Abstract
Health education is compulsory for patients with chronic and life-threatening disease, especially for those with diabetes mellitus (DM). This study aimed to examine the long-term effectiveness of the Diabetes Conversation Map Program (DCMP) among DM patients in Taiwan.
A quasi-experimental research design using convenience sampling and nonrandom group assignment was applied to recruit 95 type 2 diabetic subjects from a hospital in Taiwan. In addition to routine care, the experiment group (n = 49) received 7 sessions of DCMP that delivered over 2 months, while the control group (n = 46) received only routine care during the same period. We conducted structured questionnaire survey and reviewed medical record at 3 time points (before DCMP, 3 days after DCMP, and 3 months after DCMP completion) to collect the effectiveness data. The effectiveness was determined using the generalized estimating equation model.
We found that improvements in the body mass index, blood glucose, glycated hemoglobin, self-monitoring of blood glucose, and diabetic health literacy in the DCMP group compared with controls (all P values <.05), with no significant changes in depressive symptoms. The positive effects were further maintained for 3 months after DCMP.
The findings may serve as a reference for helping healthcare professionals provide appropriate interventions to improve adaptation processes and clinical outcomes for DM patients.
Keywords: diabetes conversation map program, diabetes mellitus, effectiveness, generalized estimating equation
1. Introduction
Diabetes mellitus (DM), characterized by hyperglycemia resulting from defects in insulin activity, is a key challenge worldwide. Diabetic adults are estimated to equal 285 million and 439 million in the years 2010 and 2030, respectively, representing an increase of nearly 55%.[1] Given the complex symptoms and long duration of DM, the high costs of disease management cannot be ignored. A report by the American Diabetes Association indicated that the direct medical costs of DM in the United States in 2012 were $69 billion, with the total societal costs (the sum of direct and indirect costs) estimated to exceed $245 billion.[2]
DM not only causes enormous economic burdens but also triggers major challenges for human health worldwide. The guidelines by American Diabetes Association suggest that target blood glucose levels for diabetes control should be 70 to 130 mg/dL before meals and <180 mg/dL after meals, and glycated hemoglobin A1c (HbA1c) concentration should be less than 7%.[3] Once DM patients exhibit poor glucose control, adverse complications can possibly occur. A study reported that, in comparison with patients with HbA1c <6%, those with HbA1c levels ranging from 7.0 to 7.9% had a 1.14-fold greater risk of cardiovascular disease, and a 25% increased risk of microvascular complications during a 4-year tracking period.[4] Andersson et al[5] also found that each unit increase in HbA1c of DM patients was related to an increase in the mortality rate by 22%. As a consequence, tight glycemic control is imperative for DM patients to improve prognosis.
Health education is the cornerstone of glycemic control for diabetic patients. The majority of conventional health education programs were developed using existing knowledge administered in a 1-way format.[6–8] These programs, however, seem to be unable to fully grasp the plight of DM patients in achieving glycemic control.[9,10] To fill this gap, Healthy Interactions, the International Diabetes Federation, and diabetes scholars have created the “Diabetes Conversation Map Program” (DCMP), a health education program that has been adopted by more than 100 countries worldwide.[11] DCMP is built on patient-centered and conversation-based approaches, encouraging DM patients to participate in the drafting of applicable glycemic control strategies in an empowered learning environment, and further strengthening patients’ self-care ability through interactive discussion.[12,13]
Despite many reports on the effects of DCMP, the effectiveness of DCMP as an education intervention remains contradictory. For example, Ciardullo et al[14] used the single-group pretest–posttest design to assess the effects of DCMP on 63 DM patients and found that the level of fasting plasma glucose (FPG) decreased from 152.9 to 138.2 mg/dL, HbA1c levels decreased from 8.2% to 7.8%, and body mass index (BMI) decreased from 27.6 to 25.5 kg/m2 (all P < .05). Another study also proposed that DCMP could boost patients’ self-care efficacy positively relative to traditional health education.[15] Nevertheless, 1 report showed that both DCMP and traditional health education were able to reduce HbA1c levels, by 0.27% and 0.24%, respectively, but with no significant differences in performing twice-daily self-monitoring of blood glucose (SMBG) (35.9% vs 33.8%).[16] Of note, the direct application to residents in Chinese may not be appropriate due to significant differences in the environment, life style, economic and physical conditions, and the existing geography.
