Abstract
To evaluate effect of sellar reconstruction during pituitary adenoma resection surgery by the endoscopic endonasal transsphenoidal approach using artificial cerebral dura mater patch.
This was a retrospective study of 1281 patients who underwent endoscopic transsphenoidal resection for the treatment of pituitary adenomas between December 2006 and May 2014 at the Neurosurgery Department of the People's Liberation Army General Hospital. The patients were classified into 4 grades according to intraoperative cerebrospinal fluid (CSF) leakage site. All patients were followed up for 3 months by telephone and outpatient visits.
One thousand seventy three (83.7%) patients underwent sellar reconstruction using artificial dura matter patched outside the sellar region (method A), 106 (8.3%) using artificial dura matter patched inside the sellar region (method B), and 102 (8.0%) using artificial dura matter and a mucosal flap (method C). Method A was used for grade 0–1 leakage, method B for grade 1 to 2 leakage, and method C for grade 2 to 3 leakage. During the 3-month follow-up, postoperative CSF leakage was observed in 7 patients (0.6%): 2 among patients who underwent method B (1.9%) and 5 among those who underwent method C (4.9%). Meningitis was diagnosed in 13 patients (1.0%): 2 among patients who underwent method A (0.2%), 4 among those who underwent method B (3.8%), and 7 among those who underwent method C (6.7%).
Compared with other reconstruction methods, sellar reconstruction surgery that only use artificial dura mater as repair material had a low rate of complications.
Keywords: artificial dura mater, cerebrospinal fluid leakage, neurosurgery, pituitary adenoma, sellar reconstruction
1. Introduction
Postoperative cerebrospinal fluid (CSF) leakage is one of the most common complications after pituitary adenoma resection by endoscopic endonasal transsphenoidal approach (0.6%–3.5%).[1,2] CSF leakage may lead to life-threatening conditions such as bacterial infection, tension pneumocephalus, and abscess.[3–7] Patients with severe CSF leakage are required to undergo repair surgery and the rate of postoperative diabetes insipidus and meningitis is significantly increased.[8]
Currently, the materials used for the sellar reconstruction surgery by endoscopic endonasal transsphenoidal approach are of two kinds: autologous tissues (subcutaneous fat, autologous bone, muscle, fascia lata, pedicle periosteal flap of turbinate mucosa, and mucosal periosteal flap located at the posterior lateral wall of the nasal cavity); and artificial patches (fibrin glue, resin plate made of silicon, titanium mesh, cyanoacrylate, hydroxyapatite cement, and titanium mesh).
Artificial dura mater provides type I collagen scaffold for the defect site, which is helpful for the growth of fibroblasts.[9] It is widely used in neurosurgery and can prevent serious complications such as CSF leakage, intracranial infections, encephalocele, cerebral adhesions, and scars. The artificial dura mater is sutured to the defect site. The edge of artificial dura mater is closed using a sealant to prevent CSF leakage from the incision sutures, or an artificial dura mater patch slightly larger than the defect is draped on the defect site with the edges over the edge of dura mater. Artificial dura matter has been shown to be nontoxic, tissue compatible, nonpermeable, without adhesion with brain tissues, resistant to mechanical stress, chemically stable, and without inflammatory response.[10,11]
Intraoperative CSF leakage can be classified according to its severity,[12] but the methods for leakage repair according to this classification are still poorly defined. Therefore, the aim of the present study was to observe the methods used for leakage repair according to leakage severity and the occurrence of complications.
2. Materials and methods
2.1. Study design
This was a retrospective study of 1281 patients who underwent endoscopic transsphenoidal resection for the treatment of pituitary adenomas between December 2006 and May 2014 at the Neurosurgery Department of the People's Liberation Army General Hospital. The study was approved by the ethics committee of the People's Liberation Army General Hospital. The need for individual consent was waived by the committee because of the retrospective nature of the study.
2.2. Patients
Inclusion criteria were: underwent endoscopic transsphenoidal surgery; and final diagnosis of pituitary adenoma. Exclusion criteria were: sellar lesions that were not pituitary adenomas; or underwent craniotomy.
Final diagnosis was based on the postoperative pathology examination of the surgical specimen. The pituitary adenomas were classified as microadenoma (<1 cm), macroadenoma (1–4 cm), and giant adenoma (>4 cm).
