Abstract
Rationale:
Hepatic angiosarcoma is a rare endothelial cell tumor that may lead to concurrent consumptive coagulopathies including disseminated intravascular coagulation (DIC). This report details a multifaceted approach to managing DIC in a patient with advanced-stage hepatic angiosarcoma, which continued to progress after a brief response to taxane-based chemotherapy.
Patient concerns:
A 55-year-old man with a recent history of hemorrhoids and hemarthroses presented with acute rectal bleeding. He was found to have concurrent hepatomegaly, abnormal liver function tests, anemia, thrombocytopenia, and coagulopathy.
Diagnoses:
DIC in the setting of hepatic angiosarcoma.
Interventions:
The patient's acute bleeding in the setting of DIC was controlled with a combination of antifibrinolytic agents to prevent clot breakdown, heparin products to prevent deposition of new clot, and romiplostim to increase platelet production. His angiosarcoma was treated with various combinations of chemotherapy, including taxane-based chemotherapy, doxorubicin, and pazopanib.
Outcomes:
The patient's DIC and acute bleeding on initial presentation improved following treatment with unfractionated heparin and low-molecular weight heparin maintenance therapy. It is unclear if the chemotherapy to treat the hepatic angiosarcoma played a significant role in the improvement of DIC.
Lessons:
Laboratory measurement of prothrombin fragment 1.2, a byproduct of prothrombin conversion to thrombin, proved to be a useful way to monitor this patient's DIC over time.
Keywords: anticoagulation, DIC, hepatic angiosarcoma
1. Introduction
Angiosarcoma is an aggressive vascular tumor of the soft tissues or viscera that is composed of malignant endothelial cells and accounts for only about 2% of all soft-tissue sarcomas.[1–3] Primary hepatic angiosarcoma is considerably less common than angiosarcoma of the skin or breast,[1] and probably has the worst prognosis of the various subtypes of angiosarcoma,[2] with a reported median survival of <6 months.[4,5] Exposure to vinyl chloride, thorium dioxide, and arsenic have been specifically associated with hepatic angiosarcoma; however, most cases of hepatic angiosarcoma are sporadic.[4] Patients with hepatic angiosarcoma typically present with abdominal pain and nonspecific signs of liver disease, which may include hepatomegaly, ascites, jaundice, and elevated liver function tests.[4] The disease is often unresectable at diagnosis, and chemotherapy is the mainstay of treatment.[1,5]
Consumptive coagulopathies, including disseminated intravascular coagulation (DIC), have been reported in the setting of various types of angiosarcoma, including primary hepatic angiosarcoma.[6–11] A retrospective study identified 7 patients with angiosarcoma and unexplained DIC (i.e., not attributable to chemotherapy or sepsis) and found that patients who had any clinical response to cancer chemotherapy had coincident improvement in their coagulopathy.[6] Conversely, disease progression led to worsening of DIC.[6]
While treatment of DIC is usually targeted at the underlying cause, challenges may arise when either the underlying cause has yet to be diagnosed or it is unresponsive to available treatment options. Here, we present a patient with primary hepatic angiosarcoma who presented with active bleeding due to DIC. We highlight a multifaceted approach to the management of ongoing DIC in this patient, whose underlying hepatic angiosarcoma has continued to progress despite treatment and an initial partial response to chemotherapy.
