Abstract
Background:
Orthostatic hypotension (OH) is a major clinical sign of cardiovascular autonomic dysfunction in diabetic patients. Our aim was to quantitatively evaluate the prevalence and risk factors of OH in patients with diabetes mellitus (DM) and assess its prognosis.
Methods:
A comprehensive search of the PubMed, Embase, China National Knowledge Infrastructure, VIP Chinese Journal, Wanfang, and SINOMED databases was conducted for related published work up to September 25, 2016, and manually searched eligible studies from the references in accordance with the inclusion criteria.
Results:
We included 21 studies in the analysis, with a total sample size of 13,772. The pooled prevalence of OH in DM was 24% (95% confidence interval [CI]: 19–28%). Potential risk factors, that is, glycosylated hemoglobin A (HbA1c) (odds ratio [OR], 1.13, 95% CI, 1.07–1.20), hypertension (OR, 1.02, 95% CI, 1.01–1.02), and diabetic nephropathy (OR, 2.37, 95% CI, 1.76–3.19), were significantly associated with OH in DM. In addition, the prognosis of OH in DM was associated with higher risk of total mortality and cardiovascular events.
Conclusion:
The pooled prevalence of OH in DM appears high. HbA1c, hypertension, and diabetic nephropathy are risk factors for OH in DM. OH indicates poor prognosis in diabetic patients. Attention should be focused on diabetic patients with the stated risk factors to prevent OH.
Keywords: diabetes mellitus, meta-analysis, orthostatic hypotension, prevalence, risk factors
1. Introduction
Orthostatic hypotension (OH) is a major clinical sign of cardiovascular dysautonomia and affects between 5% and 30% of all individuals in an age-dependent manner.[1] According to the consensus of the Committee of the American Autonomic Society and the American Academy of Neurology,[2] OH is most widely defined as a fall in systolic blood pressure (SBP) of at least 20 mm Hg or a drop in diastolic blood pressure (DBP) of at least 10 mm Hg within 3 minutes of standing. OH is easy to diagnose; however, it is often overlooked in clinical practice because it is asymptomatic in normal conditions. The risk factors associated with OH include older age; use of antihypertensive drugs; and comorbidities such as hypertension, cardiac failure, and kidney disease.[3,4] OH is associated with increased risk for cardiovascular mortality in different populations.[5–7] OH also may lead to falls and syncope.[8,9]
OH and diabetes mellitus (DM) are a dangerous combination. OH prevalence is higher in patients with DM as compared with people without DM.[10,11] Furthermore, both DM and OH are strong predictors of mortality and cardiovascular events.[12] In patients with DM, OH may indicate the increased risk of developing severe complications, and reduces quality of life.[13] Hypertension occurs in >50% of adults with DM. Although the incident rate of OH is not related to aggressive (SBP < 120 mm Hg) versus standard (SBP < 140 mm Hg) blood pressure target assignment,[14] some antihypertensive medications, including diuretics, α-blockers, and vasodilators tend to cause OH.[15] Currently, there is no satisfactory drug for treating OH. Many studies have reported the prevalence, risk factors, and prognosis of OH in patients with DM, but quantitative estimation of OH in such patients is scarce. We, therefore, conducted a systematic review and meta-analysis in patients with DM with OH to estimate its prevalence, identify the risk factors, and evaluate the prognosis. The pooled estimates could lead to insights for better prevention strategies of OH and alert clinicians to pay closer attention to the use of antihypertensive medications.
2. Methods
2.1. Ethics statement
As all analyses are based on previously published studies, ethical approval was not necessary for this review.
2.2. Search strategy
Two investigators independently searched the PubMed, Embase, China National Knowledge Infrastructure, VIP Chinese Journal, Wanfang, and SINOMED databases for published related work up to September 25, 2016, and manually searched eligible studies from the references in accordance with the inclusion criteria. The search terms were “orthostatic hypotension,” “postural hypotension,” “diabetes mellitus,” and their corresponding keywords in Chinese. Discrepancies were resolved by additional assessment by a third investigator. The corresponding authors of the selected articles were contacted for information missing from the published articles.
2.3. Inclusion and exclusion criteria
Studies potentially eligible for inclusion had to meet the following criteria: (1) written in English or Chinese; (2) investigated subjects with OH in a sample of patients with DM, with original data being derived from cross-section, case–control, and a certain phase in a longitudinal study; (3) reported estimates of prevalence or odds ratio (OR) with corresponding 95% confidence interval (CI) or reported sufficient data to calculate them; (4) for studies with overlapping data sets, the study with the most complete data was selected. We excluded: (1) case reports, conference abstracts, and drug trials (as drugs might induce OH or cause hypotension); and (2) studies that did not meet ≥1 inclusion criteria.
2.4. Data extraction and methodological quality score
Two researchers extracted the literature independently. For all included studies, we extracted the following information: first author and publication year, type of DM, quality score, sample size, country, setting, and definition of OH and prevalence. Table 1 lists the characteristics of the 21 studies included in this meta-analysis.[8,9,11,14,16–32]
Table 1.
Characteristics of the 21 studies included in this meta-analysis.
