Abstract
Distribution of different malignant lymphoma subtypes varies substantially in different geographic regions, even in different districts in China.
In order to estimate the epidemiologic characteristics of malignant lymphoma in Hubei, China, we retrospectively analyzed a total number of 2027 newly diagnosed cases from April 2009 to April 2014 in a single institution according to the 2008 WHO classification.
The median diagnosis age of all these lymphoma patients was 53 (1–99) years, and the median ages for non-(NHL) and Hodgkin lymphoma (HL) were 54 (1–99) years and 38 (5–84) years, respectively. Among the included patients, mature B-cell neoplasms occupied 61.3%, mature T- and NK-cell neoplasms accounted for 21.0%, precursor lymphoid cell neoplasms made up 4.5%, and HL constituted 8.0%. The most common subtype of NHL was diffuse large B cell lymphoma (41.3%), followed by NK/T cell lymphoma (13.4%), extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) (8.0%), follicular lymphoma (6.6%), lymphoblastic lymphoma (4.9%), and mantle cell lymphoma (4.0%). Mixed cellularity lymphoma ranked first among classical HL subtypes, and there is a bimodal median age distribution revealed by our study, which is different from results reported by other regions of China. Most subtypes revealed male predominance while MALT lymphoma showed a slight female predominance. Extranodal lymphomas most frequently involved gastrointestinal tract, sinonasal region, and Waldeyer ring.
In summary, the distribution of lymphoma subtypes in Hubei of China is similar to that of Asian populations, as well as other regions of China, but distinct from the Western countries.
Keywords: China, epidemiology, Hodgkin lymphoma, lymphoma, non-Hodgkin lymphoma
1. Introduction
The incidence of lymphoid neoplasms worldwide has been steadily increasing over last 2 decades. It is known that the incidence rates of malignant lymphoma are higher in Western countries than in Eastern Asian, however, the absolute number of estimated new cases of lymphoma is higher in China (88200) than in United States (80900) in 2015.[1,2] Nonetheless, malignant lymphoma is currently the one of the most common malignancies in China.[2] Furthermore, the incidence rates and distributions of histologic subtypes of malignant lymphoma not only show significant geographic differences but also vary in age, sex, and ethnicity.[3–7] The descriptive epidemiologic studies within defined populations help us to better understand the pathobiology of these differences. Moreover, comparison of the incidence rates and frequency trends of specific lymphoma subtypes may provide critical clues to guide future epidemiologic studies.[8]
Classification of disease is the language of medicine, categorizing known entities in a way that facilitates understanding among clinicians, pathologists, and basic scientists.[9] The 4th World Health Organization (WHO) classification of neoplasms of the hematopoietic and lymphoid tissues, published in 2008, represents a worldwide consensus on the diagnosis of these tumors.[10] Although the 4th version was updated in 2016, there are no new definite entities compared with 2008.[11] Lots of descriptive epidemiologic studies, according to the 2008 WHO classification of lymphoma, help us to learn the clinical features of defined populations.
Previous studies have demonstrated that the histological distribution of lymphoma subtypes is different between Western countries and China. The incidence of NK/T-cell lymphoma is more frequent in China than North America, while a comparatively lower proportion of follicular lymphoma (FL) and chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) in Chinese patients.[6,7,12] This suggested that environmental factors and life styles could be related to the frequency of malignant lymphoma.[13–16]
Several studies have reported the epidemiologic features of lymphoma in provinces of Southwest and Eastern China, and geographic differences are also seen in some histological subtypes.[6,7,12,14] However, the distribution features of lymphoma subtypes in Central China still remain unknown. In the present study, with the aim to gain a better insight into the subtype distribution and major clinical characteristics of lymphomas in Hubei province of Central China, 2027 consecutive patients diagnosed with malignant lymphoma according to the 2008 WHO classification from April 2009 to April 2014 were reviewed and analyzed.
2. Materials and methods
2.1. Patients and methods
The clinical data of all patients diagnosed with lymphoid neoplasms on tissue samples from April 2009 to April 2014 at Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, a most reputable hospital in Central China with 5000 beds, were respectively analyzed. Clinical information obtained from medical records including patient gender, age, pathological diagnosis, and the site of tissue sampling. Similar to previous studies,[12,17] plasmacytoma and multiple myeloma were excluded in our collection. The data collection conformed to the provisions of the Declaration of Helsinki. All investigations had been approved by the Institutional Ethics Committees. Due to the retrospective nature of the study, informed consent was not required.