2. Case report
2.1. Patient information and clinical findings
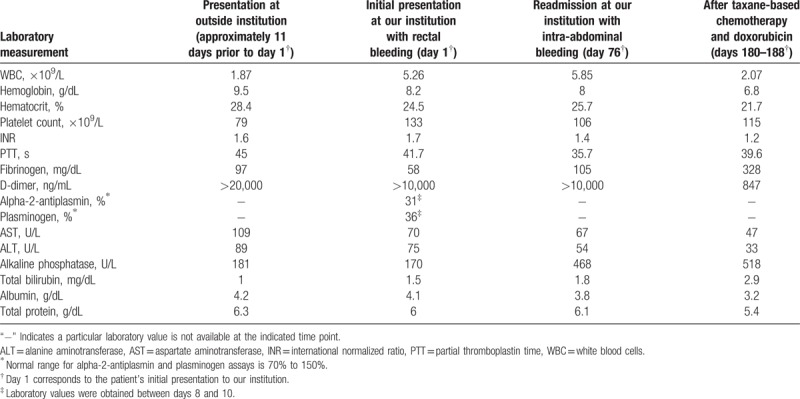
The patient is a 55-year-old man with a recent history of hemorrhoids who presented to our institution with painless, bloody bowel movements occurring every 1 to 2 hours. In the few months prior to this presentation, he had a spontaneous hematoma of the left hip followed by a spontaneous left ankle hematoma. Subsequent work-up revealed anemia, thrombocytopenia, and hepatomegaly of uncertain etiology. About 1 month after this work-up was performed, the patient underwent banding of his bleeding hemorrhoids. In the days following the procedure, he was admitted to an outside hospital with fevers and was found to have Proteus bacteremia that was treated with antibiotics. During this hospitalization, he had a hemoglobin of 9.5 g/dL, platelet count of 79 × 109/L, white blood cell count of 1.87 × 109/L, fibrinogen of 97 mg/dL, international normalized ratio (INR) of 1.6, partial thromboplastin time (PTT) of 45 seconds, aspartate aminotransferase (AST) of 109 U/L, and alanine aminotransferase (ALT) of 89 U/L (Table 1). A broad infectious work-up was unrevealing. A computed tomography (CT) scan showed hepatomegaly with heterogeneous enhancement of the liver parenchyma. A bone marrow biopsy showed maturing trilineage hematopoiesis and no evidence of malignancy, and a liver biopsy showed dilated sinusoids and atypical endothelial cells. He was discharged from the hospital and shortly thereafter began having an increasing frequency of large, bloody bowel movements, prompting him to present to our emergency department for evaluation. The patient reported no known toxic exposures and minimal alcohol consumption. Physical examination on admission was notable for marked hepatomegaly with no jaundice or asterixis. There were no ecchymoses or petechiae.
Table 1.
Key laboratory values.
Informed written consent was obtained from the patient for publication of this case report and accompanying figures.
2.2. Diagnostic assessment
Repeat CT scan of the abdomen on admission demonstrated hepatomegaly with heterogeneous enhancement of the liver parenchyma, concerning for malignancy (Fig. 1). Laboratory studies were significant for hemoglobin 8.2 g/dL, platelet count 133 × 109/L (normal: 150–400 × 109/L), INR 1.7, PTT 41.7 seconds (normal: 22–35 seconds), fibrinogen 58 mg/dL (normal: 200–375 mg/dL), D-dimer >10,000 ng/mL (normal: <500 ng/mL), as well as elevated liver function tests (AST 70 U/L, ALT 75 U/L, alkaline phosphatase 170 U/L, total bilirubin 1.5 mg/dL) (Table 1). Anoscopy revealed a bleeding rectal ulcer, which was sutured in an attempt to achieve hemostasis. He ultimately underwent a liver biopsy early during this admission. Histopathological examination showed marked dilated sinusoids lined by an atypical endothelial proliferation (Fig. 2). Specifically, in areas of azonal dilatation, the endothelial cells showed immunopositivity for P53, increased c-MYC expression, and increased Ki-67 labeling. Molecular-genetic workup included MYC fluorescence in situ hybridization that did not show gene amplification (Fig. 3), and workup using a multigene panel next-generation sequencing assay demonstrated a TP53 p.R175H variant, which was in line with the P53 immunopositivity. The morphological, immunophenotypical, and molecular-genetic features were diagnostic of a low-grade angiosarcoma. Notably, the growth pattern, demonstrating a seemingly bland endothelial proliferation along the hepatocytes, was in fact remarkable and referred to as a so-called sinusoidal, low-grade angiosarcoma. A diagnosis of DIC was also made based on clinical and laboratory findings.
