Abstract
We explored possible relationships between sudden sensorineural hearing loss (SSNHL) in patients differing in terms of audiographic data and the levels of vascular markers in routine blood data.
We included 37 patients with low-frequency SSNHL (LF-SSNHL), 28 with high-frequency SSNHL (HF-SSNHL), 32 with all-frequency SSNHL (AF-SSNHL), 32 with total-deafness SSNHL (TD-SSNHL), and 31 age- and sex-matched healthy controls. Peripheral venous blood samples were collected, and routine blood parameters including platelet and lymphocyte count, mean platelet volume (MPV), and platelet-to-lymphocyte ratio (PLR) were measured. Each group was divided into recovery subgroup and unrecovery subgroup in accordance with hearing level after 1 month therapy, then compared the difference of platelet and lymphocyte count, MPV, and PLR between the 2 subgroups.
No significant difference was observed between platelet count of all SSNHL patients and control group (all P > .05). MPV of AF-SSNHL and TD-SSNHL, PLR of all SSNHL patients were significantly higher than those of control group (all P < .05), while lymphocyte count of all audiographically distinct SSNHL patients was significantly lower than that of control group (all P < .05). However, the difference of platelet count, lymphocyte count, MPV, and PLR among audiographically distinct SSNHL patients was not significant (all P > .05). In HF-SSNHL patients, lymphocyte count of unrecovery subgroup was significantly lower, while MPV and PLR of the unrecovery subgroup were significantly higher than those of recovery subgroup (all P < .05). In AF-SSNHL patients, MPV of the unrecovery group was significantly higher than that of recovery subgroup (P < .05).
Lymphocyte count, MPV, and PLR may be relative to SSSNHL, but they could not be used to distinct SSNHL audiographically. Lower lymphocyte, higher MPV, and PLR may be indicative for the prognosis of HF-SSNHL patients; higher MPV may be related to bad treatment outcome of AF-SSNHL patients.
Keywords: lymphocyte, mean platelet volume, platelet-to-lymphocyte ratio, sudden sensorineural hearing loss
1. Introduction
Sudden sensorineural hearing loss (SSNHL) is common and is characterized by acute idiopathic hearing loss ≥20 dB at ≥2 continuous frequencies within 72 hours.[1] SSNHL is often unilateral, accompanied by tinnitus, vertigo, and a sense of ear fullness. The incidence varies geographically; the condition is much more common than reported because spontaneous recovery in the absence of treatment is common.[2,3] Although the etiologies of most cases of SSNHL remain unknown, various pathologies may underlie SSNHL, differing in terms of the audiographic features. The Chinese guidelines for the diagnosis and treatment of sudden deafness (2015) and the German guidelines for the diagnosis of sudden idiopathic SSNHL (2004) stress that membranous labyrinthine edema may contribute to low-frequency SSNHL (LF-SSNHL); hair cell damage may trigger high-frequency SSNHL (HF-SSNHL); endothelial dysfunction or vasospasm can cause all-frequency SSNHL (AF-SSNHL); and an inner ear embolism or a thrombosis may play an important role in the pathophysiology of total-deafness SSNHL (TD-SSNHL).[1,4] Therefore, audiographic data are important when planning the treatment of SSNHL.
Blood examinations are simple and inexpensive, yielding information on red and white blood cell and platelet counts, the cell subgroups present,[5] blood coagulation, inflammation, thrombosis, and atherosclerosis.[6] Recent studies have focused on the associations of these variables with middle-ear inflammation and microvascular embolisms[7]; many reports have described associations between blood routine data including the count of platelet and lymphocyte, the mean platelet volume (MPV), platelet-to-lymphocyte ratio (PLR), on the one hand, and SSNHL diagnosis and prognosis, on the other.[3,8–15] However, no work has yet explored the relationships of the count of platelet and lymphocyte, MPV, and PLR with SSNHL in patients differing audiographically. Thus, we evaluated the utility of these data in terms of SSNHL diagnosis and prognosis in such patients. Platelet is an important member of clotting system, lower lymphocyte is always associated with virus infection, PLR is a marker of inflammation, and MPV is a marker of atherothrombosis. We thus evaluated the roles played by inflammation and atherothrombosis in SSNHL patients who differed audiographically.
