Abstract
It is difficult to predict precisely whether the lesion corresponds to endoscopic resection indication. Furthermore, discrepancy may occur between endoscopic forceps biopsy (EFB) and finally resected specimen, which may be diagnosed as undifferentiated cancer and additional surgery may be required. Our study aimed to evaluate predictive factors to diagnose undifferentiated cancer after endoscopic submucosal dissection (ESD).
Among the 532 patients diagnosed by ESD between January 2009 and December 2015, 557 early gastric cancer (EGC) cases were studied. Factors predicting diagnosis of undifferentiated cancer and clinical outcomes of the lesions were retrospectively analyzed.
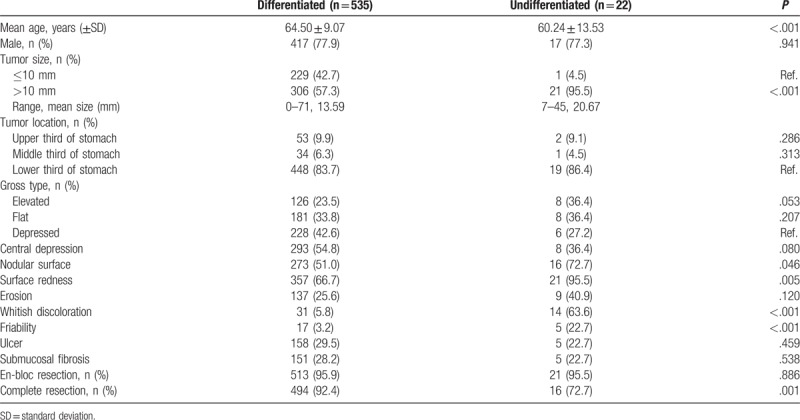
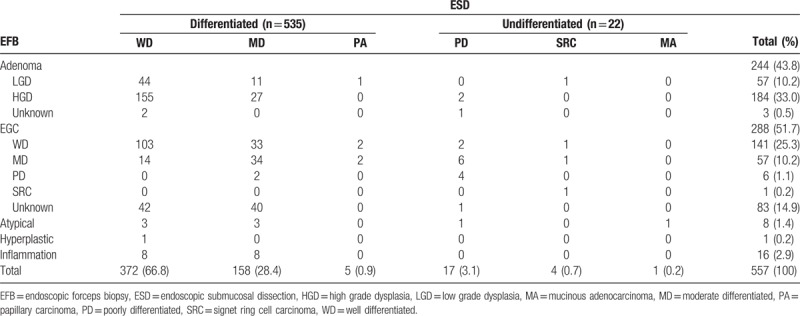
Among the 557 cases with EGC, 535 (96.1%) were diagnosed as differentiated cancer and 22 (3.9%) as the undifferentiated type with ESD. Tumor size was larger (mean size 20.67 vs 13.59 mm, P < .001) and age was lower (60.24 vs 64.50 years, P < .001) in the group with undifferentiated cancer. En bloc resection rate was similar (95.5% vs 95.9%, P = .886), but the complete resection rate was lower (72.7% vs 92.4%, P < .001) in the group with undifferentiated cancer. On multivariate analysis, tumor size ≥10 mm (OR = 11.340, P = .032), age <55 years (OR = 5.972, P = .004), surface redness (OR = 11.562, P = .024), and whitish discoloration (OR = 35.368, P < .001) were predominantly associated with undifferentiated cancer.
Young age (<55 years), large tumor size (≥10 mm), surface redness, and whitish discoloration are predictors of undifferentiated cancer, and lesions with these features detected need to be treated cautiously.
Keywords: early gastric cancer, endoscopic submucosal dissection, undifferentiated histology
1. Introduction
Owing to advanced diagnostic technology, the detection and incidence of early gastric cancer (EGC) has been increasing worldwide. Endoscopic submucosal dissection (ESD) is now accepted as a curative treatment for early gastric cancer (EGC) without lymph node metastasis, especially histologically differentiated mucosal cancer. With improvements in endoscopic techniques, it is suggested that ESD could be used for undifferentiated mucosal gastric cancers as well. A study concluded that there was no lymph node metastasis in patients with undifferentiated mucosal gastric cancer without lymphovascular invasion when the size of the tumor was 2 cm or smaller and without ulceration.[1] However, this report is not universally accepted and surgery is recommended if an undifferentiated cancer is diagnosed using endoscopic forceps biopsy (EFB).