Figure 1.
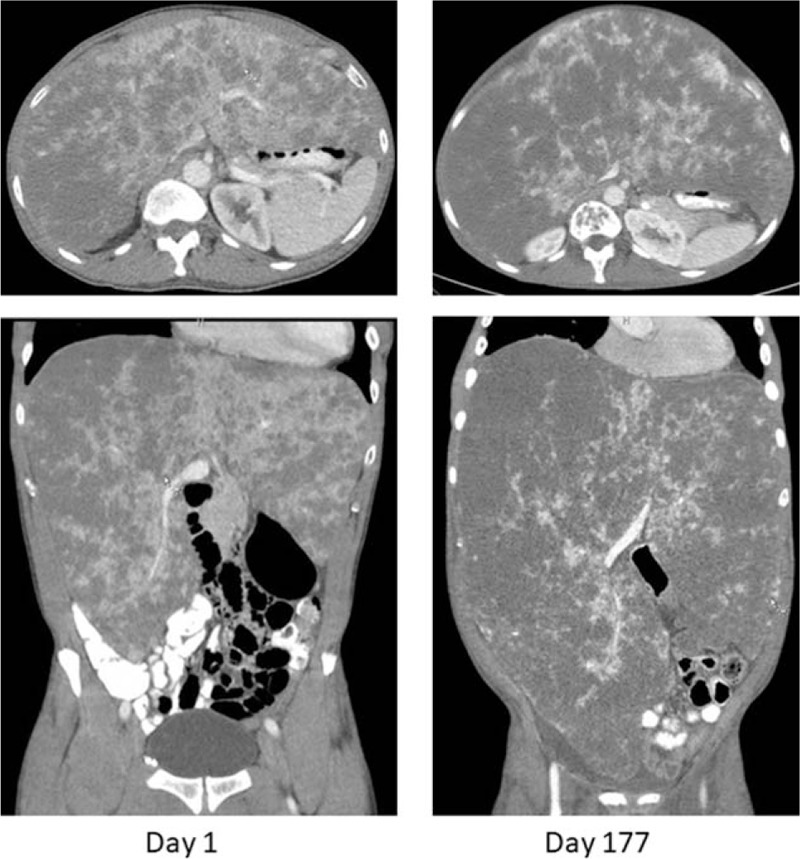
Computed tomography images of the abdomen on day 1 (initial presentation at our institution) and day 177 (after several rounds of chemotherapy). Images from day 177 show significant disease progression of the patient's hepatic angiosarcoma.
Figure 2.

Histologic findings showing angiosarcoma of the liver (A, B). The remarkable bland appearing malignant endothelial cells (arrows) growing along the intact hepatocytes (asterisks). This is characteristic of “angiosarcoma, sinusoidal pattern.”
Figure 3.

Molecular-genetic findings from liver biopsy. (A) The endothelial cells showed no MYC gene amplification or rearrangement. The image shows a dual-color fluorescent in situ hybridization against the MYC locus against a DAPI counterstained (nuclear) background. Rearrangement would be visible as split red and green probe signals whereas amplification would be visible as >4 yellow signals per nucleus. (B) Pile up view (integrated genome viewer) of a somatic TP53 p.R175H missense mutation in exon 5. Each base alteration (here G>T) is visualized in red. DAPI, 4’,6-diamidino-2-phenylindole.
2.3. Therapeutic interventions and outcome
This report will focus primarily on the management of the patient's DIC in the setting of hepatic angiosarcoma. Figure 4 provides a visual representation of the trends in important coagulation parameters over time and the treatment regimen employed at each time point. The following paragraphs will detail the disease course and management from day 1 (defined as initial presentation at our institution) to approximately day 180.
Figure 4.