2. Materials and methods
A total of 129 patients with SSNHL diagnosed at the Affiliated Sixth People's Hospital of Shanghai Jiao tong University were retrospectively enrolled. The patients were divided into 4 groups in terms of the pretreatment audiographic features defined in the Chinese guidelines for the diagnosis and treatment of sudden deafness (2015).[1] The LF-SSNHL group contained 37 patients with hearing loss at 0.125 to 1 kHz or HL ≥20 dB at both 0.25 and 0.5 kHz. The HF-SSNHL group contained 28 patients with high frequency hearing loss at 2 to 8 kHz or at both 4 and 8 kHz (≥20 dB). The AF-SSNHL group contained 32 patients with overall hearing loss at all frequencies and mean pure-tone thresholds ≤80 dB from 0.25 to 8 kHz. The TD-SSNHL group contained 32 totally deaf patients (hearing loss at all frequencies and a mean pure tone threshold ≥81 dB from 0.25 to 8 kHz). The control group contained 31 age- and sex-matched healthy individuals without disease evident on regular check-ups.
Patients with any history of otological surgery, otitis media, trauma, acute or chronic inflammation, hypertension, diabetes mellitus, metabolic syndrome, heart failure, myocardial infarction, a cerebral embolism, cancer, hepatitis, nephritis, an autoimmune disease, chronic obstructive pulmonary disease, Meniere disease, infection of the upper respiratory tract, pregnancy, cor pulmonale, or for whom data were incomplete were excluded. All enrolled patients were treated within 7 days from disease onset, had received no previous therapy, and underwent both hematological assessment and pure-tone hearing testing during the first visit.
Audiometric examinations were performed in soundproof rooms to obtain the pure-tone thresholds for both air and bone conduction at 0.125, 0.25, 0.5, 1, 2, 4, and 8 kHz. A qualified audiologist performed the testing before treatment, 3 days, 1 week, and 1 month after therapy. Fasting blood samples were obtained before treatment to ensure that steroids would not be prescribed for high-risk patients with metabolic and/or cardio-cerebrovascular disease; all samples were drawn from the antecubital vein in the morning.
All patients were prescribed dexamethasone 10 mg/day (5 mg/mL, Furuitang Drug Industry, China) through intravenous for the first 3 days, then a progressively reductive dose was maintained for at least 1 week; vasodilators (Breviscapine injection, 40 mg/day, Kunming Longjin Pharmaceutical Co, Ltd, China) was injected for 2 weeks; oral neurotrophic factor (Extract of Ginkgo Biloba Leaves Tablets, 80 mg, tid, Dr Willmar Schwabe) was given for 1 month; and hyperbaric oxygen therapy if necessary for some patients. We evaluated treatment outcomes after 1 month therapy by reference to the Chinese guidelines for the diagnosis and treatment of sudden deafness (2015)[1]: complete recovery, the pure-tone thresholds of the impaired frequencies reached those of the normal unaffected ear; notable recovery, the pure-tone thresholds of the impaired frequencies improved by ≥30 dB; partial recovery, the pure-tone thresholds of the impaired frequencies improved by ≥15 dB but <30 dB; and no improvement, the pure-tone thresholds of impaired frequencies improved by <15 dB.
2.1. Statistical analysis
SPSS for Windows software, version 20.0, was used for all data analyses. Student independent-sample t test and ANOVA were employed to evaluate the significance of differences in continuous variables that were normally distributed. The χ2 test was used to compare categorical variables. Bonferroni and LSD post hoc test was applied to explore relationships between hematological parameters and audiographic shapes when the variances were homogeneous; Dunnett post hoc test was employed when the variances were not homogeneous. A P value <.05 was considered to reflect statistical significance. GraphPad Prism version 6.0 for Windows was used to draw the figures.
3. Results
We respectively analyzed the demographic characteristics and laboratory parameters of all groups (Table 1). Neither age nor sex differed between the groups (both P > .05).
Table 1.
Demographic and laboratory data.

