Supplemental Digital Content is available in the text
Keywords: echocardiography, mortality, National Inpatient Sample, pulmonary embolism, trend
Abstract
Pulmonary embolism (PE) is a devastating diagnosis which carries a high mortality risk. Echocardiography is often performed to risk stratify patients diagnosed with PE, and guide management strategies. Trends in the performance of echocardiography among patients with PE and its role in influencing outcomes is unknown.
We analyzed the 2005 to 2014 National Inpatient Sample Database to identify patients with primary diagnosis of PE or secondary diagnosis of PE and ≥1 of the following diagnoses: syncope, thrombolysis, acute deep vein thrombosis, acute cardiorespiratory failure, and secondary pulmonary hypertension. Trends in the performance of echocardiography and in-hospital mortality were analyzed. The admissions were divided into 2 groups with echocardiography, and without echocardiography, and 1:2 propensity score matching (PSM) was performed for comparison. The primary end-point was in-hospital mortality. The secondary endpoints were length of stay and total hospitalization costs. Odd ratios (OR) with confidence intervals (CI) were reported.
A total of 299,536 unweighted PE cases were studied. Performance of echocardiography among patients with PE patients increased from 3.5% to 5.6%, whereas in-hospital mortality decreased from 4.2% to 3.7% between years 2005 and 2014. Before matching, patients who received an echocardiogram were more likely to be younger, African American, admitted to a large, urban teaching institute, and had higher rates of concurrent acute deep vein thrombosis, and acute respiratory failure. Post-PSM, patients who received echocardiography during hospitalization had lower in-hospital mortality (odds ratio 0.75, 95% confidence intervals (CI) 0.68–0.83; P < 0.001), longer length of stay (median 6 days vs 5 days; P < .001) and higher mean hospitalization costs ($34,379 vs $27,803; P < .001) compared to those without echocardiography.
Performance of echocardiography among patients with a PE is increasing and is associated with lower in-hospital mortality.
1. Introduction
Pulmonary embolism (PE) is associated with significant morbidity and mortality among affected patients. Nearly, 75 to 269 per 100,000 cases of venous thromboembolic (VTE) disease occur every year in Western Europe, North America, Australia, and southern Latin America.[1] PE contributes roughly one-thirds to this VTE burden. Short-term mortality associated an acute PE is significant with 1 out of 6 patients dying within 3 months of diagnosis.[2] An autopsy-based study showed that nearly 4.3% of the patients with sudden cardiac death suffered an acute PE.[3] Pulmonary embolism is the third leading cause of cardiovascular disease following coronary artery disease (CAD) and stroke.[2] While there has been no change in the risk of mortality at presentation for patients with acute PE, there has been an overall reduction in short-term mortality secondary to evolution in treatment practices.[4] The authors of the study noted an increase in use of low-molecular weight heparin (LMWH), direct oral anticoagulants, thrombolytics and surgical embolectomy among 23,858 patients with a symptomatic PE over time. They argued that these treatment changes were mainly responsible for the reduction in all-cause and PE-related mortality over time.
Use of computed tomographic pulmonary angiography (CTPA) has changed the landscape for management of PE by facilitating early diagnosis in suspected cases. A study by Wiener et al[3] showed that the use of CTPA may result in overdiagnosis, rising incidence of PE, minimal change in mortality, a lower case fatality but higher anticoagulation-related complications when compared to trends before the use of CTPA. Use of echocardiography has been validated to provide early risk stratification among patients presenting with acute PE, and impacts management strategies. According to the European Society of Cardiology (ESC) guidelines, performance of echocardiography among hemodynamically unstable patients has a Class I recommendation.[5] Presence of right ventricular dysfunction on echocardiography identifies patients with a poor prognosis and is consistent with the presence of larger pulmonary perfusion defects.[4] Whether use of echocardiography in PE directly impacts downstream outcomes, is unknown. We sought to assess the trends in use of echocardiography among patients admitted with a PE, and assess its role in influencing hospital mortality.
