Abstract
To analyze the superiority of wait-and-see policy and elective neck dissection in treating cN0 patients with facial cutaneous cell carcinoma (cSCC).
Patients with clinically negative parotid and neck metastasis disease were prospectively enrolled. Three groups were divided based on whether the patient received an operation of superficial parotidectomy or/and elective dissection, and regional control and disease-specific survival rates were compared.
The occult parotid and neck metastasis rate was 20% and 16%, respectively. There was neck node metastasis without parotid metastasis in only 1 patient. All the node metastasis occurred in level II. Regional recurrence was noted in 16 (16%) patients, and 6 patients died of the disease. In the group undergoing superficial parotidectomy and elective neck dissection, 2 patients had neck node metastasis, and there was no disease-related death, further survival analysis indicated it had better regional control and disease-specific survival rates compared with the other 2 groups.
Superficial parotidectomy and elective neck dissection are suggested for patients with T3–4 facial cutaneous squamous cell carcinoma.
Keywords: elective neck dissection, facial cutaneous squamous cell carcinoma, squamous cell carcinoma of head and neck, superficial parotidectomy
1. Introduction
Non-melanoma skin cancer represents one-third of all malignancies and its incidence is expected to rise until the year 2040.[1] Cutaneous squamous cell carcinoma (cSCC) represents about 20% of all non-melanoma skin cancer and is a deadly threat owing to its ability to metastasize to any organ in the body.[1,2] The true incidence of metastasis from cSCC is unknown but is felt to be in the region of 2% to 5%.[3] Despite the low metastasis possibility, the survival rate is reduced by 50% when there is pathologic node disease. Risk factors for lymphatic metastasis are thoroughly evaluated including tumor size >2 cm, tumor depth >4 mm, location on the ear or lip, poor histological differentiation, perineural and lymphovascular invasion, and patient immunosuppression.[4–11]
There is a consensus on the treatment of lymph node positive neck in the literature.[4,5] Accurate treatment consists of superficial or total parotidectomy and selective or radical neck dissection. But in cN0 patients, the optimal management of parotid and neck remains unclear. Researchers that supporting wait-and-see policy reckon its low metastasis rate and it prevents unnecessary dissections and the patient's morbidity.[12] The authors who are not in favor with this policy describes that the cure rate of salvage surgery during follow-up period is low, and there is higher possibility of distant metastasis.[10,11]
Therefore, in current study, we aimed to analyze the superiority of wait-and-see policy and elective neck dissection in treating cN0 patients with head and neck cSCC.
2. Patients and methods
The Zhengzhou University institutional research committee approved our study, and all participants signed an informed consent agreement. All methods were performed in accordance with the relevant guidelines and regulations.
From January 2008 to December 2015, patients with clinically negative parotid and neck metastasis disease were prospectively enrolled in Department of Oral Maxillofacial Surgery, The first affiliated hospital of Zhengzhou University. Detailed information and difference of wait-and-see policy and elective neck dissection including possible postoperative dysfunction and prognosis was informed to the patients before treatment, and they decided which procedure was accepted. Information including patient characteristics, TNM stage (UICC 2010), postoperative pathologic reports, follow up was reviewed and collected.
General data were analyzed by means of a Student t test or chi-squared test or Fisher exact test. Kaplan–Meier was used to compare the survival rates between different groups. All statistical analyses were performed using SPSS 13.0. A P < .05 was considered significant.
3. Results
The mean age was 67.5 (range, 42–88) years, there were 89 male and 22 female. Sixty-seven patients were staged as T1, 24 cases as T2, 20 cases as T3 or T4. Ninety-two patients had a high or middle differential degree disease, and the differential degree was unknown in 7 patients. Clear margin was achieved in all patients, but close margin was noted in 5 patients. In almost all (87.4%) the patients, the invasion depth was <4 mm. Perineural invasion and lymphovascular invasion was noted in 15 and 13 patients, respectively. Patients in group 3 tended to have a younger age (P = .088), no significant difference was found related to the aspects of tumor stage, differential degree, invasion depth, perineural invasion, lymphovascular invasion (all P > .05) (Table 1).
Table 1.
General information in the 3 groups.
