Abstract
Rationale:
Hemothorax caused by metastasis or direct invasion of hepatocellular carcinoma (HCC) in the chest is rare. We report a case of hemothorax caused by metastasis in the mediastinum and treated with transcatheter arterial embolization (TAE).
Patient concerns:
A 60-year-old woman with HCC was admitted to receive chemotherapy. Two days after admission, she complained of dyspnea, and a chest X-ray revealed right pleural effusion. Thoracentesis confirmed the diagnosis of hemothorax. Computed tomography (CT) angiography showed lung, pleural, and mediastinal metastases and contrast extravasation from the right lower mediastinal mass.
Diagnoses:
Hemothorax caused by spontaneous rupture of mediastinal metastasis of hepatocellular carcinoma.
Interventions:
During emergent angiography, contrast extravasation from the right T10 intercostal artery was observed and we performed embolization with lipiodol and gelatin sponge particles. After embolization, no active bleeding was observed.
Outcomes:
The patient died because of sepsis and multiple organ failure 22 days after admission.
Lessons:
We reviewed 21 cases of HCC with metastasis or direct invasion in the chest presenting hemothorax. The results revealed that male sex and right hemothorax were predominant in these cases. The average age of the patients was 61.24±10.82 years. The most common symptoms were dyspnea, chest wall pain, and shock. Thoracentesis can confirm the diagnosis, and CT angiography can help identify the location of contrast extravasation before TAE. The reported bleeding arteries were the intercostal, inferior phrenic, bronchial, hepatic, and superficial cervical arteries. TAE with embolic agents is a feasible treatment. The overall outcomes in these cases were poor.
Keywords: computed tomography, hemothorax, hepatocellular carcinoma, thoracic metastasis, transcatheter arterial embolization
1. Introduction
Hepatocellular carcinoma (HCC) sometimes causes hemoperitoneum. However, hemothorax caused by metastasis or direct invasion of HCC is rare.[1] We report a case of hemothorax caused by mediastinal metastasis and treated with transcatheter arterial embolization (TAE). We also review and summarize the previously reported cases of hemothorax caused by metastasis or direct invasion of HCC.
2. Case presentation
A 60-year-old woman with progression of HCC and lung metastasis was admitted to our hospital for her 12th cycle of chemotherapy with a folinic acid, fluorouracil, and oxaliplatin (FOLFOX) regimen on March 2, 2016. She had a history of HCC in segments 7 and 8 of the liver and received segmental hepatectomy in September 2009; tumor recurrence in segments 5 and 6 with tumor rupture and hemoperitoneum postemergent embolization and segmental hepatectomy in March 2011; local recurrence in the liver dome and lung metastasis after receiving sorafenib from March 2012 to February 2013; pegylated arginine deiminase (ADI-PEG20) treatment for 12 cycles (March to May 2013); fludarabine, mitoxantrone, and prednisone (FMP) therapy for 4 cycles (June to October 2013); transcatheter arterial chemoembolization (TACE) 4 times (November 2013 to July 2014); and 11 cycles of chemotherapy with the FOLFOX regimen (December 2014 to January 2016). She also had diabetes mellitus, chronic hepatitis C, liver cirrhosis, and hypertension.
On admission, physical examination revealed a body temperature of 36.6°C, a pulse rate of 106 beats/min, blood pressure of 118/80 mm Hg, and a respiration rate of 18 breaths/min. Laboratory investigations showed the following: hematocrit, 34.4%; hemoglobin, 11.2 g/dL; red blood cell count, 3.76 million/mm3; white blood cell count, 2600/mm3; platelet count, 55,000/mm3; blood urea nitrogen, 89.4 mg/dL; creatinine, 4.17 mg/dL; sodium, 131 mEq/L; and potassium, 4.0 mEq/L.
Two days after admission, she complained of dyspnea, and a chest radiography revealed right pleural effusion (Fig. 1). Thoracentesis confirmed the diagnosis of hemothorax. Computed tomography (CT) angiography showed lung, pleural, and mediastinal metastases and contrast extravasation from the right lower mediastinal mass (Fig. 2). During emergent angiography, contrast extravasation from the right T10 intercostal artery was observed and TAE with lipiodol and gelatin sponge particles was performed. After the procedure, no active bleeding was observed (Fig. 3). However, she died because of sepsis and multiple organ failure 22 days after admission.
Figure 1.