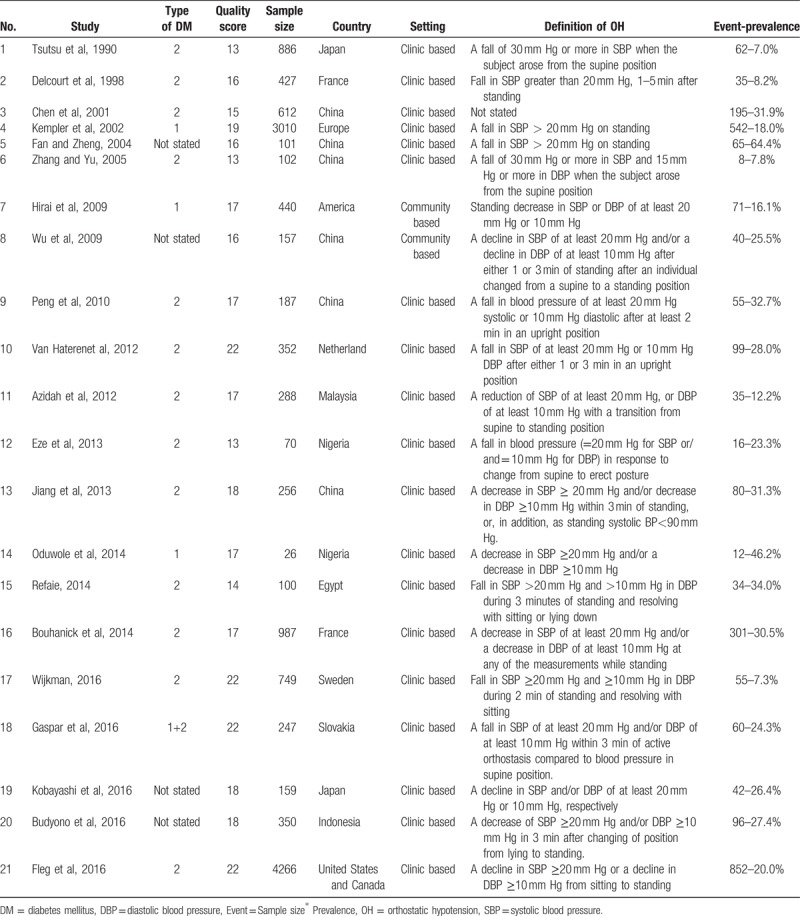
We assessed the quality of the eligible literature according to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement.[33] If a study fulfilled the item, 1 point was awarded. If it did not, 0 points were awarded. All items were assumed to be of equal importance and were not weighted. Quality was graded according to the score: a higher score indicated a higher quality study.
2.5. Statistical analysis
We quantitatively evaluated the prevalence and potential risk factors of OH in DM by meta-analysis. The correlation between risk factors and OH was examined based on the OR for case–control studies, and risk ratio (RR) or hazard ratio (HR) for cohort studies. We assumed that these effect measures would yield a similar effect estimate in the overall effect estimate[34] and would be referred to as OR. When both crude and adjusted OR were provided, the fully adjusted value for potential confounding variables was selected. When this was not possible, a qualitative descriptive analysis was performed.
Heterogeneity between studies was estimated using the Q test and I2 statistic, which show the percentage of variation between studies that is due to heterogeneity rather than chance. I2 < 25%, 25–50%, and >50% is considered low, moderate, and high-level heterogeneity, respectively.[1,35] Where there was high inter-study heterogeneity (I2 > 50%), the random effects model was used to combine results. Otherwise, the fixed effects model was used. We used the DerSimonian and Laird method in the random effects models for the pooled estimation of prevalence. Subgroup analysis was conducted to investigate the source of heterogeneity (type of DM, methodological quality score, sample size, country, and definition of OH).
Sensitivity analyses were performed by excluding the studies performed in community. Begg's and Egger's tests were used to test publication bias. A P-value of <.05 was considered statistically significant. All statistical calculations were made using STATA 12.0 (StataCorp, College Station, TX).
3. Results
3.1. Search results
A total 3679 potentially relevant studies were retrieved, of which only 21 were eventually included in the meta-analysis. Figure 1 shows the detailed flowchart of study selection. A total 28 full-text articles were selected for further review. Of these, 21 fulfilled our selection criteria. We excluded the remaining articles because of overlapping data (n = 3), article was not in English (n = 2), article was not full-text (n = 2).
Figure 1.
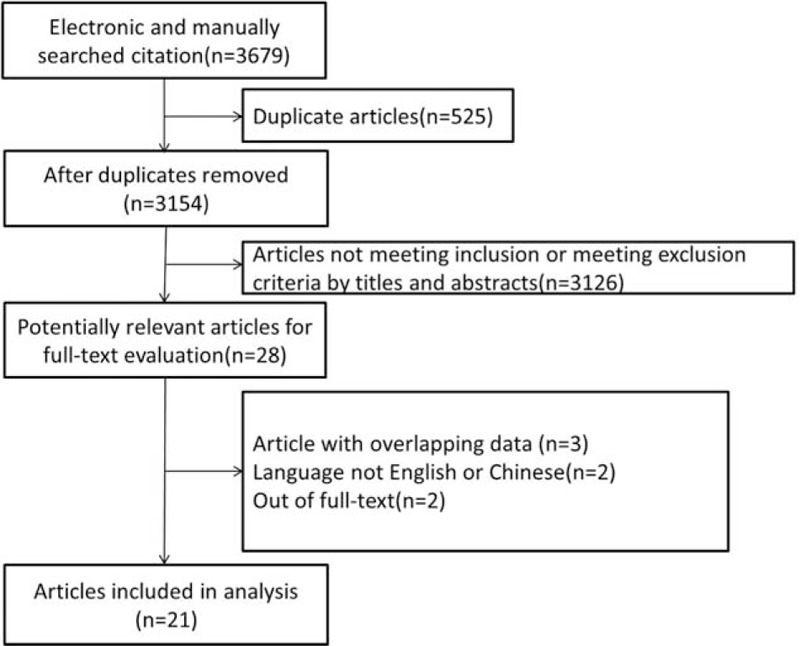
Flow diagram of study selection.