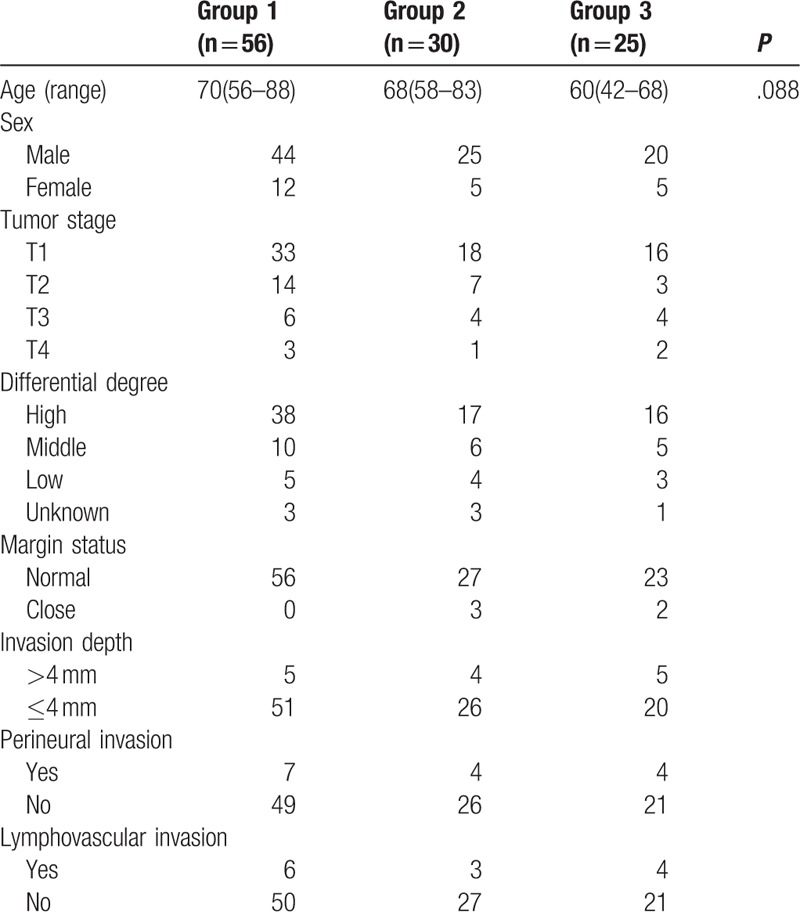
A total of 56 patients (group 1) underwent a wait-and-see policy in which patients just received an operation of tumor excision, 30 patients (group 2) received superficial parotidectomy, and 25 patients (group 3) received superficial parotidectomy and elective neck dissection (region I–III), and patients in group 2 and 3 also underwent primary tumor excision. Patients with pathologic metastatic nodes were suggested for postoperative radiotherapy. No patients received chemotherapy.
During postoperative specimen analysis, 6 (20%) patients had parotid node metastasis in group 2, and there was only 1 positive node in every patient. In group 3, 5 (20%) patients had parotid node metastasis, and all the patients had external cervical node metastasis, and 3 of the 5 cases had cervical node metastasis, moreover, there was neck node metastasis without parotid metastasis in only 1 patient. In furthermore analysis, in the 3 patients with neck disease, there was only 1 positive neck node in every patient, and all the node metastasis occurred in level II.
In our follow-up, 11 patients were lost. Regional recurrence was noted in 16 (16%) patients, and 15 (93.8%) of the patients had T3/T4 disease, there was no local recurrence, 6 patients died of the disease. In group 1, 4 patients had just parotid node metastasis and 6 patients had both parotid and neck metastasis, 5 of the 10 patients died of the disease. In group 2, 4 patients had neck node metastasis, and 1 of the 4 patients died of the disease. In group 3, 2 patients had neck node metastasis, and there was no disease-related death. Patients in group 3 had better regional control and disease-specific survival rate compared with the other 2 groups (Figs. 1 and 2).
Figure 1.
