Supplemental Digital Content is available in the text
Keywords: azithromycin, doxycycline, meta-analysis, rickettsia, scrub typhus, systematic review, tsutsugamushi
Abstract
Background:
Scrub typhus is a zoonotic disease that remains an important health threat in endemic areas. Appropriate anti-rickettsial treatment ensures a successful recovery. Doxycycline is a recommended drug, but it is contraindicated in pregnant women and young children. Azithromycin is a safer alternative drug, but its effectiveness remains largely unclear. Herein, we conducted a systematic review and meta-analysis to determine the effectiveness of azithromycin.
Methods:
Studies that investigated azithromycin in treating scrub typhus were systematically identified from electronic databases up to December 2016. Information regarding study population, disease severity, treatment protocols, and responses was extracted and analyzed.
Results:
In this review, 5 studies were included, which comprised a total of 427 patients. When comparing the treatment failure rate, we observed a favorable outcome in patients treated with azithromycin (risk ratio [RR] 0.83, 95% confidence interval [CI] 0.23–2.98). However, patients in the azithromycin group had longer time to defervescence (mean difference 4.38 hours, 95% CI −2.51 to 11.27) and higher rate of fever for more than 48 hours (RR 1.31, 95% CI 0.81–2.12). Moreover, patients treated with azithromycin had less adverse effects (RR 0.8, 95% CI 0.42–1.52).
Conclusions:
Azithromycin is as effective as other anti-rickettsial drugs with higher treatment success rates, lower frequency of adverse effects, and longer time to defervescence (GRADE 2B). Therefore, it is reasonable to use azithromycin as the first-line treatment against scrub typhus. Further studies are warranted to elucidate the effectiveness of azithromycin in specific patient groups, at high dose and influence of drug resistance.
1. Introduction
Scrub typhus is a zoonotic rickettsial disease caused by Orientia tsutsugamushi and transmitted to humans after a chigger bite.[1] It is estimated to infect 1 million people per year worldwide and may present as various clinical manifestations.[2,3] Early diagnosis of scrub typhus still remains a challenge for physicians, and the mortality of untreated cases is high.[4,5] There are several drugs to treat scrub typhus, including tetracyclines, chloramphenicol, azithromycin, and quinolones.[6] Currently, the drug of choice is doxycycline, a member of the tetracycline family, and several studies have proved its effectiveness.[6,7] However, a recent study that investigated the outcomes of mothers and fetuses in pregnancy complicated with scrub typhus revealed that approximately half of the pregnancies resulted in poor fetal outcomes.[8] Doxycycline is contra-indicated in pregnant women and young children because of potential fetotoxicity.[9] Azithromycin is classified in category B by the US Food and Drug Administration (US FDA) Pregnancy Category and is the suggested alternative drug in these patients.[10] The high rates of poor fetal outcomes in Rajan et al's report caught our attention, and the effectiveness of azithromycin was questioned.
In 2002, a meta-analysis was conducted to compare the effectiveness of anti-rickettsial drugs, and no obvious differences between tetracycline and doxycycline were found.[6] The effectiveness of azithromycin was compared with doxycycline as well; however, only 2 studies were included in the meta-analysis.[7,11] The current evidences remained largely unclear. After literature review, some additional studies have been added since the last meta-analysis. Therefore, we conducted this systematic review and meta-analysis to evaluate the effectiveness and comparability of azithromycin in treating patients with scrub typhus.
2. Materials and methods
2.1. Study selection
This study was approved by the Ethics Committees of MacKay Memorial Hospital, Taiwan (IRB No.: 16MMHIS141e) and has been registered to PROSPERO (registry number: 62102). We systematically searched for all relevant articles in 2 online databases—PubMed and EMBase—from the earliest record to December 2016. PubMed is a free database mainly derived from MEDLINE and is considered an optimal tool in biomedical electronic research. EMBase is a highly versatile, multipurpose, and up-to-date biomedical database with a broad coverage. The key terms used for the search included “scrub typhus,” “tsutsugamushi,” “azithromycin,” and “rickettsia.” Key words were combined using Boolean searches. The search was made using keywords, Boolean operators, and MeSH descriptor. Cochrane Collaboration Central Register of Controlled Clinical Trials, Cochrane Systematic Reviews, and ClinicalTrials.gov were manually searched for additional references. Studies including randomized controlled trials, quasi-experimental investigations, and prospective follow-up studies were enrolled without language restriction. Case reports without a well-designed intervention scheme, single-arm studies, and epidemiological reports were excluded.