EFB is the most important test for histological diagnosis before ESD. However, discrepancies in histological specimens obtained through EFB and ESD are common, and the causes for this might be (1) The forceps biopsy sample is too small to characterize the entire lesion. (2) The critical part of a lesion might not be accessible. (3) A targeted biopsy can be difficult because of the location of the lesions.[2] Adenomas may be diagnosed as EGC. According to previous studies, approximately 11.0% of biopsy proven low-grade adenomas are diagnosed as EGC[3] and approximately 66.5% of biopsy proven high-grade adenomas are diagnosed as EGC after ESD.[2] Likewise, a differentiated cancer might get diagnosed as an undifferentiated type. Studies have revealed that 1.5% to 8.0% of differentiated adenocarcinomas get diagnosed as undifferentiated adenocarcinomas after ESD.[4,5] Although precise diagnosis of lesions before ESD is important for proper treatment, such discrepancies make the process difficult.
The aim of our retrospective study was to identify endoscopic factors that could aid in the identification of an undifferentiated histology of a lesion before endoscopic resection
2. Materials and methods
2.1. Patients
Medical records of patients diagnosed with EGC using ESD between January 2009 and December 2015 were retrospectively reviewed at the Pusan National University Yangsan Hospital, South Korea. The indications for ESD in patient with EGC followed at our institution were well and/or moderately differentiated adenocarcinomas, tumors ≤ 2 cm in length, and absence of ulcer or ulcer-scar tissue before endoscopic resection. In some patients, regardless of these criteria, ESD was performed because of old age, severe comorbidities, patients refusing surgery, and suspicious lesions not initially diagnosed as EGC with EFB and finally diagnosed as EGC with ESD. On the contrary, there were 13 downgraded lesions (adenoma in 10 cases, negative pathology in 2 cases, and atypia in 1 case) after ESD in those initially diagnosed with EGC. In 1 patient, a piecemeal resection was performed, but the tissue was indeterminable. During the study period, 557 EGCs from 532 patients were diagnosed with ESD and divided into 2 groups, differentiated and undifferentiated (Fig. 1). Written informed consent was obtained from all patients before the procedure. The study was approved by the Ethics Committee that belongs to our Institutional Review Board.
Figure 1.
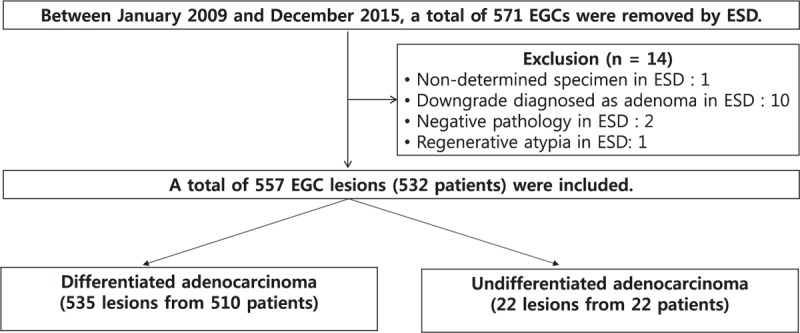
Flow chart showing lesions in patients enrolled in the study. EGC = early gastric cancer, ESD = endoscopic submucosal dissection.
2.2. Endoscopic biopsy
Diagnostic endoscopy (using GIF-H260 or GIF-H290; Olympus Optical Co., Ltd., Tokyo, Japan) and EFB were performed in all patients before ESD. Most patients were referred from other hospitals and underwent an additional EFB or a review of referred biopsy specimens. Although multiple biopsies enhance the likelihood of a better diagnosis, they lead to excessive fibrosis, which limits endoscopic resection. Thus, we performed a target biopsy only once or twice.
