Abstract
Introduction:
Metastatic spread in invasive lobular carcinoma (ILC) of breast mainly occurs in bones, gynecological organs, peritoneum, retroperitoneum, and gastrointestinal (GI) tract. Metastases to the GI tract may arise many years after initial diagnosis and can affect the tract from the tongue to the anus, stomach being the most commonly involved site. Clinical presentations are predominantly nonspecific, and rarely asymptomatic. CEA, CA 15–3, and CA 19–9 may be informative for symptomatic patients who have had a previous history of breast cancer.
Case presentation:
We introduce the case of asymptomatic colonic metastasis from breast carcinoma in a 67-year-old woman followed-up for Luminal A ILC. Diagnosis was performed through positron emission tomography/computed tomography (PET/CT) scan and contrast-enhancement spectral mammography (CESM), steering endoscopist to spot the involved intestinal tract and in ruling out further dissemination in the breast parenchyma.
Conclusion:
In colonic metastases, tumor markers might not be totally reliable. In asymptomatic cases, clinical conditions might be underappreciated, missing local or distant recurrence. CT and PET/CT scan might be useful in diagnosing small volume diseases, and steering endoscopist toward GI metastasis originating from the breast. CESM represents a tolerable and feasible tool that rules out multicentricity and multifocality of breast localization. Moreover, particular patients could tolerate it better than magnetic resonance imaging (MRI).
Keywords: breast cancer, colon, contrast-enhanced spectral mammography, lobular, metastasis, positron emission tomography
1. Introduction
Breast cancer represents the most common female malignancy. Although survival rates have been improving in the last few years, about 30% women will end up by developing a metastatic disease, irrespective of its treatment.[1,2] Bones, lungs, pleura, liver, and brain are the main areas of metastasis from breast carcinoma. A considerable difference in metastatic patterns must be underlined when comparing invasive ductal carcinoma (IDC), which commonly involves lungs, pleura, and bones, with invasive lobular carcinoma (ILC) that frequently affects bones, gynecological organs, peritoneum, retroperitoneum, and gastrointestinal (GI) tract.[3,4] Stomach and small intestine are the most frequent sites of GI tract, colonic and rectal metastases being very rare.[5,6] Detection of GI metastasis originating from breast cancer often turns out difficult, above all in asymptomatic patients.
We introduce the case report of a silent isolated colonic metastasis that originated from ILC, 12 years from the primary tumor detection, whose diagnosis followed abdomen 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) and breast contrast-enhanced spectral mammography (CESM).
2. Case presentation
A 67-year-old woman followed up for Luminal A ILC at our Hospital showed an increased CEA and CA 15–3. In 2004, when 53 years old, she underwent a left radical mastectomy for an ILC [pT2N3(6/13), ER: 70%, PgR: 40%, KI67: 2%, HER2: negative]. After surgery, we administered 4 cycles of adjuvant chemotherapy based on epirubicin and cyclophosphamide and 4 cycles of taxotere, followed by a 2-year cycle of tamoxifene, then shifting to letrozole, for a global 5-year treatment.
