Abstract
To compare the microstructure, bone quality, and the combination and penetration of cement–bone interface in tissue specimens from patients with osteoarthritis (OA) and rheumatoid arthritis (RA).
A total of 80 femoral condyle tissue specimens from 20 OA patients (40 condyles) and 20 RA patients (40 condyles) who underwent total knee arthroplasty at the Department of Orthopaedics in Tengzhou Central People's Hospital were collected between January 2017 and September 2017. According to the random number table method, 20 specimens from the OA group were defined as group A, and 20 specimens in the RA group were defined as group B. The bone quality parameters were measured by micro-CT. The remaining 20 specimens in the OA group and the remaining 20 specimens in the RA group were defined as group C and group D, the cement–bone interfaces were established by the self-made bone cement compression device, and were analyzed by micro-CT.
Micro-CT measurement revealed that the bone volume fraction (BV/TV), trabecular thickness (Tb.Th), and trabecular number (Tb.N) in group A were significantly higher than those in group B (all P < .05). The bone surface/bone volume (BS/BV), structure model index (SMI), trabecular separation (Tb.Sp), and degree of anisotropy (DA) in group A were significantly lower than those in group B (all P < .05). The penetration depth of bone cement in group D was significantly greater than those in group C via x-ray detection.
The bone quality of OA patients is better than that of RA patients, but the combination and penetration of cement–bone interface of RA patients are better than that of OA patients. The findings advance our understanding of knee prosthesis and have important clinical implications, but they require validations in future studies with larger sample sizes.
Keywords: bone–cement interface, bone quality, micro-CT, osteoarthritis, rheumatoid arthritis
1. Introduction
Osteoarthritis (OA) is a multifactorial disease whose pathogenesis is attributed to both genetic and environmental factors,[1] including cytokines, metalloproteinases, sex hormones, and intraosseous hypertension. The chondrocytes and the extracellular matrix especially the quality and quantity of the collagen I have been considered the direct reason for the loss of the biomechanics properties of articular cartilage in OA patients. As the most common arthritis in the world, OA is usually considered as a degenerative disease; however, many studies have shown different outcomes.[2–5] There is still debate about the correlation between OA and osteoporosis. Rheumatoid arthritis (RA) is a destructive, inflammatory and chronic disease that affects approximately 1% of the world's population with a high incidence of disability of 20% within 1 year.[6] It is reported that a variety of cytokines (such as interleukin family, TNF-α and its receptor superfamily, matrix metalloproteinases, etc.), microRNAs and signal transduction pathways have played an import role in the destruction of synovial and osseous in RA patients. Osteoclasts is highly related to the osteoporosis, which can further induce bone destruction in RA patients.[7]
The cartilage degeneration and bone hyperplasia of OA patients usually affect the soft tissue around the joints and further cause joint pain, especially as weight increased. The joint deformity and stiffness cannot be avoided at the end age of OA. In order to increase the content of the joint and ease the pain caused by joint swelling, RA patients usually keep the knee in the knee bending position. As the pathology progresses the inflammatory activity leads to tendon tethering and erosion and destruction of the joint surface, which impairs range of movement and leads to deformity, even bone or fiber fusion in some severe cases. Therefore, the end stages of both OA and RA can seriously affect the quality of life. Total knee arthroplasty can significantly improve the patient's pain, limited mobility, joint deformities, and significantly improve life quality. Total knee arthroplasty is considered to be an effective treatment in OA and RA patients.
In total knee arthroplasty, the prosthesis is usually fixed to the bone by bone cement that can provide good prosthetic stability by mechanical interlocking.[8] The mechanical strength of bone–cement interface that plays an important role in the life of prosthesis is mainly determined by microstructure morphology and bone quality, as well as the binding degree of bone–cement interface.[9] One study showed that bone–cement interface intensity degraded gradually over time with fatigue and load.[10] In addition, with the influence of dynamic loading, creep and fatigue damage occur in bone and bone cement, resulting in the reduction of interface intensity and causes movement of the bone–cement interface.[11–13] It is reported that simple trabecular bone is more easily damaged than bone–cement interface. However, other studies have showed that creep and fatigue cracks occur mainly in the bone cement rather than in the bone tissue.[14] The pathogenesis of OA and RA are different. Whether these differences affect the combination and penetration of the bone–cement interface are still unknown.