2.2. Data extraction and quality assessment
Two authors (C-YL and Y-JC) independently evaluated all eligible articles. These articles were scrutinized, and the data regarding study population, disease severity, treatment protocol, and details of treatment responses from the selected studies were extracted. The primary outcome was treatment success or failure. The treatment failures were defined as persistence of symptoms, fever, final laboratory abnormalities, change of treatment, or mortality. Other comparative variables included time to defervescence, rate of fever for more than 48 hours, adverse effects, and relapse. The Jadad score, ranging from 0 to 5, was used to assess the quality of the enrolled studies. Studies with scores <3 were assumed to have a lower methodological quality.[12] Discrepancies between the 2 independent evaluations for potential articles were resolved through discussion and consensus.
2.3. Data synthesis and analysis
Treatment results from all the studies were extracted, analyzed, and compared to determine differences in the effectiveness and adverse effects between azithromycin and other anti-rickettsial drugs. The effect sizes were pooled by using a random-effect model, and the results were represented by a point estimate with a 95% confidence interval (CI). The heterogeneity across studies was tested using I2 and Cochran Q tests. A P value <.10 for chi-square test of the Q statistic or an I2 >50% was considered as statistically significant heterogeneity.[13] For studies that contained continuous variables without mean and standard deviation (SD), values of case number, median, and low and high ends of the range were used to estimate the mean values.[14] A sensitivity analysis was performed by removing some studies to observe whether the action caused serious changes in the overall result. Funnel plot was performed to examine the publication bias if the number of included studies are more than 10.[15] Review Manager (version 5.3.5) was used for our analyses.
3. Results
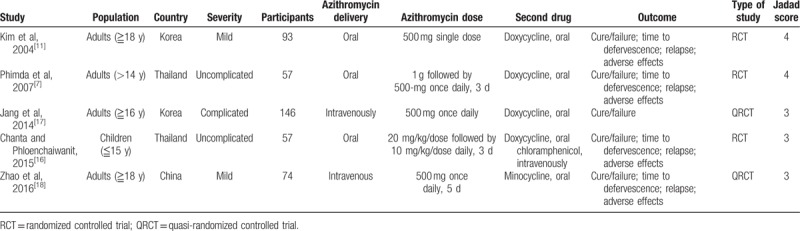
Of the 43 nonduplicate citations identified from the literature, 9 studies were included which were qualitative synthesis. Among them, 4 studies were excluded after critical review. Finally, 5 studies were enrolled for eligibility and meta-analysis (Fig. 1).[7,11,16–18] Four studies were conducted with the adult population and 1 study involved the pediatric population (Table 1). Two studies were executed in Korea, 2 in Thailand, and 1 in China. Four studies investigated the treatment in uncomplicated patients with scrub typhus, and 1 study was performed in patients with complicated scrub typhus. Oral azithromycin was used in 3 studies, and intravenous azithromycin was used in the other 2 studies. The comparative arms included oral doxycycline in 3 studies, oral minocycline in 1 study, and oral doxycycline or intravenous chloramphenicol in 1 study. In all, 427 patients were included in the analyses.
Figure 1.