She underwent annual Rx mammography, ultrasound (US) examination, and CEA and CA 15–3 markers screenings. The tests were negative until October 2016, when just a CEA and CA 15.3 increase was recorded (14.4 and 57 U/mL, with reference range 0.5–5.0 U/mL and 1–30 U/mL, respectively). No other symptoms or signs were reported by the patient. Due to negative traditional breast imaging, patient underwent total body CT scan followed by a colonoscopy, which were both negative. After a 3-month follow-up, CEA and CA 15–3 levels kept high. Therefore, the patient underwent a total body PET/CT scan, showing a FDG uptake of 4.7 and 1.9 Standardized Uptake Value (SUV), the former being suspicious for neoplastic lesion at level of ileocecal valve (Fig. 1A), the latter being nonspecific at the level of right breast axillary tail (Fig. 1B). A second colonoscopy showed a nonspecific, protruding, hyperemic, nonulcerated colonic wall area, easy to be missed by unguided colonoscopy, in proximity of ileocecal valve that underwent biopsy (Fig. 2). It showed a mild chronic inflammation of intestinal lamina propria, where epithelial cells marked by eccentric nucleus and broad vacuolated cytoplasm were detected, being consistent with localization of ILC of the breast. At immunohistochemistry, results were as follows: ER: 98% PgR: 95%, Ki67: 18%, HER2: negative. Multidisciplinary Group for Breast Cancer suggested contrast-enhancement spectral mammography (CESM) in order to exclude ILC multifocality and/or multicentricity. It showed a contrast enhancement just at level of FDG uptake (Fig. 3). At US second look of right breast, a 4-mm small ipoechoic area was detected. Biopsy result showed ILC (Fig. 4). The patient was first referred to Surgical Oncology Unit, where right hemicolectomy was performed. Later, she was referred to Breast Unit for right breast quadrantectomy and lymph-node biopsy (negative result). After 7-month follow-up, no recurrence appeared.
Figure 1.
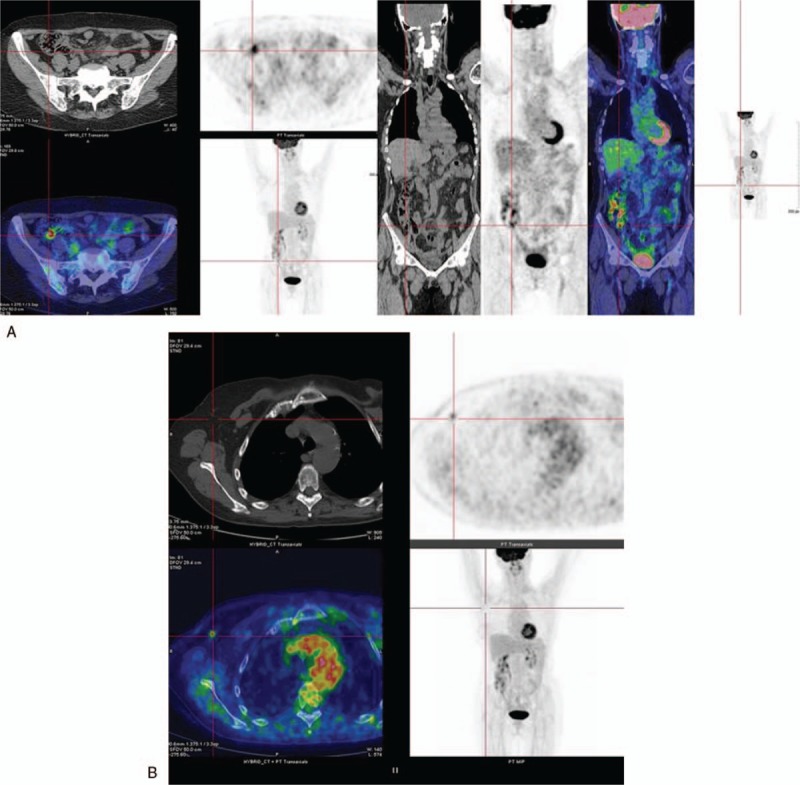
18F-FDG PET/CT: Axial and coronal 18F-FDG PET/CT images showing focal FDG uptake in the region of ileocecal valve with a maximum SUV of 4.7 (1A) and a focal FDG uptake in correspondence of right breast axillary tale with a SUV of 1.9 (1B).
Figure 2.
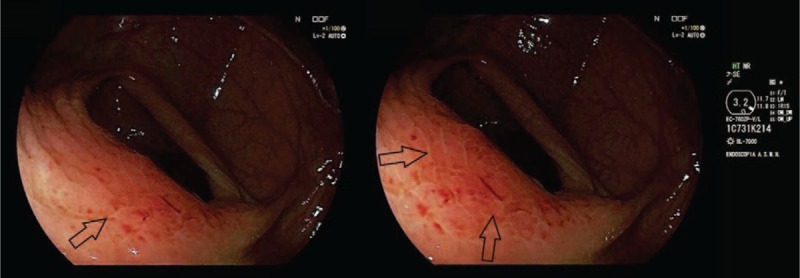
Colonoscopy: Diagnostic colonoscopy images show a nonspecific, protruding, hyperemic, nonulcerated colonic wall area, near the ileocecal valve (black arrows). The biopsy of this area revealed the presence of a lobular breast cancer metastasis.