2.3. Preoperative assessment
The sphenoid sinus, nasal septum, tumor size, tumor texture, bilateral carotid artery diameter were evaluated routinely. Magnetic resonance imaging (MRI) was analyzed carefully before surgery in order to understand the attachment point of the saddle diaphragm under pathological conditions (Fig. 1). The sellar bone window and incision range were evaluated according to the attachment point. The principle was that the incision range of sellar dura could not exceed the attachment point.
Figure 1.
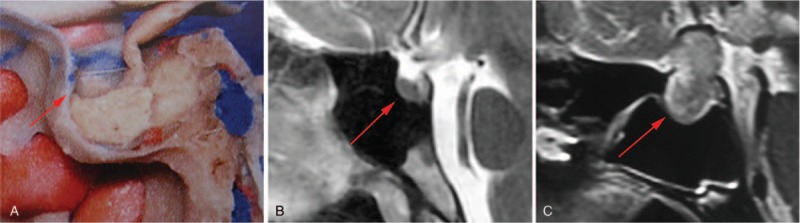
The attachment point of saddle diaphragm in different situations. (A) Normal anatomic attachment point (the picture is from Rohoton Anatomy, reproduced with permission). (B) MRI scan showing the normal attachment point. (C) MRI scan showing an abnormal attachment point under pathological condition. The red arrows indicate the attachment point of saddle diaphragm. MRI = magnetic resonance imaging.
2.4. Surgery
All patients received preoperative intravenous antibiotics (ceftriaxone 1.0 g, intravenously 30 minutes before operation). The patients were placed in a supine position under general anesthesia, with the head inclined 20° downward. The facial skin and nasal mucosa were disinfected with povidone-iodine. Sterile drapes were prepared. Saline and epinephrine were infiltrated into the mucosa of the nasal base. Under endoscopy, the opening of the sphenoid sinus was found and positioned. The mucosa in the front and outside of the sphenoid sinus was removed, and the anterior wall of the sphenoid sinus was exposed completely. The anterior wall of the sphenoid sinus was drilled with abrasion drill. The size of the bone window was about 2.0 × 1.0 cm. The local mucosa of the sphenoid sinus was cleaned and complete hemostasis was done. The dura mater was cut in a “+” shape. The open range of the dura matter was required not to exceed the attachment point of the sellar diaphragm. After removal of the lower part of the tumor with an aspirator, the endoscope entered into the tumor. The residual tumor was removed under direct vision. CSF leakage was observed closely during the process of tumor resection.
2.5. Postoperative management
Eventual fluids from the nasal cavity and body temperature were observed closely after surgery. If there was clear fluid out of the nasal cavity or the patients had symptoms such as headache and fever, the patients underwent lumbar catheter drainage. If an infection was confirmed, the patients received amikacin (20 mg 1–2 times/d) through intraspinal canals. The drainage lasted for 5 to 7 days and the patients received intravenous antibiotics (vancomycin 500 mg q8 hour or meropenem 500 mg q8 hour). Patients with poor response to conservative treatment underwent secondary endonasal transsphenoidal surgery to repair the CSF leakage.
2.6. Felice's classification system of intraoperative CSF leakage
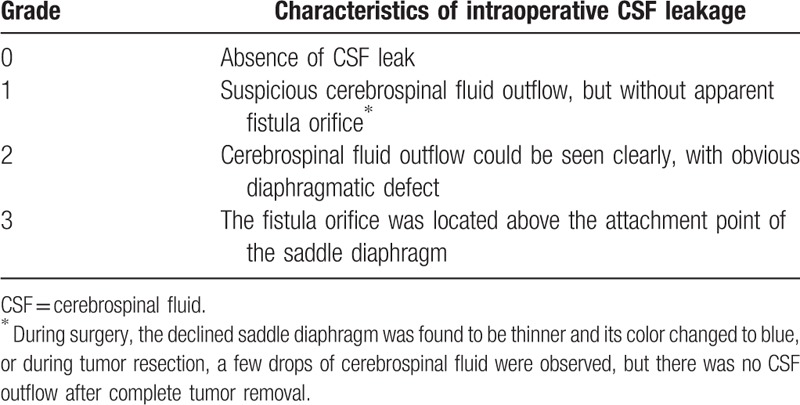
Table 1 presents the CSF leakage classification used in this study, based on previous classifications[3,12]: Grade 0, no CSF leakage; Grade 1, suspicious CSF outflow, but no leakage site could be found (in most cases, during surgery, the declined saddle diaphragm was found to be thinner and its color changed to blue, or during the process of tumor resection, a few drops of CSF were observed, but there was no CSF outflow after tumor removal); Grade 2, CSF outflow could be seen clearly and obvious defect was observed; and Grade 3, the leakage site was located above the attachment point of saddle diaphragm, including the front part of the tuberculum sellae, rear part of the sellar back, and the posterior clinoid process.