Micro-CT can detect the stereoscopic structure of trabecular bone without destroying the samples. This method can accurately measure the bone microstructural parameters and provide a new technique for bone tissue measurement.[15,16] Compared with the traditional histological measurement, micro-CT can analyze the structural parameters of bone and further shed light on the biomechanical properties of the bone via reconstructing the three-dimensional structure. Previous studies on bone–cement interface were mostly carried out in animal models. The aim of this study was to compare the microstructure, bone quality, and the combination and penetration of cement–bone interface via micro-CT in tissue specimens from OA and RA patients to provide experimental support and to identify factors that are involved in the aseptic loosening of joint prosthesis.
2. Materials and methods
2.1. Materials
Disposable pulse lavage system (model: 201-01-00) was purchased from Apex Tools and orthopaedics Company (Guangzhou, China). All other chemicals and instruments were purchased from commercial sources. Homemade bone cement pressure device was also used in this study to establish bone cement–bone interface model.
2.2. Patients and bone specimen preparation
The patients with or ever had other diseases that may affect bone metabolism were exclude from this study. The patients who are taking or have taken the drugs that affect bone metabolism (such as calcitonin, bisphosphonates, glucocorticoids, vitamin D, and estrogen) are also excluded from the study. A total of 80 femoral condyle tissue specimens from 20 OA patients (40 condyles) and 20 RA patients (40 condyles) who underwent total knee arthroplasty at the Department of Orthopaedics in Tengzhou Central People's Hospital were collected between January 2017 and September 2017. All bone tissues were repeatedly washed with normal saline to remove bone debris, fat particles and residual blood clots as well as cartilage. Then the bone tissues were wrapped in gauze with normal saline and stored at −80°C. All the specimens were marked with age, gender, disease, hospital number, and operation time. Written informed consent was obtained from patients before their bone specimens were donated. The study was approved by the Ethics Committee of Tengzhou Central People's Hospital.
2.3. Bone–cement interface models of OA and RA
Bone tissue specimens were thawed at room temperature and washed with warm saline. Then the bone tissues were placed in a self-made bone cement press device with the cancellous bone surface facing up. Four screws were used to keep the stability of bone specimens. Bone cement was mixed according to the powder monomer 10: 18.88 (weight: weight), and injected in the cylinder body of self-made pressure device by the bone cement gun in dough phase. Subsequently, the cement was injected into the self-made pressurizing device cylinder in the dough period. Two kilogram weight was placed to pressurize and maintain the pressure for about 1 minute. The screw was removed after bone cement solidified. Each model has 2 components: bone and cement. Bone specimens were trimmed and the residual bone cement removed.
2.4. Groups
As mentioned above, 40 patients (20 OA patients and 20 RA patients) met the latest diagnostic criteria developed by American Association of Orthopedic Surgeons (AAOS) and American Rheumatology Association (ACR). These specimens were categorized into 2 groups: (OA) 40 specimens from OA patients; (RA) 40 specimens from RA patients. According to the random number table method, 20 specimens from the OA group were defined as group A, and 20 specimens in the RA group were defined as group B. The bone quality parameters were measured by micro-CT. The bone quality parameters (20 mm × 15 mm × 7 mm of cancellous bone) were obtained through micro-CT for groups A and B. The remaining 20 specimens in the OA group and the remaining 20 specimens in the RA group were defined as group C and group D. Specimens in groups C and D were analyzed by micro-CT and underwent three-dimensional analysis.
