Abstract
Introduction:
Preoperative anemia and old age are independent risk factors for perioperative morbidity and mortality. However, despite the high prevalence of anemia in elderly surgical patients, there is limited understanding of the impact of anemia on postoperative complications and postdischarge quality of life in the elderly. This study aims to investigate how anemia impacts elderly patients undergoing major abdominal surgery in terms of perioperative morbidity, mortality and quality of life for 6 months postoperatively.
Methods and analysis:
We will conduct a prospective observational study over 12 months of 382 consecutive patients above 65 years old, who are undergoing elective major abdominal surgery in Singapore General Hospital (SGH), a tertiary public hospital. Baseline clinical assessment including full blood count and iron studies will be done within 1 month before surgery. Our primary outcome is presence of morbidity at fifth postoperative day (POD) as defined by the postoperative morbidity survey (POMS). Secondary outcomes will include 30-day trend of POMS complications, morbidity defined by Clavien Dindo Classification system (CDC) and Comprehensive Complication Index (CCI), 6-month mortality, blood transfusion requirements, days alive out of hospital (DaOH), length of index hospital stay, 6-month readmission rates and Health Related Quality of Life (HRQoL). HRQoL will be assessed using EuroQol five-dimensional instrument (EQ-5D) scores at preoperative consult and at 1, 3, and 6 months.
Ethics and dissemination:
The SingHealth Centralised Institutional Review Board (CIRB Ref: 2017/2640) approved this study and consent will be obtained from all participants. This study is funded by the National Medical Research Council, Singapore (HNIG16Dec003) and the findings will be published in peer-reviewed journals and presented at academic conferences. Deidentified data will be made available from Dryad Repository upon publication of the results.
Keywords: elderly, major abdominal surgery, preoperative anemia
1. Introduction
Preoperative anemia is associated with poor postoperative outcomes, such as increased mortality, length of stay, intensive care unit admissions, and complications including acute kidney injury, stroke and postoperative infection.[1–14] It is also associated with perioperative blood transfusion,[15,16] which is independently associated with increased risk of perioperative morbidity and mortality.[6,17]
Old age is another independent risk factor for perioperative morbidity, mortality and increased healthcare resource utilization,[18–21] as the elderly may have limited physiologic reserve and higher prevalence of unrecognized cardiovascular disease that renders them more vulnerable to the stress of surgery compared to younger patients.
Put together, old age and anemia may have a compounding effect, which is concerning as the prevalence of anemia increases with age in both the general and surgical population. In a large cohort of surgical patients in a tertiary hospital in Singapore, 45.0% of elderly patients aged above 70 years had preoperative anemia, which is much higher than the overall incidence of 27.8% of anemia in the surgical population.[22,23] Indeed, initial studies show that anemic elderly undergoing noncardiac surgery have increased risk of postoperative 30-day mortality and cardiac event.[13] Studies in the nonsurgical population have also suggested that anemia may negatively affect physical function, cognitive performance, mood, and quality of life of the elderly person.[24–27] However, the impact of preoperative anemia on elderly patients in terms of postsurgical recovery and short- and medium-term quality of life beyond the hospitalization period is not currently known.
In light of the above, we have designed an observational study to examine the impact of preoperative anemia on elderly surgical patients undergoing major abdominal surgery. The study will encompass a large spectrum of outcome measures that are collected during hospitalization and after discharge up to 6 months. The primary aim of this study is to determine the presence of morbidity at 5th postoperative day (POD) using the postoperative morbidity survey (POMS). Our secondary aim is to determine the trend of complications within 30 postoperative days (POD) as defined by POMS, 30-day morbidity as defined by Clavien Dindo Classification system (CDC) and Comprehensive Complication Index (CCI), perioperative transfusion requirements, length of hospital stay, 6-month mortality, 6-month readmission rates, Days alive and out of Hospital (DaOH) at 30 days and 6 months, and Health Related Quality of Life (HRQoL) outcomes as assessed by the EuroQol five-dimensional instrument (EQ-5D) questionnaire. Data from this study will encourage clinicians to investigate and manage perioperative anemia, and guide future intervention. Furthermore, the findings could inform perioperative shared decision-making process among the elderly surgical patients.[28]
2. Methods and analysis
2.1. Overview of study design
This is a single-centre prospective observational study on patients aged 65 years and above, who are undergoing elective major abdominal surgery in Singapore General Hospital (SGH), a tertiary public hospital, over a 12-month period. All patients will be followed for six months from the date of operation. We plan to recruit a minimum of 382 patients. Figure 1 shows the study schedule.