Table 1.
Classification system of CSF leakage during pituitary adenoma resection by endoscopic nasal transsphenoidal approach.
2.7. Sellar reconstruction
The appropriate sellar reconstruction approach was selected according to the intraoperative classification of intraoperative CSF leakage. The sizes of intrasellar tamponade were adjusted according to the intraoperative condition of saddle diaphragm and intrasellar situations. The objective of intrasellar tamponade was to decrease CSF outflow. Patients with Grade 0 or 1 and whose decline of sellar diaphragm was found to be good during the surgery underwent method A (Fig. 2A), in which an appropriate size of hemostatic gauze or gelatin sponge was filled into the sellar region and artificial dura mater was patched outside the sellar region. Patients with Grade 2 leakage underwent method B (Fig. 2B), in which hemostatic gauze or gelatin sponge was filled into the sellar region to block CSF leakage, the artificial dura mater was patched inside the sellar region, and hemostatic gauze or gelatin sponge was attached on the outside of the sellar region. Fibrin glue (CryoLife, Inc., Kennesaw, GA) was used for fixation. Patients with Grade 3 leakage underwent method C (Fig. 2C), in which the hemostatic gauze or gelatin sponge was filled in the CSF leakage site, the artificial dura mater was patched inside the sellar region, and the artificial dura mater patch was attached outside the sella turcica basement durendocrine. Fibrin glue was used for fixation and a mucosal flap of the nasal septum was attached.
Figure 2.
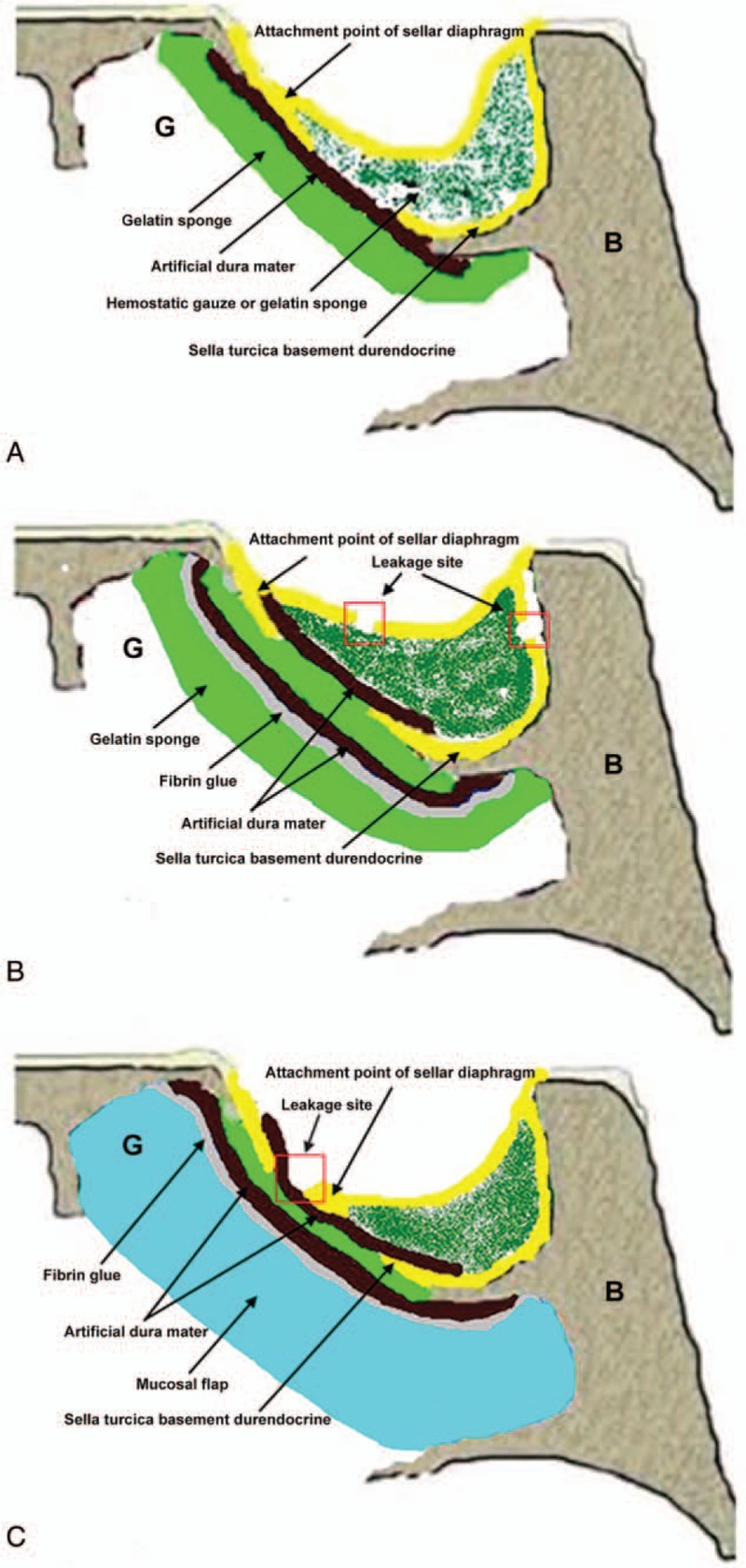
Schematic diagrams of the 3 surgical methods. (A) Method A. Hemostatic gauze or gelatin sponge was filled in the sellar region. The artificial dura mater was attached outside the sella turcica basement durendocrine. (B) Method B. Hemostatic gauze or gelatin sponge was filled in the sellar region to block the CSF leakage site. The artificial dura mater was patched inside the sellar