2.5. Micro-CT scanning
All specimens were scanned and reconstructed with micro-CT system at the condition of spatial resolution 35 μm × 35 μm × 35 μm and pixel 8000 × 8000 (Skyscan1176, Belgium). CTAn software was used to detect and analyze the three-dimensional parameters of trabecular bone, including bone volume fraction (BV/TV, %), bone surface/bone volume (BS/BV, mm−1), trabecular thickness (Tb.Th, μm), trabecular separation (Tb.Sp, μm), structure model index (SMI), trabecular number (Tb.N, mm−1), and degree of anisotropy (DA). Interest area (10 mm × 10 mm × 5 mm) was selected with CTVol software, and this area was limited to the upper surface of the specimens. The three-dimensional entity model was established in this way.
2.6. Statistical analyses
All statistical analyses were performed using SPSS 19.0 software package (SPSS Inc., Chicago, IL). Continuous variables were summarized with mean ± standard deviation (SD). Comparisons between groups A and B, and between groups C and D were performed by using the independent sample t-test. A P < .05 was considered statistically significant.
3. Results
3.1. Parameters of trabecular bone in OA and RA patients
The structural parameters, including BV/TV and Tb.Th, showed statistically significant difference between OA and RA specimens (BV/TV: 38.16% vs 19.19%; Tb.Th: 210.32 μm vs 184.52 μm, all P < .05). The results are similar with respect to Tb.N (2.10 mm−1 vs 1.11 mm−1, P < .05). Moreover, BS/BV and Tb.Sp were also significantly different between OA and RA groups (BS/BV: 18.78 mm−1 vs 24.26 mm−1; Tb.Sp:362.01 μm vs 620.71 μm, all P < .05, Table 1).
Table 1.
Parameters of trabecular bone in OA and RA patients.
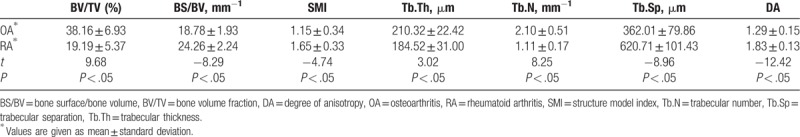
The SMI showed significant difference between OA and RA patients. OA patients showed lower SMI value than RA patients (1.15 vs 1.65, P < .05), indicating that OA patients exhibit more plate structure, while RA patients were featured by more rod structure. In addition, OA patients also had much lower DA value than RA patients (1.29 vs 1.83, P < .05).
3.2. Three-dimensional trabecular bone structure and bone–cement interface analysis
As shown in Fig. 1A, the trabecular bone of OA patients showed a plate structure, while trabecular bone of RA patients showed a rod-like structure (Fig. 1B). Three-dimensional coordinate system was established with Moldflow of Plastics Insight 6.1 software, and the x–y plane was selected for further analysis. Figure 1C showed that the density of trabecular bone in OA patients is much higher, and no tapering and breaking of trabecular bone were observed in these patients. The trabecular space was porous in OA patients. However, trabeculae bone of RA patients was very sparse and disarranged; some trabecular bones were tapered and broken. Moreover, the trabecular space was increased and the porous structure was destroyed in RA patients (Fig. 1D). Several large bone cement squeeze imprints were observed at the bone–cement interface in OA patients, and some part of the interface showed in the profile of trabecular bone (Fig. 2A). In RA patients, bone cement was evenly penetrated into the trabecular bone space and large bone cement squeeze imprints were rarely observed (Fig. 2B). The penetration depth of bone cement in the RA patients was significantly greater than those in the OA patients via x-ray detection (Fig. 3A and B).
Figure 1.
