Supplemental Digital Content is available in the text
Keywords: apatinib, gastric cancer, meta-analysis, S-1
Abstract
Background:
Apatinib-targeted therapy is considered a promising treatment option for malignancies. This study systematically evaluated the efficacy and safety of the combination of apatinib and S-1 for the treatment of patients with advanced gastric cancer (GC).
Methods:
Clinical trials were searched from the PubMed, Cochrane Library, Embase, CNKI, and Wanfang databases. Outcome measures including therapeutic efficacy, quality of life (QoL), and adverse events were extracted and evaluated.
Results:
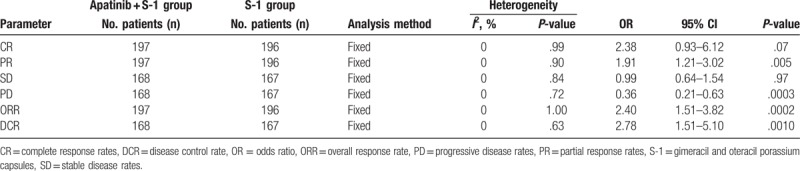
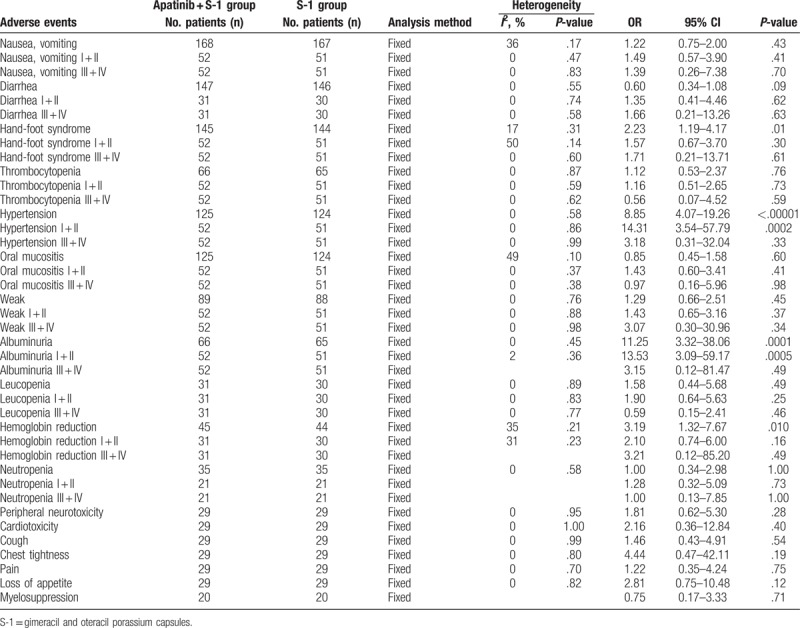
Data from 8 trials including 393 patients with advanced GC were included. The results indicated that, compared with S-1 alone, the combination of apatinib with S-1 significantly improved patient partial response rate (odds ratio [OR] = 1.91, 95% confidence interval [CI] = 1.21–3.02, P = .005), overall response rate (ORR, OR = 2.40, 95% CI = 1.51–3.82, P = .0002), and disease control rate (DCR, OR = 2.78, 95% CI = 1.51–5.10, P = .0010), whereas the rates of complete response (CR, OR = 2.38, 95% CI = 0.93–6.12, P = .07) and stable disease (SD, OR = 0.99, 95% CI = 0.64–1.54, P = .97) and QoL (OR = 1.22, 95% CI = 0.51–2.92, P = .66) did not differ significantly. Moreover, the group receiving the combined therapy had higher rates of hand-foot syndrome (OR = 2.23, 95% CI = 1.19–4.17, P = .01), hypertension (OR = 8.85, 95% CI = 4.07–19.26, P < .00001), albuminuria (OR = 11.25, 95% CI = 3.32–38.06, P = .0001), and hemoglobin reduction (OR = 3.19, 95% CI = 1.32–7.67, P = .010), whereas analysis of other adverse events did not show significant differences (P > .05).
Conclusion:
The combination of apatinib and S-1 is more effective for GC treatment than S-1 alone. However, this combined treatment could lead to increased hand-foot syndrome, hypertension, albuminuria, and hemoglobin reduction. Therefore, the benefits and risks should be considered before treatment.