2.3. ESD procedure
We performed ESD using the previously described technique.[2] After marking the lesion, normal saline with a mixture of epinephrine and indigo carmine was injected into the submucosal layer to elevate the lesion off the muscularis propria. The mucosa surrounding the lesion was then precut using an electrosurgical generator (ERBE VIO 300D, Endocut I mode, Effect 3, duration 2; Erbe Co, Tubingen, Germany) with a needle-type electrosurgical knife (dual knife or flex knife) and an insulation-tipped electrosurgical knife. The submucosal connective tissue beneath the lesion was dissected with coagulation current (Swift coagulation 60 W, ERBE VIO 300D). After removal of the lesion, hot biopsy forceps were used for preventive post-ESD coagulation of all exposed vessels. Fig. 2 illustrates a case of ESD for poorly differentiated adenocarcinoma.
Figure 2.
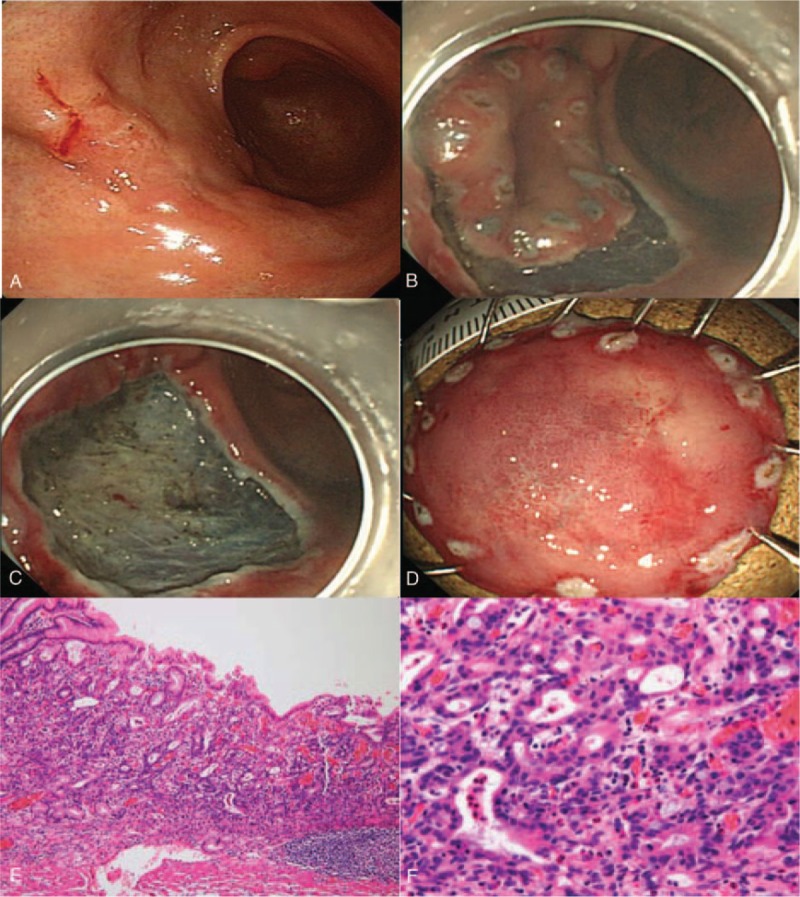
A case of poorly differentiated adenocarcinoma in a 44-year-old woman. A, Conventional endoscopic image: the lesion located in the anterior antral wall with surface redness and whitish discoloration. B,C, Endoscopic findings during ESD. D, En bloc resected ESD specimen (long diameter 3.1 cm). E, Pathologically diagnosed adenocarcinoma confined to mucosa. F, H&E stain, 400 × magnification, moderately to poorly differentiated adenocarcinoma. ESD = endoscopic submucosal dissection.