Figure 1.
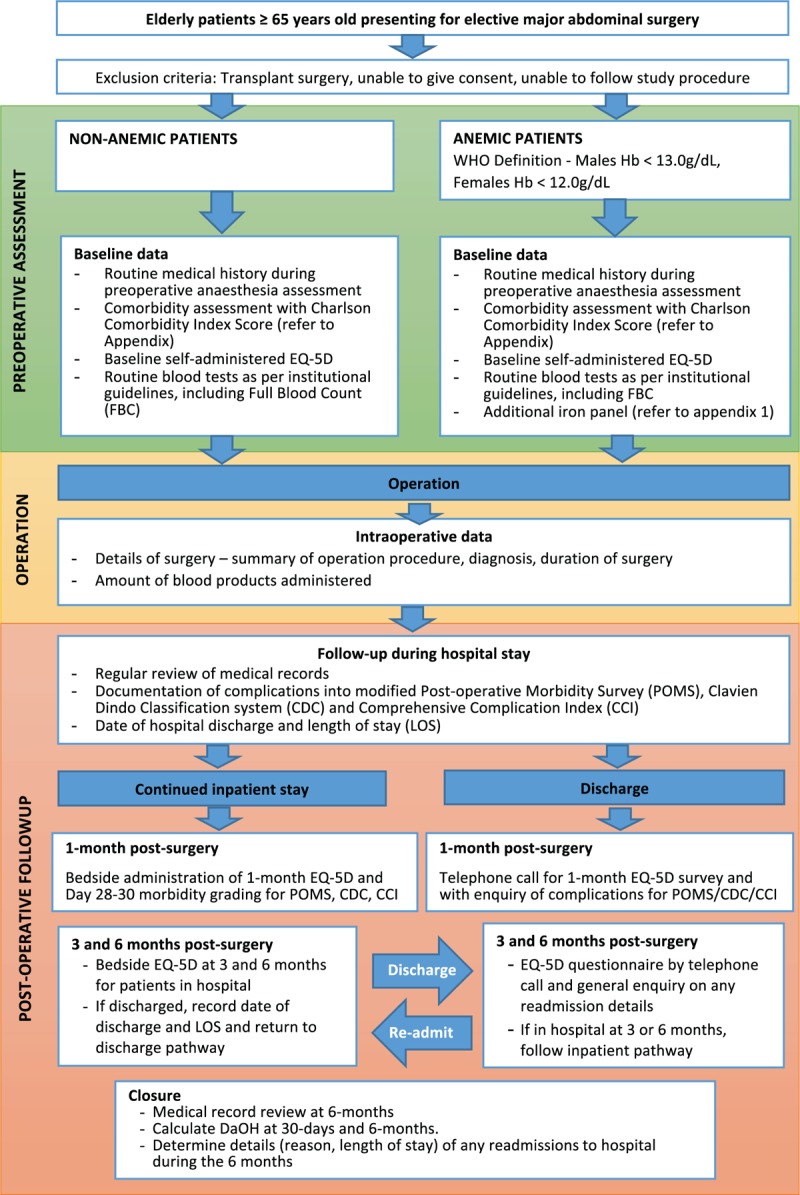
Study schedule. CCI = Comprehensive Complication Index, CDC = Clavien Dindo Classification system, DaOH = Days Alive and Out of Hospital, EQ-5D = EuroQol five-dimensional instrument, Hb = hemoglobin; LOS = length of stay, POMS = Postoperative Morbidity Survey, WHO = World Health Organization.
