Supplemental Digital Content is available in the text
Keywords: ABSSSI, antibacterial, gram-positive bacteria, linezolid, outpatients, tedizolid phosphate
Abstract
Background:
Acute bacterial skin and skin structure infections (ABSSSI) are a frequent cause of hospital admissions in the United States. Safe and effective outpatient treatments may lower ABSSSI-associated health care costs by reducing unnecessary hospital admissions. Using data from 2 phase 3 trials (ESTABLISH-1, NCT01170221; ESTABLISH-2, NCT01421511), this post-hoc analysis explored the efficacy and safety of tedizolid in an outpatient setting.
Methods:
Subgroup analysis was performed on US outpatients (defined as patients who were not in hospital at the time of treatment initiation) with ABSSSI caused by presumed or proven gram-positive pathogens. Patients were randomly assigned to receive tedizolid phosphate 200 mg once daily for 6 days (n = 403) or linezolid 600 mg twice daily for 10 days (n = 410). The primary end point was early clinical response (48–72 hours after the start of treatment). Secondary end points included investigator-assessed clinical response at end of therapy (EOT) and post-therapy evaluation (PTE; 7–14 days after therapy). Additional assessments included the patient-reported level of pain using a visual analog scale (VAS) and the per-pathogen favorable microbiological response rate at the PTE visit. Compliance with treatment and safety outcomes was also recorded.
Results:
Early clinical response was similar between treatment groups (tedizolid, 82.4%; linezolid, 79.0%), as was investigator-assessed clinical response at EOT (tedizolid, 87.1%; linezolid, 86.1%) and PTE (tedizolid, 83.1%; linezolid, 83.7%). Mean changes from baseline to days 10 to 13 in VAS scores were identical between treatment groups (tedizolid, –51.9 mm; linezolid, –51.9 mm). Microbiological eradication rates were generally similar in both treatment groups for all key pathogens. Patients in both groups had favorable response at PTE. More tedizolid-treated patients (89.3%) than linezolid-treated patients (77.3%) were compliant with treatment. The most frequently reported drug-related treatment-emergent adverse events were nausea (tedizolid, 10.7%; linezolid, 13.8%), diarrhea (tedizolid, 4.5%; linezolid, 5.9%), and headache (tedizolid, 5.5%; linezolid, 4.4%). Treatment discontinuation rates were low for both treatment groups (tedizolid, 0.7%; linezolid, 1.0%).
Conclusion:
Short-course therapy with tedizolid can successfully treat patients with ABSSSI caused by presumed or proven gram-positive pathogens in an outpatient setting.
1. Introduction
Acute bacterial skin and skin structure infections (ABSSSI) are a frequent cause of hospital admissions in the United States.[1,2] The number of admissions has been rising steadily for more than a decade,[1,2] placing increased pressure on health care resources. However, a large proportion of patients with ABSSSI may not require hospital care and could thus be suitable for outpatient treatment.[2,3] Outpatient treatment could prevent hospital stays, reduce health care costs, and decrease the incidence of nosocomial infections.[3] The decision to provide outpatient treatment is generally based on clinical assessment of the patient's severity of infection and comorbidities.[3]
Treatment agents that have long half-lives, allow for short courses of therapy, and provide good tolerability profiles are likely to be the most suitable for outpatient treatment of patients with ABSSSI.[2,3] Tedizolid phosphate, the prodrug of the oxazolidinone antibacterial tedizolid, has pharmacokinetic properties that allow once-daily dosing and has demonstrated potent activity against a wide variety of gram-positive pathogens, including methicillin-resistant Staphylococcus aureus (MRSA).[4] Clinical trials have demonstrated an at least fourfold greater potency for tedizolid over linezolid against these pathogens.[5–9] Given that MRSA is the most frequent cause of ABSSSI in the United States,[10,11] there is a need for oral antibacterials with activity against this pathogen.