2. Methods
2.1. Data source and study population
We conducted our study using a retrospective, unweighted cohort from the Nationwide Inpatient Sample (NIS), which is a nationally representative data set of hospital admissions. Patients aged 18 years and above, admitted with a primary diagnosis of PE or a secondary PE diagnosis in addition to a concurrent diagnosis of either syncope, thrombolytic use, acute deep vein thrombosis, acute respiratory failure, or secondary pulmonary hypertension between 2005 and 2014 were included. Pulmonary embolism was diagnosed using the International Classification of Diseases, Ninth Revision, Clinical Modification coding (ICD-9CM), identified under the code 415.1x. Please refer to Supplemental Table 1 for the diagnosis and procedure codes used for this study. The NIS is the largest, publicly available, all-payer inpatient database in the United States,[6] and institutional board review was not necessary. Annually, the NIS is composed of discharge-level data from roughly 8 million hospitalizations from approximately 1000 hospitals (representing about 85% of all nonfederal hospitals). It is designed to approximate a stratified probability sample of 20% of nonfederal acute care hospitals in the United States. Validation and quality control of the NIS data are performed by an independent contractor and the database has been validated extensively against the American Hospital Association Annual Survey and the National Hospital Discharge Survey.[7] Each hospitalization within the database contains clinical and resource-use information. Patients’ diagnoses are documented in parallel, as both ICD-9CM and clinically meaningful clusters of ICD-9CM's, termed Clinical Classification Software (CCS) codes. Patients who had a diagnosis of shock, cardiac arrest, acute myocardial infarction, percutaneous coronary interventions, coronary angiography and those with missing information for gender, location and teaching status, size of the hospitals, and in-hospital mortality were excluded from the study. We also excluded patients who were transferred out of the hospital. The primary outcome of our study was in-hospital mortality. Total cost of hospitalization in US dollars and length of stay in days were secondary outcomes. Comparisons were made between those who had an echocardiogram during hospitalization and those who did not, among those admitted with PE. The study was exempted from Institutional Board Review by Lehigh Valley Health Network.
2.2. Statistical analysis
Demographics, baseline characteristics, and procedures were summarized using descriptive statistics. Categorical variables were expressed as percentages and analyzed using Fischer exact test or Pearson Chi-square. Continuous variables were analyzed with nonparametric, Mann–Whitney U test. Trend analysis was performed using the Mantel–Haenszel test of trend. To address uneven baseline characteristics, propensity score matching (PSM) was used to identify similar cohorts of patients between the patients who had an echocardiogram performed to those who did not. PSM was performed using a 1:2 matching protocol without replacement and caliper width 0.01 using “performance of echocardiography” as treatment variable. Both groups were matched on several variables that included age, Charlson comorbidity index, gender, race, atrial and ventricular arrhythmias, diabetes, syncope, chronic lung and liver disease, coagulopathy, thrombolytic use, secondary pulmonary hypertension, acute deep vein thrombosis, hypertension, obesity, valvular heart disease, CAD, right heart catheterization, acute respiratory failure and/or ventilator use, history of myocardial infarction, ischemic strokes, and dyslipidemia. In addition, hospital characteristics, such as size, region, location and teaching status, and year of admission were also included in PSM model. Absolute standardized differences for the variables were calculated postmatching to confirm close matching (goal: <10%). Predictors for performance of echocardiography were evaluated using a binary logistic regression model with variables mentioned within the propensity model. Results were considered statistically significant for 2-tailed P values <.05. IBM SPSS statistics version 24 (Armonk, NY) was used to perform data analysis.
3. Results
3.1. Trends in use of echocardiography and mortality
Between 2005 and 2014, 4.6% of the patients admitted with PE underwent echocardiography during hospitalization. We calculated the yearly utilization of echocardiography by dividing number of admissions for PE with an echocardiogram by the total admissions for PE during that calendar year, and was reported as percentages (Table 1 and Fig. 1). There was an uptrend in the proportion of patients admitted with PE who had an echocardiogram performed between 2005 and 2014 (3.5–5.6%; Ptrend < .001). Similarly, we analyzed the rate of in-hospital mortality on a yearly basis (Table 1 and Fig. 1). The in-hospital mortality decreased from 2005 to 2014 (4.2–3.7%; Ptrend < .001), with a nadir (3.5%) observed in the year of 2009.