2.2. Patient recruitment
Recruitment will take place during the patient's preoperative evaluation clinic visit in SGH. This typically occurs within 4 weeks before the surgery. The study coordinators will explain in private and obtain written consent from willing participants. Inclusion criteria: aged 65 years or older and ability to sign informed consent; undergoing elective major abdominal surgery for benign or malignant disease (major defined as procedures expected to last more than 2 hours, or with an anticipated blood loss greater than 500 mL). Exclusion criteria: Organ transplant surgery (specifically, patients who are admitted to the hospital for an organ transplant; patients who had previous transplants but have been discharged can be included). Inability to comprehend and/or perform study procedures due to language barrier or other organic causes. Table 1 shows the eligibility criteria of the study.
Table 1.
Inclusion and exclusion criteria.

2.3. Sample size
The sample size was calculated based on a pilot study of this protocol conducted in our institution. In our sample of 41 patients, the incidence of anemia was 51%. The presence of postoperative morbidities as defined as Post-Operative Morbidity Survey (POMS) score ≥1 on day 5 was 7/21 (33%) in nonanemic group and 10/20 (50%) in anemic group. Thus, to have a power of 90% to detect the difference of 17% (50–33%) significantly, the number of patients needed in each arm would be 159 patients, hence 318 patients. Taking into account a potential dropout rate of 20% at 6-month follow-up, our targeted sample size would be 382.
2.4. Baseline assessment
Patient's demographic data and presence of significant comorbidities will be recorded from the routine preoperative assessment clinical notes. Comorbidity burden will be recorded and analyzed using Charlson Comorbidity Index scores.[29] The Charlson Comorbidity Index is a method of weighting comorbid conditions to measure burden of disease and case mix. The index has been widely used in both clinical and health research and has been validated for its ability to predict mortality in various disease subgroups, including colorectal cancer, general surgical and elderly patients.[30–34]
We will take preoperative Full Blood Count as per normal clinical pathway. The last laboratory value within 1 month before the index surgery will be used to determine preoperative anemia status. Anemia will be diagnosed based on the World Health Organisation gender-based stratification of anemia severity.[35] Mild anemia will be defined as hemoglobin concentration of 11 to 12.9 g/dL in males and 11 to 11.9 g/dL in females. Moderate anemia for both genders will be hemoglobin concentration between 8 and 10.9 g/dL and severe anemia will be defined as <8.0 g/dL. As per our institution guidelines, patients with anemia will undergo further investigations such as iron studies (serum ferritin, iron, total iron binding capacity, and transferrin saturation).
Self-administered baseline measures of EQ-5D will be taken during the preoperative assessment visit. The research coordinator will guide patients in filling out the questionnaire. The EQ-5D is a generic measure of HRQoL comprising of a Visual Analogue Scale (VAS) for self-rating of general health (a score of 100 is the best possible general health status) and 5 domains evaluating mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. The EQ-5D has been used widely in Singapore to examine HRQoL among different disease groups and population norms have been established for EQ-5D in Singapore.[36] Questionnaires will be made available in English, Simplified Chinese, Malay and Tamil to suit the primary language of the patient.
2.5. Intraoperative data
Intraoperative data such as surgical details (summary of operation procedure, diagnosis, surgical duration) and amount of blood products administered will be obtained via hospital electronic records.
2.6. Study outcomes and follow-up
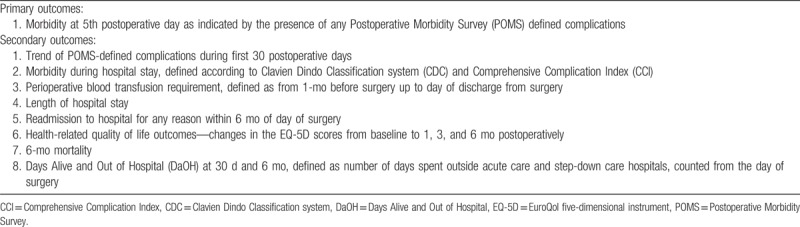
Our primary outcome is the presence of morbidity at the 5th postoperative day (POD) as indicated according to the presence of any POMS-defined complications. POMS was developed to classify in-hospital complications that a patient may encounter after surgery into nine domains—neurological, hematological, pain, gastrointestinal, wound, cardiovascular, pulmonary, infectious, and renal.[37] We will then continue to monitor the trend of POMS-defined complications for the first 30 postoperative days as part of our secondary outcomes. Patients will have their POMS complications graded on POD 3, 5, 7–8, 14–15, 21–22, and 28–30, with flexible ranges for the last three periods of assessment to ease the logistics of follow up.