Two phase 3 trials, ESTABLISH-1 and ESTABLISH-2, demonstrated the noninferiority of tedizolid phosphate (200 mg once daily for 6 days) to linezolid (600 mg twice daily for 10 days) in patients with ABSSSI caused by confirmed or suspected gram-positive pathogens.[12,13] As previously reported, the tedizolid group in ESTABLISH-1 showed early clinical response rates (the primary endpoint) of 79.5% (95% CI, 74.8%–83.7%) compared with the linezolid group (79.4% [95% CI, 74.7%–83.6%]) for a treatment difference of 0.1% (95% CI, −6.1%–6.2%). In ESTABLISH-2, 85% of patients in the tedizolid group and 83% in the linezolid group achieved early clinical response (difference 2.6%, 95% CI −3.0–8.2). The subpopulation of nonhospitalized US-based patients from both ESTABLISH trials was analyzed post hoc in this study to determine the efficacy and tolerability of tedizolid in an outpatient setting.
2. Methods
2.1. Study design
ESTABLISH-1 (ClinicalTrials.gov, NCT01170221) and ESTABLISH-2 (ClinicalTrials.gov, NCT01421511) were double-blind, double-dummy, multicenter, randomized phase 3 trials in patients with ABSSSI.[12,13] Patients were randomly assigned to receive tedizolid phosphate 200 mg once daily for 6 days (n = 664) or linezolid 600 mg twice daily for 10 days (n = 669). ESTABLISH-1 patients received oral therapy exclusively, whereas ESTABLISH-2 patients first received intravenous therapy for 24 hours and then were switched to oral study drug when prespecified clinical improvement criteria were met.
2.2. Patient selection
Patients were ≥18 (ESTABLISH-1) or ≥12 (ESTABLISH-2) years of age, and key inclusion criteria were diagnosis of ABSSSI (cellulitis/erysipelas, wound infection, or major cutaneous abscess), lesion surface area ≥75 cm2 (wound infections and abscesses also required erythema extending ≥5 cm from the edge of the wound or abscess to the lesion margin), ≥1 regional or systemic sign of infection (lymphadenopathy, temperature ≥38°C, white blood cell count ≥10,000 cells/mm3 or <4000 cells/mm3, or immature neutrophil count >10%), and suspected or documented gram-positive pathogen. Key exclusion criteria included uncomplicated or gram-negative ABSSSI, use of any systemic or topical antibiotic with activity against gram-positive bacteria within 96 hours before the first dose of study drug, previous unsuccessful treatment of the same infection site, infection close to a prosthetic device, severe sepsis or known bacteremia at the time of enrollment, or confirmed immunocompromised status.
All patients or their guardians provided written informed consent. Institutional review boards or the equivalent at each study center approved the trial. A data and safety monitoring board reviewed safety data during the conduct of the study.
2.3. Assessments
2.3.1. Efficacy
The primary end point for the integrated data analysis of the ESTABLISH trials was early clinical response, defined as a reduction in lesion area of ≥20% at 48 to 72 hours after the start of study drug. Important secondary end points were investigator-assessed clinical response at the end of therapy (EOT) and at the PTE, which was assessed 7 to 14 days after EOT. A two-sided 95% confidence interval was calculated for the observed difference in outcome rates using the method of Miettinen and Nurminen.[14]
Patient-reported level of pain was assessed using a visual analog scale (VAS) at baseline and on study days 10 to 13. The VAS is a 100-mm line on which the 0-mm point indicates no pain and the 100-mm point indicates worst pain ever; patients were required to indicate their pain levels on the VAS twice within 5 minutes. If the VAS scores were different by >10 mm, the patient was to complete the VAS a third time. The 2 closest scores were recorded. The baseline VAS pain score, the score on study days 10 to 13, and the change from baseline (visit minus baseline) were summarized.
The per-pathogen microbiological response rate at the PTE visit was determined programmatically based on the data from the central microbiology laboratory and the investigator's assessment of clinical response. A favorable response was defined as eradication or presumed eradication of the original baseline pathogen. The number and percentage of patients classified with a favorable microbiological response were tabulated for both treatment groups.
2.3.2. Compliance
Treatment-compliant patients were defined as outpatients who took all doses (100%) of their assigned treatment. For tedizolid phosphate (200 mg once daily for 6 days), compliant patients received 6 doses; for linezolid (600 mg twice daily for 10 days), compliant patients received 20 doses. Patients were categorized as noncompliant if they missed ≥1 dose.
2.3.3. Safety
Safety evaluations included assessment of treatment-emergent adverse events (TEAEs), clinical laboratory results, vital signs and electrocardiograms, and physical examinations.