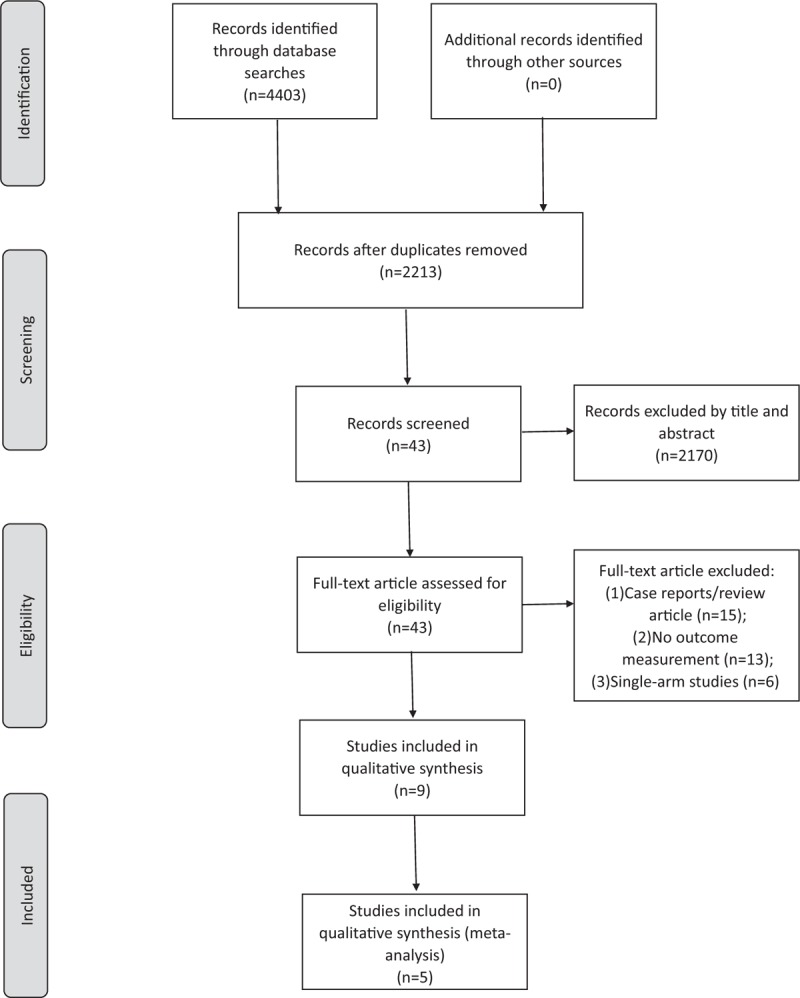
Flow diagram showing the selection of articles for review.
Table 1.
Studies comparing azithromcyin and other drugs treating scrub typhus.
By comparing the rate of treatment failures, we found a favorable outcome in patients treated with azithromycin than with other anti-rickettsial drugs (risk ratio [RR] 0.83, 95% CI 0.23–2.98) (Fig. 2). However, patients in the azithromycin group had longer time to defervescence (mean difference 4.38 hours, 95% CI −2.51 to 11.27) (Fig. 3) and higher rate of fever for more than 48 hours (RR 1.31, 95% CI 0.81–2.12) (Fig. 4). Adverse effects were reported in 3 studies, and in those, patients treated with azithromycin had less adverse effects (RR 0.8, 95% CI 0.42–1.52) (Fig. 5). No patients with relapse were found in both comparative arms.
Figure 2.
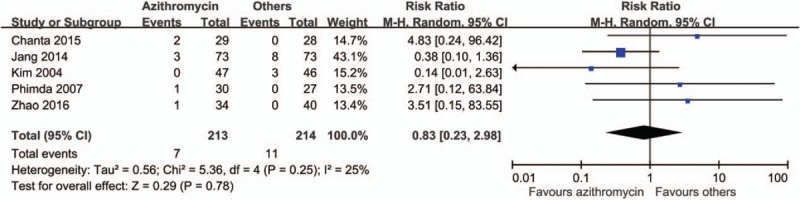
Forrest plot of the treatment failure between azithromycin and other drugs.
Figure 3.
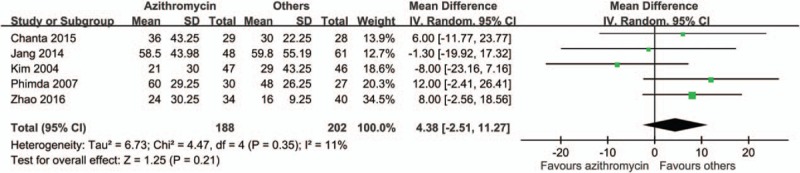
Forrest plot of the time to defervescence between azithromycin and other drugs.