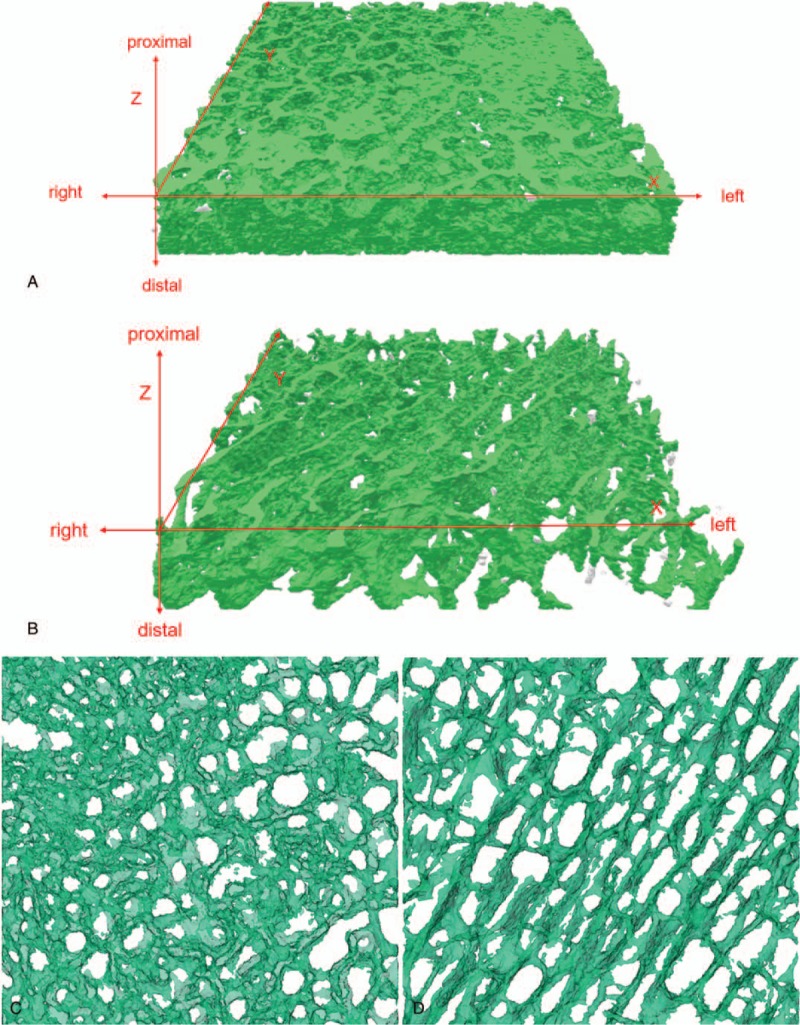
Three-dimensional reconstruction of the trabecular bone of the unilateral femoral condyle of OA and RA patients. Establishing a three-dimensional coordinate system, marking the proximal, distal, left, and right sides of the specimen. (A) A plate-like structure obtained from patients with OA; (B) A rod-like structure obtained from patient with RA. X–Y-axis: (C) Trabecular bone space was porous in OA patients. The trabecular bone was dense, with few trabecular thickness decreasing and trabecular fracture. (D) The trabecular bone was loose, messy, and fractured. Trabecular space was increased, and the porous structure was destroyed in RA patients. OA = osteoarthritis, RA = rheumatoid arthritis.
Figure 2.
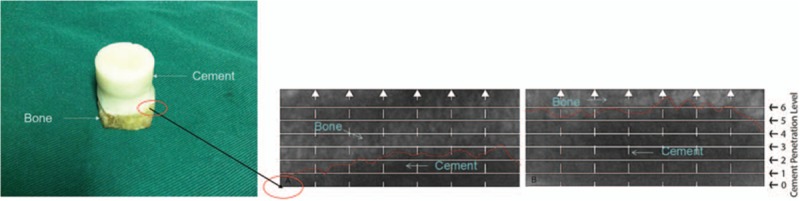
Select the Z-axis of cement–bone interface (X–Y-axis plane). Several large bone cement squeeze imprints were observed at the bone–cement interface in OA patients, and some part of the interface showed the profile of trabeculae bone (A). In RA patients, bone cement was evenly penetrated into the trabecular bone space and large bone cement squeeze imprints were rarely observed (B). OA = osteoarthritis, RA = rheumatoid arthritis.