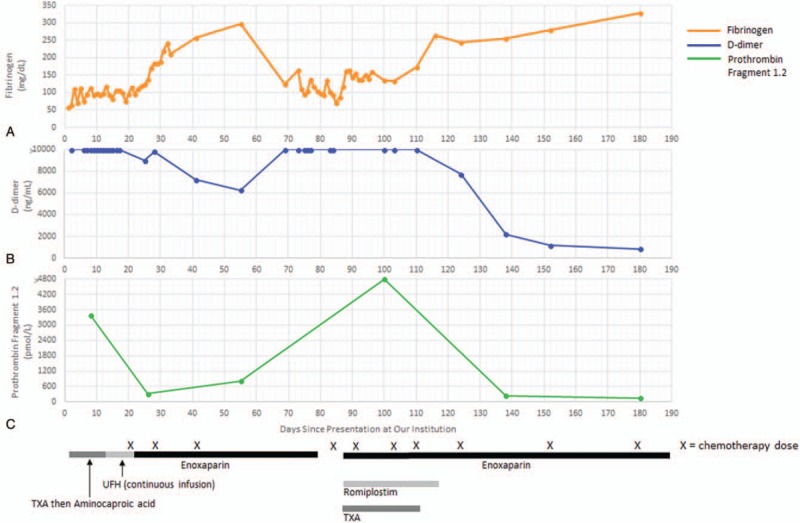
The 3 graphs depict trends in fibrinogen (A), D-dimer (B), and prothrombin fragment 1.2 (C) levels over time. The horizontal axis depicts the total number of days since the patient's initial presentation at our institution, with day 1 corresponding to the first day of his initial hospitalization. Timing of chemotherapy doses is denoted by each “X” located below the horizontal axis. The thick bars at the bottom of the figure correspond to the period of time that the patient received particular medications as indicated. TXA = tranexamic acid, UFH = unfractionated heparin.
For the first 1.5 days of hospitalization, tranexamic acid (TXA) was used to help stabilize clot formation in the setting of extensive rectal bleeding. The patient was subsequently transitioned to intravenous aminocaproic acid for hospital days 2 to 5 and then oral aminocaproic acid for hospital days 8 to 11. Prothrombin fragment 1.2 was markedly elevated at 3390 pmol/L (normal: 87–325 pmol/L) on hospital day 8, indicating high levels of thrombin activation. Given continued rectal bleeding with evidence of a high burden of thrombin formation, the decision was made to initiate a continuous infusion of unfractionated heparin (UFH). He was treated with the UFH infusion for hospital days 12 to 20 and then transitioned to enoxaparin 40 mg twice daily on hospital day 20. Of note, the patient required numerous transfusions of packed red blood cells and cryoprecipitate throughout his hospital stay until the rectal bleeding subsided and fibrinogen levels were stably above 100 mg/dL. Once the biopsy results had established a diagnosis of hepatic angiosarcoma, chemotherapy was initiated with paclitaxel on hospital day 20. By hospital days 25 to 26, the patient's prothrombin fragment 1.2 level had decreased to 317 pmol/L and the D-dimer to 9013 ng/mL (Fig. 4). On hospital day 28, the patient's chemotherapy was switched to gemcitabine and docetaxel. At the time of discharge on day 33, the patient was continued on enoxaparin twice daily with adequate control of rectal bleeding and fibrinogen stable in the 200 mg/dL range.
The patient received 1 additional dose of gemcitabine and docetaxel about 1 week after discharge but then did not receive any chemotherapy for a period of about 6 weeks during which time his diagnosis was reassessed by a laparoscopic liver biopsy to obtain additional tissue for histologic and molecular studies. Following the biopsy, he had persistent bleeding from one of the port sites, and he was subsequently readmitted to our institution (on day 76) with intra-abdominal hemorrhage thought to be secondary to the liver biopsy. He required transfusions with packed red blood cells and cryoprecipitate and ultimately underwent embolization of the hepatic artery to help control the bleeding. Enoxaparin was held for about 9 days in the setting of intra-abdominal bleeding. After stabilization, enoxaparin was restarted and up-titrated to 30 mg twice daily by the time of discharge. TXA was also initiated for clot stabilization. In addition, romiplostim was started because his platelet count, which was 106 × 109/L at the time of readmission, had gradually decreased to the 30 to 40 × 109/L range over the course of this hospitalization. Chemotherapy with gemcitabine and docetaxel was resumed (on day 84) once the pathology confirmed an unequivocal diagnosis of angiosarcoma, and he was discharged on day 96.