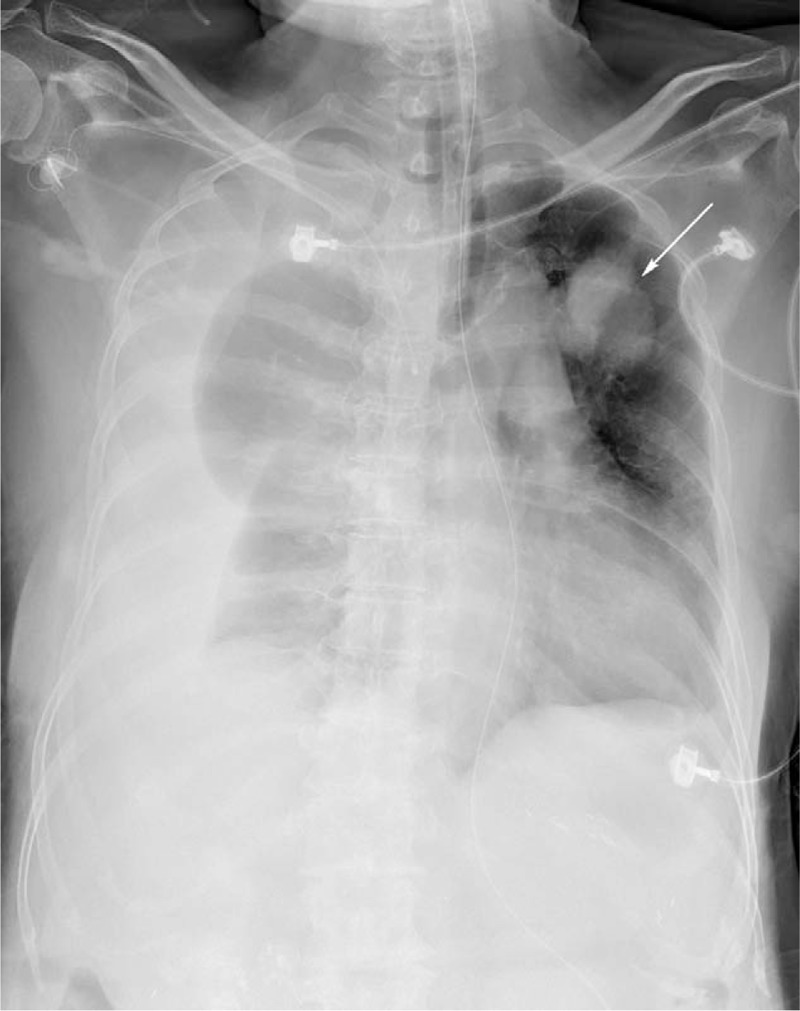
Chest radiography after intubation revealed right pleural effusion and left upper lung metastasis (arrow).
Figure 2.
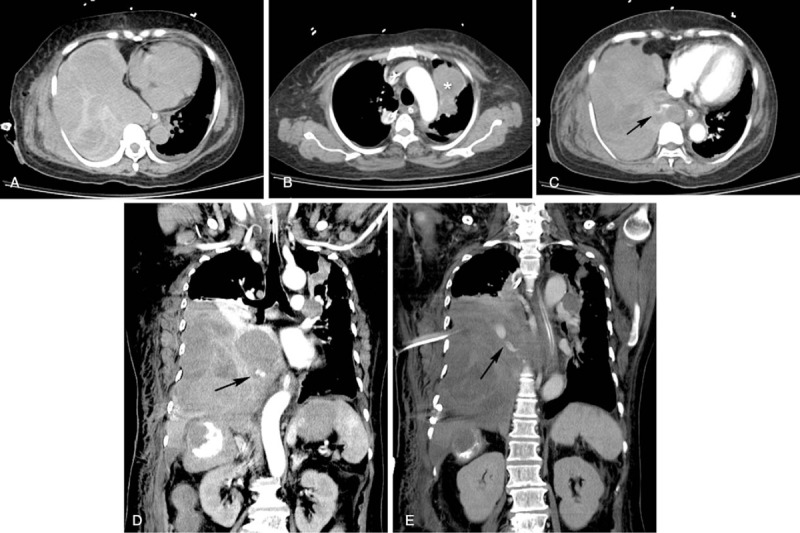
Computed tomography (CT) of the abdomen. (A) Axial noncontrast-enhanced CT revealed a hyperdense lesion in the right pleural space, consistent with hemothorax. (B) Lung metastasis was observed in the left upper lobe of the lung (∗). (C and D) Contrast extravasation was observed on axial and coronal CT angiography (arrows in C and D). (E) Coronal contrast-enhanced CT showed contrast extravasation (arrow).
