On transrectal ultrasonography, a markedly enlarged volume and irregular margins are important characters of prostate sarcoma[14]; in addition, transrectal ultrasonography is usually used to guide a biopsy to confirm the diagnosis. Unenhanced CT often demonstrates an enlarged, well or ill-defined prostate, the shape of the tumor may be round, lobular, or irregular,[15] normally necrosis and cystic changes in the tumor are common because of the high malignancy and rapid growth, but there are no evidence of cystic changes on unenhanced CT. Limited published data regarding the CT enhancement characteristics of the adult prostate sarcoma showed that tumors had a heterogeneous enhancement or delayed enhanced compared with the normal prostate tissue, whereas the necrosis and cystoids areas were unenhanced thus tend to be obvious, indicating rapid and hypervascular enhancement of these tumors.[2,15] The imaging features of magnetic resonance imaging (MRI), such as a large size, irregular margins and heterogeneity, are similar to those of transrectal ultrasonography and CT, besides that, the peripheral solid part of the lesion showed a slightly hyperintense on T1WI and T2WI, a hyperintense on DWI. The centric part of the lesion presents as a hypointense on T1WI, T2WI, and diffusion-weighted imaging; moreover, it has an advantage in detecting metastatic lesions and revealing the adjacent structures, which could show clearer than CT findings. PET/CT is not sensitive to prostate sarcoma, because it is a qualitative analysis method based on the uptake of glucose in tumors, very limited literature reported that compared with FDG-PET, PET/CT improves especially in the lesion localization as well as characterization.[5,16]
When the tumor is too large, it may invade adjacent tissues and organs, and the bladder and rectum are the most vulnerable organs, once the tumor is involved in the bladder and rectum, it can cause symptoms such as abdominal pain and frequent urination.[10,17] Early metastases are not rare in those PSS patients, and the most common one is lung metastasis. In the present case, our patient had neither metastasis nor lower abdomen pain may due to the disease duration was short and the tumor was not too large.[18]
Macroscopically, PSS can be solid or mixed with cystic areas, necrosis, and hemorrhage may also occur especially in high-grade tumors. Unexpectedly, size is not related to tumor grade and the prognosis, because tumors are graded based on the degree of cellular atypia. Microscopically, PSS is characterized by proliferation of spindle and ovoid stromal cells, some of which poses atypical nucleus and necrotic focuses. Immunochemically, PSS normally expresses a positive vimentin, CD34, and progesterone receptor, but not estrogen receptors. It was also reported that in most cases, a significant increase in the Ki-67 labeling index is observed if a high grade of intraepithelial neoplasia is associated with the tumor.[5,12] Surgical resection remains the mainstay treatment of prostate sarcoma, including radical prostatectomy, cystoprostatectomy, and total pelvic exenteration, however, except for metastatic lesions. Although it is still unclear whether the adjuvant radiotherapy and chemotherapy improve patients’ survival, they are needed to prevent local relapse and distant metastasis. In our case, a radical prostatectomy without radiotherapy or chemotherapy has been performed and the operative effect was good.
Differentiating between prostate sarcoma and other prostatic disorders is quite important since the difference of their treatments and prognoses. The differential diagnosis of prostatic stromal sarcoma should include prostate carcinoma, benign prostatic hyperplasia (BPH), and abscess of the prostate, they share similar clinical symptoms, but imaging characteristics vary.
Prostate carcinoma often occurs in the peripheral zone of the prostate, with a lower density than normal prostate tissue, CT scan shows uneven enlargement of the prostate or nodular protrusion in the prostate. The hypervascular nodules are shown clearly on enhanced CT, but the density is still lower than normal prostate tissue. The MRI detection and display of prostate carcinoma are mainly based on T2WI, which mainly manifested as a hypointense defect area in the peripheral zone, where it is significantly different from hyperintense of normal prostate tissue in peripheral zone.[16,19] However, CT and MR imaging alone are not sufficient to identify prostate sarcoma, clinical information should also be taken into account. Prostate carcinoma used to appear mostly in the aged people with obviously increased PSA, while PSS mostly in younger age with normal PSA, simply because PSA is produced by prostate epithelial cells, while sarcoma originates from stromal cells. BPH occurs in elderly men, the imaging differences from prostate sarcoma are that BPH patients show an evenly enlarged prostate with even density. On MRI, T2WI clearly shows that BPH is the hyperplasia of the central zone of the prostate, while the peripheral zone of the prostate is thinned by the extrusion of hyperplasia, besides, pseudocapsule can be seen in hyperplasia. For prostate abscess, there are different manifestations on MRI among the prostate abscess wall, abscess cavity and the separations in the cavity. On T1WI, both the prostate abscess cavity and the separations in the cavity show hypointense, and the wall of the prostate abscess shows hyperintense, the prostate abscess cavity shows hyperintense on T2WI, on dynamic contrast-enhanced MR, abscess border and partitions in the cavity show apparently enhancement.[20] In addition, patients with prostate abscess often have fever and shiver, which should not be overlooked when making the differential diagnosis, since prostate sarcoma and prostate cancer patients do not present these symptoms.