1. Introduction
Gastric cancer (GC) is the fourth most common malignant disease and the second leading cause of cancer-related death worldwide.[1,2] The incidence of GC has significantly increased, with about 990,000 new cases every year.[2] China is a high-risk area for GC and new cases of GC in this region account for about 42.5% of the worldwide total.[3,4] Early GC is easily misdiagnosed because it has fewer symptoms. Most patients with GC have advanced-stage disease, in which the 5-year survival rate is <20%.[5]
Fluoropyrimidine is one of the most extensively used drugs in the treatment of advanced GC.[6] S-1 is a novel oral fluoropyrimidine derivative containing tegafur, gimeracil, and oteracil potassium in a molar ratio of 1.0:0.4:1.0, with single-agent response rates above 40% for advanced GC.[6,7] Preclinical studies showed that S-1 is significantly effective in inhibiting tumor growth in nude mice with human GC.[8–10] In Japan, a great response and favorable safety were reported for S-1 for the treatment of several cancers.[11–16] Although appropriate chemotherapy strategies improve patient survival, most patients eventually relapse and develop resistance to treatment, which was not able to completely eradicate small lesions and metastatic cells.[5,17] Thus, more effective treatments are urgently required.
In recent years, the use of molecular-targeted therapy has been rising rapidly and it is considered a powerful therapeutic method for cancer treatment.[3,17] Angiogenesis is important for tumor growth and metastasis, in which vascular endothelial growth factor (VEGF) and its receptors (VEGFRs) play pivotal roles.[18–20] Apatinib is a novel antiangiogenic drug specifically targeting VEGFRs, especially VEGFR2 (1/3 VEGFRs), which was approved for the 2nd-line treatment of advanced GC in the People's Republic of China in 2014.[21,22]
The clinical application of apatinib-targeted therapy for malignancies has been reported and several studies found the combination of apatinib and S-1 to have better therapeutic effects than those in patients treated with S-1 alone.[23–30] However, systematic analyses assessing the therapeutic efficacy of apatinib combined with S-1 in advanced GC remain scarce. Therefore, the present meta-analysis investigated the treatment effect and safety of apatinib combined with S-1 in comparison with those of S-1 alone for advanced GC, to provide a scientific reference for the design of future clinical trials.
2. Materials and methods
2.1. Data sources and selection criteria
This meta-analysis was conducted in accordance with the PRISMA guidelines. Studies were searched across the PubMed, Cochrane Library, Embase, CNKI, and Wanfang databases using the key terms “apatinib” and “S-1” combined with “gastric cancer.” No language or date limits were applied. Studies published before March 2018 were included in our analysis. All analyses were based on previous published studies. Therefore, no ethical approval or patient consent is required.
Selection criteria: Randomized controlled trials (RCTs) of patients with advanced GC were included in our analysis. Patients in the experimental group received apatinib combined with S-1 therapy, while patients in the control group were treated with S-1 alone.
2.2. Data extraction and quality assessment
Literature screening and data extraction were carried out by 2 independent investigators (YL and CZ) and verified by the 3rd reviewer (KZ). All involved studies were summarized as follows: 1st author names, years of publication, study locations, numbers of cases, patient ages, enrollment periods, apatinib dosages, and study parameters. The quality of the included trials was evaluated as described in the Cochrane Handbook.[31]
2.3. Outcome definitions
The clinical responses included treatment efficacy, quality of life (QoL), and adverse events. Treatment efficacy was assessed in terms of the complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) rates, overall response rate (ORR, ORR = CR + PR), disease control rate (DCR, DCR = CR + PR + SD), and patient QoL. Adverse events including nausea and vomiting, diarrhea, hand-foot syndrome, thrombocytopenia, hypertension, oral mucositis, weak, albuminuria, leukopenia, hemoglobin reduction, neutropenia, peripheral neurotoxicity, cardiotoxicity, cough, chest tightness, pain, loss of appetite, and myelosuppression were also assessed.
2.4. Statistical analysis
The analysis was performed using Review Manager 5.3 (Cochrane Collaboration). P < .05 indicated statistically significant differences. Heterogeneity among studies was assessed using Cochran Q test to determine the most suitable analysis model and funnel plots were used to assess the publication bias of the included studies.[32]I2 < 50% or P > .1 indicated study homogeneity. Odds ratios (ORs) were the principal measurement of the therapeutic effect and were presented with 95% confidence interval (CI). When heterogeneity was small (I2 < 50%), a Mantel–Haenszel fixed-effects model was applied for OR estimation. When at least a moderate level of statistical heterogeneity (I2 > 50%) was detected, a Mantel–Haenszel random-effects model was selected. Subgroup analyses were conducted to evaluate the impact of apatinib dosages and sample sizes of the included studies.