2.4. Endoscopic and pathologic evaluation
We assessed baseline characteristics and endoscopic findings of lesions in all patients enrolled in our study. Endoscopic examination was performed and reported by 2 endoscopists (DGR and SJK). Both the endoscopists were trained to review about 100 typical endoscopic findings before evaluating the endoscopic images in our study. A blind review was performed on all specimens. Both endoscopists concurred on the diagnosis of 479 of 557 lesions. For the remaining 78 lesions, the diagnosis was made through discussion and consensus. The Paris classification defined the gross types of superficial lesions, which were categorized as elevated, flat, or depressed.[6] Central depression, nodularity, surface redness, erosion, ulceration, whitish discoloration, friability, and submucosal fibrosis were also evaluated. Central depression was defined as a depression in the inner part of the lesion compared with the surrounding, regardless of gross type. Surface nodularity was defined as the presence of irregularly raised or nodular mucosa. Surface redness was defined as a red discoloration of the mucosal surface of the lesion compared with the surrounding mucosa. Erosion was defined as a shallow superficial mucosal defect. On the other hand, lesions with ulcerations or scarring from previous ulceration were those with converging folds or deformity of the muscularis propria or submucosal fibrosis. Whitish discoloration was defined as discolored lesion compared with surrounding mucosa. Friability was defined as minor spontaneous bleeding. Endoscopic pictures recorded the submucosal fibrosis observed during the ESD procedure. The location of lesions was described using the Japanese Classification of Gastric Cancer whereby the gastric area is divided into three equal sections: the upper-, middle-, and lower-third of the stomach.[7]
En bloc resection was defined as resection in a one-piece fashion with no residual tumor viewed endoscopically.[8] Complete R0 resection was defined as en bloc resection without any positive resection margins or lymphovascular invasion.[9]
All endoscopically resected tissue slides were subject to a blind review by 2 pathologists. Doubtful cases were re-evaluated under a multi-headed microscope to reach a consensus. The resected specimens were stretched, pinned, and fixed with formalin. Specimens resected in a piecemeal fashion were reconstructed. The fixed specimen was sectioned at 2-mm intervals. The length of the major and minor axes of all lesions was recorded.
2.5. Statistical analysis
Individual lesions were used to analyze the data obtained because some patients had multiple lesions. Univariate analysis was performed with χ2 test or the Fisher exact test for categorical variables and Student t test for continuous variables. Multivariate analysis with multiple logistic regression model identified predictive factors for undifferentiated cancer. P < .05 was considered statistically significant. Statistical calculations were performed with SPSS version 21.0 for Windows (SPSS Inc., Chicago, IL).
3. Results
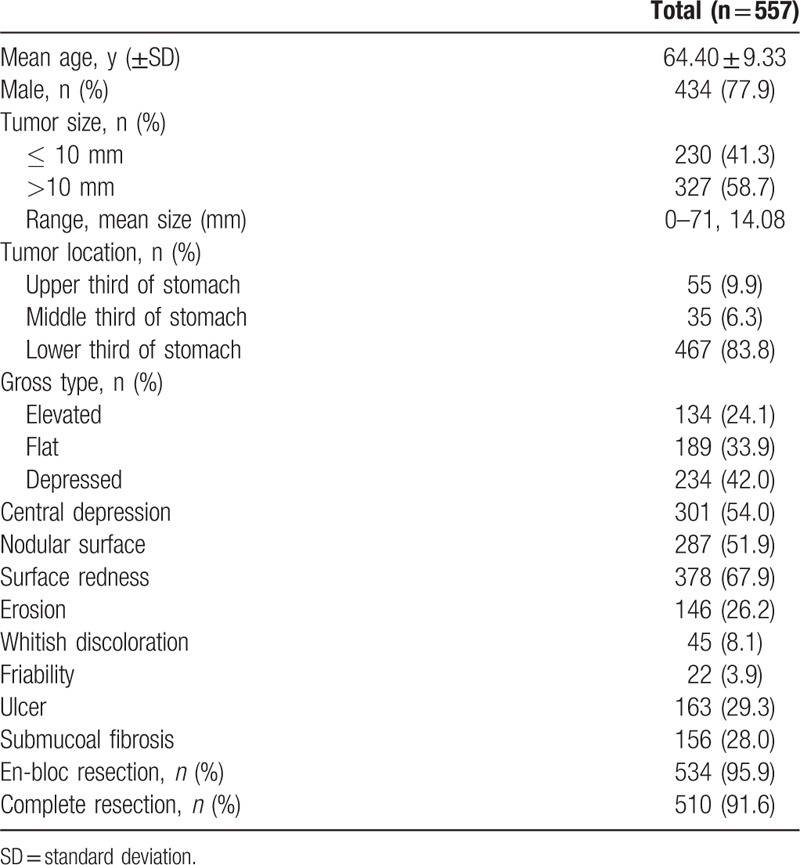
Among the 532 patients diagnosed with ESD between January 2009 and December 2015, 557 cases of EGC were enrolled in a study. The mean age was 64.40 ± 9.33 years, and the participants were predominantly men (77.9%). Table 1 shows the baseline characteristics of this study.