The pathological diagnosis was determined according to the 2008 WHO classification of lymphoid neoplasms. Hematoxylin and eosin staining and immunohistochemical (IHC) staining were routinely used for diagnosis. IHC indicators included leukocyte common antigen CD45/LCA; B-cell associated markers such as CD20, CD79a, CD19, CD20, CD22, CD23, CD38, CD138, PAX5, IgG, IgM, IgA, λ, κ; T/NK cell associated markers such as CD3, CD2, CD5, CD7, CD4, CD8, CD43, CD45RO, CD56, CD57, TIA-1, Granzyme B, perforin; cell differentiation markers such as CD30, TdT, CD99, CD10, Bcl-6, MUM1; tumor gene and proliferation related markers such as ALK, Bcl-2, Bcl-10, cyclin D1, c-myc, p53, Ki-67; and myeloid-related markers such as MPO, CD15, CD123, CD117, CD61. In situ hybridization for Epstein–Barr virus encoded small RNA (EBER) was performed in cases suspected to be EBV associated lymphomas, such as extranodal NK/T cell lymphoma (ENKTCL), angioimmunoblastic T-cell lymphoma (AITL), and mixed cellularity Hodgkin lymphoma. Fluorescence in situ hybridization for detection of MYC translocation was mandatory in cases diagnosed to be Burkitt lymphoma (BL). Polymerase chain reaction for immunoglobulin heavy chain (IgH) and T-cell receptor (TCR) gene rearrangement were used to decide lymphoma subtype while the diagnosis was unclear from the histopathologic and immunophenotypic evaluation. All the cases were reviewed by 2 pathologists (HP and XN). When there was a disagreement between the reviewers, the cases were referred to the panel of expert pathologists of the Department of Pathology, Union Hospital and the final diagnosis was reached by consensus.
2.2. Statistical analysis
Data analysis was done using SAS software version 9.4 (SAS Institute Inc, Cary, NC). Pearson chi-squared or Fisher exact tests were used when comparisons of categorical data of different regions were done. A P value less than .01 was considered to be statistically significant.
3. Results
A total number of 2027 consecutive cases from April 2009 to April 2014 were included in this study. The distributions of lymphoid neoplasm subtypes were shown in Table 1. Of the 2027 cases reviewed, 123 cases could not be definitely diagnosed as different subtype because of insufficient biopsy materials or unsatisfactory staining. Among these 123 cases, 98 could only be diagnosed as non-Hodgkin lymphoma (NHL) cases and 17 diagnosed as Hodgkin lymphoma (HL). In addition, still 8 cases could be sorted into neither NHL nor HL. Among the included patients, 1242 (61.3%) were mature B-cell neoplasms, 426 (21.0%) were mature T- and NK-cell neoplasms, 91 (4.5%) were precursor lymphoid cell neoplasms, and 162 (8.0%) had HL. Diffuse large B cell lymphoma (DLBCL) accounted for 767 (767/1857, 41.3%) ranked first among all NHL, followed by ENKTCL (n = 249, 13.4%), extranodal marginal zone lymphoma of mucosa associated lymphoid tissue (MALT lymphoma, n = 148, 8.0%), FL (n = 122, 6.6%), lymphoblastic leukemia/lymphoma (LBL, n = 91, 4.9%), mantle cell lymphoma (MCL, n = 75, 4.0%), AITL (n = 67, 3.6%), CLL/SLL (n = 65, 3.5%), PTCL NOS (n = 54, 2.9%), and anaplastic large cell lymphoma (ALCL, n = 41, 2.3%; among of them, ALK+ 31, ALK− 10). Of the 162 patients with HL, nodular lymphocyte predominant HL occupied 2.5% (n = 4) and classical HL (CHL) occupied 87.0% (n = 141), with mixed cellularity classical Hodgkin lymphoma (MC-CHL) being the most common subtype (n = 73, 45.1%) and nodular sclerosis classical Hodgkin lymphoma (NS-CHL) being the secondary subtype (n = 58, 35.8%).
Table 1.
Distribution of malignant lymphoma subtypes by age, gender, and biopsy site.