3.2. Prevalence of OH in DM
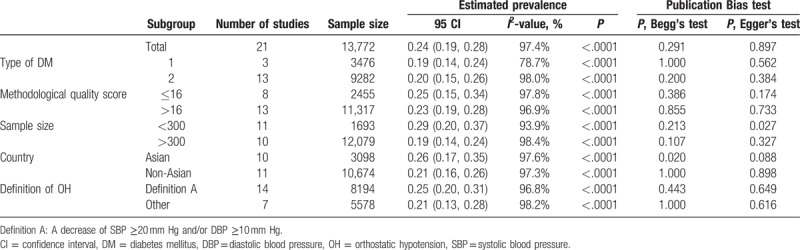
The included studies documented sample sizes ranging from 26 to 4266 subjects, with a total sample size of 13,772. The prevalence of OH in patients with DM ranged between 7.0% and 64.4%, and pooled prevalence was 24% (95% CI: 19%–28%, Fig. 2). The diabetic patient population was subgrouped according to type of DM, methodological quality score, sample size, country, and definition of OH. Table 2 presents the pooled prevalence of these subgroups, and information on the data heterogeneity and the publication bias. The pooled prevalence in the subgroups ranged from 19% to 29%, and heterogeneity was high; the I2 ranged from 78.7% to 98.4%. No significant bias in the included studies were revealed by Begg's test (P = .291) and Egger's test (P = .897). Sensitivity analysis was conducted by excluding the 2 studies performed in community, and the results of the pooled prevalence remained the same.
Figure 2.
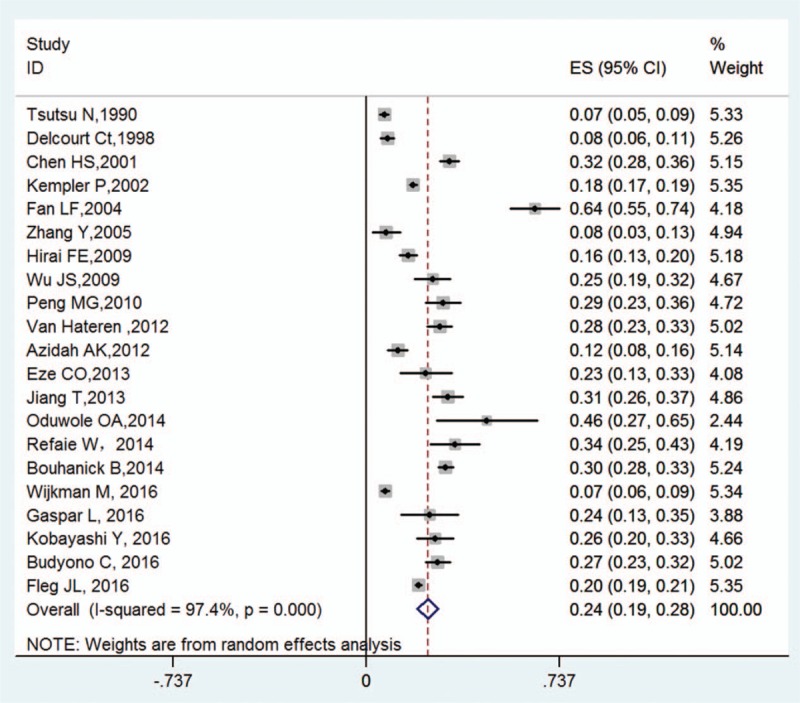
Prevalence of OH in DM and the pooled prevalence rate per study (with the corresponding 95% CI). CI = confidence interval, DM = diabetes mellitus, ES = estimated statistics, OH = orthostatic hypotension.
Table 2.
Subgroup analyses of prevalence rates of orthostatic hypotension in diabetes mellitus.
3.3. Risk factors of OH in DM
Table 3 summarizes the risk factors of statistical testing (P < .05) of the major risk factors in univariate analysis, and unadjusted and adjusted multivariable regression analysis. Of the various risk factors, only 7 risk factors from 9 studies were eligible for the exploratory meta-analysis: age, sex, HbA1c (glycosylated hemoglobin A), BMI (body mass index), supine SBP, hypertension, and diabetic nephropathy.
Table 3.
Risk factors of orthostatic hypotension in diabetes mellitus.
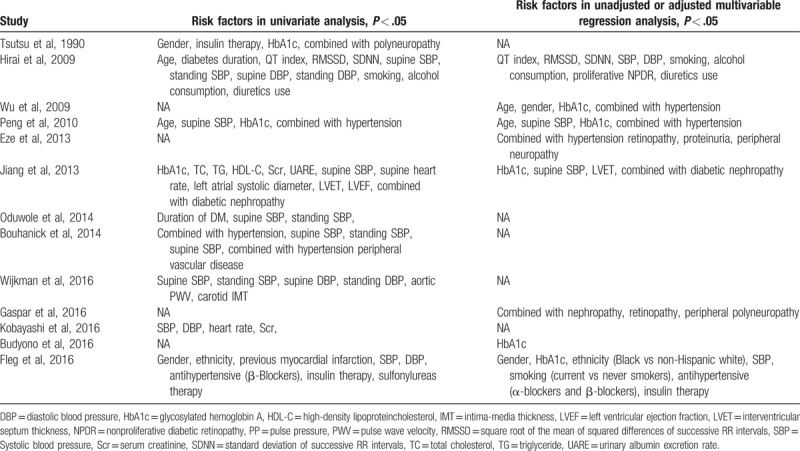
3.3.1. Age
Seven studies reported the age effect size.[11,14,20–22,29,31] The overall estimate of age as a risk factor was 1.01 (95% CI, 1.00–1.02, Z = 1.84, P = .066), suggesting no significant difference. There was low heterogeneity (I2 = 0.00%, P = .594; Fig. 3) and no publication bias as per Begg's test (P = .368) and Egger's test (P = .260).