2.4. Analysis populations
Only outpatients from the pooled US population in the ESTABLISH trials were included in this analysis; US patients who were hospitalized at the time of treatment initiation were considered inpatients and were excluded from this analysis. The intent-to-treat (ITT) population (all randomly assigned US outpatients) was the primary population for efficacy analyses. The clinically evaluable analysis set at PTE included all patients in the ITT population who completed the investigator's assessment of outcome at the PTE visit without major protocol violations or receipt of treatments that might confound outcomes. The microbiological ITT population included all US outpatients for whom the baseline pathogen had been identified. Safety analyses were performed in the safety population (all US outpatients who received ≥1 dose of study drug).
3. Results
3.1. Patient characteristics
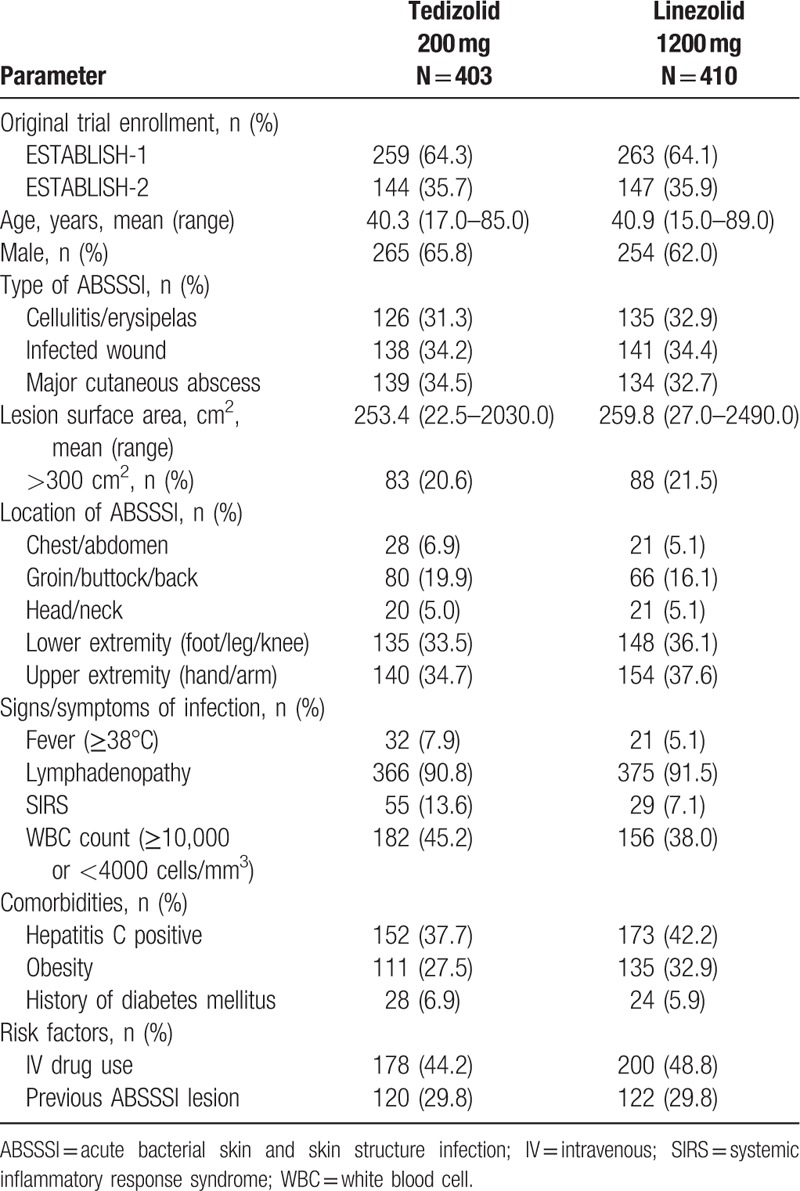
The pooled ITT population consisted of 813 outpatients from the ESTABLISH studies; 403 of these patients were randomly assigned to receive tedizolid phosphate, and 410 were randomly assigned to receive linezolid (Supplementary Figure 1). Patient demographics and clinical characteristics are described in Table 1. Demographics were comparable between both treatment groups. Severity of infection, comorbidities, and type of ABSSSI were also generally similar between tedizolid- and linezolid-treated patients. Systemic inflammatory response syndrome, however, was about twice as common in the tedizolid group. A large proportion of patients in both treatment groups currently or recently received intravenous drugs (tedizolid, 44.2%; linezolid, 48.8%). Based on their clinical characteristics, the patient population in both treatment groups was moderately to severely ill, and ∼21% had large (>300 cm3) lesions.