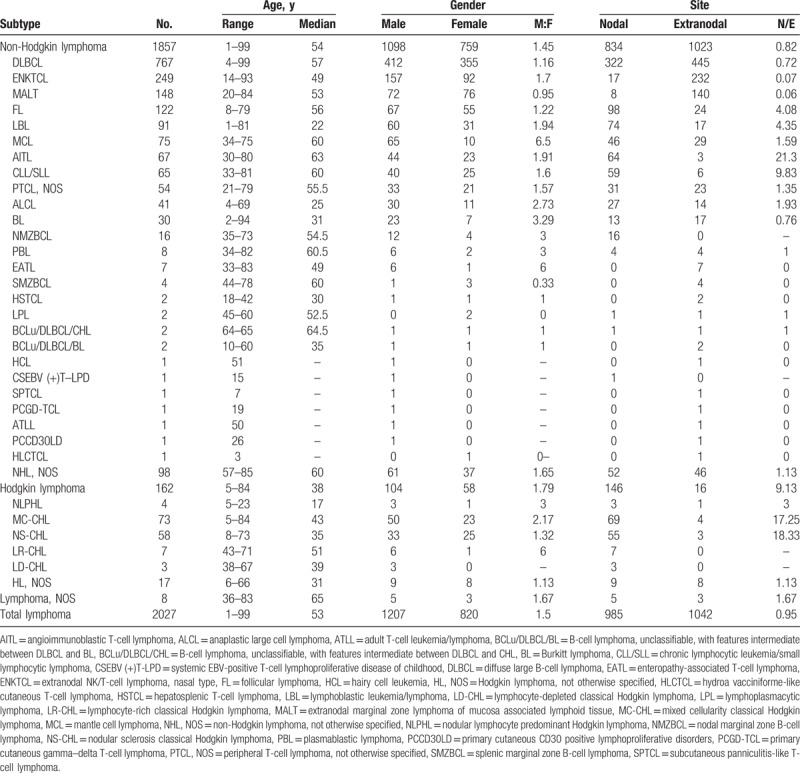
Trends in the frequency of lymphoid neoplasms by subtype, age and sex were shown in Fig. 1. The overall male to female (M/F) ratio was 1.5; and each subtype M/F ratio of most common subtypes was shown in Table 1. Except MALT lymphoma with a gender ratio of 0.95, the majority of lymphoma subtypes implied male predominance, especially in MCL which gender ratio was 6.5. Besides MCL, BL, ALCL, and AITL were more inclined to involve males than other lymphoma subtypes.
Figure 1.
Distribution of common lymphoma subtypes by gender and age. CHL = classical Hodgkin lymphoma, CLL/SLL = chronic lymphocytic leukemia/small lymphocytic lymphoma, MALT = extranodal marginal zone lymphoma of mucosa associated lymphoid tissue.
The median diagnosis age of all these lymphoma patients was 53 (1–99) years, and the median ages for NHL and HL were 54 (1–99) years and 38 (5–84) years, respectively. Patients between 55 and 64 years had higher incidence of NHL than other age groups. Of all NHL cases, AITL had the oldest median age of 63 years, while LBL with the youngest median age of 22 years was prone to involve in youth. In the age group of childhood (0–14 years), LBL had the highest incidence (25/73, 34.2%) followed by MC-CHL (11/73, 15.1%), ALCL (9/73, 12.3%), BL (8/73, 11.0%), and DLBCL (6/73, 8.2%), especially BL with the youngest age group peak of 0 to 15 years. For the elderly (≥65 years), DLBCL consisted almost half of all cases (231/479, 48.2%) and ranked first, followed by ENKTCL (37/479, 7.7%), AITL (32/479, 6.7%), MALT lymphoma (28/479, 5.8%), and CLL/SLL (22/479, 4.6%).