Figure 3.
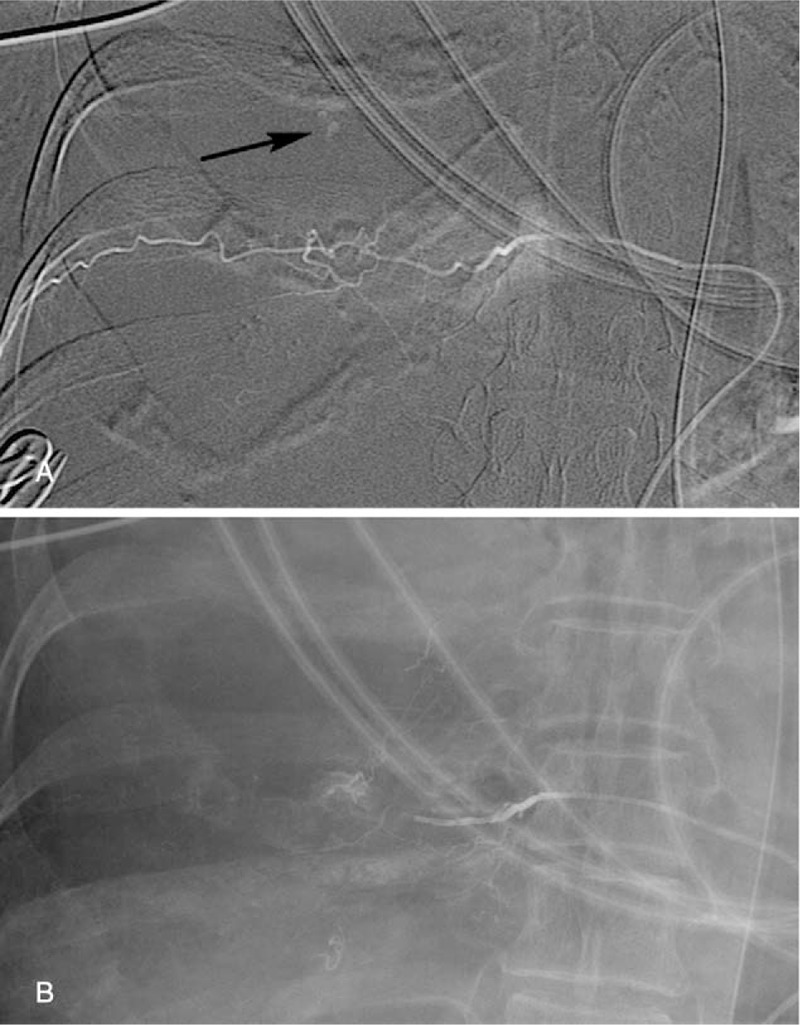
Angiography of the right T10 intercostal artery showed (A) contrast extravasation (arrow) (B) and total occlusion after embolization with lipiodol and gelatin sponge particles.
The case report has been approved by the Chang Gung Medical Foundation Institutional Review Boards (IRB), Taipei, Taiwan. The patient consent was waivered by the IRB.
3. Discussion
HCC is usually hypervascular and spontaneous rupture is not uncommon. Metastatic lesions of HCC, like their primary lesions, have the tendency to rupture.[2] Rupture of metastatic lesions have been reported in the adrenal gland,[3,4] kidneys,[5] mandible,[6] peritoneum,[7,8] and spleen,[9,10] besides chest lesions, which will be discussed below.
In 2012, Ono et al[11] reviewed 17 cases of hemothorax caused by either metastasis or direct invasion in the chest. We further reviewed previous literature and found 21 cases (including the present case) of HCC with metastasis or direct invasion in the chest presenting hemothorax (Table 1); these cases are summarized in Table 2.
Table 1.
All published cases of metastasis or direct invasion of hepatocellular carcinoma in the chest presenting hemothorax (n = 21).