Table 1.
Patient demographics and clinical characteristics of US-based outpatients (ITT population).
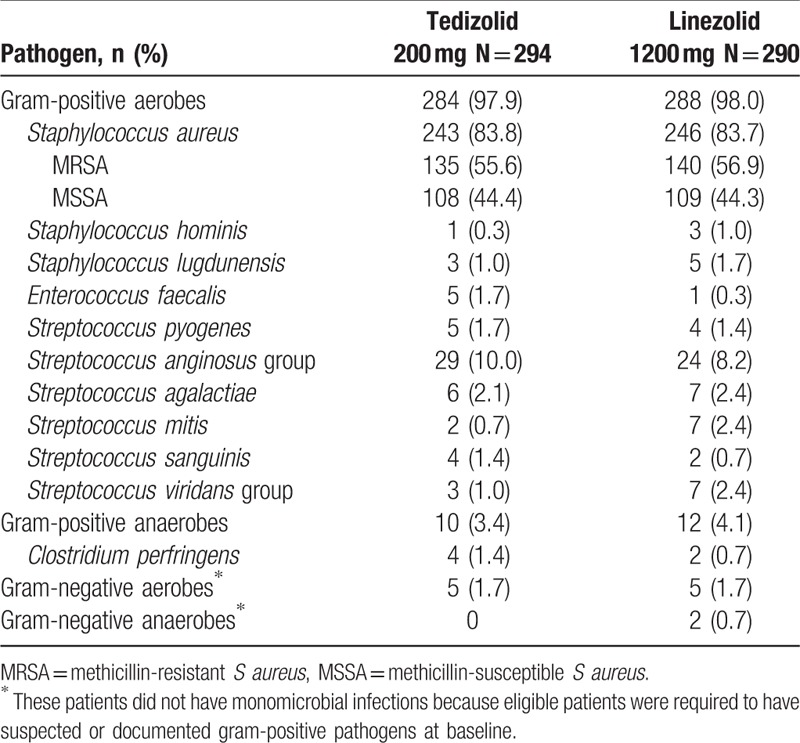
The most common pathogen isolated at baseline was S aureus (Table 2); slightly more than half the S aureus isolates were MRSA. The second most common pathogens were of the Streptococcus anginosus group (S anginosus, Streptococcus constellatus, and Streptococcus intermedius). Baseline pathogens were similar between treatment groups.
Table 2.
Baseline pathogens isolated from primary infection site or blood culture of US-based outpatients (MITT population).
3.2. Efficacy
Early clinical response (≥20% reduction in lesion size at 48–72 hours) was similar between treatment groups (tedizolid, 82.4%; linezolid, 79.0%) (Fig. 1A). Clinical success rates at EOT were slightly higher than early response rates but remained similar between the tedizolid (87.1%) and linezolid (86.1%) treatment groups (Fig. 1B). Rates of clinical success at PTE were also similar between the tedizolid (83.1%) and linezolid (83.7%) treatment groups (Fig. 1C).
Figure 1.

Clinical response in US-based outpatients (ITT population). (A) Early clinical response. (B) Clinical success at EOT evaluation. (C) Clinical success at PTE evaluation. Value above brackets indicates treatment difference (95% confidence interval). EOT = end of therapy, ITT = intent-to-treat, PTE = post-therapy evaluation.
Changes from baseline in VAS scores are shown in Figure 2. Results were similar between treatment groups. Patients recorded mean scores of 59.5 mm (tedizolid) and 58.1 mm (linezolid) at baseline, which were reduced to 7.6 mm (tedizolid) and 6.1 mm (linezolid) on study days 10 to 13.
Figure 2.