Table 1.
Trends of pulmonary embolism cases, echocardiography, and in-hospital mortality from 2005 to 2014.
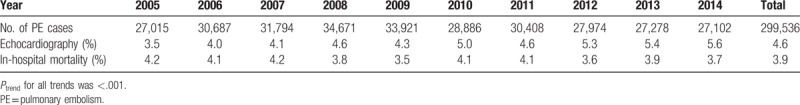
Figure 1.
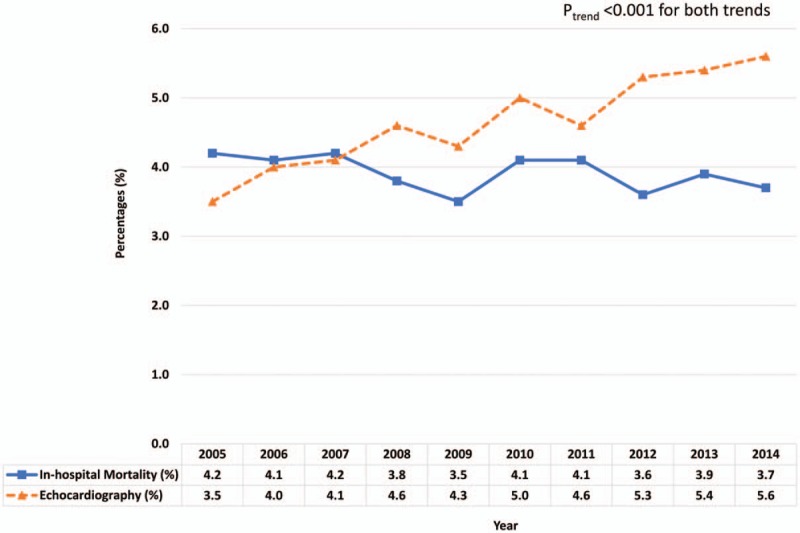
Percentage of echocardiography performance and in-hospital mortality among patients with pulmonary embolism from years 2005 to 2014.
3.2. Baseline characteristics and outcomes
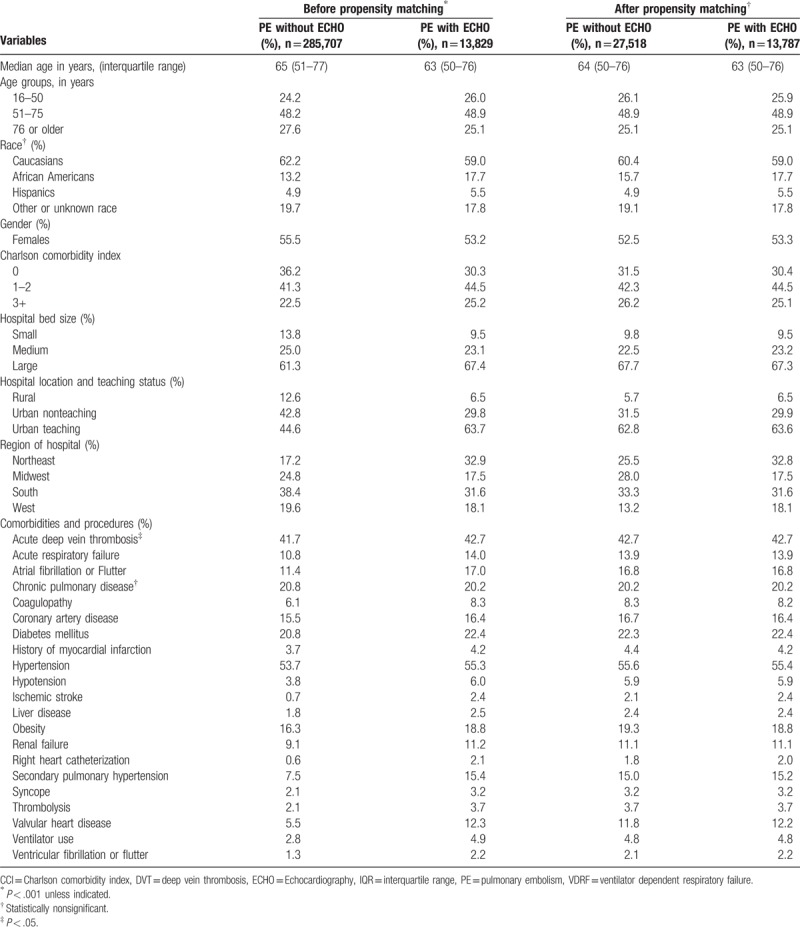
A total of 299,536 admissions for pulmonary embolism were included in the study, of which 13,829 (4.6%) admissions had an echocardiogram during hospital stay. Before matching, patients who had an echocardiogram during hospital stay were younger (63 years vs 65 years; P < .001) and less likely to be female (53.2% vs 55.5%; P < .001) compared to those without an echocardiogram (Table 2). Patients who received an echocardiogram were also more likely to be admitted to an urban teaching hospital, have acute deep vein thrombosis, acute respiratory failure, atrial or ventricular arrhythmias, coagulation disorders, ischemic stroke, renal failure, secondary pulmonary hypertension, diabetes mellitus, syncope, ventilator use and thrombolytic use among others. The overall burden of comorbidities was higher within the group of patients that received an