The patient completed a total of 4 cycles of gemcitabine and docetaxel (last cycle finished on day 110) but was then switched to doxorubicin (on day 124) after restaging scans showed progressive metastatic disease. Interestingly, his DIC remained well-controlled despite radiographically confirmed progression of the angiosarcoma. At this time, TXA was discontinued and enoxaparin was up-titrated to 60 mg twice daily. Romiplostim was also discontinued as the platelet count had recovered. After 2 cycles of doxorubicin and imaging showing continued disease progression (Fig. 1), he was transitioned to pazopanib therapy (on day 180).
3. Discussion
This case illustrates the complexities of managing DIC in the setting of an underlying hepatic angiosarcoma unresponsive to chemotherapy. To manage this patient's DIC over time, we employed a combination of the following strategies: acute stabilization with antifibrinolytic agents in the setting of active hemorrhage, prevention of new clot formation with heparin products, and stimulation of platelet production with romiplostim.
Given that the patient's tumor is comprised of malignant endothelial cells, we hypothesized that this patient's coagulopathy could be due to either a primary consumptive process (i.e., DIC) or primary hyperfibrinolysis, since endothelial cells are involved in the production of factors involved in both clot formation and clot destruction. Although our patient's initial routine coagulation studies were consistent with DIC, it is important to recognize that these tests are not specific for DIC and have imperfect sensitivity.[12,13] We therefore used prothrombin fragment 1.2 levels to help confirm the underlying pathophysiology of the patient's coagulopathy and trend it over time. Prothrombin fragment 1.2 serves as a laboratory marker of thrombin activation, as prothrombin fragment 1.2 is produced by cleavage of prothrombin into thrombin.[14] We observed enormous elevations in prothrombin fragment 1.2 coinciding with clinical manifestations hypofibrinogenemia and acute bleeding, which occurred at the patient's initial presentation to our institution with rectal bleeding and upon readmission with intra-abdominal hemorrhage after liver biopsy. Massively elevated prothrombin fragment 1.2 levels strongly supported a primary diagnosis of DIC, with aberrant activation of the coagulation cascade being the most likely inciting event, leading to subsequent increased consumption of coagulation factors and platelets.
We concluded that primary hyperfibrinolysis was unlikely to be the pathologic process causing bleeding in this patient, but it is important to recognize that DIC usually leads to a secondary increase in fibrinolytic activity.[15] Indeed, there was up-regulation of fibrinolysis and consumption of fibrinolytic factors in this patient, as evidenced by decreased plasma levels of alpha-2-antiplasmin and plasminogen, which were measured in the days after his initial presentation with rectal bleeding. Thus, antifibrinolytic agents (TXA and aminocaproic acid) were employed in an attempt to stabilize the patient in the setting of active, uncontrolled rectal bleeding and again in the period following intra-abdominal bleeding. While antifibrinolytic agents are not traditionally used to treat DIC, their use is not unprecedented, particularly in the case of trauma or prostate cancer leading to a fibrinolytic-dominant form of DIC.[15–17]
Heparin has been used to treat DIC in the past, but there are no large trials demonstrating a clear role for heparin in the routine management of DIC.[14,15] UFH and low-molecular weight heparin (LMWH) have been used in patients with cancer and DIC, especially since these patients are known to have a high risk of thrombotic events at baseline.[17,18] It has been shown that UFH and LMWH lead to improvement in numerous coagulation parameters, including prothrombin fragment 1.2 and D-dimer, in healthy volunteers treated with lipopolysaccharide to mimic the coagulation activation in DIC.[19] In addition, a small study in which a subset of pediatric patients with septic shock and DIC were treated with UFH demonstrated some improvement in laboratory coagulation studies following UFH treatment but no survival benefit.[20] Although heparin is generally considered an acceptable treatment option for DIC when its potential benefits outweigh the risk of exacerbating bleeding, its use is based on minimal evidence.