3. Results
3.1. Search results
A total of 421 articles were identified with initial search. After title and abstract review, 335 articles were excluded because they did not include clinical trials (n = 185), were unrelated studies (n = 43), or duplication or repetition (n = 107), leaving 86 studies as potentially relevant. After detailed assessments of the full texts, case reports, or reviews (n = 23), articles without a control group (n = 17) or without apatinib and S-1 combined therapy (n = 34), or with insufficient data (n = 4) were excluded. Finally, data from 8 trials,[23–30] including 393 patients with advanced GC were included in the present meta-analysis (Fig. 1).
Figure 1.
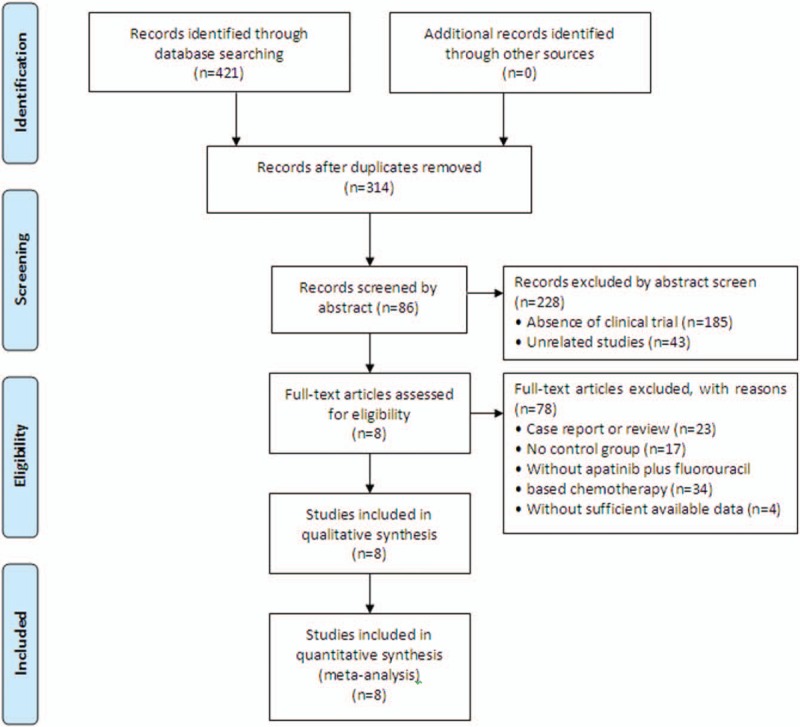
Flow diagram of the selection process.
3.2. Patient characteristics
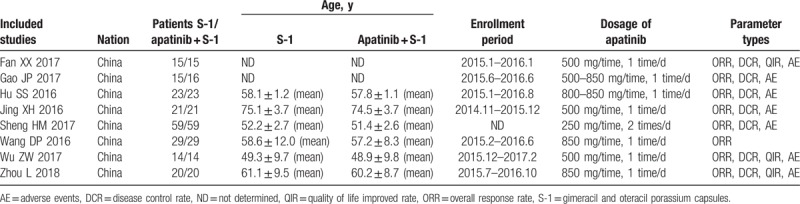
After selection, all included trials were conducted in China. In total, 197 patients with advanced GC were treated with apatinib in combination with S-1, while 196 patients were treated with S-1 alone. The detailed information on the included trials and patients is presented in Table 1 and Supplementary Table 1.
Table 1.
Clinical information from the eligible trials in the meta-analysis.
3.3. Quality assessment
The assessment of bias risk is shown in Figure 2. None of the included trials provided a clear description of the performance and detection risks. The selection and attrition risks of the included trials were low; 1 study was considered to have an unclear risk owing to selective reporting.
Figure 2.