Figure 4.
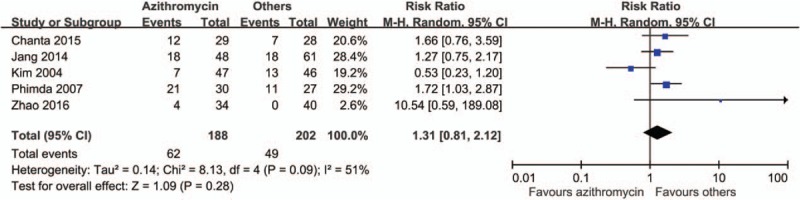
Forrest plot of patients with fever more than 48 hours between azithromycin and other drugs.
Figure 5.
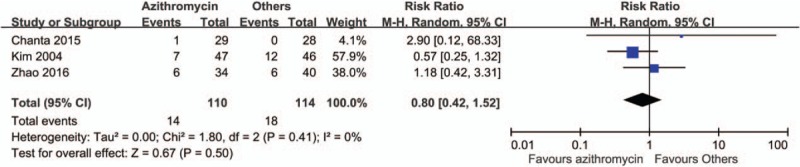
Forrest plot of the incidence of adverse effect between azithromycin and other drugs.
4. Discussion
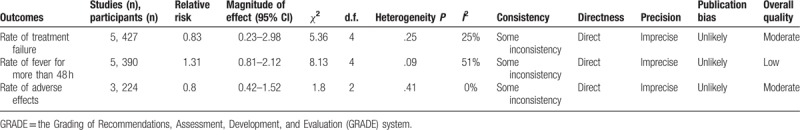
Scrub typhus still remains an important health threat in endemic areas, even though dramatic improvements have been achieved with prompt anti-rickettsial treatments.[4,5] Previous meta-analyses indicated that azithromycin was effective against scrub typhus,[6,19] and 3 additional studies were included in our analysis. We concluded that the effectiveness of azithromycin is comparable with the effectiveness of other anti-rickettsial drugs. Specifically, azithromycin treatment offered higher treatment success rates, lower adverse effects, and longer time to defervescence compared with other anti-rickettsial drugs. Our meta-analyses suggested that azithromycin may have the same effectiveness as other drugs and may serve as the first-line treatment for scrub typhus (GRADE 2B, weak recommendation with moderate evidence quality).[20]The quality of evidences is summarized in Table 2.
Table 2.
GRADE recommendation of azithromcyin for treating scrub typhus.
However, the treatment effectiveness may be affected by the disease severity. One study compared the treatment in patients with complicated scrub typhus; meanwhile, 4 studies investigated the treatment of patients with uncomplicated scrub typhus. For patients with complicated scrub typhus, Jang et al[17] found there were no significant differences in the treatment success rates and survival rates among patients treated with azithromycin and doxycycline. Similarly, the forest plots of studies investigating patients with uncomplicated patients showed analogous conclusions (Supplementary Figs. 1–3). Therefore, for infected patients with any degree of severity, azithromycin might be the first-line treatment, particularly in patients with contraindications to other rickettsial drugs. Although only very few studies have been conducted so far and further studies investigating different disease severities are required.