Because this patient continued to have active bleeding despite treatment with antifibrinolytic agents during his initial hospitalization, the decision was made to start a continuous infusion of UFH, with transition to daily enoxaparin. With a high prothrombin fragment 1.2 level indicating profoundly increased thrombin generation, heparin products were initiated with the goal of blocking thrombin formation and thereby halting the underlying process of coagulation factor consumption. Adequate control of the patient's bleeding and improvement in his coagulation laboratory studies was ultimately achieved following treatment with UFH and enoxaparin. A few months later, the prothrombin fragment 1.2 was profoundly elevated a second time in the setting of active intra-abdominal bleeding, but once again, resuming enoxaparin after acute stabilization of bleeding led to normalization of the prothrombin fragment 1.2 level, recovery of normal fibrinogen levels, and a large decrease in D-dimer.
The role of chemotherapy in controlling the coagulopathy is not clear. It is important to note that the patient began taxane-based chemotherapy around the same time that enoxaparin was first begun, so it is plausible that treatment of the underlying angiosarcoma with chemotherapy also played at role in controlling the patient's DIC, despite the fact that there was only an initial, partial response of the tumor to treatment, followed soon thereafter by tumor progression.
Overall, this case illustrates the complexity of managing DIC in the setting of a hepatic angiosarcoma, a rare tumor of malignant endothelial cells that has been previously associated with consumptive coagulopathy. With episodes of active bleeding coinciding with enormous elevations in prothrombin fragment 1.2, we found treatment with UFH and enoxaparin effective in improving coagulopathy, as evidenced by normalization of prothrombin fragment 1.2 and fibrinogen as well as a large drop in D-dimer. We also propose that prothrombin fragment 1.2 levels can be a useful tool in distinguishing DIC from primary hyperfibrinolysis and can used to help monitor the coagulopathy over time.
Author contributions
Conceptualization: Jochen K. Lennerz, David J. Kuter.
Data curation: Emily A. Rosen, Mounica Vallurupalli, Edwin Choy, Jochen K. Lennerz, David J. Kuter.
Formal analysis: Emily A. Rosen, Mounica Vallurupalli, David J. Kuter.
Investigation: Emily A. Rosen, Mounica Vallurupalli, Edwin Choy.
Methodology: David J. Kuter.
Project administration: David J. Kuter.
Supervision: Mounica Vallurupalli, David J. Kuter.
Writing – original draft: Emily A. Rosen, Mounica Vallurupalli, David J. Kuter.
Writing – review & editing: Emily A. Rosen, Mounica Vallurupalli, Edwin Choy, Jochen K. Lennerz, David J. Kuter.
Footnotes
Abbreviations: ALT = alanine aminotransferase, AST = aspartate aminotransferase, CT = computed tomography, DIC = disseminated intravascular coagulation, INR = international normalized ratio, LMWH = low-molecular weight heparin, PTT = partial thromboplastin time, TXA = tranexamic acid, UFH = unfractionated heparin.