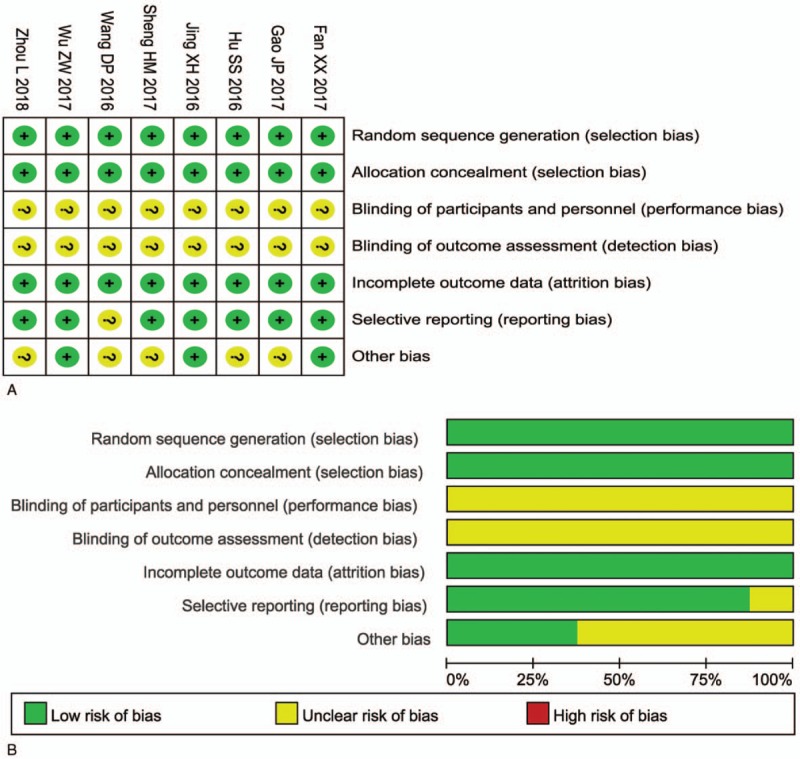
(A) Risk of bias summary: review of authors’ judgments about each risk of bias item for included studies. (B) Risk of bias graph: review of authors’ judgments about each risk of bias item presented as percentages across all included studies. Each color represents a different level of bias: red for high-risk, green for low-risk, and yellow for unclear-risk of bias.
3.4. Therapeutic efficacy assessments
As shown in Figure 3, Supplementary Figure 1, and Table 2, the pooled results showed that patients underwent combined therapy had significantly improved PR, ORR, and DCR (PR: OR = 1.49, 95% CI = 1.06–2.10, P = .02; ORR: OR = 1.69, 95% CI = 1.20–2.38, P = .003; DCR: OR = 2.33, 95% CI = 1.63–3.33, P < .00001) and significantly decreased PD (OR = 0.43, 95% CI = 0.30–0.61, P < .00001), whereas the CR and SD did not differ significantly from those in patients who received S-1 alone (CR: OR = 1.97, 95% CI = 0.85–4.54, P = .11; SD: OR = 1.31, 95% CI = 0.95–1.80, P = .10). Fixed-effect models were used to analyze the OR rates because of the low heterogeneity.
Figure 3.
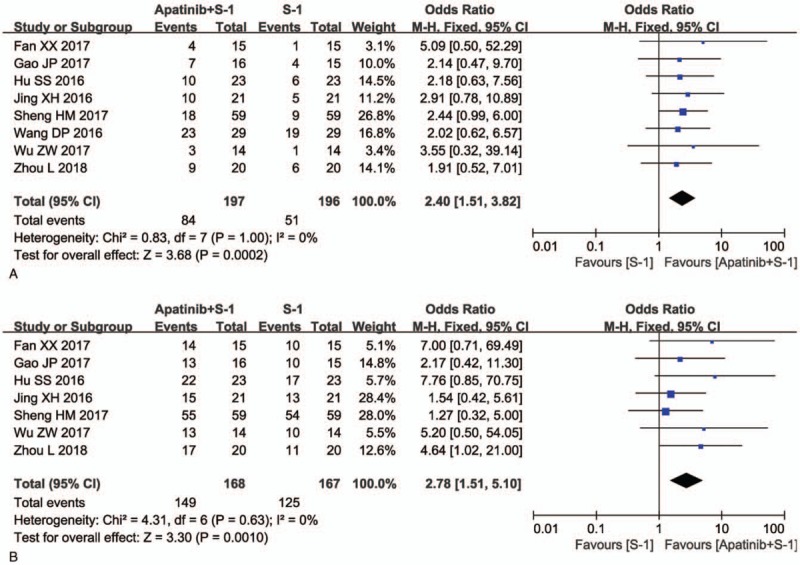
Forest plot of the comparison of overall response rate (ORR, A) and disease control rate (DCR, B) between the apatinib + S-1 and S-1 groups. S-1: gimeracil and oteracil porassium capsules. The fixed-effects meta-analysis model (Mantel–Haenszel method) was used.