Figure 3.
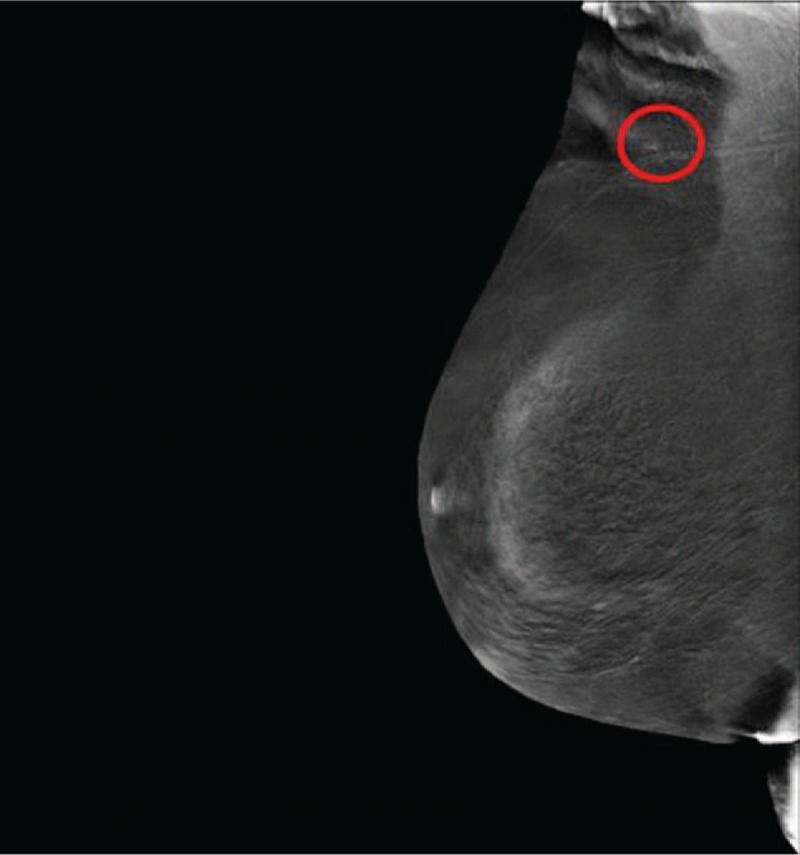
Contrast-enhanced spectral mammography: Mediolateral oblique recombined image showed faint enhancement (red circle) visible in the right axillary breast tale.
Figure 4.
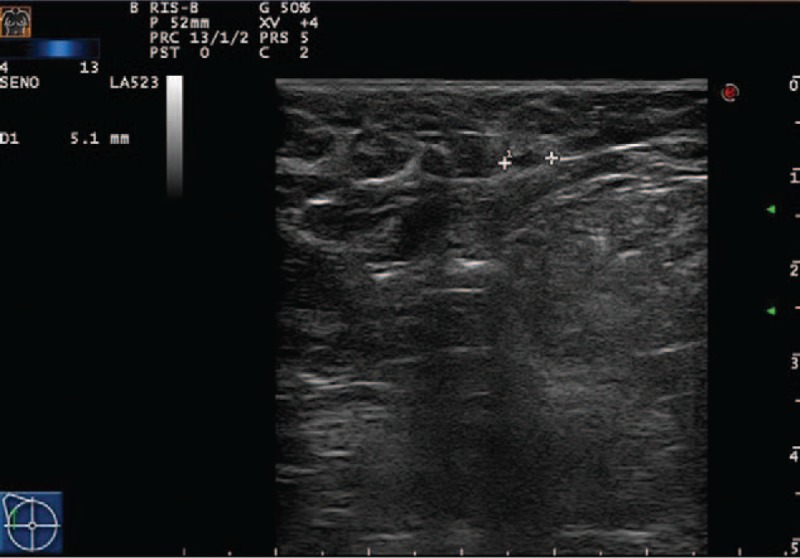
Ultrasound: Minute ipoechoic area of 4 mm visible at the second ultrasound look.