region. Gelatin sponge was attached at the outside of the sellar region. Fibrin glue was used to achieve fixation. (C) Method C. Hemostatic gauze or gelatin sponge was filled in the CSF leakage site. The artificial dura mater was patched inside the sellar region. The artificial dura mater was also attached outside the sella turcica basement durendocrine. Fibrin glue was used for fixation. A mucosal flap of nasal septum was filled into the defect. B: sphenoid; G: sphenoid sinus cavity. CSF = cerebrospinal fluid.
2.8. Follow-up
All patients were followed up by telephone and outpatient visits. The average follow-up time was 3.3 months (from 0.3 to 6.6 months). The presence or absence of fever, headache, stiff neck, and fluids discharged from nasal cavity and pharynx were recorded. Meningitis was considered in the presence of symptoms like headache, fever, nausea, and vomiting, positive meningeal irritation sign, CSF leukocyte count >1000/mm3, and positive CSF bacterial culture.
2.9. Statistical analysis
All statistical analyses were done using SPSS 20.0 software package (SPSS Inc., Chicago, IL). Chi-square tests was used to compare the incidence of intraoperative CSF leakage in different groups pituitary adenoma (microadenoma, macroadenoma, giant adenoma). A P < .05 was considered statistically significant.
3. Result
3.1. Characteristics of the patients
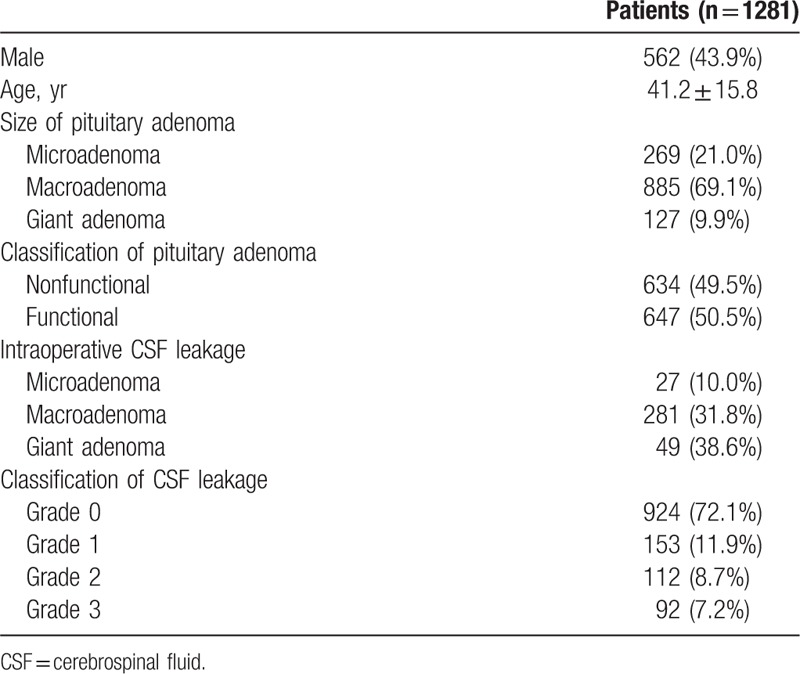
There were 568 men and 713 women, aged 42.3 ± 10.2 years. There were 269 patients with pituitary microadenoma, 885 with pituitary macroadenoma, and 127 with giant adenoma. The incidence of intraoperative CSF leakage in microadenoma, macroadenoma, giant adenoma were 10.0%, 31.8%, and 38.6%, respectively (P = .00). There were 634 cases of nonfunctioning pituitary adenomas and 647 cases of functional pituitary adenomas. There were 924 patients (72.1%) with Grade 0 leakage, 153 (11.9%) with Grade 1 leakage, 112 patients (8.7%) with Grade 2 leakage, and 92 patients (7.2%) with Grade 3 leakage (Table 2).