echocardiogram compared to those who did not, but in-hospital mortality was similar in both groups (3.9% vs 4.0%; P = .75) before the PSM. Before matching, the mean length of stay and cost of hospitalization were higher among PE patients receiving an echocardiogram. The imbalance between the covariates significantly improved between the 2 groups after PSM was conducted (Table 2). After PSM, standardized mean difference of covariates after PSM for all matched variables was <10%.
Table 2.
Baseline characteristics among patients admitted for acute PE.
3.3. Primary and secondary outcomes
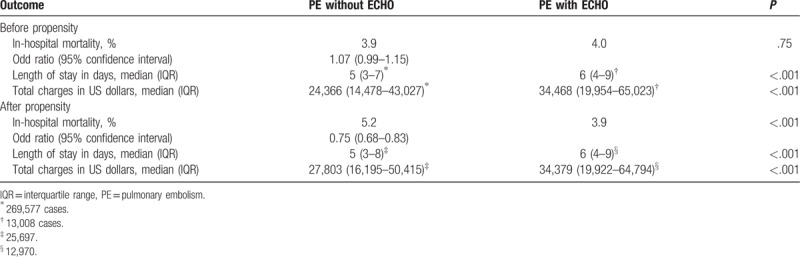
After PSM, 13,787 (>99%) admissions with an echocardiogram were retained and matched with 27,518 admissions without an echocardiogram. After PSM, the mortality rate among patients who received an echocardiogram was significantly lower than those who did not (3.9% vs 5.2%, odds ratio, OR, 0.75, 95% CI, 0.68–0.83; P < .001) as demonstrated in Table 3. The secondary outcomes such as length of stay (median 6 days vs 5 days; P < .001) and mean cost of hospitalization ($34,279 vs $27,803; P < .001) were significantly higher among the patients who received an echocardiogram.
Table 3.
Outcomes before and after propensity score matching.