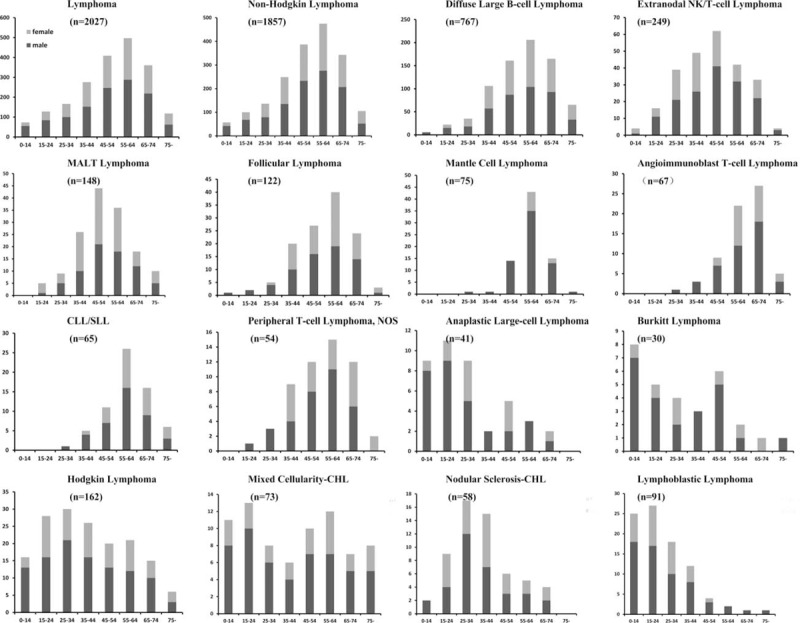
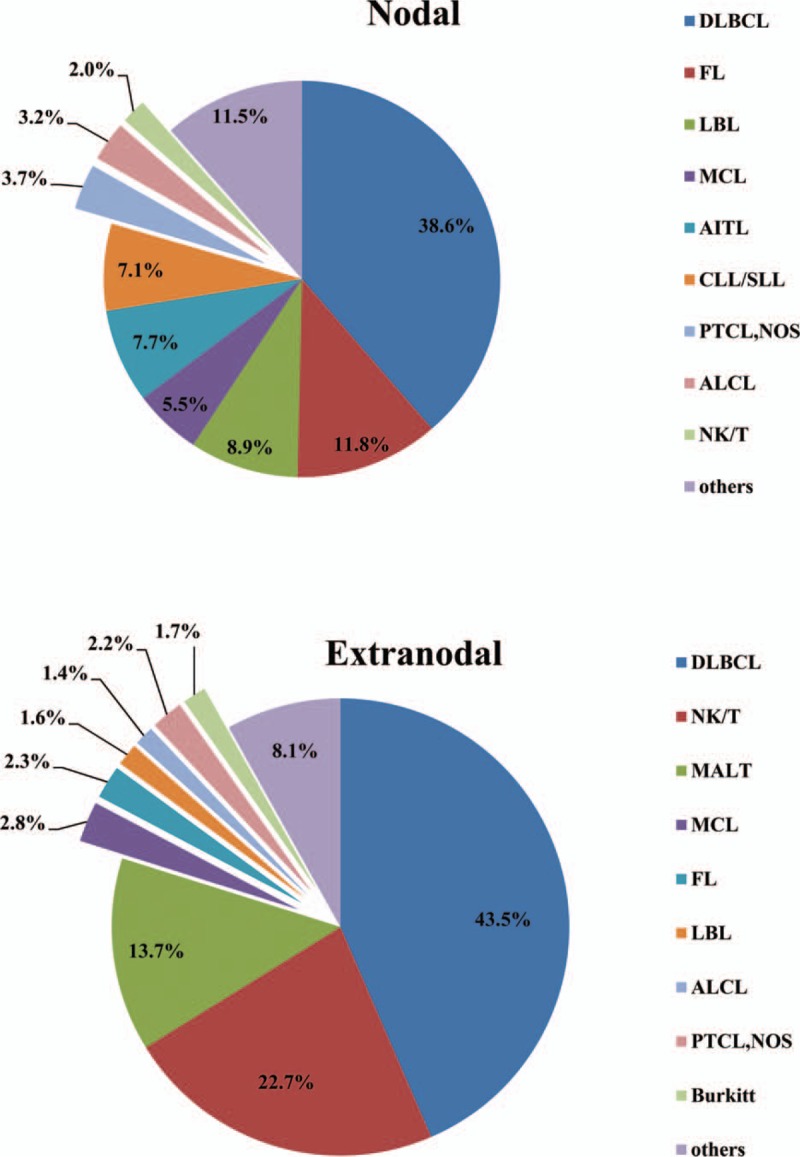
In this study, the biopsy sites of 985 cases were lymph nodes (48.6%), whereas 1042 cases (51.4%) were diagnosed on extranodal biopsies. HL was most commonly diagnosed on a lymph nodal specimen (90.1%), while NHL was commonly diagnosed on an extranodal specimen (55.1%). Different distributions of NHL subtypes in terms of nodal and extranodal sites were shown in Fig. 2. We observed that proportion of extranodal neoplasm (55.1%) was higher than that of nodal neoplasm (44.9%) in NHL. DLBCL was the most common NHL subtype in both nodal (322/834, 38.6%) and extranodal (445/1023, 43.5%) lymphomas, followed by FL and NKTCL, respectively. As shown in Fig. 3, gastrointestinal tract was the most common site involved by extranodal NHL, followed by sinonasal regions and Waldeyer ring.
Figure 2.
Distribution of pathological subtypes in nodal and extranodal non-Hodgkin lymphoma. AITL = angioimmunoblastic T-cell lymphoma, ALCL = anaplastic large cell lymphoma, CLL/SLL = chronic lymphocytic leukemia/small lymphocytic lymphoma, DLBCL = diffuse large B-cell lymphoma, FL = follicular lymphoma, LBL = lymphoblastic leukemia/lymphoma, MALT = extranodal marginal zone lymphoma of mucosa associated lymphoid tissue, MCL = mantle cell lymphoma, NK/T = extranodal NK/T-cell lymphoma, nasal type, PTCL, NOS = peripheral T-cell lymphoma, not otherwise specified.
Figure 3.
Distribution of extranodal sites involved by non-Hodgkin lymphoma.