In addition to POMS, postoperative complications up to POD 30 will be graded using the CDC and CCI.[37,38] CDC grades the severity of surgical complications based on the therapy used to treat the complication, and is scored based on the highest grade of complication.[38] The CCI at Day 30 then takes into account all Clavien Dindo complications incurred and gives different weightage to each according to their severity.[39,40]
Other secondary health-care outcomes include perioperative blood transfusion requirement, defined as blood transfusion requirements from 1 month before surgery up to day of discharge from surgery, length of hospital stay for surgery, readmission to the hospital for any reason within 6 months of date of surgery, 6-month mortality, Days Alive and Out of Hospital (DaOH), and HRQoL outcomes.
DaOH was devised as a tool to evaluate endpoints in clinical trials in heart failure[41–43] and has been further validated as a measure for postsurgical outcomes in Australia.[44] It is a patient-centric outcome that reflects the burden of length of stay of index admission, readmissions, and early death after surgery. The DaOH index in this study will be calculated by summating the total amount of time spent by the patient in acute-care hospital for the surgery and in rehabilitation in step-down hospitals after the surgery, as well as any readmissions back to hospitals, within 30 days and 6 months after the surgery. As time spent at home and out of hospital is highly valued by patients, DaOH will be a good representative of the overall postoperative experience. For this study, we estimate the DaOH index at 30 days and 6 months to reflect the short and long-term effect of anemia. The information will be obtained during telephone follow ups with the patient when we collect information about their HRQoL. Further readmission details such as length of stay will be determined by subsequent medical record review.
HRQoL outcomes are assessed by determining EQ-5D scores at baseline, 1, 3, and 6 months postoperatively. Apart from the baseline EQ-5D score obtained at the preoperative assessment clinic, the EQ-5D score at 1, 3, and 6 months will be obtained by telephone interview of the patients still hospitalized at 30 days after surgery will have EQ-5D questionnaire administered at the bedside together with postoperative day 28 to 30 POMS. If they are hospitalized at 3 or 6 months postoperatively, the EQ-5D questionnaire will be administered by their bedside.
A list of the study outcomes is shown in Table 2.
Table 2.
Primary and secondary outcomes.
2.7. Quality control
All data will be monitored and reviewed by the Principal Investigator (PI) or co-investigators (Co-I). The PI and Co-I will provide training to the research coordinators with regards to the administration and interpretation of various scoring systems used in this study. If there is any disagreement in the classification of existing comorbidities or postoperative complications, the PI or Co-I will be involved to reach an agreeable resolution.
The participants can withdraw their participation from the study at any time. While patients do not need justification for withdrawing from the study, the reasons for their withdrawal will be recorded. In addition, patients who are lost to follow up during the study will have their baseline characteristics such as demographic data, preoperative comorbidity including anemia, baseline EQ-5D and intraoperative details analyzed to see if there are significant differences between those who were lost to follow up and those who completed the study follow up.
As this is an observational study, and there is no interaction between the study team members and the patient's primary physicians that can potentially alter their management, the patients are not exposed to additional risks due to their participation in this project. Hence, no compensation is anticipated to be necessary.
2.8. Data management
Participant data confidentiality will be maintained throughout the study. Each patient will be assigned a unique study-related index number upon recruitment. This study-related index number will be used on the case report form. The key to the index numbers will be stored in a separate document and access restricted to the principal and co-investigator.
All research data will be entered into a hard-copy case report form and the deidentified data will be reentered in the institution's Research Electronic Data Capture (REDCap) System, which is a centralized secured data management platform. Records generated in this study for all participants, such as the patient's written informed consent, EQ-5D assessment forms and postoperative complication assessment forms, as well as the Singhealth Centralised Institutional Review Board (CIRB) records and other regulatory documentation will be retained by the PI and be accessible for inspection by authorities. Only study team members will have access to the research data.