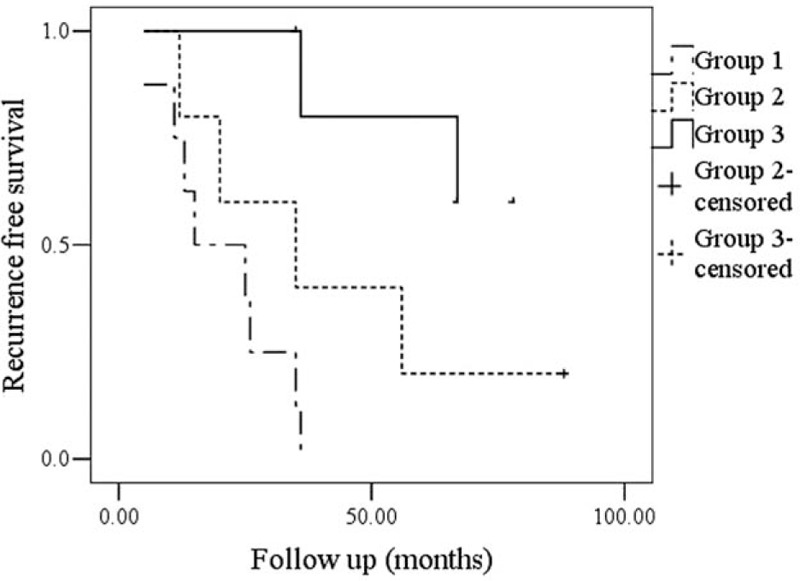
Comparison of recurrence free survival among the groups (P = .003).
Figure 2.
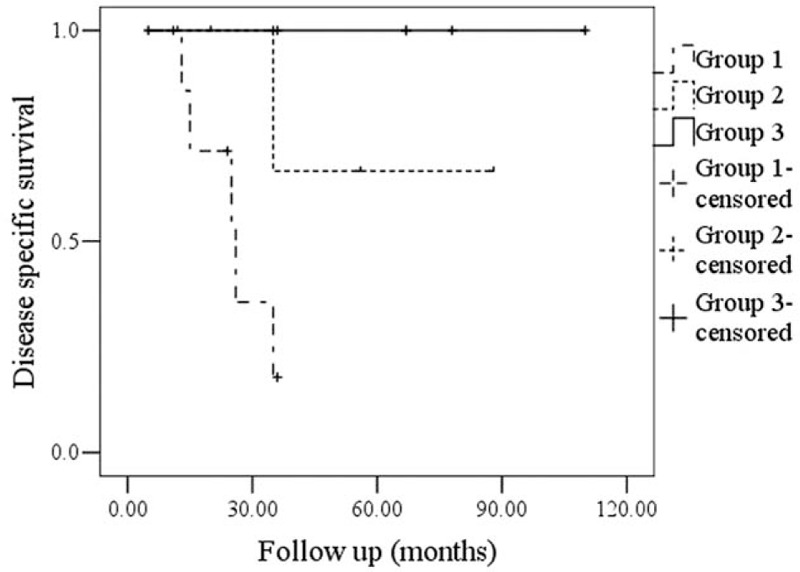
Comparison of disease specific survival among the groups (P = .007).
4. Discussion
The optimal treatment of parotid and neck in head neck cSCC without clinically positive node disease remained unclear. Previous author had pointed the high-risk cutaneous SCC patient group was defined by patient-related, tumor-related, and previous treatment-related risk factors by retrospective studies,[5] but in most cases, risk factors including differential degree, invasion depth, perineural invasion, lymphovascular invasion only could be learned by postoperative pathology analysis, which remained unknown during frozen section. Therefore, the exact risk for possible lymphatic metastasis was unclear until postoperative pathology species were evaluated, and a second operation might be required in some patients, which was associated with unnecessary pain and increased hospitalization expenses. It was urgent to accurately find out whether parotidectomy or elective dissection was suitable for the patient before operation, we were the first to analyze the possible best treatment procedure for cN0 patients with head neck cSCC by prospective design.