3.1. The platelet, lymphocyte count, MPV and PLR
Figure 1 shows that the platelet count was 218.35 ± 42.65 × 109/L, 214.32 ± 63.07 × 109/L, 224.59 ± 64.41 × 109/L, 212.44 ± 45.11 × 109/L, and 202.48 ± 46.61 × 109/L in the LF-SSNHL, HF-SSNHL, AF-SSNHL, TD-SSNHL, and control groups, respectively. There was no significant difference between each study group and control group in terms of platelet count (P = .14, P = .39, P = .10, and P = .45 for LF-, HF-, AF-, and TD-SSNHL, respectively). The lymphocyte count was 1.94 ± 0.81 × 109/L, 1.83 ± 0.89 × 109/L, 1.78 ± 0.56 × 109/L, 1.64 ± 0.68 × 109/L, and 2.21 ± 0.64 × 109/L in the LF-SSNHL, HF-SSNHL, AF-SSNHL, TD-SSNHL, and control groups, respectively. There was significant difference between each study group and control group in terms of lymphocyte count (all P < .01). The MPV values were 10.23 ± 0.94 fL, 10.04 ± 1.43 fL, 10.77 ± 1.56 fL, 10.85 ± 1.67 fL, and 9.75 ± 1.66 fL in the LF-SSNHL, HF-SSNHL, AF-SSNHL, TD-SSNHL, and control groups, respectively. The MPVs of AF-SSNHL and TD-SSNHL patients were significantly higher than that of the control group (P < .01, P < .01), however, not for LF-SSNHL and HF-SSNHL (P = .17, P = .45). The PLRs were 129.43 ± 53.35, 143.40 ± 79.64, 139.49 ± 60.34, 145.62 ± 68.88, and 98.48 ± 32.68 in the LF-SSNHL, HF-SSNHL, AF-SSNHL, TD-SSNHL, and control groups, respectively. The PLRs of all audiographically distinct SSNHL patients were significantly higher than that of the control group (P = .03, P < .01, P < .01, and P < .01 for LF-, HF-, AF-, and TD-SSNHL, respectively). There was no significant difference between each audiographically distinct SSNHL patients in terms of the platelet count, lymphocyte count, MPV, and PLR (all P > .05).
Figure 1.
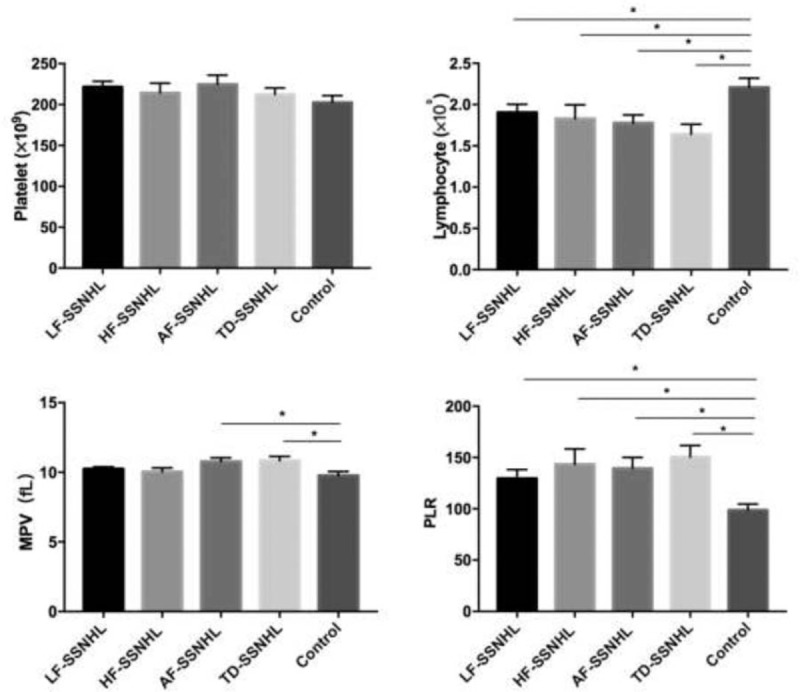
The difference of platelet, lymphocyte, MPV, and PLR between SSNHL groups and control group. AF-SSNHL = all-frequency SSNHL, HF-SSNHL = high-frequency SSNHL, LF-SSNHL = low-frequency SSNHL, MPV = mean platelet volume, PLR = platelet to lymphocyte ratio, SSNHL = sudden sensorineural hearing loss, TD-SSNHL = total deafness SSNHL. ∗Meant significant difference between 2 groups (P < .05). The error bars indicate standard errors.