3.4. Predictors for performance of echocardiography
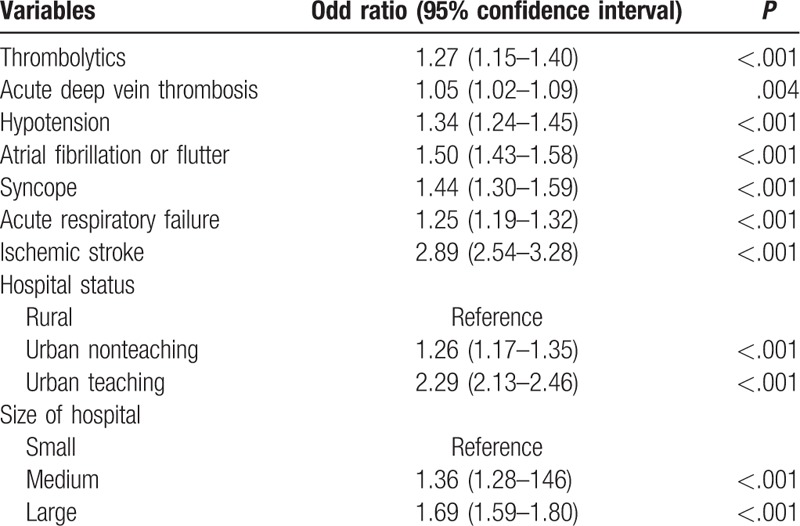
Presence of ischemic stroke (adjusted odds ratio, aOR, 2.89, 95% CI 2.54–3.28; P < .001), atrial fibrillation or flutter (aOR 1.50, 95% CI 1.43–1.58; P < .001), syncope (aOR 1.44, 95% CI 1.30–1.59; P < .001), hypotension (aOR 1.34, 95% CI 1.30–1.59; P < .001), acute respiratory failure (aOR 1.25, 95% CI 1.19–1.32; P < .001) and use of thrombolytic agents (aOR 1.27, 95% CI 1.15–1.40; P < .001) were independently associated with a higher likelihood for performance of echocardiography during hospitalization for PE (Table 4). Among hospital characteristics, admission to urban teaching centers (aOR 2.29, 95% CI 2.13–2.46; P < .001) and large bed-size hospitals (aOR 1.69, 95% CI 1.59–1.80; P < .001) predicted a higher likelihood for performance of echocardiography.
Table 4.
Independent variables predicting increased echocardiography use.
4. Discussion
To the best of our knowledge, this is the largest study to date comparing the impact of performance of echocardiography on hospital-mortality among patients admitted with a PE. The major findings of our study were as follows: While the overall in-hospital mortality among patients admitted with PE decreased from 2005 to 2014, the proportion of patients receiving an echocardiogram increased during the same time period; patients with PE who received an echocardiogram were younger with more comorbidities; performance of an echocardiogram was associated with a significantly lower hospital mortality, but longer length of stay and higher cost of stay; and admission to a large urban teaching center, presence of ischemic stroke, syncope, acute respiratory failure, syncope and thrombolytic agents were among the factors that independently predicted higher likelihood for performance of echocardiography among patients admitted with a PE.
Echocardiography provides assessment of right and left ventricular size and function, regional wall motion, valvular dysfunction, intracardiac shunting, thrombus visualization and hemodynamic assessment of right or left-sided pressures noninvasively. The role of echocardiography as a diagnostic modality for acute PE suffered from early failure secondary to its inability to maintain sufficient sensitivity.[8] Clinical practice has evolved into using alternate modalities such as either CTPA or ventilation/perfusion scanning to diagnose acute PE. In patients with nonmassive PEs, presence of right ventricular strain is prognostic and is associated with higher in-hospital mortality.[9] Kucher et al[4] evaluated 1035 patients with hemodynamically stable PE from the International Cooperative Pulmonary Embolism Registry who had echocardiograms performed within the first 24 hours of diagnosis, and found that the presence of right ventricular hypokinesis was both common (39.1% of all cases) and predictive of 30-day mortality on multivariate analysis. By excluding patients with shock, cardiac arrest, or acute myocardial infarction, our study population is likely to represent patients with hemodynamically stable PE. Echocardiography is primarily used as a tool to guide therapy in patients with known acute PE, and for reassessment of changes in right ventricular function and/or pulmonary artery pressures following thrombolysis and thrombectomy. Both the indications have been deemed appropriate for hemodynamically stable PE according to the Appropriate Use Criteria for Echocardiography[10] document in addition to other guidelines. There has been an overall uptrend in the use of noninvasive cardiac imaging, especially echocardiography during the past decade according to an analysis of Medicare claims.[11] The easy availability of echocardiography, accumulating expertise, low procedural cost and risk, backing from several guidelines and potential for guiding early appropriate management in especially submassive PE may explain the uptrend in use of echocardiography in admissions with PE over time.[12]