Compliant with CIRB policy, both soft and hard copies of the research data will be kept for at least 6 years.
2.9. Statistical methods
2.9.1. Statistical analyses
The demographic characteristic, baseline clinical features, and outcomes between anemic and nonanemic patients will be compared using the Chi-square test for categorical variables and the independent samples T test or Mann–Whitney U test for continuous variables. For binary outcomes of interest such as presence of any POMS-defined complication at 5th POD, presence of Clavien Dindo complications of grade 3 and above, death and readmission within 6 months, and any perioperative blood transfusion, univariate, and multivariate logistic regression analysis will be performed to determine the impact of relevant clinical variables as well as anemia. For continuous outcomes, such as length of stay, DaOH, CCI, and EQ-5D, we will perform linear regression with the General Linear Model or quantile regression where appropriate to estimate the slope coefficients of preoperative anemia and other relevant clinical variables. P values and 95% confidence intervals will be presented.
2.9.2. Analysis of EQ-5D data
The EQ-5D has been used widely in Singapore to examine HRQoL among different disease groups and population norms have been established for EQ-5D in Singapore, allowing a cost-utility analysis in determining quality-adjusted life years.[36,45–47] Changes of EQ-5D scores from the baseline value to values at 3 and 6 months will be compared between patients with and without preoperative anemia. Missing EQ-5D data for patients known to be alive and not hospitalized at the time point being analyzed will be imputed using the “last observation carried forward” method, for which at least one previous follow-up value is required. If no preceding values were available, the patient will be excluded from that analysis. For hospitalized patients unable to complete the questionnaires, or if the patient had died between the last time point and the planned time point, the worst reply will be used for the imputation, that is, 3 for the EQ-5D subquestions and 0 for the EQ-5D VAS score. Similar approach has been used in previous anemia-related study reporting HRQoL via EQ-5D.[48]
2.10. Data monitoring
Data monitoring will be performed by the Principal Investigator (PI) and co-investigators (Co-I). The practices are also subjected to audit and monitoring by the Division of Research at SGH as well as the CIRB. No safety monitoring will be performed as this is not an interventional trial. There are no plans for interim analyses due to the relatively low sample size and anticipated rapid recruitment rate. Data monitoring is independent from the sponsor and competing interests.
3. Ethics and dissemination
This study will be conducted in accordance with the Singapore Good Clinical Practice Guidelines, which is based on the principles enshrined in the Declaration of Helsinki. This study has been approved by the Singhealth Centralised Institutional Review Board (CIRB) (CIRB Ref: 2017/2640). Any protocol modification will be submitted to the CIRB for approval before implementation. Results of this study will be presented at international conferences and submitted to a peer-reviewed journal. Deidentified data will be made available from Dryad Repository upon publication of the results.
Acknowledgments
We would like to thank our statistician Dr. Hao Ying of Singhealth Health Services Research Unit for reviewing the statistics of the protocol.
Author contributions
Conceptualization: Hairil Rizal Abdullah, Yilin Eileen Sim, Yi Tian Mary Sim, Ecosse Lamoureux.
Data curation: Hairil Rizal Abdullah, Yilin Eileen Sim, Yi Tian Mary Sim, Ecosse Lamoureux.
Formal analysis: Hairil Rizal Abdullah, Yilin Eileen Sim, Yi Tian Mary Sim, Ecosse Lamoureux.
Funding acquisition: Hairil Rizal Abdullah, Yilin Eileen Sim, Yi Tian Mary Sim, Ecosse Lamoureux.
Investigation: Hairil Rizal Abdullah, Yilin Eileen Sim, Yi Tian Mary Sim, Ecosse Lamoureux.
Methodology: Hairil Rizal Abdullah, Yilin Eileen Sim, Yi Tian Mary Sim, Ecosse Lamoureux.
Project administration: Hairil Rizal Abdullah, Yilin Eileen Sim, Yi Tian Mary Sim, Ecosse Lamoureux.
Resources: Hairil Rizal Abdullah, Yilin Eileen Sim, Yi Tian Mary Sim, Ecosse Lamoureux.