3.2. Subgroup analysis
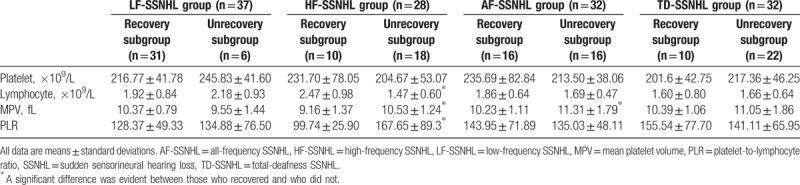
All patients were divided into subgroups based on whether or not they recovered (complete, notable, and partial recovery) after 1 months of treatment with steroids, vasodilators, neurotrophic factors, vitamins B1, and hyperbaric oxygen as required. The recovery rates were 83.78%, 35.71%, 50%, and 31.25% in the LF-SSNHL, HF-SSNHL, AF-SSNHL, and TD-SSNHL groups, respectively. Table 2 details the treatment outcomes. Table 3 lists the routine blood data of all patients. Lymphocyte count, MPV, and PLR of the HF-SSNHL subgroup (all P < .01) and MPV of the AF-SSNHL subgroup (P = .04) differed significantly from those of the other subgroups; no other parameter differed significantly among the subgroups (all P > .05).
Table 2.
Treatment outcomes of SSNHL patients with different audiograms.
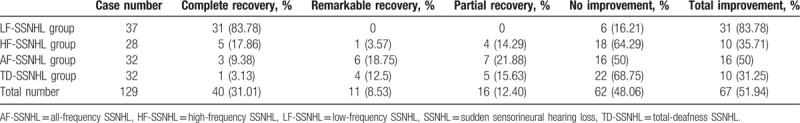
Table 3.
The blood routine parameters of the recovered and unrecovered group of 4 study groups.
4. Discussion
Both the Chinese and the German guidelines[1,4] stress that SSNHL subgrouped by reference to different audiographic features may indicate that different disease mechanisms are in play and that the optimal treatments should thus vary. Recently, many studies have shown that routine blood parameters can be used both to diagnose SSNHL and to predict prognosis. Unfortunately, only a few studies have explored the relationships between SSNHLs subgrouped by audiographic data and routine blood parameters.[16] Therefore, we determined whether significant relationships were evident between various SSNHL audiographic patterns and routine blood parameters.
Our major findings are the MPV of AF-SSNHL and TD-SSNHL, and PLR of all SSNHL patients was significantly higher than those of control group, while lymphocyte count of all audiographically distinct SSNHL patients were significantly lower than that of control group. However, no significant difference of blood parameters was observed among the 4 study groups. These indicated that although MPV and PLR may be related to SSNHL, they could not be used to classify the different type of SSNHL. Maybe we need further research to find the right biomarkers to distinguish different audiology features. Besides, the sample size we investigated was small, which may decrease the statistical power, so we need to expand the number of SSNHL patients in the further study.
Based on the therapy outcome, each SSNHL group was divided into recovery subgroup and unrecovery subgroup. Lymphocyte count, MPV, and PLR of the HF-SSNHL recovery subgroup and MPV of the AF-SSNHL recovery subgroup differed significantly from those of the unrecovery subgroups. This indicated that higher MPV and PLR, lower lymphocyte count of the HF-SSNHL, and higher MPV of the AF-SSNHL may indicate poor prognosis.
Both vascular and inflammatory factors contribute to SSNHL development.[14] The cochlea is supplied principally by the single, terminal labyrinthine artery (a terminal division of the anterioinferior cerebellar artery: the AICA), and the inner ear is thus susceptible to alterations in circulation.[17] SSNHL is associated with various clinical conditions including ischemic vascular disease, transient ischemic attacks, and amaurosis fugax.[18] In patients with vascular conditions, the walls of the blood vessels may be diseased (in those with arteritis and spasms) or intravascular conditions may be in play.[10] In addition, viral inflammation of the neural fibers and the ganglia may be important.[19]
In our data, the difference of lymphocyte count was shown significantly between audiographically distinct patients and controls; and lymphocyte count of the HF-SSNHL unrecovery subgroup was significantly higher than that of recovery subgroup. Both we and Seo et al[14] found that the levels of lymphocytes were decreased to levels just below the normal value; Seo et al emphasized that these findings could not be considered abnormal. Furthermore, İkinciogullari et al[20] and Masuda et al[21] found no correlation between the lymphocyte count and the recovery rate. Nonetheless, others have suggested that a reduced lymphocyte count is associated with poorer recovery from hearing loss.[10,21,22]
Platelets play active roles in the pathophysiology of thrombosis, coagulation, inflammation, and vessel atherosclerosis.[6,23] The MPV is a measure of platelet activation.[24] Larger platelets produce proteins efficiently exerting hemostatic, vasomotor, and proinflammatory functions.[25] An elevated MPV is a risk factor for thrombotic vascular disease.[3] Lower lymphocyte count is associated with virus inflammation; PLR is used to evaluate the extent of systemic inflammation[5] and indicator of the extent of endothelial injury to the peripheral vascular system. Higher PLR indicates that the extent of platelet adhesion to recently damaged vessels is elevated.[26] Both MPV and PLR are recognized as independent predictors of cardiovascular disease.[11,15,27,28] SSNHL has been considered to be associated with inflammation, ischemia, thrombosis, and embolisms. Recently, attention has been focused on the relationships between SSNHL and MPV and PLR.