By the end of 2012, at least 90 hospitals in Taiwan had offered DCMP courses.[17] Although several studies have been conducted in Taiwan to measure the effectiveness of DM health education, these studies were limited to traditional health education strategies and only focused on changes in patients’ self-care knowledge or behavior.[6,18,19] In addition, they mostly ignored the assumption of independence among the patients’ responses and did not consider maturation effects over time, possibly blunting effectiveness of the interventions.[20,21] To narrow this gap in the literature, this pretest–posttest study aimed to evaluate the effects of DCMP for DM patients in Taiwan by using a generalized estimating equations (GEE) model, with the intent that the findings could serve as a reference for providing empirically robust grounds for healthcare providers to formulate more appropriate interventions for DM patients.
2. Methods
2.1. Study design and participants
A quasi-experimental research design using convenience sampling and nonrandom group assignment was used to recruit participants from the endocrinology department of a hospital in Taiwan from January to May of 2014. The inclusion criteria were being at least 20 years old at the time of recruitment, having no cognitive impairment and severe complications, being able to express opinions in either Mandarin or Taiwanese, and having been diagnosed with DM by an endocrinologist. To ensure participants’ anonymity, all questionnaires were marked with an encryption code to facilitate data analysis, but with no personal identifiers.
2.2. Sample size calculation
The sample size needed for this study of repeated measures was estimated by using the methodology of Cohen,[22] where α was set to 0.05, power was set to 0.8, and the effect size was set to 0.2 that focused on the changes in HbA1c level between 2 groups.[14,16] It was determined, based on these psychometrics, that a sample of at least 68 patients (each group including 34 cases) was required for sound data analysis (based on the G- POWER 3.1 analytical software, Franz Faul, Universitat Kiel, Kiel, Germany).
2.3. Intervention
Because continued participation was essential for this study, participants were divided into experiment or control groups in accordance with their personal preference. Study flowchart of this work is shown as Fig. 1. Participants in the control group received usual health education lasting for about 20 minutes after per medical visit which consisted of consultation from ward nurses on disease symptoms, related treatment options, and clarification of doctors’ orders. They were also encouraged to ask questions of the research team, their ward nurses and physicians at any time within the duration of the study.
Figure 1.
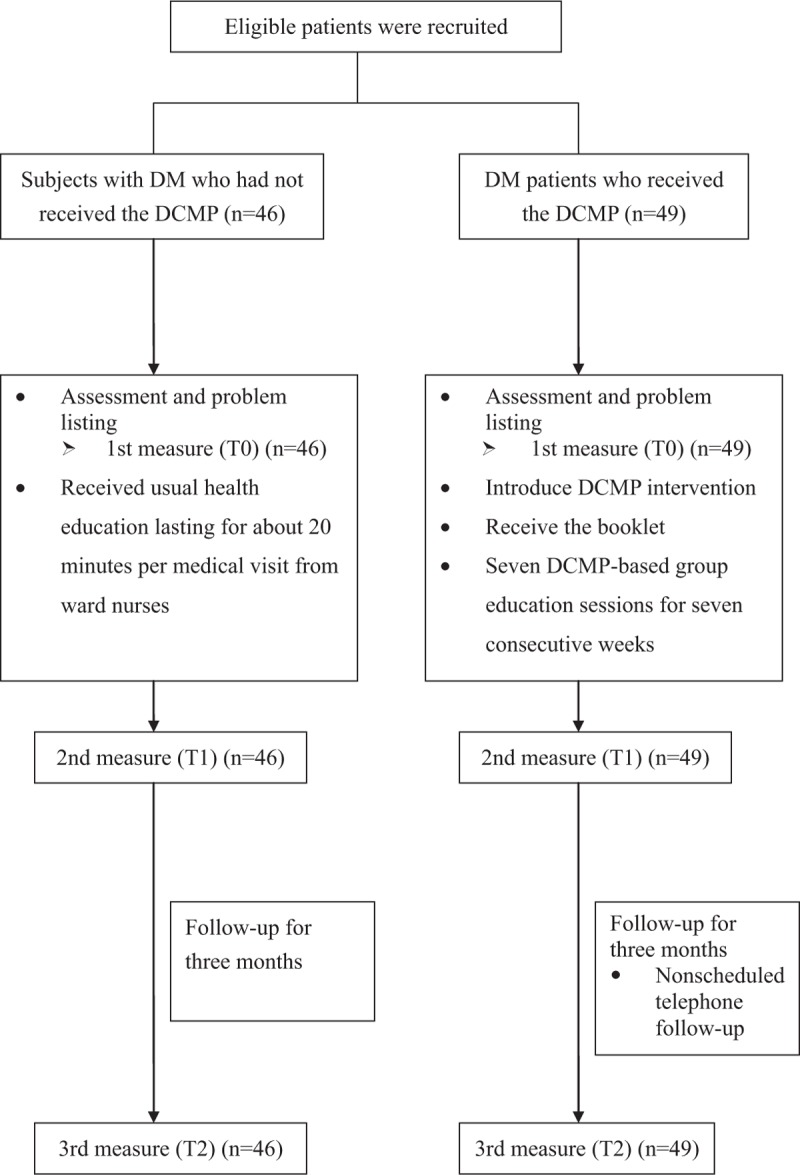
Study flow diagram.