Figure 3.
Forest plot of age and OH in DM. DM = diabetes mellitus, OH = orthostatic hypotension.
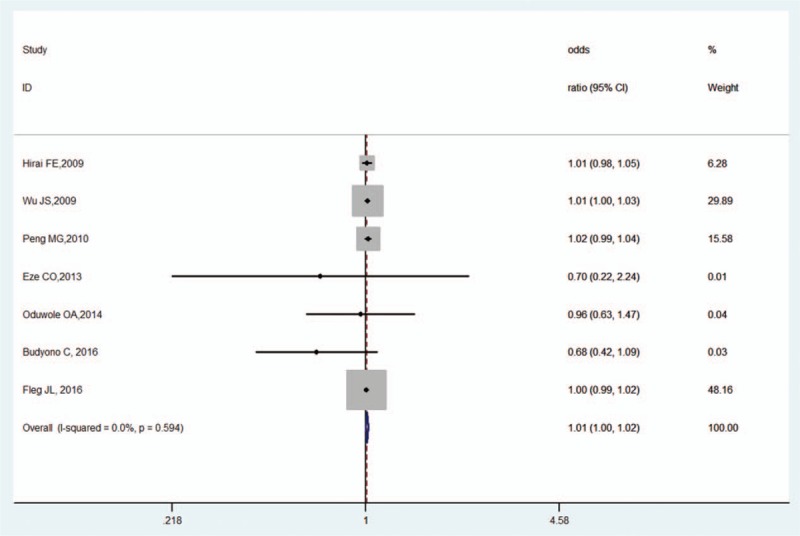
3.3.2. Sex
Three studies assessed the effects of sex.[11,20,21] Compared with female patients, male patients did not have significantly higher risk for OH in DM (OR, 0.96, 95% CI, 0.58–1.60, Z = 0.15, P = .881). There was low heterogeneity (I2 = 0.00%, P = .537; Fig. 4) and no publication bias as per Begg's test (P = 1.000) and Egger's test (P = .911).
Figure 4.
Forest plot of sex and OH in DM. DM = diabetes mellitus, OH = orthostatic hypotension.
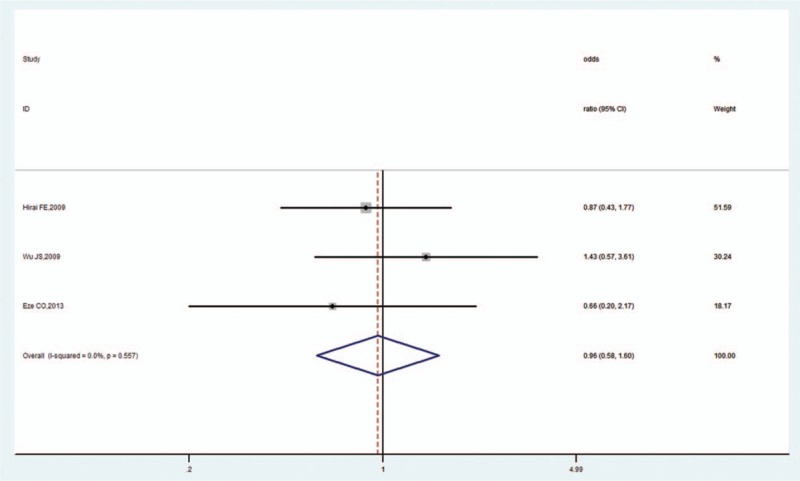
3.3.3. HbA1c
Seven studies evaluated the influence of HbA1c on OH in DM.[11,14,20,22,29,31,32] The pooled OR was 1.13 (95% CI, 1.07–1.20, Z = 4.34, P < .001), which indicated that high HbA1c increased the risk of OH in DM. There was low heterogeneity (I2 = 38.9%, P = .133; Fig. 5) and no publication bias as per Begg's test (P = .368) and Egger's test (P = .224).
Figure 5.
Forest plot of HbA1c and OH in DM. DM = diabetes mellitus, OH = orthostatic hypotension.
3.3.4. BMI
Three studies reported the effect size of BMI.[14,20,22] The pooled OR was 1.00 (95% CI, 0.98–1.01, Z = 0.07, P = .948), revealing that BMI and OH in DM were not correlated. There was low heterogeneity (I2 = 9.7%, P = .330; Fig. 6) and no publication bias as per Begg's test (P = 1.000) and Egger's test (P = .530).
Figure 6.
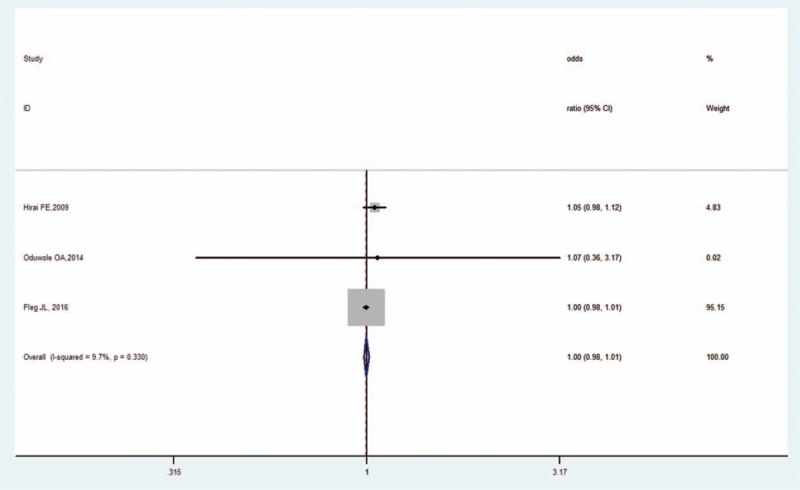
Forest plot of BMI and OH in DM. BMI = body mass index, DM = diabetes mellitus, OH = orthostatic hypotension.