Durmus et al[8] found that both MPV and PLR were significantly higher in SSNHL patients who did not recover than in those who did, emphasizing the predictive utilities of these parameters in terms of prognosis. In addition, Sagit et al[9] and Ulu et al[13] found that the MPV was significantly higher in SSNHL group than in controls, and that vascular impairment, ischemia, and thrombosis contributed to the pathogenesis of SSNHL. However, no significant correlation between MPV and the severity of hearing loss was apparent.[9,13] Moreover, Karli et al[3] and Ozturk et al[29] found no significant difference between either the MPV or the platelet level when an SSNHL group and a control group were compared. Neither these clinical observations nor the results of other clinical and experimental studies support the notion that SSNHL is of vascular etiology; the MPV may be influenced by many factors including septum deviation, major depression, and insulin resistance.[30–32] In our study, SSNHL patients were grouped by audiographic curves, the results showed that MPV of AF-SSNHL and TD-SSNHL group were significantly higher than that of control group, which may explain the conflicting data in the literature.
Moreover, Seo et al found that PLR was elevated in patients with both recurrent and nonrecurrent SSNHL, but were higher in the latter patients. Endothelial dysfunction was thought to play an important role in hearing loss physiopathology, associated with microvascular disturbance and inflammation.[33]
Recently, Salvago et al[16] compared differences in routine blood parameters (fibrinogen level; activated partial thromboplastin time; prothrombin time; hemoglobin, white blood cell, neutrophil, and lymphocyte levels; platelet count; and hematocrit) among SSNHL patients whose audiograms differed. The lymphocyte percentage was lower and the platelet count was higher in those with down-sloping HL than in others.
Overall, we found that lymphocyte count and PLR of all audiographically distinct SSNHL, MPV of AF-SSNHL, and TD-SSNHL were significantly higher than in controls, suggesting that inflammation, platelet activity, and thrombosis play major roles in SSNHL patients. The MPV of recovering HF-SSNHL and AF-SSNHL patients were significantly lower than those of patients who did not recover. Therefore, the MPV may be useful to predict the prognosis of HF-SSNHL and TD-SSNHL patients. Besides, the lymphocyte and PLRs of recovering HF-SSNHL were significantly different from those of patients who did not recover, thus, lymphocyte and PLR may indicate the prognosis of HF-SSNHL.
5. Conclusion
The most important findings are that lymphocyte count, MPV, and PLR may be relative to SSSNHL, but they could not be used to distinct SSNHL audiographically. However, platelet count did not show any relationship between SSNHL and controls, or among each SSNHL group. Furthermore, MPV and PLR in HF-SSNHL patients and MPV in AF-SSNHL patients could be used to predict the prognosis of therapy outcomes.
5.1. Ethical approval
All work with humans adhered to the ethical standards of Shanghai Jiao Tong University Affiliated Sixth People's Hospital and conformed to the dictates of the 1964 Helsinki Declaration and later amendments, or comparable ethical standards.
Acknowledgments
The authors thank grants from the Shanghai Municipal Education Commission/Gaofeng Clinical Medicine Grant Support (no. 20152526) and promote the Municipal Hospital Clinical Skills and Clinical Innovation Action Plan for three years (16CR4027A) to YF, the National Natural Science Foundation of China (no. 81530029) to SY, and the Shanghai Pujiang Program (no. 15PJD030) to ZC for the support.
Footnotes
Abbreviations: AF-SSNHL = all-frequency SSNHL, HF-SSNHL = high-frequency SSNHL, LF-SSNHL = low-frequency SSNHL, MPV = mean platelet volume, PLR = platelet-to-lymphocyte ratio, SSNHL = sudden sensorineural hearing loss, TD-SSNHL = total deafness SSNHL.