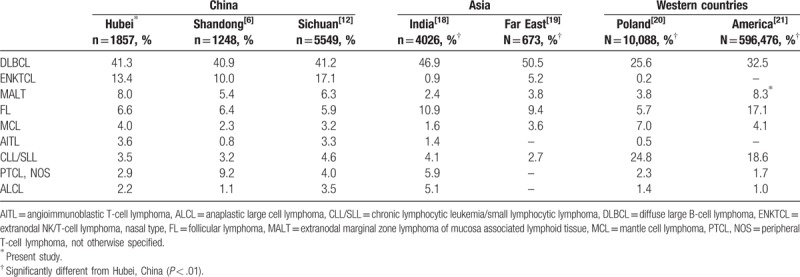
We compared the distribution of NHL subtypes in Hubei with that in other regions of China,[6,12] Asia,[18,19] and Western countries.[20,21] Pearson chi-squared and Fisher exact tests were used for data comparing. A P value less than .01 was considered to be statistically significant and the results were shown in Table 2. DLBCL is always the most frequent subtype whether in Asia, Europe, or America. NK/T cell lymphoma is more common in China and other regions in Asia than in Western countries. However, the frequency of NK/T cell lymphoma in India is pretty low. CLL/SLL is much less common in China and other regions in Asia than in Western countries.
Table 2.
Distribution of non-Hodgkin lymphoma common subtypes in different regions.
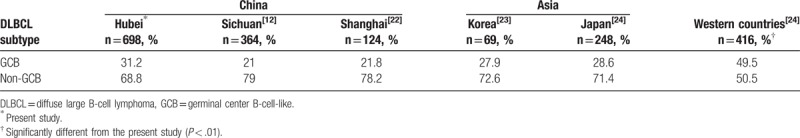
According to the results of CD10, BCL-6, and MUM1 staining, 698 cases of DLBCL from this study were further classified as germinal center B-cell-like (GCB) and non-GCB subtypes using the Hans algorithm. The results showed that 218 cases were classified as GCB subtype (31.2%) and 480 as non-GCB subtype (68.8%). As is shown in Table 3, we compared our data with that of other regions in China,[12,22] Asia,[23,24] and Western countries[24] using the same statistical methods. The results showed that DLBCL subtype is similar in Asian population, whereas the frequency of non-GCB is much higher in Asian countries than in Western countries.
Table 3.
Distribution of DLBCL subtypes in China and other countries.
4. Discussion
Numerous studies have studied the geographic variations of lymphoid neoplasms worldwide.[3–7] To gain a better insight into the epidemiologic features of lymphoid neoplasms according to the WHO classification in Hubei province of Central China, we examined the comprehensive cases of malignant lymphoma diagnosed at a single hospital over 5 years. Currently, of the 2027 enrolled cases, 730 (36.0%) individuals were consulted patients who came from the districts of Hubei province, which, to an extent, represents the present conditions of Central China.
In the present study, HL constituted 8.0% of all diagnosed lymphomas, which is similar to previous reports in China (8.5%),[7,17,25] Japan (7%),[5] and America (8.8%),[8] but is much lower than the frequency of HL reported in India (21.3%).[18] Previously, it has been suggested that MC-CHL is the most common subtype of HL in China,[25] and our results confirm this, as the proportion of MC-CHL in all diagnosed HL was 45.1%, whereas in developing countries NS-CHL is the predominant subtype.[8] Interestingly, as for age distribution, HL showed a bimodal age curve (a peak age of 25–34 years and a second lower peak age of 55–64 years) in our study, which is similar to that of North America and Europe,[26] but is different from previous results of China. The bimodal age curve might be formed by the different peak ages of the 2 main subtypes (MC-CHL and NS-CHL).[27]
DLBCL is the most common subtype of NHL worldwide.[28] In the present study, DLBCL accounted for 41.3% ranking first among all NHL, which is consistent with previous studies in China[7,8] and Western countries.[29,30] Immunohistochemical staining was performed on 698 cases of DLBCL using antibodies against CD10, BCL10, and MUM1. According to Hans algorithm, the frequency of the non-GCB subtype was much higher than that of the GCB subtype (68.8% vs 31.2%), which coincided with other studies.[31,32] As shown in Table 3, the comparison results with other regions in China, Korea, Japan, and Western countries[12,22–24] revealed that GCB subtype of DLBCL is less common in China and other Asian countries than in the West. Although the Hans algorithm was commonly used to determine the cell of origin (COO) for its accessibility and low cost, the concordance with gold standard of gene expression profiling is poor.[33,34] Therefore, the prognostic value of COO determined by immunohistochemistry methods is questionable.[33,35]
In our study, mature T- and NK-cell lymphomas accounted for 21.0% of all lymphoid neoplasms, which is much higher than the proportions in Western countries,[36,37] but consistent with reports in other regions of China.[6] ENKTCL was the most common subtype of T/NK cell lineage lymphoma, which made up almost half of all mature T/NK-cell lymphomas, and 13.4% of all diagnosed NHL in our study. ENKTCL is prevalent in East Asia (especially China) and South America, but its incidence is relatively low in Western countries.[31,36] AITL also has a higher incidence in Asian countries than that in Western countries. The proportion of AITL was 3.6% in our study, which is slightly less than that in Hong Kong (8%).[36] Both ENKTCL and AITL were reported to associate strongly with Epstein–Barr virus (EBV) infection in Asian populations,[38,39] and EBV-DNA level was a good indicator for response, disease progression, and prognosis in ENKTCL.[40] In our study, EBV was detected in 98.4% (245/249) ENKTCL patients by EBER in situ hybridization which was mandatory in all ENTKCL suspected samples.