3.3.5. Supine SBP
Four studies reported the supine SBP effect size.[14,20,31,32] The pooled OR was 1.39 (95% CI, 0.95–2.03, Z = 1.68, P = .093), indicating that supine SBP and OH in DM were not correlated. There was high heterogeneity (I2 = 71.2%, P = .008; Fig. 7) but no publication bias as per Begg's test (P = 1.000) and Egger's test (P = .834).
Figure 7.
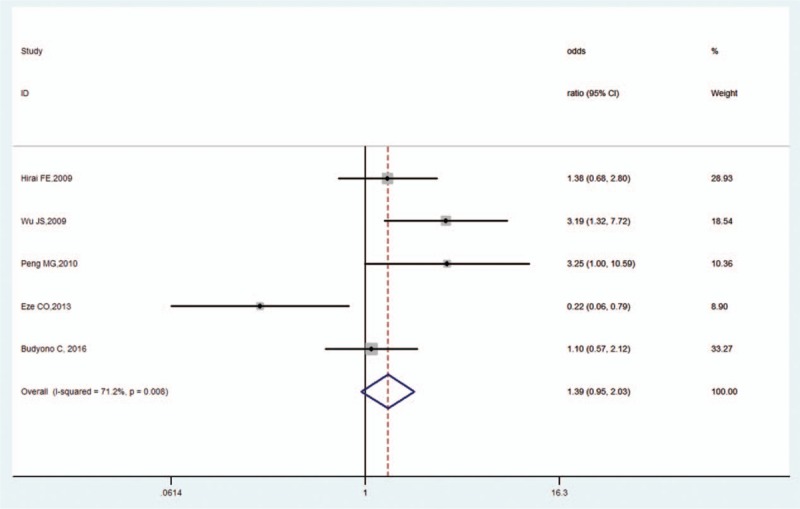
Forest plot of supine SBP and OH in DM. DM = diabetes mellitus, OH = orthostatic hypotension, SBP = systolic blood pressure.
3.3.6. Hypertension
Five studies analyzed the impact of hypertension on OH in DM.[11,20,21,29,31] Compared to nonhypertensive patients, patients with hypertension showed the increased risk for OH in DM (OR, 1.02, 95% CI, 1.01–1.02, Z = 6.13, P < .001). There was high heterogeneity (I2 = 77.5%, P = .004; Fig. 8) and no publication bias as per Begg's test (P = .734). However, Egger's test revealed publication bias (P = .038).
Figure 8.
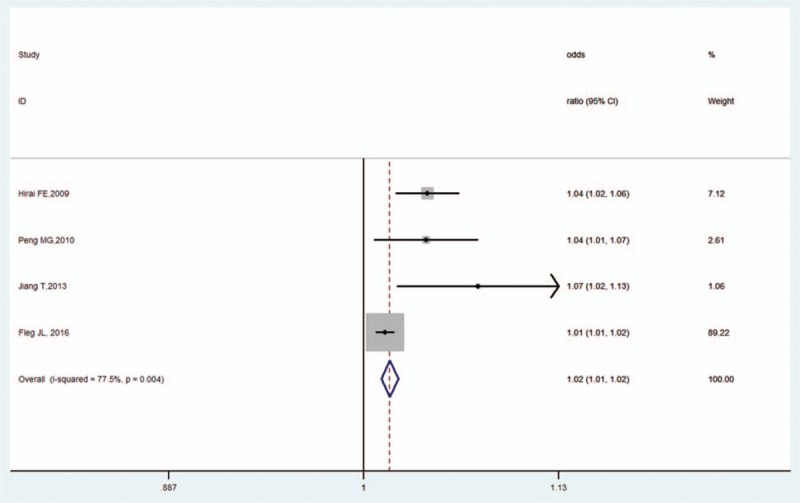
Forest plot of hypertension and OH in DM. DM = diabetes mellitus, OH = orthostatic hypotension.
3.3.7. Diabetic nephropathy
Five studies reported the diabetic nephropathy effect size.[20,21,27,29,32] The pooled OR was 2.37 (95% CI, 1.76–3.19, Z = 5.69, P < .001), revealing a correlation between diabetic nephropathy and OH in DM. There was high heterogeneity (I2 = 75.5%, P < .001; Fig. 9) and significant publication bias as per Begg's test (P = .009) and Egger's test (P = .002).
Figure 9.
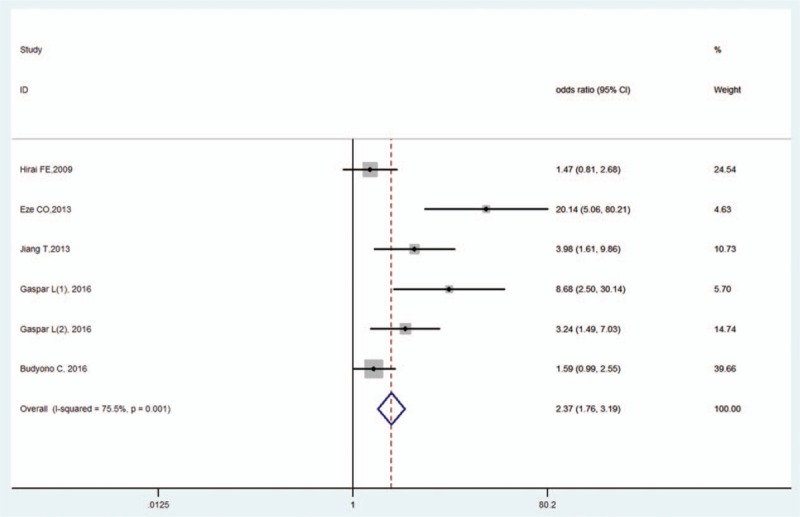
Forest plot of diabetic nephropathy and OH in DM. DM = diabetes mellitus, OH = orthostatic hypotension.