Table 1.
Baseline characteristics in this study.
These EGCs were divided into 2 groups, differentiated (n = 535) and undifferentiated (n = 22). On univariate analysis, the undifferentiated group showed larger tumor size (P < .001) and younger age (P < .001). En bloc resection rate was similar (P = .886), but complete resection rates was lower (P < .001) in the undifferentiated group (Table 2).
Table 2.
Baseline characteristics and endoscopic features comparing differentiated and undifferentiated cancer in univariate analysis.
Clinical and endoscopic characteristics associated with the undifferentiated histology were analyzed. Multivariate analysis revealed that tumor size ≥10 mm (OR = 11.340, P = .032), age <55 years (OR = 5.972, P = .004), surface redness (OR = 11.562, P = .024), and whitish discoloration (OR = 35.368, P < .001) were predominantly associated with undifferentiated cancer (Table 3). Figure 2 illustrates a case of ESD for poorly differentiated adenocarcinoma with surface redness and whitish discoloration. Each undifferentiated cancers with surface redness and whitish discoloration are shown in Figure 3.
Table 3.
Clinical and endoscopic characteristics associated with undifferentiated histology in endoscopic submucosal dissection in univariate and multivariate analysis.
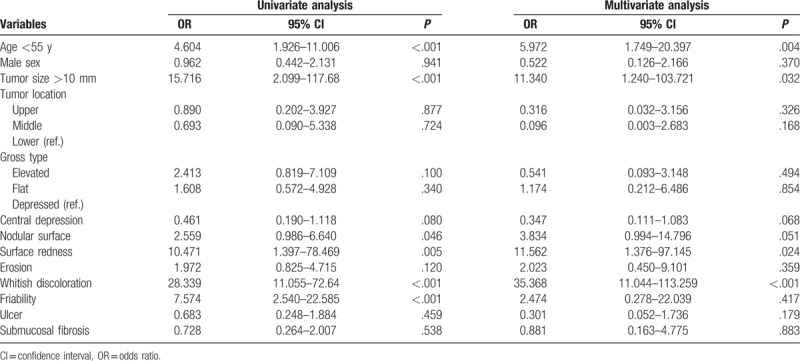
Figure 3.
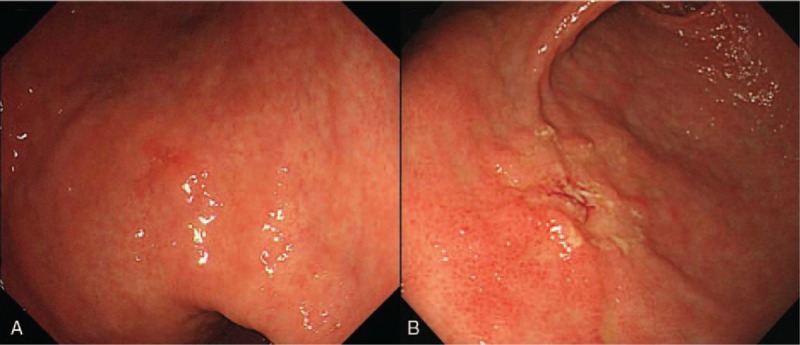
Undifferentiated cancers with surface redness (A) and whitish discoloration (B). A, The lesion located at lower body lesser curvature in lower body with surface redness. B, The lesion located at anterior wall in antrum with whitish discoloration.
Overall diagnostic discrepancy rate was 49.4% (282/571). Of the 198 lesions initially diagnosed as differentiated cancer on EFB, 94.9% (188/198) were eventually diagnosed as differentiated cancer and 5.1% (10/198) as undifferentiated type. Four adenomas initially diagnosed using EFB were finally diagnosed as undifferentiated cancer after ESD. On the contrary, of the seven lesions initially diagnosed as undifferentiated cancer on EFB, 71.4% (5/7) were eventually confirmed as undifferentiated cancer and 28.6% (2/7) as a differentiated type (Table 4).