Figure 3.
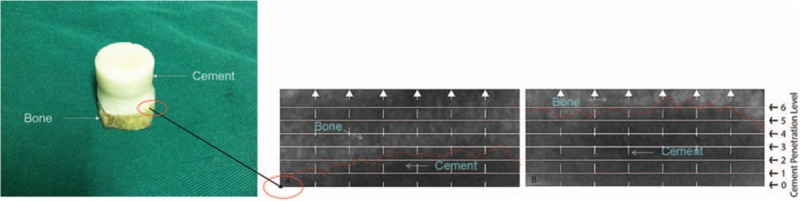
The left showed the established cement–bone interface model. Meanwhile, the x-ray scan of the longitudinal section of the model was performed. The cement–bone interface was set as level 0, and the depth of bone cement penetration into the bone tissue is measured. The right showed that he penetration depth of bone cement in the RA patients (B) was significantly greater than these in the OA patients (A) by x-ray detection. OA = osteoarthritis, RA = rheumatoid arthritis.
4. Discussion
To the best of our knowledge, this the first study to compare the microstructure, bone quality, the combination and penetration of cement–bone interface simultaneously in tissue specimens from OA and RA patients through micro-CT. We found that the bone quality parameters of OA patients including BV/TV, Tb.Th, and Tb.N are better than that of RA patients, but the analysis of three-dimensional structure suggested the combination and penetration of cement–bone interface of RA patients are better than that of OA patients. During the development of OA and RA, the structure of trabecular bone was changed due to the pathological process of the disease and the new stress distribution, resulting in the changes in the trabecular bone morphology, and further changed the mechanical features of trabecular bone. Micro-CT study of the trabecular microstructure and cement–bone interface of these 2 diseases can help the designing, installation and obtain the long-term stable mechanical properties of orthopedic surgical prostheses. It can also improve the reliability of the artificial joint replacement and reduce the postoperative revision rate.
The findings advance our understanding of knee prosthesis and have important clinical implications. The Tb.N is the number of trabecular per mm length, and the reciprocal of the mean distance of the central axis of the structure is derived by the distance transformation method.[17] The Tb.Th is the average thickness of trabecular bone, evaluated by calculating the 3D distance of the fitted sphere inside the trabecular bone.[18] Li and colleagues[19] found that the average trabecular bone thickness and the average number of trabecular bone were positively associated with trabecular bone volume. Zhang and colleagues[20] found that the number of trabecular bone in OA patients and healthy individuals shows no significant difference; however, the prevalence of osteoporosis in the RA patients was 1.9 times higher than in healthy individuals.[21] Fazzalari and Parkinson[22] found that compared with normal persons, OA patients had a different trabecular bone shape; more specifically, the Tb.N was increased, the Tb.Sp was smaller, and the Tb.Th showed no change. Chappard and colleagues[23] found that in the early stage of OA, the main change of trabecular bone was increment in Tb.Th; and in the late stage, the changes of subchondral trabecular bone was replaced by the increase in the Tb.N. Here, we found that Tb.Th and Tb.N in OA patients were significantly higher than those in RA patients, confirming that OA patients have lower corresponding parameters compared with normal individuals.
There are 3 major microstructures of trabeculae bone in patients: the rod structure, plate structure, and spherical structure. The SMI is used to evaluate the characteristics of rod and plate structure.[24] The index ranges from 0 to 4, and it is 0 for the ideal plate structure, 3 for the ideal rod structure, and 4 for the ideal spherical structure. Because most of bone tissues have plate structure (also called lamellar structure), and the increasing SMI for RA suggests that the trabecular bone structure is transforming from plate structure to rod structure, which can be clearly observed by CTAn software.