We draw a conclusion that adult prostatic stromal sarcoma is rare and high aggressive malignant tumor. Though the clinical manifestations of prostatic stromal sarcoma are unspecific, imaging results may provide a clue for discovering the tumor and distinguishing it from other similar lesions before surgery. When an adult male shows the same CT or MRI findings as mentioned above, and symptoms like dysuria, hematuria, PSS should be taken into consideration.
Author contributions
Formal analysis: Jingjun Wu, Miao Niu.
Supervision: Ailian Liu.
Writing – original draft: Weiping Yang.
Writing – review & editing: Weiping Yang.
Supplementary Material
Footnotes
Abbreviations: BPH = benign prostatic hyperplasia, CT = computed tomography, MRI = magnetic resonance imaging, PSA = prostate-specific antigen, PSS = prostatic stromal sarcoma.
Even though the patient has been anonymized in the case, we have tried to obtain patient's permission to be included in this manuscript, unfortunately, the patient has passed away, so we could only got the informed consent from his wife. The document has been attached under “OA_Supplemental Digital Content,”.
This article does not involve ethical committee approval.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Gaudin PB, Rosai J, Epstein JI. Sarcomas and related proliferative lesions of specialized prostatic stroma: a clinicopathologic study of 22 cases. Am J Surg Pathol 1998;22:148–62. [DOI] [PubMed] [Google Scholar]
- [2].Ren FY, Lu JP, Wang J, et al. Adult prostate sarcoma: radiological-clinical correlation. Clin Radiol 2009;64:171–7. [DOI] [PubMed] [Google Scholar]
- [3].Kim JY, Cho YM, Ro JY. Prostatic stromal sarcoma with rhabdoid features. Ann Diagn Pathol 2010;14:453–6. [DOI] [PubMed] [Google Scholar]
- [4].Froehner M, Bartholdt E, Meye A, et al. Adult prostate sarcoma diagnosed from tissue spontaneously excreted through the urethra. Urol Oncol 2004;22:119–20. [DOI] [PubMed] [Google Scholar]
- [5].Rojas-Jimenez A, Otero-Garcia M, Mateos-Martin A. Stromal prostatic sarcoma: a rare tumor with rare clinical and imaging presentation. J Radiol Case Rep 2013;7:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tamada T, Sone T, Miyaji Y, et al. MRI appearance of prostatic stromal sarcoma in a young adult. Korean J Radiol 2011;12:519–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Venyo AK. A review of the literature on primary leiomyosarcoma of the prostate gland. Adv Urol 2015;2015:485786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Muglia VF, Saber G, Maggioni G, Jr, et al. MRI findings of prostate stromal tumour of uncertain malignant potential: a case report. Br J Radiol 2011;84:e194–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Morikawa T, Goto A, Tomita K, et al. Recurrent prostatic stromal sarcoma with massive high-grade prostatic intraepithelial neoplasia. J Clin Pathol 2007;60:330–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chang YS, Chuang CK, Ng KF, et al. Prostatic stromal sarcoma in a young adult: a case report. Arch Androl 2005;51:419–24. [DOI] [PubMed] [Google Scholar]
- [11].Huang YC, Wang JY, Lin PY, et al. Synchronous prostate stromal sarcoma and gastrointestinal stromal tumor of rectum: case report and review of the literature. Urology 2006;68:672 e11–3. [DOI] [PubMed] [Google Scholar]
- [12].Colombo P, Ceresoli GL, Boiocchi L, et al. Prostatic stromal tumor with fatal outcome in a young man: histopathological and immunohistochemical case presentation. Rare Tumors 2010;2:e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Osaki M, Osaki M, Takahashi C, et al. Prostatic stromal sarcoma: case report and review of the literature. Pathol Int 2003;53:407–11. [DOI] [PubMed] [Google Scholar]
- [14].Stilgenbauer R, Benedict M, Bamshad R, et al. Sarcoma of the prostate: sonographic findings and pathologic correlation. J Ultrasound Med 2007;26:1789–93. [DOI] [PubMed] [Google Scholar]
- [15].Lee CH, Lin YH, Lin HY, et al. Gastrointestinal stromal tumor of the prostate: a case report and literature review. Hum Pathol 2006;37:1361–5. [DOI] [PubMed] [Google Scholar]
- [16].Dong A, Gong J, Wang Y, et al. MRI and FDG PET/CT findings of malignant fibrous histiocytoma of the prostate. Clin Nucl Med 2014;39:889–91. [DOI] [PubMed] [Google Scholar]
- [17].Tazi K, Moudouni SM, Elfassi J, et al. Leiomyosarcoma of the prostate: a study of two cases. Ann Urol (Paris) 2001;35:56–9. [DOI] [PubMed] [Google Scholar]
- [18].Zhang Q, Wang H, Ren L, et al. Primary synovial sarcoma of the prostate metastatic to the liver and lung: a case report. World J Surg Oncol 2014;12:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dorsam J, Kalble T, Riedasch G, et al. The value of diagnostic imaging in benign prostatic hyperplasia and prostatic cancer. Radiologe 1994;34:101–8. [PubMed] [Google Scholar]
- [20].Aphinives C, Pacheerat K, Chaiyakum J, et al. Prostatic abscesses: radiographic findings and treatment. J Med Assoc Thai 2004;87:810–5. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