Temporal trends in PE-related outcomes suggest overall decline in all cause and PE-related mortality in the most recent decade. This is in contrast to earlier studies which did not show significant changes in mortality following a diagnosis of PE despite lowering incidence of disease in the population.[13] Our study is consistent with overall improvement in hospital-mortality between 2005 and 2014, however it must be noted that absolute variation in year to year mortality was only 0.7%. The reductions in hospital mortality seen within our study are not as marked as seen in prior studies probably secondary to the low event rates, exclusion of massive PEs or hemodynamically unstable patients from the analysis and reporting of only hospital mortality which may relatively underestimate the impact of disease on short-term outcomes. The overall hospital mortality seen within our patients is similar to what has been reported (2–5%) in more recent observational and randomized control trials including submassive PEs.[14] As mentioned in the introduction, more effective therapies and interventions such as greater use of low-molecular-weight heparins, direct oral anticoagulants, thrombolysis, and surgical embolectomy in practice have contributed to the decline in death, nonfatal recurrence of disease and nonfatal bleeding, none of the studies have tried to evaluate the direct impact of using echocardiography among patients. Our study offers a unique perspective where we show that performance of echocardiography in nonmassive PE was associated with reduction in mortality. We believe that the additional hemodynamic information obtained following performance of an echocardiogram directs more judicious treatment strategies (use of fluids, vasopressors, anticoagulation techniques, appropriate level of care, frequency of monitoring, repeat echocardiography, dosing and delivery of thrombolytics) and indirectly favors improved outcomes. Thrombolytic use was closely matched post-PSM in both study groups and appears not to have played a major role in influencing above mentioned outcomes, however the database did not provide crucial treatment details. Within the pre-PSM analysis, despite the higher comorbidities among echocardiogram recipients and the longer need for inpatient stay with higher costs, in-hospital mortality was no different irrespective of the use of echocardiogram. The current landscape in treatment of submassive PEs with thrombolytics and thrombectomy/embolectomy still constitutes a relatively small proportion (<2% in prior registries) of patients presenting with PE. We are, however, unable to evaluate specific changes in treatment patterns following the use of echocardiography due to the limitations of our database, and further elaboration of these changes may hold the key to explaining the survival benefit. By matching for several hospital related characteristics, and year of admission we have attempted to account for changes in management strategies and potential for a lower mortality occurring with time secondary to overdiagnosis from more widespread use of diagnostic modalities especially CTPA.
Current guidelines are unclear on which patients with hemodynamically stable PE should undergo echocardiography. There is a consensus that echocardiography should be performed when thrombolysis is contemplated. Development of Pulmonary Embolism Response Teams at institutes has also facilitated early echocardiography use in PE to quickly risk stratify patients, with the intention to improve outcomes.[15] Our study shows a very low but increasing rate of echocardiography use among patients with PE. While prior studies do not report rate of echocardiography use specifically, it can be assumed that the absolute rates noted in our study may be underestimated considering the high rates of right ventricular strain reported previously.[16] Prior studies also suggest underestimation of echocardiograms using the NIS database.[12] Our findings are consistent with the fact that higher risk patients underwent echocardiography as evidenced by the increased comorbidity burden seen within that population in addition to the higher incidence of acute events such as stroke, acute respiratory failure/ventilator use, syncope and atrial or ventricular arrhythmias among others. Among the factors that independently predicted performance of echocardiography among patients admitted with a PE, were presence of an ischemic stroke, syncope, atrial arrhythmias, hypotension, thrombolytic use, and admission to urban nonteaching or teaching institutes, and medium to large sized hospitals.