The most important advantage of using azithromycin in clinical application is its safety in pregnant women and children. Scrub typhus complicating pregnancy is uncommon, but if it happens, it may lead to serious unfavorable outcomes to mothers and fetuses.[21,22] The use of azithromycin in pregnant women is believed to be safe for both mothers and fetuses, and it is classified in category B by the US FDA Pregnancy Category.[9] Physicians should prescribe a category B drug to pregnant women if the benefit outweighs the risk. However, other available anti-rickettsial drugs are not recommended because of possible fetotoxicity. Chloramphenicol, ciprofloxacin, and rifampin are classified in category C, and tetracycline, doxycycline, and minocycline are classified in category D.[9] Mounting evidences showed that azithromycin use in pregnant women is safe and well-tolerated without adverse effects to fetuses.[22–28] Kim et al[10] have reviewed the literature regarding scrub typhus in pregnant women, and they concluded that azithromycin was effective against scrub typhus with favorable pregnancy outcomes. In the study reported by Rajan et al, 33 pregnant women with scrub typhus were treated with azithromycin. The maternal mortality was low (3%), but poor fetal outcomes were observed in half of the fetuses.[8] The effectiveness of azithromycin was called into question. Although the effectiveness of azithromycin has been confirmed by our meta-analysis, subgroup analysis of pregnant women is unavailable. On the contrary, Kumar et al[22] reported that pregnancies of 14 women treated with azithromycin were uneventful. Doxycycline is cheap, effective, easily available, and it is the preferable drug for treating scrub typhus.[29] However, in view of potential fetotoxicity, azithromycin is the recommended first-line anti-rickettsial drug in pregnant patients.[10,22,30]
In addition to common gastrointestinal adverse effects, the life-threatening risk of association between azithromycin and cardiovascular death with conflicting evidences has been reported.[31,32] In the present systematic review and meta-analysis, adverse effects occurred in 14% patients, and no cardiovascular adverse effects were reported.
Different routes of delivery and dosages of azithromycin may contribute to the different outcomes. Although the route of delivery and dosage of azithromycin were not emphasized in current treatment guidelines, we hypothesize that a higher transplacental concentration may increase the success rate of treatment. Oral administration of azithromycin is convenient with high compliance, but not available in some areas. In the studies reported by Jang et al[17] and Zhao et al,[18] intravenous azithromycin was administered. The oral bioavailability of azithromycin is approximately 38%, and a single high-dose regimen (1 or 2 g) has been applied in several diseases, such as gonococcal urethritis.[31–35] Higher dose of azithromycin was considered an effective and safe regimen. The use of intravenous azithromycin in children and pregnant sheep model was reported as a safe treatment.[34,36] Therefore, a high-dose intravenous injection of azithromycin in pregnant women may be a reasonable strategy. However, further studies are warranted to test the hypothesis.
In addition to route of delivery and dosage, resistance of scrub typhus to some antibiotics, including doxycycline and fluoroquinolones, was reported to be associated with treatment failure.[37,38] Higher resistance of doxycycline in some areas may contribute to treatment failure. In the present meta-analyses, azithromycin was favored in the study by Kim et al[11] and the study by Jang et al.[17] Both studies were conducted in Korea, and the possible role of a geographic difference in the resistance is unclear. However, resistance was not tested in most studies. Therefore, surveillance for antibiotic resistance is required to clarify the effectiveness of anti-rickettsial treatment.
Our study was subjective to some limitations. First, the case numbers and study numbers were limited. Scrub typhus is a well-known disease with huge burden and azithromycin has been widely used for decades. However, only few well-designed studies investigating the effectiveness of azithromycin in treating scrub typhus are currently available. Only 5 studies including 427 participants were eligible to be enrolled in our meta-analysis. Although the heterogeneity between studies was mild to moderate, further large-scale studies are warranted. Second, subgroup analyses in specific patients such as pregnant women and children are valuable and are able to answer our clinical question. However, it is unable to perform the analyses due to lack of studies. It is unethical to conduct a randomized controlled study to apply potentially fetotoxic drugs in pregnant women. Third, the doses of azithromycin were similar, but the routes of delivery were not the same in different studies. Oral bioavailability may affect the treatment outcomes. Furthermore, the severity of scrub typhus of most patients was uncomplicated, and a majority of comparative arms was done with doxycycline. Well-designed randomized controlled studies are required to clarify the influences of disease severity and comparative drugs. Finally, resistance was not measured, and the association between resistance and treatment failure was not investigated.