Software: Hairil Rizal Abdullah, Yilin Eileen Sim, Yi Tian Mary Sim, Ecosse Lamoureux.
Supervision: Hairil Rizal Abdullah, Yilin Eileen Sim, Yi Tian Mary Sim, Ecosse Lamoureux.
Validation: Hairil Rizal Abdullah, Yilin Eileen Sim, Yi Tian Mary Sim, Ecosse Lamoureux.
Visualization: Hairil Rizal Abdullah, Yilin Eileen Sim, Yi Tian Mary Sim, Ecosse Lamoureux.
Writing – original draft: Hairil Rizal Abdullah, Yilin Eileen Sim, Yi Tian Mary Sim, Ecosse Lamoureux.
Writing – review & editing: Hairil Rizal Abdullah, Yilin Eileen Sim, Yi Tian Mary Sim, Ecosse Lamoureux.
Footnotes
Abbreviations: CCI = Comprehensive Complication Index, CDC = Clavien Dindo Classification, CIRB = Singhealth Centralised Institutional Review Board, Co-I = co-investigator, DAOH = days alive out of hospital, EQ-5D = EuroQol five-dimensional instrument, HRQoL = Health Related Quality of Life, PI = Principal Investigator, POD = postoperative day, POMS = postoperative morbidity survey, REDCap = Research Electronic Data Capture system, SGH = Singapore General Hospital, VAS = Visual Analogue Scale.
Trial registration number and registry name: ClinicalTrials.gov ID NCT03497416.
Funding: This work is supported by funds from the Singapore Ministry of Health National Medical Research Council (NMRC) Health Services Research New Investigator Grant (HNIG) (no. NMRC/HNIG/0004/2017). Apart from reviewing the study design during the grant selection process, the NMRC have no role in the analysis and interpretation of the results.
HRA and YES are the principal and co-investigator, respectively. All authors contributed to design of study and draft of the protocol. All authors made revisions to the manuscript and have read and approved the final version.
Ethics approval: SingHealth Centralised Institutional Review Board (CIRB Ref: 2017/2640).
Data sharing: The deidentified full dataset of this study will be made available from Dryad Repository upon publication of the results.
The authors have no conflicts of interest to disclose.
References
- [1].Baron DM, Hochrieser H, Posch M, et al. Preoperative anaemia is associated with poor clinical outcome in non-cardiac surgery patients. Br J Anaesth 2014;113:416–23. [DOI] [PubMed] [Google Scholar]
- [2].Fowler AJ, Ahmad T, Phull MK, et al. Meta-analysis of the association between preoperative anaemia and mortality after surgery. Br J Surg 2015;102:1314–24. [DOI] [PubMed] [Google Scholar]
- [3].Abdullah HR, Sim YE, Hao Y, et al. Association between preoperative anaemia with length of hospital stay among patients undergoing primary total knee arthroplasty in Singapore: a single-centre retrospective study. BMJ Open 2017;7:e016403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chan DXH, Sim YE, Chan YH, et al. Development of the Combined Assessment of Risk Encountered in Surgery (CARES) surgical risk calculator for prediction of postsurgical mortality and need for intensive care unit admission risk: a single-center retrospective study. BMJ Open 2018;8:e019427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Abdullah HR, Sim YE, Sim YT, et al. Preoperative Red Cell Distribution Width and 30-day mortality in older patients undergoing non-cardiac surgery: a retrospective cohort observational study. Sci Rep 2018;8:6226doi:10.1038/s41598-018-24556-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Beattie WS, Karkouti K, Wijeysundera DN, et al. Risk associated with preoperative anemia in noncardiac surgery: a single-center cohort study. Anesthesiology 2009;110:574–81. [DOI] [PubMed] [Google Scholar]
- [7].Jans Ø, Jørgensen C, Kehlet H, et al. Role of preoperative anemia for risk of transfusion and postoperative morbidity in fast-track hip and knee arthroplasty. Transfusion 54, 2014, 717–726. [DOI] [PubMed] [Google Scholar]
- [8].Musallam KM, Tamim HM, Richards T, et al. Preoperative anaemia and postoperative outcomes in non-cardiac surgery: a retrospective cohort study. Lancet 2011;378:1396–407. [DOI] [PubMed] [Google Scholar]