Table 2.
Characteristics of the patients.
3.2. Surgery
As shown in Table 3, 1074 patients underwent reconstruction according to method A (924 patients with Grade 0 leakage and 149 with Grade 1); 106 patients underwent reconstruction according to method B (4 patients with Grade 1 leakage and 102 with Grade 2); and 102 patients underwent reconstruction according to Method C (10 patients with Grade 2 leakage and 92 with Grade 3).
Table 3.
Surgical approaches.
One patient with Grade 2 leakage suffered from CSF leakage during tumor resection. The site of the leakage was located in the saddle diaphragm. After resection of the tumor, the saddle diaphragm was folded to block the leak. Then, sellar reconstruction was done according to method A (Fig. 3).
Figure 3.
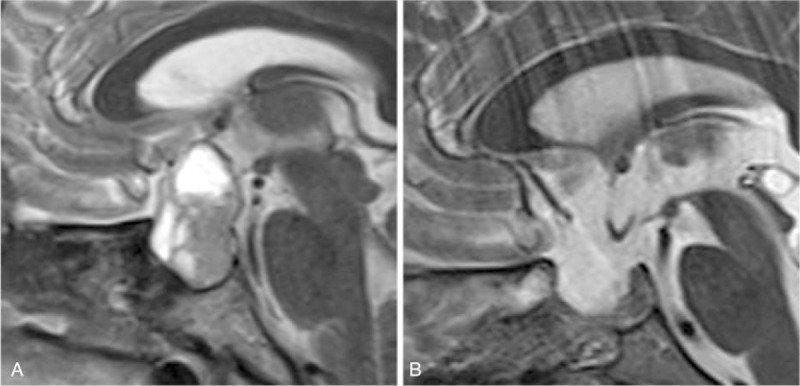
Method A. (A) MRI of the tumor before surgery. (B) MRI scan after sellar reconstruction. MRI = magnetic resonance imaging.
In 4 patients with Grade 1 CSF leakage, during surgery, decline of saddle diaphragms was observed to extrude the sellar bone window, with part of them entering into sphenoid sinus. Then, sellar reconstruction was done according to method B (Fig. 4).
Figure 4.
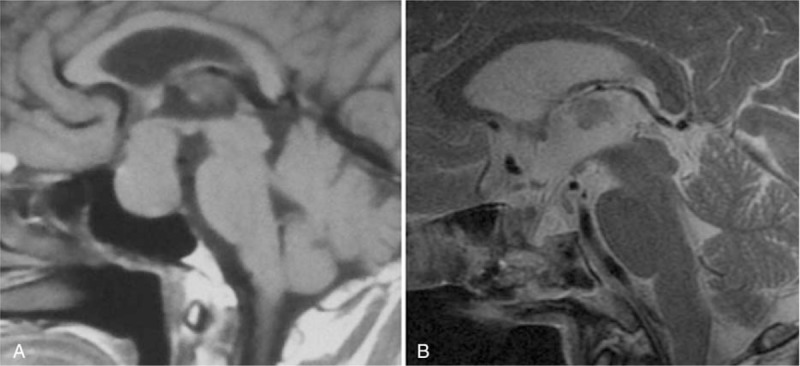
Method B. (A) MRI of the tumor before surgery. (B) MRI scan of the sellar reconstruction. MRI = magnetic resonance imaging.