Participants of the experimental group received 7 DCMP-based group education sessions (10–12 participants, 1.5 hours each) for 7 consecutive weeks in addition to routine health education at a private room in the outpatient unit. One registered nurse who received DCMP facilitator training, from the Taiwanese Association of Diabetic Educators, served as the class facilitator. DCMP sessions were conducted using 7 colored “maps” covering different topics, including “walk with diabetes,” “what is diabetes?”, “a healthy diet and exercise,” “walk with insulin,” “diabetes complications and related risk factors,” “foot care for diabetes patients,” and “a type 1 diabetes patient in the home.” Each map, a laminated 3-by-5-foot table-top visual with colorful drawings as metaphors of situations familiar to DM patients, was placed on a table with participants gathered around it. This procedure served to create mutual imagery to be shared with participants. In addition to using these colored maps, the facilitator further offered participants self-designed conversation cards and asked them to share individual glycemic control experiences based on the questions shown on the cards. This interactive learning environment allowed patients to discuss the effects of the illness, its treatment, and changes in the self-image, roles, and relationships with family members, friends or coworkers; and furthermore to distinguish related diabetic care facts from myths.[23] All the strategies were rationally systematically planned and, when needed, modified in accordance with the individualized therapeutic regimen offered to the patient, and in consultation with his or her family, the physician, or any other members of the medical team. Additionally, the nonscheduled telephone follow-up calls during the study period were also implemented to assess the patients’ condition and to discuss any problems that may have arisen, depending on participants’ needs.
2.4. Outcome measures
The primary outcomes comprised the weekly SMBG times and several biochemical parameters containing BMI, HbA1c, and FPG through patients’ medical records review. In addition, the depressive symptoms as well as the level of DM health literacy were also measured, which were determined by the Taiwanese Depression Questionnaire (TDQ) and a self-administered questionnaire regarding DM health literacy.
TDQ, created by Lee et al,[24] is a structured interview that has been validated against the Diagnostic and Statistical Manual of Mental Disorders (Third Edition). It assesses depressive symptom in the preceding week with 18 self-reported items that are rated on a Likert scale ranging from 0 to 3. Thus, the TDQ total score ranges from 0 to 54, where higher scores indicate a higher level of depressive symptom. We adopted the TDQ because it is easy to administer, well adapted to the Asian culture, and uses the Taiwanese language. TDQ has been shown to have good internal consistency by various previous studies in assessing the depressive symptom, with Cronbach α values between 0.89 and 0.92.[25–27] The Cronbach α in our study was 0.93.
The DM health literacy questionnaire was developed by the primary investigators. It contained 20 items and was scored using a 5-point Likert scale, with higher total scores indicating more positive diabetic health literacy. Regarding psychometric properties, the questionnaire was assessed for content validity by 5 experts from several disciplines, including 3 endocrinologists, a nurse supervisor, and a nursing professor in the endocrinology field. The questionnaire was reviewed for clarity and appropriateness and revised based on the experts’ feedback and suggestions. The questionnaire was acceptable if the index of content validity was >0.80. Scale reliability was indicated by Cronbach α of 0.90.
2.5. Covariates
Additional items addressing demographic and disease characteristics were developed based on clinical experience and literature review,[28] and were collected at study entry via patient interviews and medical records. Demographic data included sex, age, marital status, education level, religion, household status, monthly income, and certain lifestyle factors, such as smoking, exercise habits, and presence of sleep disturbances. Smoking status was recorded as “non-smoker” or “current or ex-smoker.” Those who exercised regularly (i.e., weekly) were classified as having “exercise habits.” Sleep disturbances were defined as waking up at night more than twice without external factors during the week before the interview. Disease characteristics included the presence of chronic disease (i.e., stroke, hypertension, heart disease, renal disease, or cancer), medication regimen, and duration of DM.