Funding/support: This study was supported by grants from the Shanghai Municipal Education Commission/Gaofeng Clinical Medicine Grant Support (no. 20152526) and promote the Municipal Hospital Clinical Skills and Clinical Innovation Action Plan for three years (16CR4027A) to YF, the National Natural Science Foundation of China (no. 81530029) to SY, and the Shanghai Pujiang Program (no. 15PJD030) to ZC.
The authors have no conflicts of interest to disclose.
References
- [1].Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery; Society of Otorhinolaryngology Head and Neck Surgery, Chinese Medical Association. [Guideline of diagnosis and treatment of sudden deafness (2015)]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2015;50:443–7. [PubMed] [Google Scholar]
- [2].Nosrati-Zarenoe R, Arlinger S, Hultcrantz E. Idiopathic sudden sensorineural hearing loss: results drawn from the Swedish national database. Acta Otolaryngol 2007;127:1168–75. [DOI] [PubMed] [Google Scholar]
- [3].Karli R, Alacam H, Unal R, et al. Mean platelet volume: is it a predictive parameter in the diagnosis of sudden sensorineural hearing loss? Indian J Otolaryngol Head Neck Surg 2013;65:350–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Michel O. Deutsche Gesellschaft für Hals-Nasen-Ohren-Heilkunde, Kopf- und Hals-Chirurgie. [The revised version of the german guidelines “sudden idiopathic sensorineural hearing loss”]. Laryngorhinootologie 2011;90:290–3. [DOI] [PubMed] [Google Scholar]
- [5].Balta S, Demirkol S, Fau-Kucuk U, et al. The platelet lymphocyte ratio may be useful inflammatory indicator in clinical. Hemodial Int 2013;17:668–9. [DOI] [PubMed] [Google Scholar]
- [6].Coppinger JA, Cagney G, Toomey S, et al. Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood 2004;103:2096–104. [DOI] [PubMed] [Google Scholar]
- [7].Hiramatsu M, Teranishi M, Uchida Y, et al. Polymorphisms in genes involved in inflammatory pathways in patients with sudden sensorineural hearing loss. J Neurogenet 2012;26:387–96. [DOI] [PubMed] [Google Scholar]
- [8].Durmus K, Terzi H, Karatas TD, et al. Assessment of hematological factors involved in development and prognosis of idiopathic sudden sensorineural hearing loss. J Craniofac Surg 2016;27:e85–91. [DOI] [PubMed] [Google Scholar]
- [9].Sagit M, Kavugudurmaz M, Guler S, et al. Impact of mean platelet volume on the occurrence and severity of sudden sensorineural hearing loss. J Laryngol Otol 2013;127:972–6. [DOI] [PubMed] [Google Scholar]
- [10].Kum RO, Ozcan M, Baklaci D, et al. Investigation of neutrophil-to-lymphocyte ratio and mean platelet volume in sudden hearing loss. Braz J Otorhinolaryngol 2015;81:636–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Huczek Z, Kochman J, Filipiaket KJ. Mean platelet volume on admission predicts impaired reperfusion and long-term mortality in acute myocardial infarction treated with primary percutaneous coronary intervention. ACC Curr J Rev 2005;14:1. [DOI] [PubMed] [Google Scholar]
- [12].Li H, Zhao D, Diao M, et al. Hyperbaric oxygen treatments attenuate the neutrophil-to-lymphocyte ratio in patients with idiopathic sudden sensorineural hearing loss. Otolaryngol Head Neck Surg 2015;153:606–12. [DOI] [PubMed] [Google Scholar]
- [13].Ulu S, Ulu MS, Ahsen A, et al. Increased levels of mean platelet volume: a possible relationship with idiopathic sudden hearing loss. Eur Arch Otorhinolaryngol 2013;270:2875–8. [DOI] [PubMed] [Google Scholar]
- [14].Seo YJ, Jeong JH, Choi JY, et al. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio: novel markers for diagnosis and prognosis in patients with idiopathic sudden sensorineural hearing loss. Dis Markers 2014;2014:702807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Papa A, Emdin M, Passino C, et al. Predictive value of elevated neutrophil-lymphocyte ratio on cardiac mortality in patients with stable coronary artery disease. Clin Chim Acta 2008;395:27–31. [DOI] [PubMed] [Google Scholar]