The most important finding in current study was that superficial parotidectomy associated with elective dissection increased regional control rate and DSS rate especially in patients with T3/T4 tumors regardless of the status of perineural or lymphovascular invasion or tumor thickness. This finding provided us with more clear preoperative plan. No similar literature was available for comparing, but there was accurate retrospective evidence suggesting that tumor size >2 cm was an risk factor for lymphatic metastasis.[4–11] In other subsites of head and neck, previous authors had compared wait-and-see policy and elective neck dissection in early stage tongue cancer. Orabona et al[13] reported in their research 66 patients received an elective neck dissection owing to early squamous cell carcinoma of the tongue (stages I–II), and 61 patients underwent “watchful waiting” observation. During the follow-up, a significant difference was found between the 2 groups as concerns tumor stage and pathologic tumor classification (P < .001). In T1 stage tumors with depth of infiltration ≤4 mm, or low grade, the “watchful waiting” strategy for cervical metastases was appropriate, given the low regional recurrence rate (15%) and overall survival of 100%. In case of T2 lesions with depth of infiltration ≥4 mm or high grade, it would be better to perform the elective neck dissection, with 13% of local recurrence and 100% of survival at 6 years.
Current study suggested the occult parotid and neck metastasis rate was 20% and 16%, respectively, the finding was consistent with previous reports.[8,9] Moreover, it was noted that the incidence of occult neck nodal disease was as high as 60% in patients with known parotid metastasis, similar results were also presented by previous authors,[14,15] and only 1 patient had positive neck disease without parotid involved. The finding indicted that the parotid status might be reliable for predicting neck metastasis. In a study reported by Sweeny et al[9] the authors also presented as high as 28.6% of the patients with positive parotid metastasis had pathologic neck disease, and only 5% had negative parotid nodes and positive cervical nodes.
Significance of external jugular node and level II was well described. Vauterin et al[16] described that in their pathologically positive neck dissections, level II was involved in 79%, and the external jugular lymph node was involved in almost all the patients. Similar finding was also seen in current study. This partly reflected the routine inclusion of the external jugular node, which lies superficial to the anterior border of the sternocleidomastoid muscle.
Efficacy of radiotherapy in treating head and neck cSCC had been reported. A single-institution study published by Wray et al[7] described that in 71 consecutive patients undergoing elective nodal radiotherapy, median followup was 4.5 years for all patients. The actuarial regional control rate at 5 years was 96%. There were no (0%) grade 3 or higher complications from elective nodal irradiation, therefore, the authors concluded that elective nodal irradiation in patients with high-risk cSCC of head and neck was safe and effective. Usually in our cancer center, primary radiotherapy was not suggested for any patients. An important fact was that different regional or free flaps were available for repairing any size defects,[17–19] and it was totally acceptable that there was no positive margin.
There were some limitations in current study. First, it was retrospective and there was selective bias which might affect the finding; second, the relative small sample size might vacillate our conclusions. More large sample size prospective study was needed to clarify the question.
In summary, superficial parotidectomy and elective neck dissection are suggested for patients with T3–4 facial cutaneous squamous cell carcinoma without taking other risk factors including invasion depth and perineural invasion into consideration.
Author contributions
Data curation: Yan Xiao.
Funding acquisition: Yan Xiao.
Visualization: Yan Xiao.
Writing – original draft: Yan Xiao.
Writing – review & editing: Shuai Yuan, Fei Liu, Bing Liu, Wei He, Wenlu Li, Quancheng Kan.
Methodology: Juanfang Zhu, Quancheng Kan.
Resources: Juanfang Zhu.
Supervision: Wei He, Quancheng Kan.
Formal analysis: Quancheng Kan.
Footnotes
Abbreviation: cSCC = facial cutaneous cell carcinoma.
YX and SY are the first two authors and made the same contribution.
The authors have no conflicts of interest to disclose.