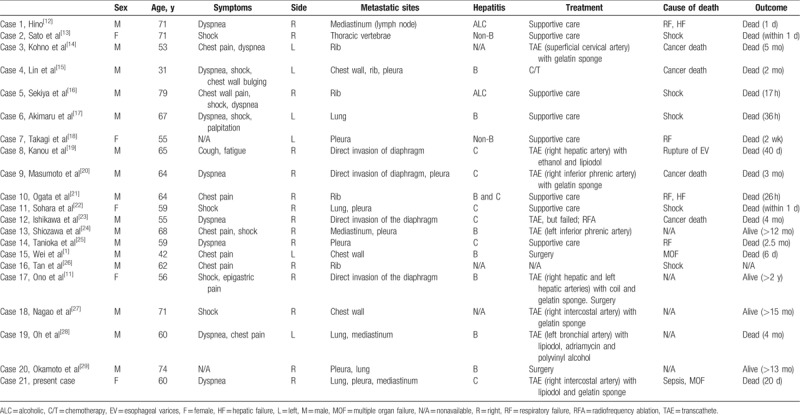
Table 2.
Summary table of all published cases.
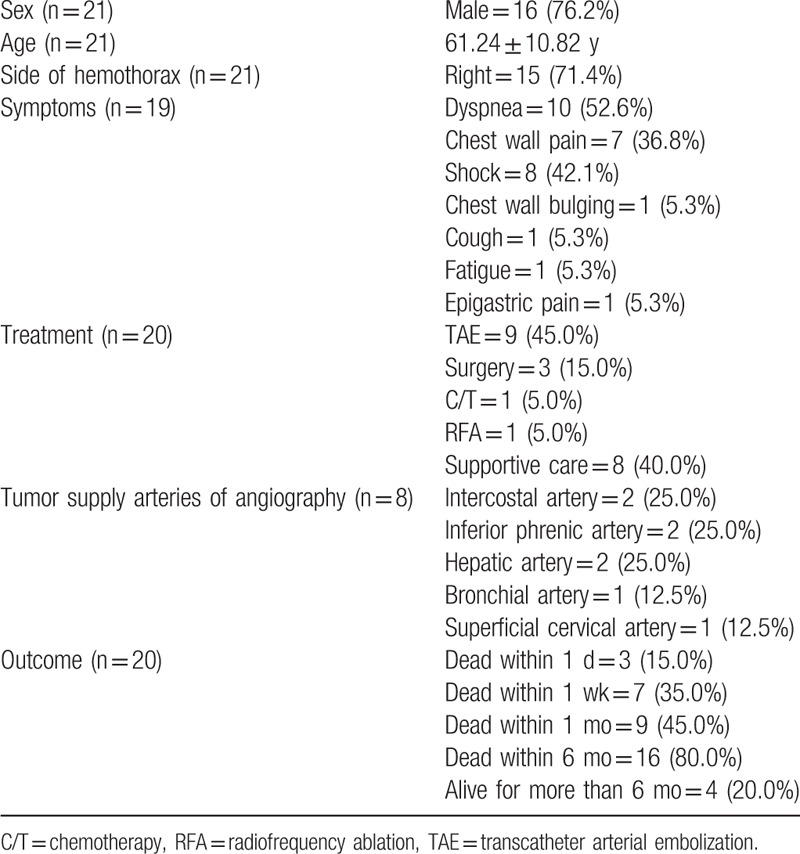
According to Table 2, these cases predominantly involved men (76.2%) and right hemothorax (71.4%). The average age of affected patients is 61.24 ± 10.82 years. The most common symptoms are dyspnea (52.6%), chest pain (36.8%), and shock (42.1%). Patients may have acute onset of pleural effusion and symptoms. Pleural tapping can confirm the diagnosis.
Nine patients (45.0%) were treated with TAE, and procedural failure occurred in one case. The reported bleeding arteries were the intercostal, inferior phrenic, bronchial, hepatic, and superficial cervical arteries. These arteries were related to the locations of contrast extravasation. The patients who exhibited bleeding in 2 cases with direct diaphragmatic invasion involved bleeding from the hepatic arteries. To identify the bleeding arteries, CT angiography can be helpful. A gelatin sponge was most commonly used, and other embolic agents, including ethanol, lipiodol, and polyvinyl alcohol particles were also used. Other treatment modalities, including chemotherapy, surgery, and radiofrequency ablation have been adopted. However, there are too few cases to analyze and more cases are needed for further evaluation.