The DA can describe the symmetry and directionality of trabecular bone. Elevation of DA suggests an increase in osteoporosis, which cannot be effectively reflected by two-dimensional histomorphology. The DA and BV/TV can be used to reflect the mechanical properties of trabecular bone. In this study, we found that OA patients had increased DA but decreased BV/TV. Arlot and colleagues[25] found that the trabeculae bone with rod structure was prone to fracture when the BV/TV was less than 15%. Large-scale population based studies suggest that RA patients have lower BMD and higher risk of osteoporosis.[26–28] Bone strength depends on the bone mineral content and the degree of mineralization of organic matrix. The bone cannot bear load when bone mineral content of bone matrix is 100%.[29] Mineralization rate of osteoarthritis is low, which also can explain that trabeculae of RA are more likely to fracture than that of OA.
The BMD influences the penetration of bone cement, although a larger penetration depth correlates with the interface strength in unidirectional loading.[30] This experimental study confirms that trabecular bone spacing (Tb.Sp) and the penetration depth of bone cement in RA patients are greater compared with those in OA patients. Bone mineral density was negatively correlated with bone cement penetration.[31] In addition, bone ingrowth into cement could decrease the possibility of bone resorption and promote the formation of a stable interface.[32] Currently, it is believed that later loosening of the cement–bone interface is closely related to the bone cement particles on the interface. Fatigue fragmentation and joint micromovement can make artificial joints fixed with bone cements to wear and produce many particles. These bone cements particles can lead to foreign body reaction that mainly involves macrophages and giant cells. These cells can release some media after the phagocytosis of bone cements particles, leading to thickening of the membrane and loosening of the prosthesis. When a stable closed interface is formed between the bone and bone cements, the particles that cause inflammation, bone dissolution and loosening of bone cannot enter the interface, thereby reducing occurrence of prosthesis loosening. The micro-CT scan images of this experiment show that the bonding degree of cement–bone interface in RA patients is better than that in OA patients.
Despite the strict study design, this study cannot completely simulate cement–bone interface to carry out the biomechanical study due to the limit of bone specimen strength. In human environment, bleeding of the bone bed and bone regeneration all affect the morphology of cement–bone interface.[33] In addition, the sample size was small. Future studies with large sample sizes are required to validate our findings.
In summary, this study found that the bone quality of OA patients is better than that of RA patients, but the combination and penetration of cement–bone interface of RA patients are better than that of OA patients. The findings provide a deeper understanding of knee prosthesis in OA and RA patients, but require validations in future studies with larger sample sizes.
Acknowledgments
We would like to thank Prof Zhao JN of the Department of Joint Surgery, Jinling Hospital for his some valuable suggestions to improve the process in the analysis and interpretation of the results. We are grateful to all of the patients who have participated in this study.
Author contributions
Conceptualization: Yuanzheng Song, Fahao Zhu, Feng Lin, Feng Zhang, Shuaigong Zhang.
Data curation: Yuanzheng Song, Fahao Zhu, Feng Lin, Feng Zhang, Shuaigong Zhang.
Formal analysis: Yuanzheng Song, Fahao Zhu, Feng Lin, Feng Zhang, Shuaigong Zhang.
Funding acquisition: Yuanzheng Song, Fahao Zhu, Feng Lin, Feng Zhang, Shuaigong Zhang.
Investigation: Yuanzheng Song, Fahao Zhu, Feng Lin, Feng Zhang, Shuaigong Zhang.
Methodology: Yuanzheng Song, Fahao Zhu, Feng Lin, Shuaigong Zhang.
Project administration: Yuanzheng Song, Fahao Zhu, Shuaigong Zhang.
Resources: Yuanzheng Song, Fahao Zhu.
Software: Yuanzheng Song.
Supervision: Yuanzheng Song.
Validation: Yuanzheng Song.
Visualization: Yuanzheng Song.
Writing – original draft: Yuanzheng Song.
Writing – review & editing: Yuanzheng Song.