The incidence of PE within the general population appears to be increasing per most but not all epidemiological estimates.[17] In addition, there is a very high prevalence of comorbidities among those who survive including recurrence of venous thromboembolic events, chronic thromboembolic pulmonary hypertension, bleeding, and late death. These put added strain on the healthcare system in terms of costs and resources.[18] We found that the average cost of hospitalization and length of stay were higher among patients receiving an echocardiogram during hospitalization. This may be secondary to the fact that echocardiographic findings of right ventricular strain might have led to additional testing, and closer monitoring. It is unclear if cost effectiveness would eventually favor those getting an echocardiogram, as the impact on longer term PE-related comorbidities mentioned above is unknown. Jiminez et al[19] showed that the mean length of stay among patients with PE decreased from 13.6 to 9.3 days from 2001 to 2013 on retrospective analysis of the RIETE registry, but included more severe PE cases. Earlier data from the NIS (1998–2005) also showed a longer length of stay for PE admissions compared to our finding.[20] They noted a significant increase in hospital charges during the same period despite a minimal decline in overall length of stay. Another study conducted among 991 patients hospitalized at Brigham and Women's Hospital between 2003 and 2010 reported high costs related to treatment of pulmonary embolism. The average cost per hospitalization in a smaller study was lower ($8764) than noted in our analysis.[21] One reason for this difference may be that the NIS does not report actual amount paid for by insurance or patient.
Our study has important limitations, some of which are inherent to study design and use of an administrative database. Our study heavily relies on accuracy of coding for procedure and diagnosis. The procedure code for echocardiography (88.72) does not distinguish between transthoracic from transesophageal echocardiogram, however both tests can appropriately detect right ventricular strain. Our inability to characterize the extent and location of PE may have an impact as asymptomatic subsegmental PEs are likely to have a better prognosis than those with larger clot burden. By excluding shock, acute myocardial infarction and cardiac arrest among, we attempted to restrict our analysis to lower-risk and submassive PEs, but the database does not provide direct information on severity of PE. Our study is unable to identify the indications for which echocardiography was performed, and how treatment was impacted. From a statistical aspect, the biggest limitation of PSM is that it cannot account for unmeasured confounding variables that could impact outcomes. However, we mitigated some of these limitations by studying a very large population of patients and matching for several important patients, and hospital characteristics.
5. Conclusion
Although the use of echocardiograms among patients with PEs is increasing, overall rate of echocardiography use is still low. Recent trends suggest an overall decline in hospital mortality. Performance of echocardiography was associated with a reduction in hospital mortality among patients with PE, possibly secondary to timely improvement in downstream care. Additional studies are needed to guide appropriate patient selection for echocardiography use in hemodynamically stable PE.
Author contributions
Conceptualization: Brijesh Patel, Mahek Shah, Matthew Martinez, Raman Dusaj.
Formal analysis: Brijesh Patel, Mahek Shah, Lohit Garg, Manyoo Agarwal.
Funding acquisition: Brijesh Patel, Raman Dusaj.
Supervision: Raman Dusaj.
Writing – original draft: Brijesh Patel, Mahek Shah, Lohit Garg, Manyoo Agarwal, Matthew Martinez, Raman Dusaj.
Writing – review & editing: Brijesh Patel, Mahek Shah, Lohit Garg, Manyoo Agarwal, Matthew Martinez, Raman Dusaj.
Supplementary Material
Footnotes
Abbreviations: aOR = adjusted odd ratio, CAD = coronary artery disease, CCS = Clinical Classification Software, CI = confidence interval, CTPA = computer tomography of pulmonary artery, ESC = European Society of Cardiology, ICD 9 = International Classification of Diseases, 9th revision Clinical modification, IQR = interquartile range, LMWH = low molecular weight heparin, NIS = National Inpatient Sample, OR = odd ratio, PE = pulmonary embolism, PSM = propensity score matching, VTE = venous thromboembolism.
BP and MS contributed equally to this work.