2.6. Data collection procedure
The study protocol was approved by the Research Ethics Committee of Dalin Tzuchi Hospital (No. B10002009) and registered in the ClinicalTrials.gov (NCT02733315). At study's initiation, researchers explained the purpose of study and its procedure to all participants. Signed informed consent was obtained after the patients understood and agreed to participate in this study. Thereafter, we applied an observer-blind approach for data collection. A trained interviewer, who was not familiar with participants and with the study design, was assigned to collect the information pertaining to the outcome variables and covariates. All data were obtained at 3 time points: before DCMP (T0), 3 days after DCMP (T1), and 3 months after DCMP (T2). To reduce the dropout rate, researchers asked participants to return the hospital for the completion of assessments via phone reminders. All the participants were followed from the date of enrollment until the end of the follow-up, but they were still given the option to withdraw from the study at any time without any penalty.
2.7. Statistical analysis
Descriptive and inferential statistical analyses were conducted in accordance with the study aims and the nature of the variables. Descriptive parameters, including mean, standard deviation, and percentage, were used to describe the distributions of demographic and disease data. Differences between the 2 groups were compared initially using t test and χ2 test as appropriate. For inferential analysis, we used GEE procedure with identity link function with normal distribution to assess the long-term effects of DCMP, while taking into account within-subject correlations between measurements over time and the influence of potential confounding covariates. All the analyses were conducted using SAS version 9.3 (SAS Institute Inc, Cary, NC), and P < .05 was considered significant.
3. Results
3.1. Demographic and disease characteristics of participants
A total of 95 patients with type 2 DM were enrolled in the study, with 49 in the experiment group and 46 in the control group. All of them completed the follow-up of this study. Overall, patients aged from 39 to 80 years with a mean of 61.3 (SD = 8.0) years. Most patients were male (51.6%), married (80.0%), and cohabitating with other people (84.2%) and had a low level of education (54.7%, defined as below 9th grade). Additionally, most participants reported having religious beliefs (72.6%), a monthly income ≤30,000 New Taiwan Dollar (69.5%), exercise habits (64.2%), and sleep disturbances (55.8%). Over 80% of the participants were nonsmokers (82.1%). In terms of disease characteristics, the majority of the participants had diabetes for more than 5 years (76.8%), and used oral hypoglycemic agents and insulin injections (89.5%). Most patients presented with comorbidities (70.5%). Mean scores of depressive symptoms and DM health literacy were 8.6 and 65.2, respectively. The average number of SMBG in 1 week was 1.7, and the mean levels of BMI, HbA1c, and FPG were 27.2 kg/m2, 8.0%, and 164.9 mg/dL, respectively.
3.2. Baseline comparison of demographic and disease characteristics between 2 groups
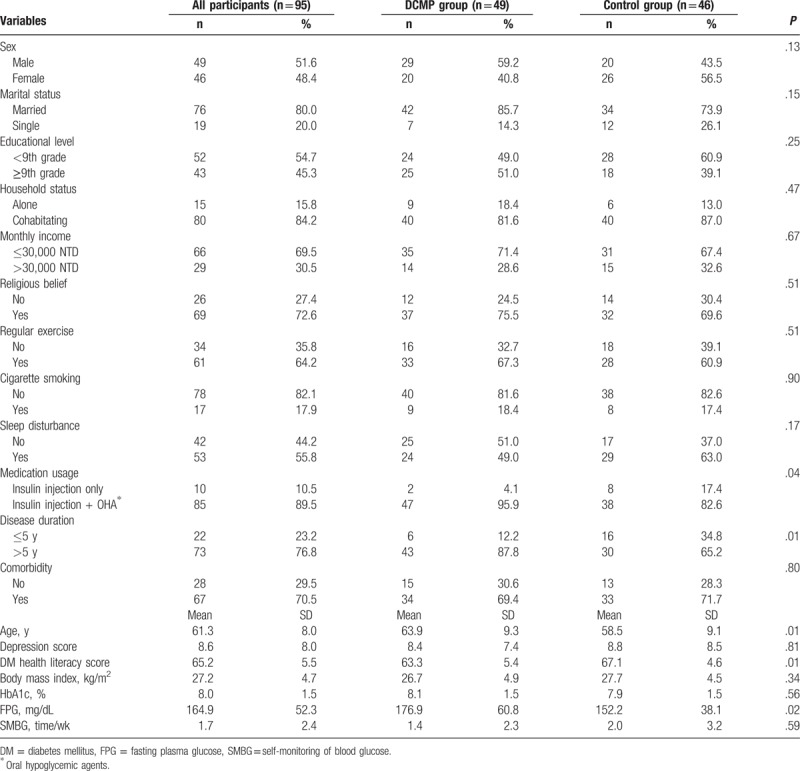
Significant differences were found between the 2 groups in age (P = .01), medication usage (P = .04), disease duration (P = .01), DM health literacy (P = .01), and FPG (P = .02) (Table 1).