FL is the second common histological subtype in Western countries, accounting for 22% to 35% of all NHL, but in China, the proportion is less than that in Western countries.[41] The frequency of FL was only 2.3% reported by Wang et al[7] in Sichuang, 7% reported by Gross et al[42] in Shanghai, 8.6% reported by Wang et al[43] in Shanxi, and 6.6% reported by the present study in Hubei. The other obvious difference in the subtype distributions observed in our study, as compared with those in America, was for CLL/SLL. The proportion of CLL/SLL was 3.5% in our study, which is similar to that in Sichuan (3.7%),[7] Central and South America (4.1%),[29] but is much lower than that in USA (18.6%).[21] On the other hand, some other subtypes, such as BL and cutaneous lymphoma, presented similar frequencies in China and Western countries.[7,12,13] The geographic disparities in distributions of specific subtypes suggest a complex etiological profile for lymphoma that likely varies by disease subtype.[7,15]
Lymphomas could involve various organs and tissues. In this study, we defined the biopsy site for establishing the diagnosis as primary site, although they were not totally identical. Thus, the statistic bias might be caused by the biopsy site selection of different surgeons. The proportion of extranodal NHL was 55.1% in our study, which is similar to the reports in other regions of China (54%–61%),[7,12,17] but is much higher than that in US (27%).[44] Our data showed extranodal involvements were more frequently observed in DLBCL, ENKTCL, and MALT lymphoma than other subtypes, and gastrointestinal tract was the most common site involved by extranodal NHL. However, Waldeyer ring was most commonly involved extranodal site reported by Yang et al[12] in Southwest China. Relatively high frequency of DLBCL and MALT involved with gastrointestinal tract in our study may contribute to this difference.
In consistent with previous studies, most subtype of malignant lymphoma in our study showed a male predominance, with an average M/F ratio of 1.5. However, our study showed that the numbers of male patients were slightly less than that of female patients (M/F: 72/76) diagnosed with MALT lymphoma, which is different from the reports in other regions of China.[12,17,25] This may be explained by the smaller samples of ours. With regard to age, the present findings are consistent with reports in literature from China.[6,12,17] The peak ages of NHL were concentrated in those 55 to 64 years, which is slightly younger than that of American and European patients.[4,21]
The main limitation of this study was the data were collected from only one institution, which could be accompanied with an inherited selection bias. However, Cancer Center of Union Hospital, Wuhan as one of the leading hospitals in Central China, serving both adults and children, is an ideal surrogate for population-based data.
In conclusion, the distribution of lymphoma subtypes in Hubei of China is similar to that of Asian populations, as well as other regions of China, but distinct from the Western countries. Our findings suggest that additional studies are needed for pursuit of epidemiologic analysis by subtype in China.
Acknowledgements
The authors thank the National Natural Science Foundation of China (81101573 and 81672940 to LZ) for the support.
Author contributions
Conceptualization: Gang Wu, Liling Zhang.
Data curation: Jingshu Meng, Chan Chang, Yin Xiao, Tao Liu.
Formal analysis: Chan Chang.
Funding acquisition: Liling Zhang.
Methodology: Jingshu Meng, Chan Chang, Huaxiong Pan, Fang Zhu.
Project administration: Liling Zhang.
Resources: Huaxiong Pan, Fang Zhu, Yin Xiao, Tao Liu.
Supervision: Xiu Nie, Gang Wu, Liling Zhang.
Validation: Huaxiong Pan, Xiu Nie, Gang Wu.
Writing – original draft: Jingshu Meng.
Writing – review & editing: Liling Zhang.
Footnotes
Abbreviations: AITL = angioimmunoblastic T-cell lymphoma, ALCL = anaplastic large cell lymphoma, BL = Burkitt lymphoma, CLL/SLL = chronic lymphocytic leukemia/small lymphocytic lymphoma, DLBCL = diffuse large B-cell lymphoma, ENKTCL = extranodal NK/T-cell lymphoma, FL = follicular lymphoma, LBL = lymphoblastic leukemia/lymphoma, MALT = extranodal marginal zone lymphoma of mucosa associated lymphoid tissue, MC-CHL = mixed cellularity classical Hodgkin lymphoma, MCL = mantle cell lymphoma, NS-CHL = nodular sclerosis classical Hodgkin lymphoma.
JM, CC, and HP contributed equally to this work.
Funding/support: This work was funded by the National Natural Science Foundation of China (81101573 and 81672940 to LZ).
The authors have no conflicts of interest to disclose.