Funding: The study was supported by Dorothy Rider Pool Trust Fund Grant #1573-007.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Konstantinides SV. Trends in pulmonary embolism outcomes: are we really making progress? J Am Coll Cardiol 2016;67:171–3. [DOI] [PubMed] [Google Scholar]
- [2].Belohlavek J, Dytrych V, Linhart A. Pulmonary embolism, part I: epidemiology, risk factors and risk stratification, pathophysiology, clinical presentation, diagnosis and nonthrombotic pulmonary embolism. Exp Clin Cardiol 2013;18:129–38. [PMC free article] [PubMed] [Google Scholar]
- [3].Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med 2011;171:831–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kucher N, Rossi E, De Rosa M, et al. Prognostic role of echocardiography among patients with acute pulmonary embolism and a systolic arterial pressure of 90 mm Hg or higher. Arch Intern Med 2005;165:1777–81. [DOI] [PubMed] [Google Scholar]
- [5].Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014;35:3033–69. 3069a–3069k. [DOI] [PubMed] [Google Scholar]
- [6].HCUP Databases. 2005–2014. Healthcare Cost and Utilization Project (HCUP). February 2016; 2016. [Google Scholar]
- [7].Wann LS, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (update on dabigatran). A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Heart Rhythm 2011;8:e1–8. [DOI] [PubMed] [Google Scholar]
- [8].Miniati M, Monti S, Pratali L, et al. Value of transthoracic echocardiography in the diagnosis of pulmonary embolism: results of a prospective study in unselected patients. Am J Med 2001;110:528–35. [DOI] [PubMed] [Google Scholar]
- [9].ten Wolde M, Sohne M, Quak E, et al. Prognostic value of echocardiographically assessed right ventricular dysfunction in patients with pulmonary embolism. Arch Intern Med 2004;164:1685–9. [DOI] [PubMed] [Google Scholar]
- [10]. American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 Appropriate Use Criteria for Echocardiography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance American College of Chest Physicians. J Am Soc Echocardiogr 2011; 24: 229–267. [DOI] [PubMed] [Google Scholar]
- [11].Andrus BW, Welch HG. Medicare services provided by cardiologists in the United States: 1999–2008. Circ Cardiovasc Qual Outcomes 2012;5:31–6. [DOI] [PubMed] [Google Scholar]
- [12].Papolos A, Narula J, Bavishi C, et al. U.S. Hospital Use of Echocardiography: insights from the Nationwide Inpatient Sample. J Am Coll Cardiol 2016;67:502–11. [DOI] [PubMed] [Google Scholar]
- [13].Konstantinides S, Geibel A, Heusel G, et al. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med 2002;347:1143–50. [DOI] [PubMed] [Google Scholar]
- [14].Sharifi M, Bay C, Skrocki L, et al. Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol 2013;111:273–7. [DOI] [PubMed] [Google Scholar]
- [15].Mahar J, Sadana D, Nguyen N, et al. A pulmonary embolism response team (pert) approach: early experience from the CLEVELAND CLINIC. Chest 2016;150(4 suppl):1157A. [DOI] [PubMed] [Google Scholar]
- [16].Grifoni S, Olivotto I, Cecchini P, et al. Short-term clinical outcome of patients with acute pulmonary embolism, normal blood pressure, and echocardiographic right ventricular dysfunction. Circulation 2000;101:2817–22. [DOI] [PubMed] [Google Scholar]
- [17].Huang W, Goldberg RJ, Anderson FA, et al. Secular trends in occurrence of acute venous thromboembolism: the Worcester VTE study (1985–2009). Am J Med 2014;127: 829.e5–839.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kroger K, Kupper-Nybelen J, Moerchel C, et al. Prevalence and economic burden of pulmonary embolism in Germany. Vasc Med 2012;17:303–9. [DOI] [PubMed] [Google Scholar]
- [19].Jiménez D, de Miguel-Díez J, Guijarro R, et al. Trends in the management and outcomes of acute pulmonary embolism: analysis from the RIETE registry. J Am Coll Cardiol 2016;67:162–70. [DOI] [PubMed] [Google Scholar]
- [20].Park B, Messina L, Dargon P, et al. Recent trends in clinical outcomes and resource utilization for pulmonary embolism in the United States: findings from the nationwide inpatient sample. Chest 2009;136:983–90. [DOI] [PubMed] [Google Scholar]
- [21].Fanikos J, Rao A, Seger AC, et al. Hospital costs of acute pulmonary embolism. Am J Med 2013;126:127–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