Table 1.
Demographic and clinical characteristics of study participants by group.
3.3. Effects of DCMP on depressive symptoms, DM health literacy, and SMBG
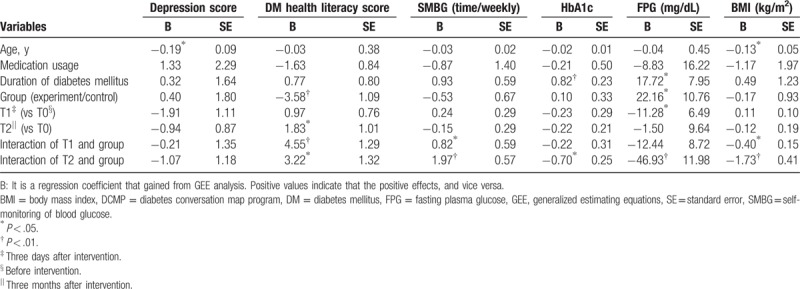
Table 2 displays the GEE analysis regarding the long-term effectiveness of DCMP for DM patients. First, no significant differences were found in baseline depression scores between the experiment and control groups. The depression scores at T1 and T2 were also not statistically different from those at T0, revealing that no maturation effect occurred in this model. The DCMP intervention did not reduce the level of depressive symptoms in either group after adjusting for baseline differences such as age, medication usage, and disease duration. Regarding DM health literacy, a maturation effect was found at T2 that was significantly higher than that initially measured in the control group (P = .02). In addition, the initial DM health literacy scores were significantly lower in the experiment group than in the control group (P < .01). After controlling for age, medication usage, disease duration, and maturation effect, we found DCMP was more beneficial to increase DM health literacy in the experiment group than that in the control group at T1 and T2, with the B values of 4.55 and 3.22 (all P values < .05). For SMBG, multivariate analyses using the GEE model revealed that the maturation effect did not occur and that the 2 groups appeared to begin with equivalent levels of SMBG at baseline. DCMP significantly enhanced the frequency of weekly SMBG in DM patients at T1 and T2. In particular, the SMBG times of the experimental group, at T2, were significantly higher than these of the control group by nearly twice, weekly (B = 1.97; P = .001).
Table 2.
GEE analysis of the effectiveness of DCMP for diabetic patients (n = 95).
3.4. Effects of DCMP on biochemical parameters
No significant differences were found in the BMI between the 2 groups at T0. Additionally, the control group showed no maturation effect at either T1 or T2. Multivariate analyses revealed that the reduction slope of BMI in the experiment group was greater than that in the control group at T1 and T2 after adjusting for age, medication usage, and disease duration, with the B values of −0.40 and −1.73, respectively. (Both P values < .05). Also, no significant differences were found in HbA1c levels between the 2 groups at T0, and no maturation effect was found in the control group. After controlling for age, medication usage, and disease duration by the GEE model, we found that the reduction slope of HbA1c was significantly greater in the experiment group than that in the control group at T2 (B = −0.70; P = .01). On the other hand, the GEE model indicated that the difference of FPG between 2 groups at T0, and a maturation effect, might have occurred when considering if DCMP was beneficial in glycemic control. After adjusting for age, medication usage, disease duration, and maturation effect, DCMP was still found to be beneficial in reducing the FPG level of DM patients at T2 as compared with the control group, yielding the B value of −46.93 (P < .01).
4. Discussion
Although some previous studies have been conducted to determine the effectiveness of traditional health education programs for DM, the results of these studies may have been biased due to inadequate samples, short follow-up periods, or inappropriate statistical methods. To the best of our knowledge, this is the first study to assess the effectiveness of DCMP from a long-term tracking perspective. In contrast to prior research, the GEE model used in this study provided further control of participants’ attributes at baseline and of temporal maturation effect, allowing us to better determine the effectiveness of DCMP.