Footnotes
Abbreviations: BS/BV = bone surface/bone volume, BV/TV = bone volume fraction, DA = degree of anisotropy, micro-CT = micro-computed tomography, OA = osteoarthritis, RA = rheumatoid arthritis, SMI = structure model index, Tb.N = trabecular number, Tb.Sp = trabecular separation, Tb.Th = trabecular thickness.
This study was supported by a grant from Jining Medical University (Grant No. JY2017FS039).
The authors have no conflicts of interest to disclose.
References
- [1].Lohmander LS, Englund PM, Dahl LL, et al. The long-term consequence of anterior cruciate ligament and meniscus injuries. Am J Sports Med 2007;35:1756–69. [DOI] [PubMed] [Google Scholar]
- [2].Dieppe P, Cushnaghan J, Tucker M, et al. The Bristol ‘OA500 study’: progression and impact of the disease after 8 years. Osteoarthritis Cartilage 2000;8:63–8. [DOI] [PubMed] [Google Scholar]
- [3].Dieppe PA, Cushnaghan J, Shepstone L. The Bristol ‘OA500'study: progression of osteoarthritis (OA) over 3 years and the relationship between clinical and radiographic changes at the knee joint. Osteoarthritis Cartilage 1997;5:87–97. [DOI] [PubMed] [Google Scholar]
- [4].Paradowski PT, Englund M, Roos EM, et al. Similar group mean scores, but large individual variations, in patient-relevant outcomes over 2 years in meniscectomized subjects with and without radiographic knee osteoarthritis. Health Quality Life Outcomes 2004;2:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sahlström A, Johnell O, Redlund-Johnell I. The natural course of arthrosis of the knee. Clin Orthop Relat Res 1997;340:152–7. [DOI] [PubMed] [Google Scholar]
- [6].Firestein GS. Evolving concepts of rheumatoid arthritis. Nature 2003;423:356–61. [DOI] [PubMed] [Google Scholar]
- [7].Romas E, Gillespie M, Martin T. Involvement of receptor activator of NFκB ligand and tumor necrosis factor-α in bone destruction in rheumatoid arthritis. Bone 2002;30:340–6. [DOI] [PubMed] [Google Scholar]
- [8].Charnley J. Anchorage of the femoral head prosthesis to the shaft of the femur. Bone Joint J 1960;42:28–30. [DOI] [PubMed] [Google Scholar]
- [9].Mann KA, Allen MJ, Ayers DC. Pre-yield and post-yield shear behavior of the cement–bone interface. J Orthop Res 1998;16:370–8. [DOI] [PubMed] [Google Scholar]
- [10].White A, Best S. Properties and Characterisation of Bone Repair Materials. 2009;Cambridge: Bone Repair Biomaterials Woodhead Publishing Limited, 121–153. [Google Scholar]
- [11].Waanders D, Janssen D, Miller MA, et al. Fatigue creep damage at the cement–bone interface: an experimental and a micro-mechanical finite element study. J Biomech 2009;42:2513–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mann KA, Miller MA, Race A, et al. Shear fatigue micromechanics of the cement–bone interface: an in vitro study using digital image correlation techniques. J Orthop Res 2009;27:340–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yang D, Zhang D, Arola DD. Fatigue of the bone/cement interface and loosening of total joint replacements. Int J Fatigue 2010;32:1639–49. [Google Scholar]
- [14].Kim DG, Miller MA, Mann KA. A fatigue damage model for the cement–bone interface. J Biomech 2004;37:1505–12. [DOI] [PubMed] [Google Scholar]
- [15].Borah B, Gross GJ, Dufresne TE, et al. Three-dimensional microimaging (MRμI and μCT), finite element modeling, and rapid prototyping provide unique insights into bone architecture in osteoporosis. Anatomical Record 2001;265:101–10. [DOI] [PubMed] [Google Scholar]
- [16].Gross G, Dufresne T, Smith T, et al. Bone architecture and image synthesis. Morphologie 1999;83:21–4. [PubMed] [Google Scholar]