3.4. Prognosis of OH in DM
Four cohort studies reported the prognosis of OH in DM.[14,25–27] Bouhanick et al[25] showed that, during a 5-year follow-up, OH was associated with severe hypertension and amputations in patients with type 2 DM. Fleg et al[14] demonstrated that the occurrence of OH was an independent marker of total mortality and heart failure death or hospitalization but not for nonfatal myocardial infarction (MI), stroke, cardiovascular death, or their composite over a median follow-up of 46.9 months. In a 10-year follow-up retrospective study, Gaspar et al[27] reported that the 10-year mortality rate was higher in diabetic patients with OH. However, a prospective cohort study by Wijkman et al[26] showed that OH in DM was not significantly associated with risk of combined end-point (the first nonfatal or fatal event of hospitalization for acute MI, stroke, or cardiovascular mortality).
4. Discussion
This is the first meta-analysis to date to investigate the prevalence of OH in DM. We also evaluated the risk factors and prognosis of OH in DM. The OH prevalence we detected is within in the range reported in previous nonsystematic reviews on the subject. Although out meta-analysis revealed much inter-study variation, most studies had large sample sizes (>100) that could produce precise estimates.
The prevalence of OH in DM varied widely across studies. It is worth noting that 2 important factors can influence the results. First, the definition of OH in the studies differed slightly from the criteria in the consensus statement. For example, Bouhanick et al[25] defined OH as a decrease in SBP of at least 20 mm Hg and/or a decrease in DBP of at least 10 mm Hg at any of the measurements while standing. If the consensus definition had been adopted, the prevalence of OH in DM would have been 26.8% instead of 30.5%. Second, elderly patients with DM are often unable to stand up; therefore, the sitting BP is measured instead of standing BP. Chen et al[18] reported that standing tests did not yield reliable data for 60 patients (9.8%), which is considered an underestimation for OH. These factors led to the inconsistent prevalence of OH in DM. In our study, meta-analysis determined that the pooled prevalence of OH in DM was 24% (95% CI: 19%–28%) after the above comprehensive factors had been considered. The pooled prevalence in the subgroups ranged from 19% to 29%. This indicates high OH prevalence, which warrants further study.
In the diabetic population, OH is associated with conventional diabetes risk factors, such as age, duration of DM, hypertension, smoking, hyperlipidemia, and obesity.[36,37] However, the risk factors in each study were not uniform. In the meta-analysis, we quantitatively evaluated 7 potential risk factors of OH in DM derived from 9 included studies, which were free from the variation of a single study sample. Among the 7 factors, we found that HbA1c, hypertension, and diabetic nephropathy were significantly associated with the increased risk of OH in DM.
OH occurs because of impaired sympathetic response to postural change secondary to poor norepinephrine response and abnormalities in baroreceptor sensitivity, resulting in the inadequate heart rate response and peripheral vasoconstriction.[36,37] HbA1c levels can reflect the general condition of blood sugar within 2 to 3 months. Poor glycemic control, as shown by high HbA1c levels, can affect vascular elasticity and reduce extravascular volume due to osmotic dieresis, eventually inducing OH.[38] Furthermore, poor glycemic control may cause decreased vasodilatation, leading to diminished blood flow in nerve fibers by reducing myo-inositol content. Autonomic denervation causes endothelial dysfunction and reduces neuropeptide responses, volume depletion caused by nephropathy, and osmotic dieresis, which may induce OH.[39,40] Hypertension can decrease carotid sinus baroreflex and β-adrenergic sensitivity and impair arterial elasticity and myocardial compliance. These physiopathological changes are closely associated with OH.[13] Here, we observed a higher prevalence of diabetic nephropathy in patients with OH. OH is a common feature of diabetic cardiac autonomic neuropathy (CAN). The impact of CAN in the pathogenesis of diabetic nephropathy is that it alters kidney glomeruli hemodynamics mainly through endothelial dysfunction, albuminuria, and erythropoietin insufficiency-induced anemia.[41] Therefore, it is vital to control the blood glucose and BP and delay the progression of diabetic nephropathy in diabetic patients.
We also determined that the prognosis of OH in DM is associated with higher risk of total mortality and cardiovascular events. These results were not surprising and mainly confirm that OH is an expression of an advanced CAN correlated with MI, stroke, and macrovascular and microvascular complications. A recent review has indicated that the presence of OH may complicate the treatment of hypertension, heart failure, and coronary heart disease.[42] Therefore, orthostatic evaluation should also be a part of the diagnostic workup in diabetic patients. However, randomized trials have shown that only a few pharmacological agents, including droxidopa[43] and midodrine,[44] demonstrate a moderate effect as compared to the placebo group. We do not know if these OH therapies will in any way alter mortality rates. More studies on the potential mechanisms of OH are needed.