Present address: Chan Chang, Department of Respiratory, Medicine, Taihe Hospital, Hubei University of Medicine, Shiyan, China.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- [2].Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- [3].Roman E, Smith AG. Epidemiology of lymphomas. Histopathology 2011;58:4–14. [DOI] [PubMed] [Google Scholar]
- [4].Smith A, Howell D, Patmore R, et al. Incidence of haematological malignancy by sub-type: a r-eport from the Haematological Malignancy Research Network. Br J Cancer 2011;105:1684–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Aoki R, Karube K, Sugita Y, et al. Distribution of malignant lymphoma in Japan: analysis of 2260 cases, 2001-2006. Pathol Int 2008;58:174–82. [DOI] [PubMed] [Google Scholar]
- [6].Liu J, Song B, Fan T, et al. Pathological and clinical characteristics of 1,248 non-Hodgkin's lymphomas from a regional cancer hospital in Shandong, China. Asian Pac J Cancer Prev 2011;12:3055–61. [PubMed] [Google Scholar]
- [7].Wang XM, Bassig BA, Wen JJ, et al. Clinical analysis of 1629 newly diagnosed malignant lymphomas in current residents of Sichuan province, China. Hematol Oncol 2016;34:193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Morton LM, Wang SS, Devesa SS, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood 2006;107:265–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Campo E, Swerdlow SH, Harris NL, et al. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood 2011;117:5019–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Swerdlow SH, Campo E, Harris NL, et al. WHO Classification: Pathology and Genetics of Tumors of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: IARC Press; 2008. [Google Scholar]
- [11].Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127:2375–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yang QP, Zhang WY, Yu JB, et al. Subtype distribution of lymphomas in Southwest China: analysis of 6,382 cases using WHO classification in a single institution. Diagn Pathol 2011;6:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Muller AM, Ihorst G, Mertelsmann R, et al. Epidemiology of non-Hodgkin's lymphoma (NHL): trends, geographic distribution, and etiology. Ann Hematol 2005;84:1–2. [DOI] [PubMed] [Google Scholar]
- [14].Fan R, Zhang LY, Wang H, et al. Multicentre hospital-based case-control study of diffuse large B-cell lymphoma in Shanghai, China. Asian Pac J Cancer Prev 2012;13:3329–34. [DOI] [PubMed] [Google Scholar]
- [15].Morton LM, Slager SL, Cerhan JR, et al. Etiologic heterogeneity among non-Hodgkin lymphoma subtypes: the InterLymph Non-Hodgkin Lymphoma Subtypes Project. J Natl Cancer Inst Monogr 2014;2014:130–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chiu BC, Hou N. Epidemiology and etiology of non-hodgkin lymphoma. Cancer Treat Res 2015;165:1–25. [DOI] [PubMed] [Google Scholar]
- [17].Sun J, Yang Q, Lu Z, et al. Distribution of lymphoid neoplasms in China: analysis of 4,638 cases according to the World Health Organization classification. Am J Clin Pathol 2012;138:429–34. [DOI] [PubMed] [Google Scholar]
- [18].Arora N, Manipadam MT, Nair S. Frequency and distribution of lymphoma types in a tertiary care hospital in South India: analysis of 5115 cases using the World Health Organization 2008 classification and comparison with world literature. Leuk Lymphoma 2013;54:1004–11. [DOI] [PubMed] [Google Scholar]
- [19].Perry AM, Diebold J, Nathwani BN, et al. Non-Hodgkin lymphoma in the Far East: review of 730 cases from the international non-Hodgkin lymphoma classification project. Ann Hematol 2016;95:245–51. [DOI] [PubMed] [Google Scholar]
- [20].Szumera-Cieckiewicz A, Galazka K, Szpor J, et al. Distribution of lymphomas in Poland according to World Health Organization classification: analysis of 11718 cases from National Histopathological Lymphoma Register project – the Polish Lymphoma Research Group study. Int J Clin Exp Pathol 2014;7:3280–6. [PMC free article] [PubMed] [Google Scholar]
- [21].Al-Hamadani M, Habermann TM, Cerhan JR, et al. Non-Hodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: A longitudinal analysis of the National Cancer Data Base from 1998 to 2011. Am J Hematol 2015;90:790–5. [DOI] [PubMed] [Google Scholar]
- [22].Chen Y CH, Fu K, Zhu XZ, et al. Prevalence of germinal center B-cell-like and non-germinal center B-cell-like types of diffuse large B-cell lymphoma in Shanghai, China. Chin J Pathol 2010;39:313–8. [PubMed] [Google Scholar]