Regarding the outcomes, 45% of patients died within a month, 80% died within 6 months, and only 20% of cases survived for more than 6 months. Most deaths within a day were caused by shock, and the causes of deaths from several days to 6 months included respiratory failure, hepatic failure, multiple organ failure, and cancer death. The outcomes of 8 patients undergoing successful TAE varied greatly; the survival time ranged from 40 days to more than 15 months. One patient who received combined TAE and surgery even survived for more than 2 years.
In conclusion, thoracic metastasis or direct invasion of HCC presenting with hemothorax is rare, and cases involving men and right hemothorax are predominant. If a patient with thoracic metastasis of HCC has acute pleural effusion, dyspnea, chest pain, or shock, tumor bleeding should be taken into consideration. CT angiography can identify the locations of contrast extravasation. The bleeding arteries that have been reported are the intercostal, inferior phrenic, bronchial, hepatic, and superficial cervical arteries. TAE with embolic agents is a feasible treatment. The overall outcomes of these cases are poor.
Author contributions
Writing – original draft: Chih-Wei Yen.
Writing – review & editing: Li-Sheng Hsu, Chien-Wei Chen, Wei-Hsiu Lin.
Footnotes
Abbreviations: ADI-PEG20 = pegylated arginine deiminase, CT = computed tomography, FMP = fludarabine, mitoxantrone, and prednisone, FOLFOX = folinic acid, fluorouracil, and oxaliplatin, HCC = hepatocellular carcinoma, TACE = transcatheter arterial chemoembolization, TAE = transcatheter arterial embolization.
The authors have no conflicts of interest to disclose.
References
- [1].Wei YF, Wang HC, Chang YC. Hemothorax due to metastatic hepatocellular carcinoma presenting with massive hemoptysis. J Formos Med Assoc 2006;105:346–8. [DOI] [PubMed] [Google Scholar]
- [2].Chen MF, Jan YY, Lee TY. Transcatheter hepatic arterial embolization followed by hepatic resection for the spontaneous rupture of hepatocellular carcinoma. Cancer 1986;58:332–5. [DOI] [PubMed] [Google Scholar]
- [3].Fukuoka K, Funatomi T, Ikegami F, et al. A case of hepatocellular carcinoma associated with multiple pulmonary tumor thrombi and rupture of its right adrenal metastasis. Gan No Rinsho 1987;33:199–204. [PubMed] [Google Scholar]
- [4].Yang PW, Wang WY, Yang CH, et al. Treatment of massive retroperitoneal hemorrhage from adrenal metastasis of hepatoma. J Chin Med Assoc 2007;70:126–31. [DOI] [PubMed] [Google Scholar]
- [5].Kinoshita O, Ichijo Y, Yoneda M, et al. Spontaneous rupture of renal metastasis from hepatocellular carcinoma. Case Rep Surg 2017;2017:8607061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Huang SF, Wu RC, Chang JT, et al. Intractable bleeding from solitary mandibular metastasis of hepatocellular carcinoma. World J Gastroenterol 2007;13:4526–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yeh CN, Chen HM, Chen MF, et al. Peritoneal implanted hepatocellular carcinoma with rupture after TACE presented as acute appendicitis. Hepatogastroenterology 2002;49:938–40. [PubMed] [Google Scholar]
- [8].Ishikawa T, Yamasaki T, Isobe Y, et al. Rupture of peritoneal metastatic tumor that developed due to needle-tract implantation of hepatocellular carcinoma. J Gastroenterol 2005;40:547–8. [DOI] [PubMed] [Google Scholar]
- [9].Horie Y, Suou T, Hirayama C, et al. Spontaneous rupture of the spleen secondary to metastatic hepatocellular carcinoma: a report of a case and review of the literature. Am J Gastroenterol 1982;77:882–4. [PubMed] [Google Scholar]
- [10].Sumiya H, Takayama H, Arai H, et al. A survival case of hepatocellular carcinoma with splenic metastasis: metachronous rupture at the liver primary and splenic metastatic lesion. Nihon Shokakibyo Gakkai Zasshi 2007;104:1639–44. [PubMed] [Google Scholar]