3. Discussion
ILC of the breast accounts for 6% to 10% of all breast cancers. It has a different metastatic pattern if compared with IDCs, probably due to the loss of E-cadherin expression on tumor cell membrane that promotes tumor cell migration, preventing a cell-to-cell adhesion.[7] From the radiology point of view, the loss of E-cadherin in ILC is also responsible for a difficult tumor detection, particularly in mammography, due to the absence of breast mass growth.[8] GI tract represents a rare site for metastatic breast disease, marked by lobular histology in most cases. Any GI tract can be involved, from the tongue to the anus.[9,10] Clinical presentations are predominantly nonspecific (abdominal pain, diarrhea, inappetence, and nausea), peculiar (bleeding, vomiting) just in advanced cases, and rarely they are asymptomatic.[11]
After a review of the literature up to 2017, stomach turned out as the most commonly affected site (65%) while only 14.9% metastases involved colon (32 in 214 reported cases). Only our case and case reported by Uskent et al[12] were found to be clinically symptomless (6.2%). In both cases, however, an increase of the screening markers was recorded (CEA and CA-15–3 in our case; just CA19–9 in Uskent et al).
Asymptomatic cases, where not only traditional imaging but also second-level techniques give negative results (such as CT and colonoscopy, as in our case), might lead to an underrating of clinical situation, missing the presence of a local or distant recurrence. To avoid this situation, we suggest to always perform 18F-FDG PET/CT scan. In our case, 18F-FDG PET/CT allowed to steer endoscopist to detect GI localization of the previously unnoted tumor, thus ruling out further dissemination in the remaining GI tract. CESM advantages over breast MRI are shorter examination times and lower costs.[13] Furthermore, CESM might be effective, when MRI is not available or in patients with contraindications (eg pacemakers or claustrophobia). Moreover, it represents a valuable tool for patients bearing the heavy psychological burden of examination for local or distant recurrence, after a first diagnosis and tumor treatment. CESM is more acceptable and feasible than MRI, as it reduces patient's stress.[14] In our case, CESM allowed a more accurate US second look of the breast, thus ruling out multicentricity and multifocality of breast localization, which are typical ILC features.
Acknowledgments
We thank Daniela Masi (IRCCS-AUSL Reggio Emilia) for editing.
Author contributions
Conceptualization: Giuseppe Falco, Simone Mele, Maurizio Zizzo.
Data curation: Giuseppe Falco, Simone Mele, Maurizio Zizzo, Saverio Coiro, Gianluca Gatta.
Formal analysis: Giuseppe Falco, Simone Mele, Maurizio Zizzo, Graziella Di Grezia, Saverio Coiro, Gianluca Gatta.
Investigation: Giuseppe Falco, Simone Mele, Graziella Di Grezia, Paolo Cecinato, Giulia Besutti, Saverio Coiro, Rita Vacondio.
Methodology: Giuseppe Falco, Simone Mele, Maurizio Zizzo, Graziella Di Grezia, Saverio Coiro.
Writing – original draft: Giuseppe Falco, Simone Mele, Maurizio Zizzo.
Writing – review & editing: Giuseppe Falco, Simone Mele, Maurizio Zizzo.
Supervision: Maurizio Zizzo, Guglielmo Ferrari.
Validation: Maurizio Zizzo.