Although this meta-analysis includes more studies with larger sample sizes than individual studies, there are some limitations. First, the meta-analysis of OH prevalence in DM showed large inter-study heterogeneity. The subgroup and sensitivity analyses were not much different from the meta-analysis results. Moreover, we did not perform specific statistical comparisons with the previous results in all subgroups owing to data limitations, different grouping methods, and nonspecific analysis of the previous data. We also believe that drug factors may have influenced heterogeneity. Many medications commonly used by patients with DM, such as diuretics, vasodilators, tricyclic antidepressants, and insulin, can aggravate OH.[45] However, we did not acquire sufficient information about these aspects for subsequent analysis. Second, the OR, RR, and HR were treated in the same way in our systematic review. Generally, it is reasonable to combine the OR with RR (or HR) when the incidence is very low (<10%).[46] With a higher incidence, combining OR and RR is more likely to generate bias. Third, not all the studies reported estimates of risk factors (OR, RR, or HR value).
In conclusion, this meta-analysis reveals that the pooled prevalence of OH in DM is high and that HbA1c, hypertension, and diabetic nephropathy are risk factors for OH in DM. More attention should be paid to diabetic patients with these risk factors in order to prevent OH, and more studies on the potential mechanisms of OH are needed.
Acknowledgments
The authors thank all individuals that contributed to data or additional information about the study.
Footnotes
Abbreviations: CAN = diabetic cardiac autonomic neuropathy, CI = confidence interval, DBP = diastolic blood pressure, DM = diabetes mellitus, HbA1c = glycosylated hemoglobin A, HDL-C = high-density lipoproteincholesterol, HR = hazard ratio, IMT = intima-media thickness, LVEF = left ventricular ejection fraction, LVET = interventricular septum thickness, NPDR = nonproliferative diabetic retinopathy, OH = orthostatic hypotension, PP = pulse pressure, PWV = pulse wave velocity, RMSSD = square root of the mean of squared differences of successive RR intervals, RR = risks ratio, SBP = systolic blood pressure, Scr = serum creatinine, SDNN = standard deviation of successive RR intervals, TC = total cholesterol, TG = triglyceride, UARE = urinary albumin excretion rate.
Funding: This work was supported by the Key Clinical Specialty Discipline Construction Program of Fujian, China (Grant Number 2013544).
The authors have no conflicts of interest to disclose.
References
- [1].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ (Clinical Research Ed) 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Neurology 1996;46:1470. [DOI] [PubMed] [Google Scholar]
- [3].Mathias CJ. Orthostatic hypotension: causes, mechanisms, and influencing factors. Neurology 1995;45(4 suppl 5):S6–11. [PubMed] [Google Scholar]
- [4].Zhu QO, Tan CS, Tan HL, et al. Orthostatic hypotension: prevalence and associated risk factors among the ambulatory elderly in an Asian population. Singapore Med J 2016;57:444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fedorowski A, Wahlstrand B, Hedner T, et al. Systolic and diastolic component of orthostatic hypotension and cardiovascular events in hypertensive patients: the Captopril Prevention Project. J Hypertens 2014;32:75–81. [DOI] [PubMed] [Google Scholar]
- [6].Fagard RH, De Cort P. Orthostatic hypotension is a more robust predictor of cardiovascular events than nighttime reverse dipping in elderly. Hypertension 2010;56:56–61. [DOI] [PubMed] [Google Scholar]
- [7].Fedorowski A, Stavenow L, Hedblad B, et al. Orthostatic hypotension predicts all-cause mortality and coronary events in middle-aged individuals (The Malmo Preventive Project). Eur Heart J 2010;31:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].van Hateren KJ, Kleefstra N, Blanker MH, et al. Orthostatic hypotension, diabetes, and falling in older patients: a cross-sectional study. Brit J General Pract 2012;62:e696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Azidah AK, Hasniza H, Zunaina E. Prevalence of falls and its associated factors among elderly diabetes in a tertiary center, Malaysia. Curr Gerontol Geriatr Res 2012;2012:539073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yoshinari M, Wakisaka M, Nakamura U, et al. Orthostatic hypertension in patients with type 2 diabetes. Diabetes Care 2001;24:1783–6. [DOI] [PubMed] [Google Scholar]
- [11].Wu JS, Yang YC, Lu FH, et al. Population-based study on the prevalence and risk factors of orthostatic hypotension in subjects with pre-diabetes and diabetes. Diabetes Care 2009;32:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ricci F, Fedorowski A, Radico F, et al. Cardiovascular morbidity and mortality related to orthostatic hypotension: a meta-analysis of prospective observational studies. Eur Heart J 2015;36:1609–17. [DOI] [PubMed] [Google Scholar]
- [13].Low PA, Tomalia VA. Orthostatic hypotension: mechanisms, causes, management. J Clin Neurol (Seoul, Korea) 2015;11:220–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fleg JL, Evans GW, Margolis KL, et al. Orthostatic hypotension in the ACCORD (Action to Control Cardiovascular Risk in Diabetes) blood pressure trial: prevalence, incidence, and prognostic significance. Hypertension (Dallas, Tex: 1979) 2016;68:888–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Shibao C, Lipsitz LA, Biaggioni I. Evaluation and treatment of orthostatic hypotension. J Am Soc Hypertens 2013;7:317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tsutsu N, Nunoi K, Yokomizo Y, et al. Relationship between glycemic control and orthostatic hypotension in type 2 diabetes mellitus—a survey by the Fukuoka Diabetes Clinic Group. Diabetes Res Clin Pract 1990;8:115–23. [DOI] [PubMed] [Google Scholar]