Table 4.
Histologic comparison between endoscopic forceps biopsy and final endoscopic submucosal dissection.
Curative resection of the undifferentiated cancer was defined as complete resection with no submucosal invasion, no ulceration, and a diameter lesser than 2 cm. Nine patients underwent curative resection of the undifferentiated cancer. Among these, 2 patients underwent surgery and showed no lymph node metastasis. Local recurrence was found in 1 patient and additional surgery was performed, whereas the remaining patients were regularly followed up with endoscopy and abdominal computed tomography (CT) during a mean follow up of 30.2 months (range, 15–43 months). Among the patients who did not meet the criteria for curative resection, 4 patients underwent surgery and 1 had a N2 stage lymph node metastasis with the remaining patients undergoing regular follow up, and 1 patient showing liver metastasis (Fig. 4).
Figure 4.
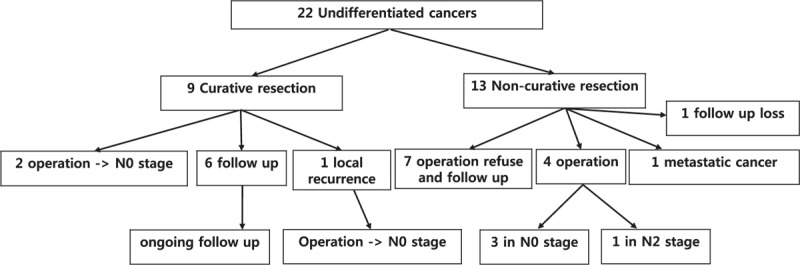
Flow diagram showing the undifferentiated cancer group.
4. Discussion
ESD has been widely accepted as a treatment modality for EGC with negligible risk of lymph node metastasis, especially in Japan and South Korea. Japanese[10] and South Korean[11] gastric cancer treatment guidelines are almost the same for the endoscopic treatment of EGC. According to these guidelines, ESD is an absolute indication for lesions meeting the following criteria: (1) lesions limited to the mucosal layer, (2) well and/or moderately differentiated adenocarcinomas, (3) tumors ≤ 2 cm in length, (4) absence of ulceration or ulcer scar tissue, and (5) tumors without lymphovascular involvement. Recently, owing to advanced technology and the increase in the number of ESD cases, these criteria have been expanded[12] and sometimes, depending on the situation, it is even applied beyond the expanded criteria. And if there is a discrepancy between the initial EFB and final ESD result that too would eventually exceed the indication for ESD. In this case, the risk of lymph node metastasis is higher than conventional lesions and the prognosis poorer. Thus, it is important to predict lesion size, depth, and degree of differentiation before ESD. The size of the lesion can be determined by endoscopic observation, chromoendoscopy, and magnifying endoscopy.[13] Although there are studies relating to conventional endoscopy to predict the depth of submucosal invasion, in addition to the use of endoscopic ultrasonography,[14–16] prediction based on histological differentiation is yet lacking.
In a recent study, endoscopic predictors for undifferentiated histology before endoscopic resection were reported as: large tumor size (>10 mm), depressed type, nodularity, and whitish discoloration—all related to undifferentiated histology after ESD.[17] In our study, large tumor size (≥10 mm) and whitish discoloration were also associated with undifferentiated histology, along with the additional predictor of surface redness, as determined on multivariate analysis. Large tumor size is a well-known factor associated with disease progression and advanced histology in EGC.[18] Whitish discoloration is associated with histological features of undifferentiated cancer. The tumor vessels were abundant and dense in well-differentiated or moderately differentiated adenocarcinoma, but were scanty and loose in poorly differentiated adenocarcinoma. These findings correlate with the redness of the carcinomatous mucosa of well-differentiated or moderately differentiated adenocarcinoma and the paleness of undifferentiated cancer.[19] Surface redness is a characteristic of differentiated histology. Although we do not know why exactly surface redness is associated with undifferentiated histology, we presume that a mixed histological type of cancer constituted about half (47.8%) the number of cases in this study, and surface redness is also associated with disease progression.[20] In our study, young age (<55 years) was also related to undifferentiated histology. Young age is well-known factor associated with undifferentiated cancer.[21] It is known that the depressed type usually exhibits more advanced lesions when classified according to the Paris classification which is most widely used in the gross classification of gastric superficial lesions.[22] In the above-mentioned study, depressed lesion was a risk factor for undifferentiated cancer.[17] However, the undifferentiated cancers in our study did not show a significant difference in Paris classification. (8 elevated type, 8 flat type, and 6 depressed type). Undifferentiated cancers in our study, color changes such as surface redness or whitish discoloration are more characteristic than classification of Paris classification.