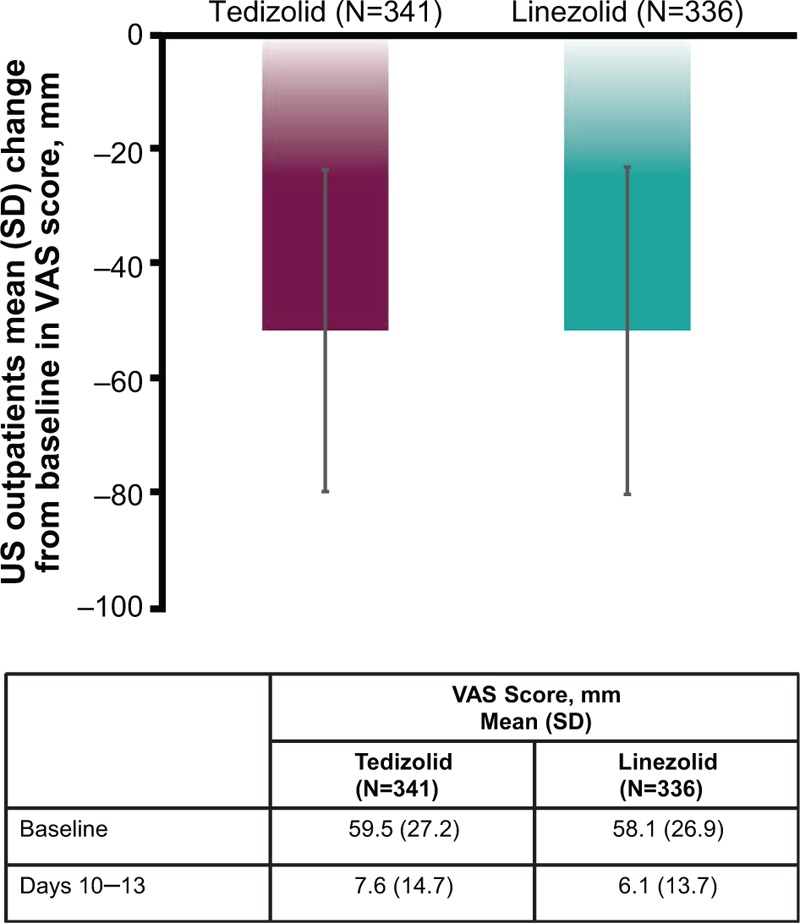
VAS scores in US-based outpatients at baseline and at days 10 to 13 (ITT population). ITT = intent-to-treat; SD = standard deviation; VAS = visual analog scale.
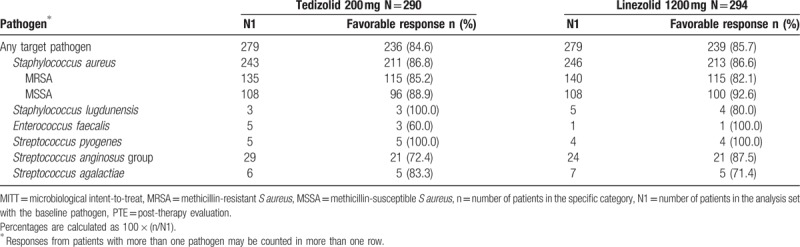
Microbiological response rates for key pathogens are shown in Table 3. Eradication rates were generally similar in both treatment groups for all key pathogens, and most patients in both groups had a favorable response at PTE. More than 86% of patients in both treatment groups had a favorable response for S aureus.
Table 3.
Microbiological response for key pathogens from primary infection site or blood culture at the PTE visit (MITT population).
Both test agents demonstrated a relatively narrow range of MIC values for S aureus (including methicillin-sensitive S aureus and MRSA), which was the most commonly isolated pathogen in the studies in the full patient population of the phase 3 studies (0.12 to 0.5 μg/mL for tedizolid, 1.0 to 4.0 μg/mL for linezolid).[12,13] Other pathogens were found in much lower numbers but were also highly susceptible to both tedizolid and linezolid.
3.3. Compliance
More patients receiving tedizolid phosphate (89.3%) than those receiving linezolid (77.3%) were compliant with treatment (Fig. 3). The difference in compliance may be attributable to both shorter duration of treatment and fewer doses associated with tedizolid phosphate compared with linezolid as a result of the once-daily and twice-daily dosing regimens. The mean (±standard deviation) number of doses taken was 5.7 (±1.0) for tedizolid phosphate and 18.0 (±4.7) for linezolid.
Figure 3.