Despite the experiment group showing a larger reduction slope in depression scores than the control group, following the implementation of DCMP, the improvement did not reach statistical significance. We speculate that the initial depressive symptom levels among the participants were not severe, possibly reducing the influence of DCMP. Our findings, however, differed from earlier reports that supported the alleviation of depressed mood in DM patients following DCMP.[16,23] This inconsistency may be associated with the difference in the screening tools for depression and the statistical analysis methods used. Some previous studies used PHQ-9[16] and Problem Areas in Diabetes Questionnaire [23] to examine the level of depressive symptoms, which are distinctly different from the scale (TDQ) used in the present study. Furthermore, previous studies did not take into consideration the interfering influence of maturation effect on the effectiveness of the intervention studied. In the present study, apart from adjusting for baseline differences between the 2 groups using the GEE model, this analytic method further took into consideration the impact of maturation effect over time, thus allowing for more robust conclusions.[20,21]
DCMP enhanced the level of DM health literacy in the experiment group compared with that in the control group, who only received routine care and did not participate in an education program. This effect was maintained for 3 months following the completion of DCMP sessions, echoing with the findings in some previous studies of DCMP in Western populations.[16,23,29] Furthermore, our study further supports that DM health literacy positively influenced the health-promoting behaviors of DM patients.[30] For instance, following DCMP, the frequency of weekly SMBG in the experiment group was higher than that in the control group. It can be inferred that most traditional health education programs merely rely on established structured processes and proceed with educational leaflets. This type of 1-direction administration may lead to the quick dissipating of the learning effect. In contrast, the facilitators of DCMP introduce the concepts, pathologies, and treatment regimens of DM through a coordinated use of colored maps and group discussion. This process may insidiously strengthen knowledge and behaviors in diabetic care among DM patients.[13,31] Nevertheless, a previous study observed a different effect on SMBG from that in the present study,[16] and this inconsistency in findings may be because that the previous study classified SMBG into 2 parts based on whether SMBG exceeded twice a week, while the present study treated SMBG as a continuous variable.
In the present study, the experiment group had a significantly greater reduction in BMI than the control group, and this effect was maintained for 3 months following completion of DCMP. This could be attributable to the inclusion, in the DCMP sessions, of the topic “healthy diet and exercise.” Patients with DM were encouraged in this class to lose weight through regular exercise and diet, which is beneficial in reducing the subsequent risk of cardiovascular diseases. However, the finding contradicts those in some reports from Western countries,[16,23,29] which may stem from the difference in the level of awareness of health burdens caused by obesity. In fact, at similar BMI levels, a higher body fat percentage was found in Asians in comparison to Caucasians, and consequently, a greater risk of death by 10% for Asians.[32] Furthermore, this inconsistency may also be associated with the statistical analyses employed in these studies. Previous studies relied mostly on univariate analyses to compare effects of pre–post education intervention, rather than adopting a more comprehensive multivariate model that controls for potentially intervening covariates (maturation effect, in particular), thus failing to accurately verify the effectiveness of DCMP.
The results of the present study demonstrated that DCMP has a positive effect on glycemic control, echoing the results of some earlier reports.[16,23,29] DCMP differs from traditional methods by using a highly interactive approach and colored images to more actively engage patients with the health information they are learning and to help them make appropriate plans for glycemic control. It is noteworthy that most participants in this study were older adults, and nearly 60% had low educational levels. Previous studies have demonstrated that older patients and those from low education levels often have poorer medical adherence.[33,34] Therefore, DCMP may be a useful approach when health education is provided to seniors or to patients with low education levels.