Prognosis in patients with undifferentiated-type gastric cancer is poorer when compared with those with the differentiated type because the undifferentiated type is associated with frequent metastasis to the lymph nodes.[23] The risk of lymph node metastasis is higher in undifferentiated EGC with lymphovascular invasion[24] or submucosal invasion.[25] However, even in undifferentiated EGC lesions without lymphovascular invasion and ulcer size less than 20 mm, there is almost no lymph node metastasis.[1] Curative ESD for undifferentiated EGC had an excellent 5-year mortality rate as seen in previous studies;[26] in our study, 2 of 9 undifferentiated EGCs achieved curative resection were operated and all were in the N0 stage. One patient had recurrence and underwent operation, but N0 stage. The remaining patients showed no recurrence during follow up.
This study has some limitations. First, it was a retrospective study conducted in a single center. Second, the sample size might be too small to conclusively support the role of these risk factors in undifferentiated EGC. Third, there was a possibility of a discrepancy by performing once or twice biopsy in consideration of the endoscopic resection. But multiple biopsies may results in excessive fibrosis, which is a serious problem during ESD.
Our study can help to overcome discrepancy that may occur before and after ESD by evaluating predictive factors with undifferentiated histology in EGC. And if curative resection could be achieved, undifferentiated EGC was also found to be not worse prognosis in this study. In our study, young age (<55 years) and endoscopic characteristics such as large tumor size (≥10 mm), whitish discoloration, and surface redness were independently associated with undifferentiated histology after ESD. Therefore, for such lesions, physicians have exercise caution before performing ESD, and patients need to be informed about the risks of surgical gastrectomy.
Footnotes
Abbreviations: CI = confidence interval, EFB = endoscopic forceps biopsy, EGC = early gastric cancer, ESD = endoscopic submucosal dissection, HGD = high-grade dysplasia, LGD = low-grade dysplasia, MA = mucinous adenocarcinoma, MD = moderate differentiated, OR = odds ratio, PA = papillary carcinoma, PD = poorly differentiated, SRC = signet ring cell carcinoma, WD = well differentiated.
Written informed consent was obtained from all patients before the procedure. The study was approved by the Ethics Committee of the Institutional Review Board (Institutional Review Board no. L-2017–19).
The authors declare no conflicts of interest.
References
- [1].Hirasawa T, Gotoda T, Miyata S, et al. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer 2009;12:148–52. [DOI] [PubMed] [Google Scholar]
- [2].Ryu DG, Choi CW, Kang DH, et al. Clinical outcomes of endoscopic submucosa dissection for high-grade dysplasia from endoscopic forceps biopsy. Gastric Cancer 2016;20:671–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Choi CW, Kim HW, Shin DH, et al. The risk factors for discrepancy after endoscopic submucosal dissection of gastric category 3 lesion (low grade dysplasia). Dig Dis Sci 2014;59:421–7. [DOI] [PubMed] [Google Scholar]
- [4].Takao M, Kakushima N, Takizawa K, et al. Discrepancies in histologic diagnoses of early gastric cancer between biopsy and endoscopic mucosal resection specimens. Gastric Cancer 2012;15:91–6. [DOI] [PubMed] [Google Scholar]
- [5].Shim CN, Kim H, Kim DW, et al. Clinicopathologic factors and outcomes of histologic discrepancy between differentiated and undifferentiated types after endoscopic resection of early gastric cancer. Surg Endosc 2014;28:2097–105. [DOI] [PubMed] [Google Scholar]
- [6].The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003;58:S3–43. [DOI] [PubMed] [Google Scholar]