- [17].Delcourt C, Vauzelle-Kervroedan F, Cathelineau G, et al. Low prevalence of long-term complications in non-insulin-dependent diabetes mellitus in France: a multicenter study. CODIAB-INSERM-ZENECA Pharma Study Group. J Diabetes Complications 1998;12:88–95. [DOI] [PubMed] [Google Scholar]
- [18].Chen HS, Hwu CM, Kuo BI, et al. Abnormal cardiovascular reflex tests are predictors of mortality in Type 2 diabetes mellitus. Diabet Med 2001;18:268–73. [DOI] [PubMed] [Google Scholar]
- [19].Kempler P, Tesfaye S, Chaturvedi N, et al. Autonomic neuropathy is associated with increased cardiovascular risk factors: the EURODIAB IDDM Complications Study. Diabet Med 2002;19:900–9. [DOI] [PubMed] [Google Scholar]
- [20].Hirai FE, Moss SE, Klein BE, et al. Postural blood pressure changes and associated factors in long-term type 1 diabetes: Wisconsin Epidemiologic Study of Diabetic Retinopathy. J Diabetes Complications 2009;23:83–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Eze CO, Onwuekwe IO, Agu CE, et al. The prevalence of orthostatic hypotension in type 2 diabetes mellitus patients in a diabetic clinic in Enugu South-East Nigeria. Nigerian J Med 2013;22:175–80. [PubMed] [Google Scholar]
- [22].Oduwole OA, Adeniyi OF, Esezebor CI, et al. Postural hypotension in type 1 diabetes: the influence of glycemic control and duration of illness. Nigerian J Clin Pract 2014;17:140–4. [DOI] [PubMed] [Google Scholar]
- [23].Refaie W. Assessment of cardiac autonomic neuropathy in long standing type 2 diabetic women. Egypt Heart J 2014;66:63–9. [Google Scholar]
- [24].Fan LFLJ, Zheng YG. Analysis of related factors of falls in elderly patients with diabetes. Chin J Gerontol 2004;11:1009–11. [Google Scholar]
- [25].Bouhanick B, Meliani S, Doucet J, et al. Orthostatic hypotension is associated with more severe hypertension in elderly autonomous diabetic patients from the French Gerodiab study at inclusion. Ann Cardiol D’angeiol 2014;63:176–82. [DOI] [PubMed] [Google Scholar]
- [26].Wijkman M, Lanne T, Ostgren CJ, et al. Diastolic orthostatic hypertension and cardiovascular prognosis in type 2 diabetes: a prospective cohort study. Cardiovasc Diabetol 2016;15:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gaspar L, Kruzliak P, Komornikova A, et al. Orthostatic hypotension in diabetic patients-10-year follow-up study. J Diabetes Complications 2016;30:67–71. [DOI] [PubMed] [Google Scholar]
- [28].Kobayashi Y, Fujikawa T, Kobayashi H, et al. Relationship between arterial stiffness and blood pressure drop during the sit-to-stand test in patients with diabetes mellitus. J Atheroscler Thromb 2016;24:147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Budyono C, Setiati S, Purnamasari D, et al. The proportion of orthostatic hypotension and its relationship with HbA1c levels in elderly patients with diabetes. Acta Medica Indonesiana 2016;48:122–8. [PubMed] [Google Scholar]
- [30].Zhang Y. Clinical analysis of diabetic orthostatic hypotension. Chin J Synthetical Med 2005;6:212. [Google Scholar]
- [31].Peng MGZQ, Deng XF, Ye HL, et al. The analysis of clinical factors about orthostatic hypotension in type 2 diabetes. Chin J Arterioscler 2010;18:889–91. [Google Scholar]
- [32].Jiang T, Song LG, Yao XX, XA The prevalence and related risk factors of orthostatic hypotention in patients with type 2 diabetes mellitus. Chin Circ J 2013;28:55–7. [Google Scholar]
- [33].von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344–9. [DOI] [PubMed] [Google Scholar]
- [34].Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 1987;9:1–30. [DOI] [PubMed] [Google Scholar]
- [35].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [36].Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation 2007;115:387–97. [DOI] [PubMed] [Google Scholar]
- [37].Low PA, Benrud-Larson LM, Sletten DM, et al. Autonomic symptoms and diabetic neuropathy: a population-based study. Diabetes Care 2004;27:2942–7. [DOI] [PubMed] [Google Scholar]
- [38].Doelman CJ, Oude Elberink JN, Miedema K, et al. Orthostatic hypotension in poorly regulated NIDDM. Diabetes Care 1996;19:542. [DOI] [PubMed] [Google Scholar]
- [39].Fleischer J. Diabetic autonomic imbalance and glycemic variability. J Diabetes Sci Technol 2012;6:1207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Iovino M, Triggiani V, Licchelli B, et al. Vasopressin release induced by hypothension is blunted in patients with diabetic autonomic neuropathy. Immunopharmacol Immunotoxicol 2011;33:224–6. [DOI] [PubMed] [Google Scholar]
- [41].Beijers HJ, Ferreira I, Bravenboer B, et al. Microalbuminuria and cardiovascular autonomic dysfunction are independently associated with cardiovascular mortality: evidence for distinct pathways: the Hoorn Study. Diabetes Care 2009;32:1698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ricci F, De Caterina R, Fedorowski A. Orthostatic hypotension: epidemiology, prognosis, and treatment. J Am Coll Cardiol 2015;66:848–60. [DOI] [PubMed] [Google Scholar]
- [43].Keating GM. Droxidopa: a review of its use in symptomatic neurogenic orthostatic hypotension. Drugs 2015;75:197–206. [DOI] [PubMed] [Google Scholar]
- [44].Izcovich A, Gonzalez Malla C, Manzotti M, et al. Midodrine for orthostatic hypotension and recurrent reflex syncope: a systematic review. Neurology 2014;83:1170–7. [DOI] [PubMed] [Google Scholar]
- [45].Kuehl M, Stevens MJ. Cardiovascular autonomic neuropathies as complications of diabetes mellitus. Nat Rev Endocrinol 2012;8:405–16. [DOI] [PubMed] [Google Scholar]
- [46].Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998;280:1690–1. [DOI] [PubMed] [Google Scholar]