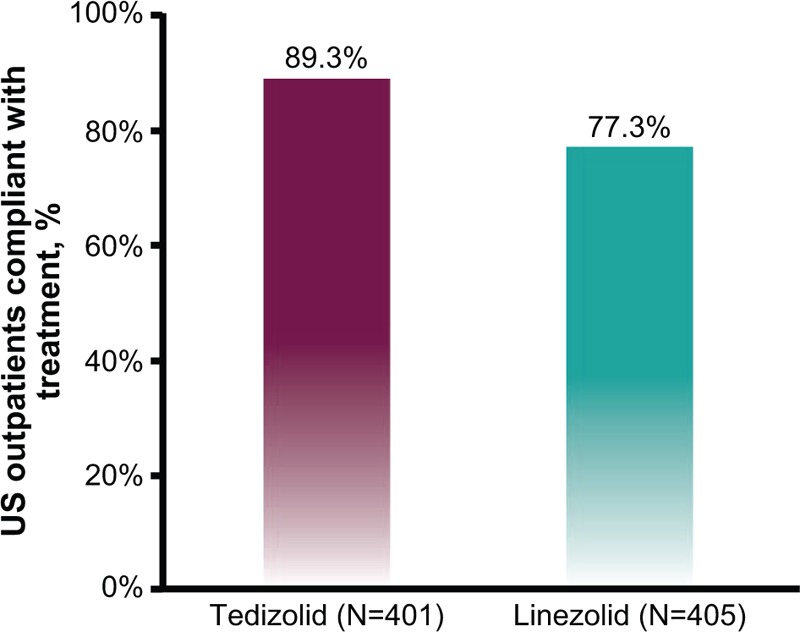
Treatment compliance of US-based outpatients (safety population).
3.4. Safety
The overall incidence of TEAEs was comparable between tedizolid and linezolid (Table 4). Fewer gastrointestinal adverse events (AEs) were reported with tedizolid than with linezolid. Moreover, the incidence of abnormal platelet counts (<112 × 103/mm3) was lower in the tedizolid group (11 patients; 2.7%) than in the linezolid group (20 patients; 4.9%). Overall, 5 (1.2%) patients in the tedizolid group and 7 (1.7%) patients in the linezolid group experienced serious TEAEs (Table 4). Serious TEAEs in the tedizolid group included abscess, dehydration, endophthalmitis, nephrolithiasis, septic shock, 7th nerve paralysis, vomiting, and weight decrease. Serious TEAEs in the linezolid group included acute coronary syndrome, anaphylactic reaction, blood glucose increase, cellulitis, diabetic ketoacidosis, major depression, suicidal ideation, and urinary tract infection.
Table 4.
TEAEs experienced by US-based outpatients (safety population).

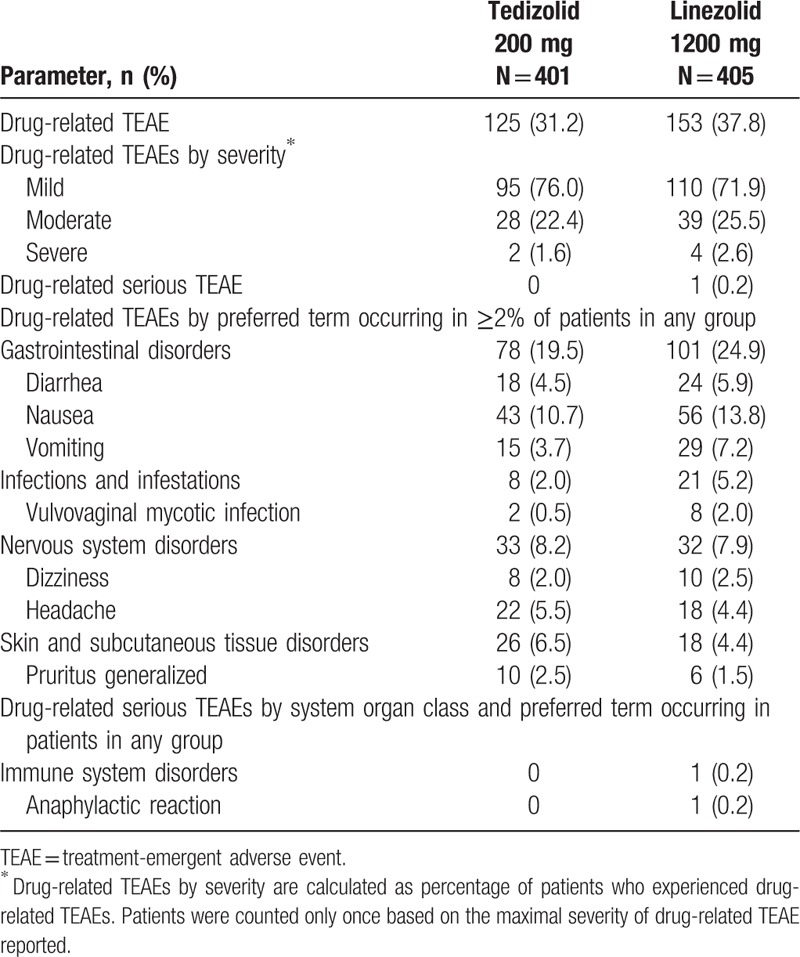
The number of patients who discontinued treatment because of a TEAE was similar between groups: 3 (0.7%) patients in the tedizolid group and 4 (1.0%) patients in the linezolid group. Drug-related TEAEs occurred at a similar frequency between treatment groups (Table 5). The majority of drug-related TEAEs were mild for both tedizolid (76.0%) and linezolid (71.6%). The most frequently reported drug-related TEAEs were nausea (tedizolid, 10.7%; linezolid, 13.8%), diarrhea (tedizolid, 4.5%; linezolid, 5.9%), and headache (tedizolid, 5.5%; linezolid, 4.4%). Only one patient experienced a serious drug-related TEAE, an anaphylactic reaction in the linezolid treatment group.
Table 5.
Drug-related TEAEs experienced by US-based outpatients (safety population).
4. Discussion
In this post hoc analysis, tedizolid and linezolid were evaluated for the treatment of ABSSSI in an outpatient population that was moderately to severely ill and had a high level of complicating factors. Both tedizolid and linezolid proved efficacious in outpatients with ABSSSI. Clinical response was achieved by ≥79% of patients in both groups at the time points evaluated. In addition, both treatment groups demonstrated similar rates of early, EOT, and late clinical response in the treatment of outpatients with ABSSSI. These data are further supported by a pooled analysis of ESTABLISH-1 and ESTABLISH-2 results, which demonstrated that treatment success at 48 to 72 hours after the first dose of oxazolidinones is a good indicator of late clinical cure.