In 10 patients with Grade 2 leakage and saddle diaphragms obviously elevated during the surgery, the size of leakage site was >5 mm, and CSF flow was important. Therefore, they underwent reconstruction according to method C (Fig. 5).
Figure 5.
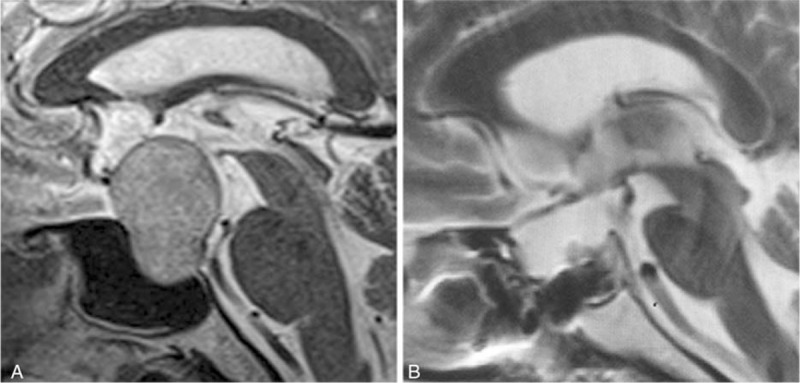
Method C. (A) MRI of the tumor before surgery. (B) MRI scan after sellar reconstruction. MRI = magnetic resonance imaging.
3.3. Postoperative follow-up and complications
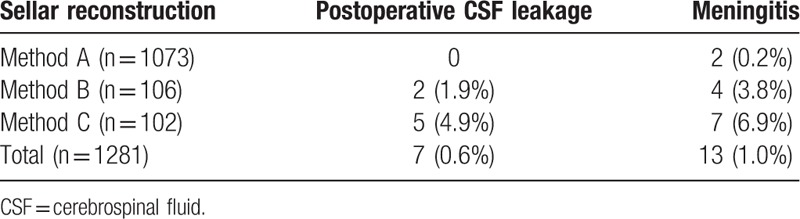
During follow-up, meningitis was diagnosed in 13 patients (1.0%): 2 among patients who underwent method A (0.2%), 4 among those who underwent method B (3.8%), and 7 among those who underwent method C (6.7%). The postoperative CSF leakage was observed in 7 patients (0.6%): 2 among patients who underwent method B (1.9%) and 5 among those who underwent method C (4.9%). Four patients of those got meningitis, 1 with taphylococcus epidermidis, 1 Bauman Acinetobacter. The patients without microbiological diagnosis were given the lumbar drainage and received amikacin (20 mg 1–2 times/d). The patients with bacteria were given antibiotics according to the drug sensitivity test and received amikacin (20 mg 1–2 times/d) through intraspinal canals. Meningitis was diagnosed in 13 patients (1.0%): 2 among patients who underwent method A (0.2%), 4 among those who underwent method B (3.8%), and 7 among those who underwent method C (6.7%) (Table 4).
Table 4.
Postoperative complications.
4. Discussion
This study summarized the characteristics of intraoperative CSF leakage of 1281 patients who underwent endoscopic resection of pituitary adenoma. According to the intraoperative CSF leakage degree and the location of leakage site, a classification system was made, and different procedures of sellar reconstruction were applied accordingly. The occurrence rate of postoperative CSF leakage was 0.6%, and the occurrence rate of postoperative meningitis was 1.0%.
A classification system was used according to the size and location of intraoperative CSF leakage site, based on suggested systems, and sellar reconstruction was conducted according to this system.[3,12] Esposito et al[12] reported the rate of postoperative CSF leakage was 2.5%. Kong et al[13] established a classification system based on the characteristics of intraoperative CSF leakage in order to avoid the subjective judgment of CSF leakage degree. The patients were graded according to the size of arachnoid leakage and sellar reconstruction was conducted. Their rate of CSF leakage was 10.5%. Jalessi et al[14] suggested a classification system based on the intraoperative CSF leakage and the size of leakage site. Their follow-up was 18 months and the rate of CSF leakage was 0.8%. They recommended that CSF leakage sites >1 mm should undergo intensive sellar reconstruction. Compared with these previous classification systems, the classification system proposed here emphasizes the relationship between the CSF leakage site and the attachment point of the saddle diaphragm.