- [17].Danielsson PE. Euclidean distance mapping. Comput Graph Image Process 1980;14:227–48. [Google Scholar]
- [18].Hildebrand T, Rüegsegger P. A new method for the model-independent assessment of thickness in three-dimensional images. J Microsc 1997;185:67–75. [Google Scholar]
- [19].Li B, Aspden RM. Composition and mechanical properties of cancellous bone from the femoral head of patients with osteoporosis or osteoarthritis. J Bone Miner Res 1997;12:641–51. [DOI] [PubMed] [Google Scholar]
- [20].Zhang ZM, Li ZC, Jiang LS, et al. Micro-CT and mechanical evaluation of subchondral trabecular bone structure between postmenopausal women with osteoarthritis and osteoporosis. Osteoporosis Int 2010;21:1383–90. [DOI] [PubMed] [Google Scholar]
- [21].Lee SG, Park YE, Park SH, et al. Increased frequency of osteoporosis and BMD below the expected range for age among South Korean women with rheumatoid arthritis. Int J Rheum Dis 2012;15:289–96. [DOI] [PubMed] [Google Scholar]
- [22].Fazzalari N, Parkinson I. Fractal properties of subchondral cancellous bone in severe osteoarthritis of the hip. J Bone Miner Res 1997;12:632–40. [DOI] [PubMed] [Google Scholar]
- [23].Chappard C, Peyrin F, Bonnassie A, et al. Subchondral bone micro-architectural alterations in osteoarthritis: a synchrotron micro-computed tomography study. Osteoarthritis Cartilage 2006;14:215–23. [DOI] [PubMed] [Google Scholar]
- [24].Hildebrand T, Rüegsegger P. Quantification of bone microarchitecture with the structure model index. Comput Meth Biomech Biomed Eng 1997;1:15–23. [DOI] [PubMed] [Google Scholar]
- [25].Arlot ME, Burt PB, Roux JP, et al. Microarchitecture influences microdamage accumulation in human vertebral trabecular bone. J Bone Miner Res 2008;23:1613–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kröger H, Honkanen R, Saarikoski S, et al. Decreased axial bone mineral density in perimenopausal women with rheumatoid arthritis—a population based study. Ann Rheum Dis 1994;53:18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Haugeberg G, Uhlig T, Falch JA, et al. Reduced bone mineral density in male rheumatoid arthritis patients: frequencies and associations with demographic and disease variables in ninety-four patients in the Oslo county rheumatoid arthritis register. Arthritis Rheumatol 2000;43:2776–84. [DOI] [PubMed] [Google Scholar]
- [28].Haugeberg G, Uhlig T, Falch JA, et al. Bone mineral density and frequency of osteoporosis in female patients with rheumatoid arthritis: results from 394 patients in the Oslo County Rheumatoid Arthritis register. Arthritis Rheumatol 2000;43:522–30. [DOI] [PubMed] [Google Scholar]
- [29].Seeman E, Delmas PD. Bone quality–the material and structural basis of bone strength and fragility. N Engl J Med 2006;354:2250–61. [DOI] [PubMed] [Google Scholar]
- [30].Waanders D, Janssen D, Mann KA, et al. The mechanical effects of different levels of cement penetration at the cement–bone interface. J Biomech 2010;43:1167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Raiss P, Pape G, Kleinschmidt K, et al. Bone cement penetration pattern and primary stability testing in keeled and pegged glenoid components. J Shoulder Elbow Surg 2011;20:723–31. [DOI] [PubMed] [Google Scholar]
- [32].Pettit AR, Ji H, von Stechow D, et al. TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am J Pathol 2001;159:1689–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mann KA, Miller MA, Cleary RJ, et al. Experimental micromechanics of the cement–bone interface. J Orthop Res 2008;26:872–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