- [7].Japanese Gastric Cancer A. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011;14:101–12. [DOI] [PubMed] [Google Scholar]
- [8].Farhat S, Chaussade S, Ponchon T, et al. Endoscopic submucosal dissection in a European setting. A multi-institutional report of a technique in development. Endoscopy 2011;43:664–70. [DOI] [PubMed] [Google Scholar]
- [9].Oka S, Tanaka S, Kaneko I, et al. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc 2006;64:877–83. [DOI] [PubMed] [Google Scholar]
- [10].Japanese Gastric Cancer A. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011;14:113–23. [DOI] [PubMed] [Google Scholar]
- [11].Lee JH, Kim JG, Jung HK, et al. Clinical practice guidelines for gastric cancer in Korea: an evidence-based approach. J Gastric Cancer 2014;14:87–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000;3:219–25. [DOI] [PubMed] [Google Scholar]
- [13].Nagahama T, Yao K, Maki S, et al. Usefulness of magnifying endoscopy with narrow-band imaging for determining the horizontal extent of early gastric cancer when there is an unclear margin by chromoendoscopy (with video). Gastrointest Endosc 2011;74:1259–67. [DOI] [PubMed] [Google Scholar]
- [14].Kwee RM, Kwee TC. The accuracy of endoscopic ultrasonography in differentiating mucosal from deeper gastric cancer. Am J Gastroenterol 2008;103:1801–9. [DOI] [PubMed] [Google Scholar]
- [15].Choi J, Kim SG, Im JP, et al. Comparison of endoscopic ultrasonography and conventional endoscopy for prediction of depth of tumor invasion in early gastric cancer. Endoscopy 2010;42:705–13. [DOI] [PubMed] [Google Scholar]
- [16].Tsujii Y, Kato M, Inoue T, et al. Integrated diagnostic strategy for the invasion depth of early gastric cancer by conventional endoscopy and EUS. Gastrointest Endosc 2015;82:452–9. [DOI] [PubMed] [Google Scholar]
- [17].Choi JM, Kim SG, Yang HJ, et al. Endoscopic predictors for undifferentiated histology in differentiated gastric neoplasms prior to endoscopic resection. Surg Endosc 2016;30:89–98. [DOI] [PubMed] [Google Scholar]
- [18].Iwamoto J, Mizokami Y, Ito M, et al. Clinicopathological features of undifferentiated mixed type early gastric cancer treated with endoscopic submucosal dissection. Hepatogastroenterology 2010;57:185–90. [PubMed] [Google Scholar]
- [19].Mori M, Adachi Y, Kakeji Y, et al. Superficial flat-type early carcinoma of the stomach. Cancer 1992;69:306–13. [DOI] [PubMed] [Google Scholar]
- [20].Goldstein NS, Lewin KJ. Gastric epithelial dysplasia and adenoma: historical review and histological criteria for grading. Hum Pathol 1997;28:127–33. [DOI] [PubMed] [Google Scholar]
- [21].Eguchi T, Takahashi Y, Yamagata M, et al. Gastric cancer in young patients. J Am Coll Surg 1999;188:22–6. [DOI] [PubMed] [Google Scholar]
- [22].Endoscopic Classification Review G. Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy 2005;37:570–8. [DOI] [PubMed] [Google Scholar]
- [23].Zheng H, Takahashi H, Murai Y, et al. Pathobiological characteristics of intestinal and diffuse-type gastric carcinoma in Japan: an immunostaining study on the tissue microarray. J Clin Pathol 2007;60:273–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Park YD, Chung YJ, Chung HY, et al. Factors related to lymph node metastasis and the feasibility of endoscopic mucosal resection for treating poorly differentiated adenocarcinoma of the stomach. Endoscopy 2008;40:7–10. [DOI] [PubMed] [Google Scholar]
- [25].Hanaoka N, Tanabe S, Mikami T, et al. Mixed-histologic-type submucosal invasive gastric cancer as a risk factor for lymph node metastasis: feasibility of endoscopic submucosal dissection. Endoscopy 2009;41:427–32. [DOI] [PubMed] [Google Scholar]
- [26].Abe S, Oda I, Suzuki H, et al. Short- and long-term outcomes of endoscopic submucosal dissection for undifferentiated early gastric cancer. Endoscopy 2013;45:703–7. [DOI] [PubMed] [Google Scholar]