In assessing the 3-month effectiveness of DCMP, we used the GEE model to adjust for baseline differences and temporal maturation effect, allowing a more robust determination. Several limitations, however, should be noted when interpreting the results. First, all the participants were drawn from a single hospital in southern Taiwan, so inferences drawn from the results might not be generalizable to populations in other geographic regions. Previous research, however, has also often been limited by such factors as participants’ ethnicity, geographic location, nationality, and the nature of the medical data available, suggesting that this limitation is not unique to our study. Second, the application of a quasi-experimental comparative research design, rather than conducting a randomized clinical trial, may have weakened the internal validity of the research because of the presence of potentially confounding variables. Nevertheless, we have employed the GEE model to control for baseline differences between the 2 groups, and further considered the potential maturation effects, which would likely reduce the probability of inflated type I error to some extent.[20] Third, the data on the adherence were unavailable for the study and, accordingly, caution should be exercised when interpreting these findings. However, former studies indicated that HbA1c, implying individual blood glucose (blood sugar) control for the past 2 to 3 months, could be assumed as a surrogate variable for the compliance level because the poor glycemic control is more common among patients with low adherence to medications.[35,36] To address this issue, we performed a sensitivity analysis that further controlled for the HbA1c while assessing the DCMP intervention effectiveness. Similar to the initial results of Table 2, DCMP still exerted positive effects on diabetic prognostics with the exception of depressive level of DM patients, suggesting that the level of compliance did not appreciably influence the relationships reported earlier. Fourth, the possible existence of the Hawthorne effect should also be considered. Nonetheless, all the participants were divided into the 2 groups and then the bias (if any) was likely to lead to underestimation of effects. The long-term measurement employed helped in reducing the influence of the Hawthorne effect in light of the law of diminishing marginal utility.[36]
5. Conclusion
In conclusion, an appropriate health education intervention can assist DM patients in coping with their condition and enhancing their self-care ability. This study demonstrated DCMP could effectively increase the frequency of weekly SMBG and the DM health literacy levels among Taiwanese DM patients. Additionally, DCMP had positive effects on biochemical parameters such as BMI, FPG, and HbA1c. These effects further persisted for 3 months following the completion of DCMP. Data from the long-term measurement allow healthcare providers to more confidently consider the practicability of DCMP for DM patients. The concepts of DCMP might be applicable to participants with other chronic diseases, which may be beneficial in progressively adapting to their disease and improving their clinical outcomes.
Footnotes
Abbreviations: DCMP = Diabetes Conversation Map Program, DM = diabetes mellitus.
J-YH, P-FC, and HL contributed equally to this work.
This research was supported by Dalin Tzuchi Hospital (Grant Number DTCRD103-I-04).
The authors have no conflicts of interest to disclose.
References
- [1].Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010;87:4–14. [DOI] [PubMed] [Google Scholar]
- [2].American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].American Diabetes Association. Standards of medical care in diabetes-2010. Diabetes Care 2010;33:S11–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Huang ES, Liu JY, Moffet HH, et al. Glycemic control, complications, and death in older diabetic patients. Diabetes Care 2011;34:1329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Andersson C, van Gaal L, Caterson ID, et al. Relationship between HbA1c levels and risk of cardiovascular adverse outcomes and all-cause mortality in overweight and obese cardiovascular high-risk women and men with type 2 diabetes. Diabetologia 2012;55:2348–55. [DOI] [PubMed] [Google Scholar]
- [6].Kuo YU, Chang SC, Chang M, et al. The effects of multi-approach health education on people with pre-diabetes. J Evid Based Nurs 2008;4:297–306. [Google Scholar]
- [7].Lu KY, Lin PL, Tzeng LC, et al. Effectiveness of case management for community elderly with hypertension, diabetes mellitus, and hypercholesterolemia in Taiwan: a record review. Int J Nurs Stud 2006;43:1001–10. [DOI] [PubMed] [Google Scholar]
- [8].Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Eng J Med 2001;344:1343–50. [DOI] [PubMed] [Google Scholar]
- [9].LaSalle JR. Empowering patients during insulin initiation: a real-world approach. J Am Osteopath Assoc 2010;110:69–78. [PubMed] [Google Scholar]