[15] Rapid, sustained clinical response will reduce the spread of lesions, thus reducing the need for hospital admission and further decreasing the need for additional care after antimicrobial therapy has been discontinued.
Although mean VAS scores ≥58 mm at baseline indicated a fairly high level of pain, both treatments were able to noticeably reduce pain scores (to <8 mm) by study days 10 to 13. Similarly, microbiological responses for key pathogens were generally comparable in both treatment groups, with most patients having favorable response at PTE.
Overall, AEs were comparable between treatment groups, though patients treated with tedizolid had fewer gastrointestinal AEs and fewer abnormalities in their platelet counts. Fewer tedizolid-treated patients (n = 125) than linezolid-treated patients (n = 153) experienced drug-related TEAEs. The most frequently reported treatment-related TEAEs were similar between both treatment groups. Tedizolid-treated patients were more compliant with their medication (89.3%) than linezolid-treated patients (77.3%), possibly because of the combination of shorter duration of treatment and fewer doses. Both treatment groups had a low incidence of discontinuations due to AEs (tedizolid, n = 3; linezolid, n = 4).
With use of the clinical assessment of infection severity, stable underlying comorbidities, and reliable home and social support networks, patients can be selected for outpatient treatment of ABSSSI. This may prevent or reduce hospital admissions, which leads to reduced treatment costs, decreased incidence of nosocomial infections, and decreasing readmission rates.[3]
The findings of this study are subject to the limitations inherent to all post hoc analyses and should, therefore, be interpreted with caution. However, until such time as robust prospective data are available, the results presented herein provide clinicians with additional information to assist with therapeutic decision-making in the outpatient setting.
5. Conclusion
In conclusion, these results, which were observed in a population with significant comorbidities, suggest that ABSSSI caused by confirmed or suspected gram-positive pathogens can be successfully treated in an outpatient setting with short course tedizolid therapy.