- [9].Richards T, Musallam KM, Nassif J, et al. Impact of preoperative anaemia and blood transfusion on postoperative outcomes in gynaecological surgery. PLoS One 2015;10:e0130861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Leichtle SW, Mouawad NJ, Lampman R, et al. Does preoperative anemia adversely affect colon and rectal surgery outcomes? J Am Coll Surg 2011;212:187–94. [DOI] [PubMed] [Google Scholar]
- [11].Ranucci M, Di Dedda U, Castelvecchio S, et al. Impact of preoperative anemia on outcome in adult cardiac surgery: a propensity-matched analysis. Ann Thorac Surg 2012;94:1134–41. [DOI] [PubMed] [Google Scholar]
- [12].Shander A, Javidroozi M, Ozawa S, et al. What is really dangerous: anaemia or transfusion? Br J Anaesth 2011;107(suppl 1):i41–59. [DOI] [PubMed] [Google Scholar]
- [13].Wu W-C, Schifftner TL, Henderson WG, et al. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing noncardiac surgery. JAMA 2007;297:2481–8. [DOI] [PubMed] [Google Scholar]
- [14].Spahn DR. Anemia and patient blood management in hip and knee surgery: a systematic review of the literature. Anesthesiology 2010;113:482–95. [DOI] [PubMed] [Google Scholar]
- [15].Carabini LM, Zeeni C, Moreland NC, et al. Development and validation of a generalizable model for predicting major transfusion during spine fusion surgery. J Neurosurg Anesthesiol 2014;26:205–15. [DOI] [PubMed] [Google Scholar]
- [16].Rosencher N, Kerkkamp HEM, Macheras G, et al. Orthopedic Surgery Transfusion Hemoglobin European Overview (OSTHEO) study: blood management in elective knee and hip arthroplasty in Europe. Transfusion 2003;43:459–69. [DOI] [PubMed] [Google Scholar]
- [17].Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999;340:409–17. [DOI] [PubMed] [Google Scholar]
- [18].Finlayson EV, Birkmeyer JD. Operative mortality with elective surgery in older adults. Eff Clin Pract 2001;4:172–7. [PubMed] [Google Scholar]
- [19].Jin F, Chung F. Minimizing perioperative adverse events in the elderly†. Br J Anaesth 2001;87:608–24. [DOI] [PubMed] [Google Scholar]
- [20].Pedersen T, Eliasen K, Henriksen E. A prospective study of mortality associated with anaesthesia and surgery: risk indicators of mortality in hospital. Acta Anaesthesiol Scand 1990;34:176–82. [DOI] [PubMed] [Google Scholar]
- [21].Manski RJ, Moeller JF, Chen H, et al. Patterns of older Americans’ health care utilization over time. Am J Public Health 2013;103:1314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kassebaum NJ, Jasrasaria R, Naghavi M, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014;123:615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sim YE, Wee HE, Ang AL, et al. Prevalence of preoperative anemia, abnormal mean corpuscular volume and red cell distribution width among surgical patients in Singapore, and their influence on one year mortality. PLoS One 2017;12:e0182543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lucca U, Tettamanti M, Mosconi P, et al. Association of mild anemia with cognitive, functional, mood and quality of life outcomes in the elderly: the “Health and Anemia” study. PLoS One 2008;3:e1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chaves PHM. Functional outcomes of anemia in older adults. Semin Hematol 2008;45:255–60. [DOI] [PubMed] [Google Scholar]
- [26].Chaves PHM, Carlson MC, Ferrucci L, et al. Association between mild anemia and executive function impairment in community-dwelling older women: the Women's Health and Aging Study II. J Am Geriatr Soc 2006;54:1429–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hong CH, Falvey C, Harris TB, et al. Anemia and risk of dementia in older adults: findings from the Health ABC study. Neurology 2013;81:528–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yek JLJ, Lee AKY, Tan JAD, et al. Defining reasonable patient standard and preference for shared decision making among patients undergoing anaesthesia in Singapore. BMC Med Ethics 2017;18:6doi:10.1186/s12910-017-0172-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- [30].Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011;173:676–82. [DOI] [PubMed] [Google Scholar]