It has been suggested that maintaining the integrity degree of saddle diaphragm during surgery and avoiding that the incision of the sellaturcica basement durendocrine exceeds the attachment point of sellar diaphragm should decrease the occurrence of CSF leakage.[2] After sellar reconstruction, 2 barriers which prevent postoperative CSF leakage. The dura mater above the attachment point of the saddle diaphragm was closely attached to the bone of the skull base. If the leakage site was located above the attachment point, artificial dura mater and mucosal flap were required for sellar reconstruction, combined with sphenoid sinus tamponade.
Thorp et al[15] used a vascular mucosal flap to repair the skull base defects of 152 patients and their rate of CSF leakage was 3.3%. They considered that a vascular mucosal flap was suitable for CSF leakage with high flow. Nevertheless, autologous tissue repair has the following disadvantages: surgery is longer; additional incisions are required on the body; the normal nasal mucosa is destroyed; and the bone tissue of the sella bottom is damaged, resulting in a lack of support surrounding sella bottom, making difficult to achieve osseous reconstruction of sella bottom.
Therefore, the use of an artificial dura mater patch have some advantages as it provides type I collagen scaffold that allows the growth of fibroblasts and derivation of novel dura mater to the defect site. This material has some beneficial features such as absorbability and antibacterial effect, and it also induces the formation of epithelial cells, fibroblasts, and collagen layer.[11,16–18] It also has excellent histocompatibility and biodegradability, and will not increase the occurrence of infection.[9,17–21] Sellar reconstruction using an artificial dura mater patch should decrease the occurrence of CSF leakage,[2] but compared with a mucosal flap combined with vascular pedicle, the artificial dura mater patch itself is avascular and the duration of scar formation is relatively longer.
The following points warrant attention during reconstruction surgery. First, the residual tumor cavity must be filled with gelatin sponge in order to decrease the CSF outflow. The amount of intrasellar gelatin sponge should not be excessive, in order to avoid the increased pressure of intrasellar region. Second, the size of the artificial dura mater patch that is attached in the sellar region should be larger than the opening in the sellaturcica basement durendocrine in order to avoid the artificial dura mater patch dropping into the sphenoid sinuses. Third, fibrin glue was used to fix the artificial dura mater patch in order to ensure no CSF leakage. In addition, the other objective of fibrin glue usage was to avoid the shifting of the artificial dura mater patch. Finally, if the tumor was huge, a mucosal flap should be preserved during surgery in order to make a more solid sellar reconstruction.
The present study has some limitations. The number of patients in each group was different. The reconstruction technique was dependent upon the leakage grade, introducing a bias. In addition, there was no control group. Additional studies are necessary to address adequately the outcomes of CSF leakage after reconstruction.
5. Conclusions
In conclusion, the incision of sellaturcica basement durendocrine should avoid exceeding the attachment point of sellar diaphragm. Compared with other reconstruction methods, sellar reconstruction surgery that only uses artificial dura mater as repair material had a low rate of complications. This could prevent the occurrence of postoperative CSF leakage and meningitis.
Acknowledgments
The authors acknowledge the PLA 301 Hospital for its help during manuscript writing.
Footnotes
Abbreviation: CSF = cerebrospinal fluid.
YY and FW have contributed equally to the work.
The authors report no conflicts of interest.