- [16].Salvago P, Rizzo S, Bianco A, et al. Sudden sensorineural hearing loss: is there a relationship between routine haematological parameters and audiogram shapes? Int J Audiol 2016;1–6. [DOI] [PubMed] [Google Scholar]
- [17].Mosnier I, Stepanian A, Baron G, et al. Cardiovascular and thromboembolic risk factors in idiopathic sudden sensorineural hearing loss: a case-control study. Audiol Neurootol 2011;16:55–66. [DOI] [PubMed] [Google Scholar]
- [18].Ballesteros F, Alobid I, Tassies D, et al. Is there an overlap between sudden neurosensorial hearing loss and cardiovascular risk factors? Audiol Neurotol 2009;14:139–45. [DOI] [PubMed] [Google Scholar]
- [19].Stokroos RJ, Albers FW, Schirm J. The etiology of idiopathic sudden sensorineural hearing loss. Experimental herpes simplex virus infection of the inner ear. Ann Otol Rhinol Laryngol 1998;108:423–8. [DOI] [PubMed] [Google Scholar]
- [20].İkinciogullari A, Koseoglu S, Kilic M, et al. New inflammation parameters in sudden sensorineural hearing loss: neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio. J Int Adv Otol 2015;10:197–200. [Google Scholar]
- [21].Masuda M, Kanzaki S, Minami S, et al. Correlations of inflammatory biomarkers with the onset and prognosis of idiopathic sudden sensorineural hearing loss. Otol Neurotol 2012;33:1142–50. [DOI] [PubMed] [Google Scholar]
- [22].Kanzaki S, Sakagami M, Hosoi H, et al. High fibrinogen in peripheral blood correlates with poorer hearing recovery in idiopathic sudden sensorineural hearing loss. Plos One 2013;9:e104680–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gawaz M, Langer H, May AE, et al. Platelets in inflammation and atherogenesis. J Thromb Haemost 2006;115:3378–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gasparyan AY, Ayvazyan L, Fau-Mikhailidis DP, et al. Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des 2011;17:47–58. [DOI] [PubMed] [Google Scholar]
- [25].Bath PM, Butterworth RJ. Platelet size: measurement, physiology and vascular disease. Blood Coagul Fibrinolysis 1996;7:157–61. [PubMed] [Google Scholar]
- [26].Gary T, Pichler M, Belaj K, et al. Platelet-to-lymphocyte ratio: a novel marker for critical limb ischemia in peripheral arterial occlusive disease patients. Plos One 2013;8:e67688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Varol E, Aksoy F, Ozaydin M, et al. Relationship between mean platelet volume and mitral annular calcification. Blood Coagul Fibrinolysis 2013;24:189–93. [DOI] [PubMed] [Google Scholar]
- [28].Acet H, Ertas F, Akil MA, et al. Relationship between hematologic indices and global registry of acute coronary events risk score in patients with ST-segment elevation myocardial infarction. Clin Appl Thromb Hemost 2016;22:60–8. [DOI] [PubMed] [Google Scholar]
- [29].Ozturk M, Kara A, Fau-Dasli S, et al. Mean platelet volume: is it a parameter associated with idiopathic sensorineural. Eur Arch Otorhinolaryngol 2014;271:2595–6. [DOI] [PubMed] [Google Scholar]
- [30].Varol E, Akcay S, Ozaydin M, et al. Mean platelet volume is associated with insulin resistance in non-obese, non-diabetic patients with coronary artery disease. J Cardiol 2010;56:154–8. [DOI] [PubMed] [Google Scholar]
- [31].Varol E, Akpinar A. Relationship between mean platelet volume and major depression. J Clin Psychopharmacol 2013;33:723–823. [DOI] [PubMed] [Google Scholar]
- [32].Sagit M, Korkmaz F, Kavugudurmaz M, et al. Impact of septoplasty on mean platelet volume levels in patients with marked nasal septal deviation. J Craniofac Surg 2012;23:974–6. [DOI] [PubMed] [Google Scholar]
- [33].Seo YJ, Park YA, Bong JP, et al. Predictive value of neutrophil to lymphocyte ratio in first-time and recurrent idiopathic sudden sensorineural hearing loss. Auris Nasus Larynx 2015;42:438–42. [DOI] [PubMed] [Google Scholar]