- [31].Erichsen R, Horváth-Puhó E, Iversen LH, et al. Does comorbidity interact with colorectal cancer to increase mortality? A nationwide population-based cohort study. Br J Cancer 2013;109:2005–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yurtlu DA, Aksun M, Ayvat P, et al. Comparison of risk scoring systems to predict the outcome in ASA-PS V patients undergoing surgery: a retrospective cohort study. Medicine 2016;95:e3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Havens JM, Olufajo OA, Cooper ZR, et al. Defining rates and risk factors for readmissions following emergency general surgery. JAMA Surg 2016;151:330–6. [DOI] [PubMed] [Google Scholar]
- [34].Robinson TN, Wu DS, Pointer L, et al. Simple frailty score predicts postoperative complications across surgical specialties. Am J Surg 2013;206:544–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].World Health Organization. Nutritional anaemias: report of a WHO scientific group [meeting held in Geneva from 13 to 17 March 1967]. 1968. Available at: http://apps.who.int/iris/handle/10665/40707. March 1, 2018. [PubMed] [Google Scholar]
- [36].Abdin E, Subramaniam M, Vaingankar JA, et al. Population norms for the EQ-5D index scores using Singapore preference weights. Qual Life Res 2015;24:1545–53. [DOI] [PubMed] [Google Scholar]
- [37].Grocott MPW, Browne JP, Van der Meulen J, et al. The Postoperative Morbidity Survey was validated and used to describe morbidity after major surgery. J Clin Epidemiol 2007;60:919–28. [DOI] [PubMed] [Google Scholar]
- [38].Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Slankamenac K, Nederlof N, Pessaux P, et al. The comprehensive complication index: a novel and more sensitive endpoint for assessing outcome and reducing sample size in randomized controlled trials. Ann Surg 2014;260:757–62. discussion 762–763. [DOI] [PubMed] [Google Scholar]
- [40].Slaman AE, Lagarde SM, Gisbertz SS, et al. A quantified scoring system for postoperative complication severity compared to the Clavien-Dindo classification. Dig Surg 2015;32:361–6. [DOI] [PubMed] [Google Scholar]
- [41].Allen LA, Hernandez AF, O’Connor CM, et al. End points for clinical trials in acute heart failure syndromes. J Am Coll Cardiol 2009;53:2248–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wasywich CA, Gamble GD, Whalley GA, et al. Understanding changing patterns of survival and hospitalization for heart failure over two decades in New Zealand: utility of “days alive and out of hospital” from epidemiological data. Eur J Heart Fail 2010;12:462–8. [DOI] [PubMed] [Google Scholar]
- [43].Cleland JGF. How to assess new treatments for the management of heart failure: composite scoring systems to assess the patients’ clinical journey. Eur J Heart Fail 2002;4:243–7. [DOI] [PubMed] [Google Scholar]
- [44].Myles PS, Shulman MA, Heritier S, et al. Validation of days at home as an outcome measure after surgery: a prospective cohort study in Australia. BMJ Open 2017;7:e015828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Luo N, Wang P, Thumboo J, et al. Valuation of EQ-5D-3L health states in Singapore: modeling of time trade-off values for 80 empirically observed health states. Pharmacoeconomics 2014;32:495–507. [DOI] [PubMed] [Google Scholar]
- [46].Wee H-L, Loke W-C, Li S-C, et al. Cross-cultural adaptation and validation of Singapore Malay and Tamil versions of the EQ-5D. Ann Acad Med Singapore 2007;36:403–8. [PubMed] [Google Scholar]
- [47].Wang Y, Tan N-C, Tay E-G, et al. Cross-cultural measurement equivalence of the 5-level EQ-5D (EQ-5D-5L) in patients with type 2 diabetes mellitus in Singapore. Health Qual Life Outcomes 2015;13:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Comin-Colet J, Lainscak M, Dickstein K, et al. The effect of intravenous ferric carboxymaltose on health-related quality of life in patients with chronic heart failure and iron deficiency: a subanalysis of the FAIR-HF study. Eur Heart J 2013;34:30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