References
- [1].Dehdashti AR, Ganna A, Karabatsou K, et al. Pure endoscopic endonasal approach for pituitary adenomas: early surgical results in 200 patients and comparison with previous microsurgical series. Neurosurgery 2008;62:1006–15. discussion 1015-7. [DOI] [PubMed] [Google Scholar]
- [2].Wang F, Zhou T, Wei S, et al. Endoscopic endonasal transsphenoidal surgery of 1,166 pituitary adenomas. Surg Endosc 2015;29:1270–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Awad AJ, Rowland NC, Mian M, et al. Etiology, prognosis, and management of secondary pituitary abscesses forming in underlying pituitary adenomas. J Neurooncol 2014;117:469–76. [DOI] [PubMed] [Google Scholar]
- [4].Berker M, Hazer DB, Yucel T, et al. Complications of endoscopic surgery of the pituitary adenomas: analysis of 570 patients and review of the literature. Pituitary 2012;15:288–300. [DOI] [PubMed] [Google Scholar]
- [5].Kassam AB, Prevedello DM, Carrau RL, et al. Endoscopic endonasal skull base surgery: analysis of complications in the authors’ initial 800 patients. J Neurosurg 2011;114:1544–68. [DOI] [PubMed] [Google Scholar]
- [6].Wang L, Yao Y, Feng F, et al. Pituitary abscess following transsphenoidal surgery: the experience of 12 cases from a single institution. Clin Neurol Neurosurg 2014;124:66–71. [DOI] [PubMed] [Google Scholar]
- [7].Thakur B, Jesurasa AR, Ross R, et al. Transnasal trans-sphenoidal endoscopic repair of CSF leak secondary to invasive pituitary tumours using a nasoseptal flap. Pituitary 2011;14:163–7. [DOI] [PubMed] [Google Scholar]
- [8].Jahangiri A, Wagner J, Han SW, et al. Morbidity of repeat transsphenoidal surgery assessed in more than 1000 operations. J Neurosurg 2014;121:67–74. [DOI] [PubMed] [Google Scholar]
- [9].Barbolt TA, Odin M, Leger M, et al. Biocompatibility evaluation of dura mater substitutes in an animal model. Neurol Res 2001;23:813–20. [DOI] [PubMed] [Google Scholar]
- [10].Kawai H, Nakagawa I, Nishimura F, et al. Effectiveness of a new gelatin sealant system for dural closure. Neurol Res 2014;36:866–72. [DOI] [PubMed] [Google Scholar]
- [11].Wang H, Dong H, Kang CG, et al. Preliminary exploration of the development of a collagenous artificial dura mater for sustained antibiotic release. Chin Med J (Engl) 2013;126:3329–33. [PubMed] [Google Scholar]
- [12].Esposito F, Dusick JR, Fatemi N, et al. Graded repair of cranial base defects and cerebrospinal fluid leaks in transsphenoidal surgery. Neurosurgery 2007;60(Suppl):295–303. discussion 303-4. [DOI] [PubMed] [Google Scholar]
- [13].Kong DS, Kim HY, Kim SH, et al. Challenging reconstructive techniques for skull base defect following endoscopic endonasal approaches. Acta Neurochir (Wien) 2011;153:807–13. [DOI] [PubMed] [Google Scholar]
- [14].Jalessi M, Sharifi G, Mirfallah Layalestani MR, et al. Sellar reconstruction algorithm in endoscopic transsphenoidal pituitary surgery: experience with 240 cases. Med J Islam Repub Iran 2013;27:186–94. [PMC free article] [PubMed] [Google Scholar]
- [15].Thorp BD, Sreenath SB, Ebert CS, et al. Endoscopic skull base reconstruction: a review and clinical case series of 152 vascularized flaps used for surgical skull base defects in the setting of intraoperative cerebrospinal fluid leak. Neurosurg Focus 2014;37:E4. [DOI] [PubMed] [Google Scholar]
- [16].Abla AA, Link T, Fusco D, et al. Comparison of dural grafts in Chiari decompression surgery: review of the literature. J Craniovertebr Junction Spine 2010;1:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Huang YH, Lee TC, Chen WF, et al. Safety of the nonabsorbable dural substitute in decompressive craniectomy for severe traumatic brain injury. J Trauma 2011;71:533–7. [DOI] [PubMed] [Google Scholar]
- [18].Parlato C, di Nuzzo G, Luongo M, et al. Use of a collagen biomatrix (TissuDura) for dura repair: a long-term neuroradiological and neuropathological evaluation. Acta Neurochir (Wien) 2011;153:142–7. [DOI] [PubMed] [Google Scholar]
- [19].Wong CY, Khairi MD, Mohamed SA, et al. Dural tear post mastoidectomy repaired with Dura Gen. Med J Malaysia 2010;65:307–8. [PubMed] [Google Scholar]
- [20].Kaplan M, Akgun B, Demirdag K, et al. Use of antibiotic - impregnated DuraGen (R) to reduce the risk of infection in dura repair: an in vitro study. Cent Eur Neurosurg 2011;72:75–7. [DOI] [PubMed] [Google Scholar]
- [21].Sade B, Oya S, Lee JH. Non-watertight dural reconstruction in meningioma surgery: results in 439 consecutive patients and a review of the literature. Clinical article. J Neurosurg 2011;114:714–8. [DOI] [PubMed] [Google Scholar]