References
- [1].Burton KA, Ashack KA, Khachemoune A. Cutaneous squamous cell carcinoma: a review of high-risk and metastatic disease. Am J Clin Dermatol 2016;17:491–508. [DOI] [PubMed] [Google Scholar]
- [2].Aslam AM, Patel AN. Facial cutaneous squamous cell carcinoma. BMJ 2016;352:i1513. [DOI] [PubMed] [Google Scholar]
- [3].O’Hara J, Ferlito A, Takes RP, et al. Cutaneous squamous cell carcinoma of the head and neck metastasizing to the parotid gland–a review of current recommendations. Head Neck 2011;33:1789–95. [DOI] [PubMed] [Google Scholar]
- [4].Košec A, Svetina L, Lukšić I. Significance of clinical stage, extent of surgery and outcome in cutaneous squamous cell carcinoma of the head and neck. Int J Oral Maxillofac Surg 2013;42:82–8. [DOI] [PubMed] [Google Scholar]
- [5].Yilmaz M, Eskiizmir G, Friedman O. Cutaneous squamous cell carcinoma of the head and neck: management of the parotid and neck. Facial Plast Surg Clin North Am 2012;20:473–81. [DOI] [PubMed] [Google Scholar]
- [6].Connolly KL, Nehal KS, Disa JJ. Evidence-based medicine: cutaneous facial malignancies: nonmelanoma skin cancer. Plast Reconstr Surg 2017;139:181e–90e. [DOI] [PubMed] [Google Scholar]
- [7].Wray J, Amdur RJ, Morris CG, et al. Efficacy of elective nodal irradiation in skin squamous cell carcinoma of the face, ears, and scalp. Radiat Oncol 2015;10:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ebrahimi A, Moncrieff MD, Clark JR, et al. Predicting the pattern of regional metastases from cutaneous squamous cell carcinoma of the head and neck based on location of the primary. Head Neck 2010;32:1288–94. [DOI] [PubMed] [Google Scholar]
- [9].Sweeny L, Zimmerman T, Carroll WR, et al. Head and neck cutaneous squamous cell carcinoma requiring parotidectomy: prognostic indicators and treatment selection. Otolaryngol Head Neck Surg 2014;150:610–7. [DOI] [PubMed] [Google Scholar]
- [10].Shao A, Wong DK, McIvor NP, et al. Parotid metastatic disease from cutaneous squamous cell carcinoma: prognostic role of facial nerve sacrifice, lateral temporal bone resection, immune status and P-stage. Head Neck 2014;36:545–50. [DOI] [PubMed] [Google Scholar]
- [11].Haksever M, Akduman D, Demir M, et al. The treatment of neck and parotid gland in cutaneous squamous cell carcinoma of face and forehead and the review of literature. Ann Med Surg (Lond) 2015;4:48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fang QG, Shi S, Zhang X, et al. Upper extremity morbidity after radial forearm flap harvest: a prospective study. J Int Med Res 2014;42:231–5. [DOI] [PubMed] [Google Scholar]
- [13].Orabona GD, Bonavolontà P, Maglitto F, et al. Neck dissection versus “watchful-waiting” in early squamous cell carcinoma of the tongue our experience on 127 cases. Surg Oncol 2016;25:401–4. [DOI] [PubMed] [Google Scholar]
- [14].Moore BA, Weber RS, Prieto V, et al. Lymph node metastases from cutaneous squamous cell carcinoma of the head and neck. Laryngoscope 2005;115:1561–7. [DOI] [PubMed] [Google Scholar]
- [15].Veness MJ, Morgan GJ, Palme CE, et al. Surgery and adjuvant radiotherapy in patients with cutaneous head and neck squamous cell carcinoma metastatic to lymph nodes: combined treatment should be considered best practice. Laryngoscope 2005;115:870–5. [DOI] [PubMed] [Google Scholar]
- [16].Vauterin TJ, Veness MJ, Morgan GJ, et al. Patterns of lymph node spread of cutaneous squamous cell carcinoma of the head and neck. Head Neck 2006;28:785–91. [DOI] [PubMed] [Google Scholar]
- [17].Fang QG, Shi S, Zhang X, et al. Total lower lip reconstruction with a double mental neurovascular V-Y island advancement flap. J Oral Maxillofac Surg 2014;72:834.e1–6. [DOI] [PubMed] [Google Scholar]
- [18].Fang QG, Shi S, Li M, et al. Free flap reconstruction versus non-free flap reconstruction in treating elderly patients with advanced oral cancer. J Oral Maxillofac Surg 2014;72:1420–4. [DOI] [PubMed] [Google Scholar]
- [19].Fang QG, Shi S, Zhang X, et al. Assessment of the quality of life of patients with oral cancer after pectoralis major myocutaneous flap reconstruction with a focus on speech. J Oral Maxillofac Surg 2013;71:2004.e1–5. [DOI] [PubMed] [Google Scholar]