- [23].Hwang HS, Yoon DH, Suh C, et al. Prognostic value of immunohistochemical algorithms in gastrointestinal diffuse large B-cell lymphoma. Blood Res 2013;48:266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Shiozawa E, Yamochi-Onizuka T, Takimoto M, et al. The GCB subtype of diffuse large B-cell lymphoma is less frequent in Asian countries. Leuk Res 2007;31:1579–83. [DOI] [PubMed] [Google Scholar]
- [25].Li X, Li G, Gao Z, et al. Distribution pattern of lymphoma subtypes in China: a nationwide multicenter study of 10 002 cases. J Diagn Concepts Pract 2012;11:111–5. [Google Scholar]
- [26].Townsend W, Linch D. Hodgkin's lymphoma in adults. Lancet 2012;380:836–47. [DOI] [PubMed] [Google Scholar]
- [27].Nakatsuka S, Aozasa K. Epidemiology and pathologic features of Hodgkin lymphoma. Int J Hematol 2006;83:391–7. [DOI] [PubMed] [Google Scholar]
- [28].Sehn LH, Gascoyne RD. Diffuse large B-cell lymphoma: optimizing outcome in the context of clinical and biologic heterogeneity. Blood 2015;125:22–32. [DOI] [PubMed] [Google Scholar]
- [29].Laurini JA, Perry AM, Boilesen E, et al. Classification of non-Hodgkin lymphoma in Central and South America: a review of 1028 cases. Blood 2012;120:4795–801. [DOI] [PubMed] [Google Scholar]
- [30].Combariza JF, Lombana M, Torres AM, et al. General features and epidemiology of lymphoma in Colombia. A multicentric study. Ann Hematol 2015;94:975–80. [DOI] [PubMed] [Google Scholar]
- [31].Ding W, Zhao S, Wang J, et al. Gastrointestinal Lymphoma in Southwest China: Subtype Distribution of 1,010 Cases Using the WHO (2008) Classification in a Single Institution. Acta Haematol 2016;135:21–8. [DOI] [PubMed] [Google Scholar]
- [32].Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004;103:275–82. [DOI] [PubMed] [Google Scholar]
- [33].Gutierrez-Garcia G, Cardesa-Salzmann T, Climent F, et al. Gene-expression profiling and not immunophenotypic algorithms predicts prognosis in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Blood 2011;117:4836–43. [DOI] [PubMed] [Google Scholar]
- [34].Scott DW, Wright GW, Williams PM, et al. Determining cell-of-origin subtypes of diffuse large B-cell lymphoma using gene expression in formalin-fixed paraffin-embedded tissue. Blood 2014;123:1214–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gleeson M, Hawkes EA, Cunningham D, et al. Caution in the use of immunohistochemistry for determination of cell of origin in diffuse large B-cell lymphoma. J Clin Oncol 2015;33:3215–6. [DOI] [PubMed] [Google Scholar]
- [36].Anderson JR, Armitage JO, Weisenburger DD. Epidemiology of the non-Hodgkin's lymphomas: distributions of the major subtypes differ by geographic locations. Non-Hodgkin's Lymphoma Classification Project. Ann Oncol 1998;9:717–20. [DOI] [PubMed] [Google Scholar]
- [37].Vose J, Armitage J, Weisenburger D, et al. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol 2008;26:4124–30. [DOI] [PubMed] [Google Scholar]
- [38].Harabuchi Y, Yamanaka N, Kataura A, et al. Epstein-Barr virus in nasal T-cell lymphomas in patients with lethal midline granuloma. Lancet 1990;335:128–30. [DOI] [PubMed] [Google Scholar]
- [39].Attygalle AD, Kyriakou C, Dupuis J, et al. Histologic evolution of angioimmunoblastic T-cell lymphoma in consecutive biopsies: clinical correlation and insights into natural history and disease progression. Am J Surg Pathol 2007;31:1077–88. [DOI] [PubMed] [Google Scholar]
- [40].Suzuki R, Yamaguchi M, Izutsu K, et al. Prospective measurement of Epstein-Barr virus-DNA in plasma and peripheral blood mononuclear cells of extranodal NK/T-cell lymphoma, nasal type. Blood 2011;118:6018–22. [DOI] [PubMed] [Google Scholar]
- [41].Jiang WQ, Huang HQ. Guidelines for the diagnosis and treatment of follicular lymphoma in China. Cancer Biol Med 2013;10:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gross SA, Zhu X, Bao L, et al. A prospective study of 728 cases of non-Hodgkin lymphoma from a single laboratory in Shanghai, China. Int J Hematol 2008;88:165–73. [DOI] [PubMed] [Google Scholar]
- [43].Wang F, Xu RH, Han B, et al. High incidence of hepatitis B virus infection in B-cell subtype non-Hodgkin lymphoma compared with other cancers. Cancer 2007;109:1360–4. [DOI] [PubMed] [Google Scholar]
- [44].Groves FD, Linet MS, Travis LB, et al. Cancer surveillance series: non-Hodgkin's lymphoma incidence by histologic subtype in the United States from 1978 through 1995. J Natl Cancer Inst 2000;92:1240–51. [DOI] [PubMed] [Google Scholar]