- [11].Ono F, Hiraga M, Omura N, et al. Hemothorax caused by spontaneous rupture of hepatocellular carcinoma: a case report and review of the literature. World J Surg Oncol 2012;10:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hino K. A case of hepatocellular carcinoma associated with hemothorax from spontaneous rupture of the mediastinal metastasis. Acta Hepatol Jpn 1987;28:1383–8. [Google Scholar]
- [13].Sato T, Noguchi S, Kanda K, et al. A case of primary hepatocellular carcinoma with metastasis to the thoracic vertebra causing fetal hemorrhage. Rinsho to Kenkyu (Jpn J Clin Exp Med) 1988;65:165–8. [Google Scholar]
- [14].Kohno T, Yamamoto M, Iizuka H, et al. Three cases of recurrent hepatocellular carcinoma with bone metastasis appearing with particular symptomes. Jpn J Gastroenterol Surg 1990;23:1887–91. [Google Scholar]
- [15].Lin HH, Liew CT, Liaw YF. Hepatocellular carcinoma presenting as acute spontaneous haemothorax. J Gastroenterol Hepatol 1990;5:362–4. [DOI] [PubMed] [Google Scholar]
- [16].Sekiya Y, Yamada H, Yoshitsugu M, et al. [An autopsy case of hepatocellular carcinoma associated with right hemothorax from spontaneous rupture of the rib metastasis]. Nihon Shokakibyo Gakkai Zasshi 1992;89:1310–3. [PubMed] [Google Scholar]
- [17].Akimaru K, Miyairi K, Tanaka H, et al. A rupture of lung metastasis of hepatocellular carcinoma causing haemothorax. J Gastroenterol Hepatol 1993;8:613–5. [DOI] [PubMed] [Google Scholar]
- [18].Takagi H, Shimoda R, Uehara M, et al. Hepatocellular carcinoma with pleural metastasis complicated by hemothorax. Am J Gastroenterol 1996;91:1865–6. [PubMed] [Google Scholar]
- [19].Kanou S, Yamada T, Hiramatsu N, et al. A case of hepatocellular carcinoma with direct invasion into the pleural cavity presenting as hemorrhage achieved hemostasis with intra-arterial injection of ethanol. Nippon Shokakibyo Gakkai Zasshi 1996;93:753–7. [PubMed] [Google Scholar]
- [20].Masumoto A, Motomura K, Uchimura K, et al. Case report: haemothorax due to hepatocellular carcinoma rupture successfully controlled by transcatheter arterial embolization. J Gastroenterol Hepatol 1997;12:156–8. [DOI] [PubMed] [Google Scholar]
- [21].Ogata H, Tsuji H, Hashiguchi M, et al. Hepatocellular carcinoma with metastasis to the rib complicated by hemothorax. An autopsy case. Fukuoka Igaku Zasshi 1999;90:342–6. [PubMed] [Google Scholar]
- [22].Sohara N, Takagi H, Yamada T, et al. Hepatocellular carcinoma complicated by hemothorax. J Gastroenterol 2000;35:240–4. [DOI] [PubMed] [Google Scholar]
- [23].Ishikawa T, Kohno T, Teratani T, et al. Thoracoscopic radiofrequency ablation therapy for hepatocellular carcinoma above the diaphragm associated with intractable hemothorax. Endoscopy 2002;34:843. [DOI] [PubMed] [Google Scholar]
- [24].Shiozawa K, Fushida S, Tani T, et al. A case of hemothorax secondary to rupture of mediastinal lymph node metastasis of hepatocellular carcinoma. J Jpn Surg Assoc 2003;64:1358–61. [Google Scholar]
- [25].Tanioka Y, Mizumachi S, Kimura T, et al. [An autopsy case of hepatocellular carcinoma with pleural metastasis]. Nihon Shokakibyo Gakkai Zasshi 2004;101:1320–4. [PubMed] [Google Scholar]
- [26].Tan CK, Wu KC, Wu RH, et al. Spontaneous hemothorax caused by metastasis of a rib tumour. CMAJ 2008;178:679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nagao E, Hirakawa M, Soeda H, et al. Transcatheter arterial embolization for chest wall metastasis of hepatocellular carcinoma. World J Radiol 2013;5:45–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Oh SY, Seo KW, Jegal Y, et al. Hemothorax caused by spontaneous rupture of a metastatic mediastinal lymph node in hepatocellular carcinoma: a case report. Korean J Intern Med 2013;28:622–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Okamoto K, Kataoka Y, Motoishi M, et al. A case of relapsed hepatocellular carcinoma with pleural and pulmonary metastasis diagnosed by thoracoscopy. J Jpn Soc Respir Endoscopy 2014;36:525–9. [Google Scholar]