- [10].Skovlund SE, Peyrot M. The diabetes attitudes, wishes, and needs (DAWN) program: a new approach to improving outcomes of diabetes care. Diabetes Spectr 2005;18:136–42. [Google Scholar]
- [11].International Diabetes Federation. Annual Report 2011. Available at: http://www.idf.org/sites/default/files/IDF-AR2011-EN.pdf [accessed Jun 12, 2016]. [Google Scholar]
- [12].Grenci A. Applying new diabetes teaching tools in health-related extension programming. JOE 2010;48:1–6. [Google Scholar]
- [13].Reaney M, Eichorst B, Gorman P. From acorns to oak trees: the development and theoretical underpinnings of diabetes conversation map education tools. Diabetes Spectr 2012;25:111–6. [Google Scholar]
- [14].Ciardullo AV, Daghio MM, Fattori G, et al. Effectiveness of the kit conversation map in the therapeutic education of diabetic people attending the diabetes unit in Carpi, Italy. Rec Prog Med 2010;101:471–4. [PubMed] [Google Scholar]
- [15].Shi LG, Zhang LJ, Wu YP, et al. Effects on conversation maps on health education type 2 diabetes mellitus patients. Nurs J Chin People's Liberation Army 2011;28:6–9. [Google Scholar]
- [16].Sperl-Hillen J, Beaton S, Fernandes O, et al. Comparative effectiveness of patient education methods for type 2 diabetes: a randomized controlled trial. Arch Intern Med 2011;171:2001–10. [DOI] [PubMed] [Google Scholar]
- [17].Taiwanese Associaiton of Diabetes Educators. Diabetes conversations. Available at: http://www.tade.org.tw/upload/News/file/328-1.doc[accessed Aug 12, 2016]. [Google Scholar]
- [18].Hsiao YC, Huang SJ. Analysis of literature on applied health education in nursing research in Taiwan. J Nurs 2003;50:48–56. [Google Scholar]
- [19].Wu SF, Liang SY, Wang TJ, et al. A self-management intervention to improve quality of life and psychosocial impact for people with type 2 diabetes. J Clin Nurs 2011;20:2655–65. [DOI] [PubMed] [Google Scholar]
- [20].Overall JE, Tonidandel S. Robustness of Generalized Estimating Equation (GEE) tests of significance against misspecification of the error structure model. Biomet J 2004;46:203–13. [Google Scholar]
- [21].Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986;42:121–30. [PubMed] [Google Scholar]
- [22].Reaney M, Zorzo EG, Golay A, et al. Impact of Conversation Map™ education tools versus regular care on diabetes-related knowledge of people with type 2 diabetes: a randomized, controlled study. Diabetes Spectr 2013;26:236–45. [Google Scholar]
- [23].Lee Y, Yang MJ, Lai TJ, et al. Development of the Taiwanese depression questionnaire. Chang Gung Med J 2000;23:688–94. [PubMed] [Google Scholar]
- [24].Chiang HH, Guo HR, Livneh H, et al. Increased risk of progression to dialysis or death in CKD patients with depressive symptoms: a prospective 3-year follow-up cohort study. J Psychosom Res 2015;79:228–32. [DOI] [PubMed] [Google Scholar]
- [25].Lee Y, Lin PY, Hsu ST, et al. Comparing the use of the Taiwanese Depression Questionnaire and Beck Depression Inventory for screening depression in patients with chronic pain. Chang Gung Med J 2008;31:369–77. [PubMed] [Google Scholar]
- [26].Liu CN. The association of depressive symptoms with socioeconomic status, general health conditions, and health behaviors in community-dwelling adults. Taiwan J Public Health 2009;28:300–11. [Google Scholar]
- [27].Huang YM, Chang TK. The outcomes and factors associated with diabetes care: the diabetes shared care program in a regional hospital in Taitung, Taiwan. Taiwan J Public Health 2011;30:19–28. [Google Scholar]
- [28].Penalba M, Moreno L, Cobo A, et al. Impact of “Conversation Map™” tools on understanding of diabetes by Spanish patients with type 2 diabetes mellitus: a randomized, comparative study. Endocrinol Nutr 2014;61:505–15. [DOI] [PubMed] [Google Scholar]
- [29].Al Sayah F, Majumdar SR, Williams B, et al. Health literacy and health outcomes in diabetes: a systematic review. J Gen Intern Med 2013;28:444–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Belton AB. Conversation maps in Canada: the first 2 years. Diabetes Spectr 2008;21:139–42. [Google Scholar]
- [31].Wen CP, David Cheng TY, Tsai SP, et al. Are Asians at greater mortality risks for being overweight than Caucasians? Redefining obesity for Asians. Public Health Nutr 2009;12:497–506. [DOI] [PubMed] [Google Scholar]
- [32].Delamater AM. Improving patient adherence. Clin Diabetes 2006;24:71–7. [Google Scholar]
- [33].Weinger K, Beverly EA, Smaldone A. Diabetes self-care and the older adult. Western J Nurs Res 2014;36:1272–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bayer FJ, Galusha D, Slade M, et al. Process of care compliance is associated with fewer diabetes complications. Am J Manag Care 2014;20:41–52. [PMC free article] [PubMed] [Google Scholar]
- [35].Tiv M, Viel JF, Mauny F, et al. Medication adherence in type 2 diabetes: the ENTRED study, a French population-based study. PLoS One 2012;7:e32412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Fehr E, Goette L. Do workers work more if wages are high? Evidence from a randomized field experiment. Am Econ Rev 2007;97:298–317. [Google Scholar]