5. Conclusions
In conclusion, scrub typhus remains an important health issue in endemic areas. Our study suggests that azithromycin is as effective as other anti-rickettsial drugs with higher treatment success rates, lower adverse effects, and longer time to defervescence. It is reasonable to use azithromycin as first-line treatment against scrub typhus. Further studies are warranted to elucidate the effectiveness in specific patient groups, of high-dose azithromycin, and the influence of drug resistance.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, FDA = Food and Drug Administration, GRADE = the Grading of Recommendations, Assessment, Development, and Evaluation system, RR = risk ratio.
S-CL and Y-JC contribute to this work equally.
The authors state that there is no conflict of interests related to this paper.
Supplemental Digital Content is available for this article.
References
- [1].Aung AK, Spelman DW, Murray RJ, et al. Rickettsial infections in Southeast Asia: implications for local populace and febrile returned travelers. Am J Trop Med Hyg 2014;91:451–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rosenberg R. Drug-resistant scrub typhus: paradigm and paradox. Parasitol Today 1997;13:131–2. [DOI] [PubMed] [Google Scholar]
- [3].Weng SC, Lee HC, Chen JJ, et al. Eschar: a stepping stone to scrub typhus. J Pediatr 2017;181:320–.e1. [DOI] [PubMed] [Google Scholar]
- [4].Peter JV, Sudarsan TI, Prakash JA, et al. Severe scrub typhus infection: clinical features, diagnostic challenges and management. World J Crit Care Med 2015;4:244–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Taylor AJ, Paris DH, Newton PN. A systematic review of mortality from untreated scrub typhus (Orientia tsutsugamushi). PLoS Negl Trop Dis 2015;9:e0003971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Panpanich R, Garner P. Antibiotics for treating scrub typhus. Cochrane Database Syst Rev 2002;CD002150. [DOI] [PubMed] [Google Scholar]
- [7].Phimda K, Hoontrakul S, Suttinont C, et al. Doxycycline versus azithromycin for treatment of leptospirosis and scrub typhus. Antimicrob Agents Chemother 2007;51:3259–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rajan SJ, Sathyendra S, Mathuram AJ. Scrub typhus in pregnancy: maternal and fetal outcomes. Obstet Med 2016;9:164–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Food and Drug Administration, HHS. Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling. Final Rule Fed Regist 2014;79:72063–103. [PubMed] [Google Scholar]
- [10].Kim YS, Lee HJ, Chang M, et al. Scrub typhus during pregnancy and its treatment: a case series and review of the literature. Am J Trop Med Hyg 2006;75:955–9. [PubMed] [Google Scholar]
- [11].Kim YS, Yun HJ, Shim SK, et al. A comparative trial of a single dose of azithromycin versus doxycycline for the treatment of mild scrub typhus. Clin Infect Dis 2004;39:1329–35. [DOI] [PubMed] [Google Scholar]
- [12].Chang KV, Hung CY, Wu WT, et al. Comparison of the effectiveness of suprascapular nerve block with physical therapy, placebo, and intra-articular injection in management of chronic shoulder pain: a meta-analysis of randomized controlled trials. Arch Phys Med Rehabil 2016;97:1366–80. [DOI] [PubMed] [Google Scholar]
- [13].Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:1–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- [16].Chanta C, Phloenchaiwanit P. Randomized controlled trial of azithromycin versus doxycycline or chloramphenicol for treatment of uncomplicated pediatric scrub typhus. J Med Assoc Thai 2015;98:756–60. [PubMed] [Google Scholar]
- [17].Jang MO, Jang HC, Kim UJ, et al. Outcome of intravenous azithromycin therapy in patients with complicated scrub typhus compared with that of doxycycline therapy using propensity-matched analysis. Antimicrob Agents Chemother 2014;58:1488–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhao M, Wang T, Yuan X, et al. Comparison of minocycline and azithromycin for the treatment of mild scrub typhus in northern China. Int J Antimicrob Agents 2016;48:317–20. [DOI] [PubMed] [Google Scholar]