All of the authors participated in the design of the study, evaluation of the data, and the writing of the manuscript.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol 2010;11:983–91. [DOI] [PubMed] [Google Scholar]
- [2].Fayette J, Martin E, Piperno-Neumann S, et al. Angiosarcomas, a heterogeneous group of sarcomas with specific behavior depending on primary site: a retrospective study of 161 cases. Ann Oncol 2007;18:2030–6. [DOI] [PubMed] [Google Scholar]
- [3].Mark RJ, Poen JC, Tran LM, et al. Angiosarcoma: a report of 67 patients and a review of the literature. Cancer 1996;77:2400–6. [DOI] [PubMed] [Google Scholar]
- [4].Locker GY, Doroshow JH, Zwelling LA, et al. The clinical features of hepatic angiosarcoma: a report of four cases and a review of the English literature. Medicine (Baltimore) 1979;58:48–64. [DOI] [PubMed] [Google Scholar]
- [5].Molina E, Hernandez A. Clinical manifestations of primary hepatic angiosarcoma. Dig Dis Sci 2003;48:677–82. [DOI] [PubMed] [Google Scholar]
- [6].Farid M, Ahn L, Brohl A, et al. Consumptive coagulopathy in angiosarcoma: a recurrent phenomenon? Sarcoma 2014;2014:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wadhwa S, Kim TH, Lin L, et al. Hepatic angiosarcoma with clinical and histological features of Kasabach-Merritt syndrome. World J Gastroenterol 2017;23:2443–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rowe K, Nehme F, Wallace J, et al. Primary hepatic angiosarcoma mimicking multifocal liver abscess with disseminated intravascular coagulation and hemoperitoneum. Cureus 2017;9:e1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Habringer S, Boekstegers A, Weiss L, et al. Kasabach-Merritt phenomenon in hepatic angiosarcoma. Br J Haematol 2014;167:716–8. [DOI] [PubMed] [Google Scholar]
- [10].Honda K, Ando M, Sugiyama K, et al. Successful treatment of cardiac angiosarcoma associated with disseminated intravascular coagulation with Nab-Paclitaxel: a case report and review of the literature. Case Rep Oncol 2017;10:863–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Alexandrova E, Sergieva S, Mihaylova I, et al. Primary angiosarcoma of the breast complicated by the syndrome of disseminated intravascular coagulation (DIC): case report and literature review. Rep Pract Oncol Radiother 2014;19:221–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kitchens CS. Thrombocytopenia and thrombosis in disseminated intravascular coagulation (DIC). Hematol Am Soc Hematol Educ Program 2009;2:240–6. [DOI] [PubMed] [Google Scholar]
- [13].Levi M, Toh CH, Thachil J, et al. Guidelines for the diagnosis and management of disseminated intravascular coagulation. Br J Haematol 2009;145:24–33. [DOI] [PubMed] [Google Scholar]
- [14].Levi M. Diagnosis and treatment of disseminated intravascular coagulation. Int J Lab Hematol 2014;36:228–36. [DOI] [PubMed] [Google Scholar]
- [15].Toh CH, Alhamdi Y. Current consideration and management of disseminated intravascular coagulation. Hematol Am Soc Hematol Educ Program 2013;2013:286–91. [DOI] [PubMed] [Google Scholar]
- [16].Palma Anselmo M, Nobre de Jesus G, Lopes JM, et al. Massive bleeding as the first clinical manifestation of metastatic prostate cancer due to disseminated intravascular coagulation with enhanced fibrinolysis. Case Rep Hematol 2016;2016:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Levi M. Management of cancer-associated disseminated intravascular coagulation. Thromb Res 2016;140:S66–70. [DOI] [PubMed] [Google Scholar]
- [18].Feinstein DI. Treatment of disseminated intravascular coagulation. Semin Thromb Hemost 1988;14:351–62. [DOI] [PubMed] [Google Scholar]
- [19].Pernerstorfer T, Hollenstein U, Hansen JB, et al. Heparin blunts endotoxin-induced coagulation activation. Circulation 1999;100:2485–90. [DOI] [PubMed] [Google Scholar]
- [20].Corrigan JJ, Jordan CM. Heparin therapy in septicemia with disseminated intravascular coagulation. N Engl J Med 1970;283:778–82. [DOI] [PubMed] [Google Scholar]