Footnotes
Abbreviations: 18F-FDG PET/CT = 18F-fluorodeoxyglucose positron emission tomography/computed tomography, CA 15–3 = carcinoma antigen 15–3, CEA = carcinoembryonic antigen, CESM = contrast-enhanced spectral mammography, ER = estrogen receptor, GI = gastrointestinal, HER2 = human epidermal growth factor receptor 2, IDC = invasive ductal carcinoma, ILC = invasive lobular carcinoma, PgR = progesterone receptor, SUV = standardized uptake value, US = ultrasound.
GF, SM, and MZ contributed to the conceptualization. GF, SM, MZ, SC, and GG did the data curation. GF, SM, MZ, GDG, SC, and GG did the formal analysis. GF, SM, GDG, PC, GB, SC, and RV contributed to the investigation. GF, SM, MZ, GDG, and SC contributed to the methodology. MZ and GF did the supervision. MZ did the validation. GF, SM, and MZ prepared the original draft and reviewed and edited the manuscript.
The authors declare that they have no conflict of interest or financial ties to disclose.
References
- [1].Eljabu W, Finch G, Nottingham J, et al. Metastatic deposits of breast lobular carcinoma to small bowel and rectum. Int J Breast Cancer 2011;2011:413949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Signorelli C, Pomponi-Formiconi D, Nelli F, et al. Single colon metastasis from breast cancer: a clinical case report. Tumori 2005;91:424–7. [DOI] [PubMed] [Google Scholar]
- [3].Arrangoiz R, Papavasiliou P, Dushkin H, et al. Case report and literature review: metastatic lobular carcinoma of the breast an unusual presentation. Int J Surg Case Rep 2011;2:301–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Borst MJ, Ingold JA. Metastatic patterns of invasive lobular versus invasive ductal carcinoma of the breast. Surgery 1993;114:637–41. [PubMed] [Google Scholar]
- [5].McLemore EC, Pockaj BA, Reynolds C, et al. Breast cancer: presentation and intervention in women with gastrointestinal metastases and carcinomatosis. Ann Surg Oncol 2005;12:886–94. [DOI] [PubMed] [Google Scholar]
- [6].Taal BG, Peterse H, Boot H. Clinical presentation, endoscopic features and treatment of gastric metastases from breast carcinoma. Cancer 2000;89:2214–21. [PubMed] [Google Scholar]
- [7].Ciriello G, Gatza ML, Beck AH, et al. Comprehensive molecular portraits of invasive lobular breast cancer. Cell 2015;163:506–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Manucha V, Khilko N, Reilly K, et al. Invasive pleomorphic lobular carcinoma, negative for ER, PR and HER/2neu—a case report. Int J Clin Exp Pathol 2011;4:200–5. [PMC free article] [PubMed] [Google Scholar]
- [9].Cormier WJ, Gaffey TA, Welch JM. Linitis plastica caused by metastatic lobular carcinoma of the breast. Mayo Clin Proc 1980;55:747–53. [PubMed] [Google Scholar]
- [10].Puglisi M, Varaldo E, Assalino M, et al. Anal metastasis from recurrent breast lobular carcinoma: a case report. World J Gastroenterol 2009;15:1388–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ambroggi M, Stroppa EM, Mordenti P, et al. Metastatic breast cancer to the gastrointestinal tract: report of five cases and review of the literature. Int J Breast Cancer 2012;2012:439023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Uskent N, Baloğlu H, Çakmakçi M, et al. Breast cancer metastases to the stomach and colon mimicking primary gastrointestinal cancer: four cases and literature review. Adv Mod Oncol Res 2016;2:204–10. [Google Scholar]
- [13].Martincich L, Montemurro F, De Rosa G, et al. Monitoring response to primary chemotherapy in breast cancer using dynamic contrast-enhanced magnetic resonance imaging. Breast Cancer Res Treat 2004;83:67–76. [DOI] [PubMed] [Google Scholar]
- [14].Iotti V, Ravaioli S, Vacondio R, et al. Contrast-enhanced spectral mammography in neoadjuvant chemotherapy monitoring: a comparison with breast magnetic resonance. Breast Cancer Res 2017;19:106. [DOI] [PMC free article] [PubMed] [Google Scholar]