- [19].Fang Y, Huang Z, Tu C, et al. Meta-analysis of drug treatment for scrub typhus in Asia. Intern Med 2012;51:2313–20. [DOI] [PubMed] [Google Scholar]
- [20].Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chansamouth V, Thammasack S, Phetsouvanh R, et al. The aetiologies and impact of fever in pregnant inpatients in Vientiane, Laos. PLoS Negl Trop Dis 2016;10:e0004577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kumar R, Thakur S, Bhawani R, et al. Clinical profile of scrub typhus in pregnancy in sub-Himalayan region. J Obstet Gynaecol India 2016;66:82–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Choi EK, Pai H. Azithromycin therapy for scrub typhus during pregnancy. Clin Infect Dis 1998;27:1538–9. [DOI] [PubMed] [Google Scholar]
- [24].Nahum GG, Uhl K, Kennedy DL. Antibiotic use in pregnancy and lactation: what is and is not known about teratogenic and toxic risks. Obstet Gynecol 2006;107:1120–38. [DOI] [PubMed] [Google Scholar]
- [25].Ramsey PS, Vaules MB, Vasdev GM, et al. Maternal and transplacental pharmacokinetics of azithromycin. Am J Obstet Gynecol 2003;188:714–8. [DOI] [PubMed] [Google Scholar]
- [26].Baldwin EA, Walther-Antonio M, MacLean AM, et al. Persistent microbial dysbiosis in preterm premature rupture of membranes from onset until delivery. PeerJ 2015;3:e1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhou YH, Xia FQ, Van Poucke S, et al. Successful treatment of scrub typhus-associated hemophagocytic lymphohistiocytosis with chloramphenicol: report of 3 pediatric cases and literature review. Medicine 2016;95:e2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tita ATN, Szychowski JM, Boggess K, et al. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med 2016;375:1231–41.27682034 [Google Scholar]
- [29].Chang K, Lee NY, Ko WC, et al. Identification of factors for physicians to facilitate early differential diagnosis of scrub typhus, murine typhus, and Q fever from dengue fever in Taiwan. J Microbiol Immunol Infect 2017;50:104–11. [DOI] [PubMed] [Google Scholar]
- [30].Cross R, Ling C, Day NP, et al. Revisiting doxycycline in pregnancy and early childhood: time to rebuild its reputation? Expert Opin Drug Saf 2016;15:367–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gordon EM, Blumer JL. Rationale for single and high dose treatment regimens with azithromycin. Pediatr Infect Dis J 2004;23:S102–7. [DOI] [PubMed] [Google Scholar]
- [32].Yasuda M, Ito S, Kido A, et al. A single 2 g oral dose of extended-release azithromycin for treatment of gonococcal urethritis. J Antimicrob Chemother 2014;69:3116–8. [DOI] [PubMed] [Google Scholar]
- [33].Mitja O, Houinei W, Moses P, et al. Mass treatment with single-dose azithromycin for yaws. N Engl J Med 2015;372:703–10. [DOI] [PubMed] [Google Scholar]
- [34].Jacobs RF, Maples HD, Aranda JV, et al. Pharmacokinetics of intravenously administered azithromycin in pediatric patients. Pediatr Infect Dis J 2005;24:34–9. [DOI] [PubMed] [Google Scholar]
- [35].Sutton AL, Acosta EP, Larson KB, et al. Perinatal pharmacokinetics of azithromycin for cesarean prophylaxis. Am J Obstet Gynecol 2015;212: 812.e811-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kemp MW, Miura Y, Payne MS, et al. Maternal intravenous administration of azithromycin results in significant fetal uptake in a sheep model of second trimester pregnancy. Antimicrob Agents Chemother 2014;58:6581–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Watt G, Chouriyagune C, Ruangweerayud R, et al. Scrub typhus infections poorly responsive to antibiotics in northern Thailand. Lancet 1996;348:86–9. [DOI] [PubMed] [Google Scholar]
- [38].Tantibhedhyangkul W, Angelakis E, Tongyoo N, et al. Intrinsic fluoroquinolone resistance in Orientia tsutsugamushi. Int J Antimicrob Agents 2010;35:338–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