Supplementary Material
Footnotes
Abbreviations: ABSSSI = acute bacterial skin and skin structure infections, AEs = = adverse events, CI = confidence interval, EOT = end of therapy, ITT = intent to treat, MRSA = methicillin-resistant Staphylococcus aureus, PTE = post-therapy evaluation, TEAEs = treatment-emergent adverse events, VAS = visual analog scale.
Funding for this research was provided by Merck & Co., Inc., Kenilworth, NJ, USA. Medical writing and editorial assistance was provided by Sally Mitchell, PhD, and Sarah Utley, PhD, of ApotheCom, Yardley, PA, USA. This assistance was funded by Merck & Co., Inc., Kenilworth, NJ, USA.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Edelsberg J, Taneja C, Zervos M, et al. Trends in US hospital admissions for skin and soft tissue infections. Emerg Infect Dis 2009;15:1516–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kaye KS, Patel DA, Stephens JM, et al. Rising United States hospital admissions for acute bacterial skin and skin structure infections: recent trends and economic impact. PLoS One 2015;10:e0143276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Moran GJ, Abrahamian FM, Lovecchio F, et al. Acute bacterial skin infections: developments since the 2005 Infectious Diseases Society of America (IDSA) guidelines. J Emerg Med 2013;44:e397–412. [DOI] [PubMed] [Google Scholar]
- [4].Shorr AF, Lodise TP, Corey GR, et al. Analysis of the phase 3 ESTABLISH trials: tedizolid versus linezolid in acute bacterial skin and skin structure infections. Antimicrob Agents Chemother 2015;59:864–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Shaw KJ, Poppe S, Schaadt R, et al. In vitro activity of TR-700, the antibacterial moiety of the prodrug TR-701, against linezolid-resistant strains. Antimicrob Agents Chemother 2008;52:4442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Brown SD, Traczewski MM. Comparative in vitro antimicrobial activities of torezolid (TR-700), the active moiety of a new oxazolidinone, torezolid phosphate (TR-701), determination of tentative disk diffusion interpretive criteria, and quality control ranges. Antimicrob Agents Chemother 2010;54:2063–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Schaadt R1, Sweeney D, Shinabarger D, et al. In vitro activity of TR-700, the active ingredient of the antibacterial prodrug TR-701, a novel oxazolidinone antibacterial agent. Antimicrob Agents Chemother 2009;53:3236–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Thomson KS, Goering RV. Activity of tedizolid (TR-700) against well-characterized methicillin-resistant Staphylococcus aureus strains of diverse epidemiological origins. Antimicrob Agents Chemother 2013;57:2892–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Prokocimer P, Bien P, Deanda C, et al. In vitro activity and microbiological efficacy of tedizolid (TR-700) against Gram-positive clinical isolates from a phase 2 study of oral tedizolid phosphate (TR-701) in patients with complicated skin and skin structure infections. Antimicrob Agents Chemother 2012;56:4608–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mistry RD, Shapiro DJ, Goval MK, et al. Clinical management of skin and soft tissue infections in the U.S. emergency departments. West J Emerg Med 2014;15:491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].King MD, Humphrey BJ, Wang YF, et al. Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft-tissue infections. Ann Intern Med 2006;144:309–17. [DOI] [PubMed] [Google Scholar]
- [12].Prokocimer P, De Anda C, Fang E, et al. Tedizolid phosphate vs linezolid for treatment of acute bacterial skin and skin structure infections: the ESTABLISH-1 randomized trial. JAMA 2013;309:559–69. [DOI] [PubMed] [Google Scholar]
- [13].Moran GJ, Fang E, Corey GR, et al. Tedizolid for 6 days versus linezolid for 10 days for acute bacterial skin and skin-structure infections (ESTABLISH-2): a randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis 2014;14:696–705. [DOI] [PubMed] [Google Scholar]
- [14].Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med 1985;4:213–26. [DOI] [PubMed] [Google Scholar]
- [15].Nathwani D, Corey R, Das AF, et al. Early clinical response as a predictor of late treatment success in patients with acute bacterial skin and skin structure infections: retrospective analysis of two randomized controlled trials. Clin Infect Dis 2017;64:214–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