Table 2.
Comparison of CR, PR, SD, PD, ORR, and DCR between the apatinib + S-1 and S-1 groups.
The QoL was also evaluated. The result showed no significant difference in patient QoL between the 2 groups (Fig. 4, OR = 1.77, 95% CI = 0.94–3.33, P = .08).
Figure 4.
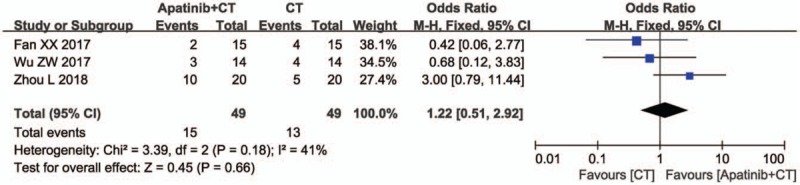
Forest plot of the comparison of quality of life (QoL) between the apatinib + S-1 and S-1 groups. S-1: gimeracil and oteracil porassium Capsules. The fixed-effects meta-analysis model (Mantel–Haenszel method) was used.
3.5. Adverse events assessment
This analysis also evaluated the safety of apatinib-targeted therapy. As shown in Figure 5, Supplementary Figures 3–13, and Table 3, the group that received apatinib plus S-1 combined therapy had higher rates of hand-foot syndrome (OR = 2.23, 95% CI = 1.19–4.17, P = .01 [Supplementary Fig. 2, hand-foot syndrome I + II: OR = 1.57, 95% CI = 0.67–3.70, P = .30; hand-foot syndrome III + IV: OR = 1.71, 95% CI = 0.21–13.71, P = .61]), hypertension (OR = 8.85, 95% CI = 4.07–19.26, P < .00001 (Supplementary Fig. 3, hypertension I + II: OR = 14.31, 95% CI = 3.54–57.79, P = .0002; hypertension III + IV: OR = 3.18, 95% CI = 0.31–32.04, P = .33]), albuminuria (OR = 11.25, 95% CI = 3.32–38.06, P = .0001 [Supplementary Fig. 4, albuminuria I + II: OR = 13.53, 95% CI = 3.09–59.17, P = .0005; albuminuria III + IV: OR = 3.15, 95% CI = 0.12–81.47, P = .49]), and hemoglobin reduction (OR = 3.19, 95% CI = 1.32–7.67, P = .010 [Supplementary Fig. 5, hemoglobin reduction I + II: OR = 2.10, 95% CI = 0.74–6.00, P = .16; hemoglobin reduction III + IV: OR = 3.21, 95% CI = 0.12–85.20, P = .49]), whereas analysis on nausea and vomiting (OR = 1.22, 95% CI = 0.75–2.00, P = .43 [Supplementary Fig. 6, nausea and vomiting I + II: OR = 1.49, 95% CI = 0.57–3.90, P = .41; nausea and vomiting III + IV: OR = 1.39, 95% CI = 0.26–7.38, P = .70]), diarrhea (OR = 0.60, 95% CI = 0.34–1.08, P = .09 [Supplementary Fig. 7, diarrhea, I + II: OR = 1.35, 95% CI = 0.41–4.46, P = .62; diarrhea, III + IV: OR = 1.66, 95% CI = 0.21–13.26, P = .63]), thrombocytopenia (OR = 1.12, 95% CI = 0.53–2.37, P = .76 [Supplementary Fig. 8, thrombocytopenia I + II: OR = 1.16, 95% CI = 0.51–2.65, P = .73; thrombocytopenia III + IV: OR = 0.56, 95% CI = 0.07–4.52, P = .59]), oral mucositis (OR = 0.85, 95% CI = 0.45–1.58, P = .60 [Supplementary Fig. 9, oral mucositis I + II: OR = 1.43, 95% CI = 0.60–3.41, P = .41; oral mucositis III + IV: OR = 0.97, 95% CI = 0.16–5.96, P = .98]), weak (OR = 1.29, 95% CI = 0.66–2.51, P = .45 [Supplementary Fig. 10, weak I + II: OR = 1.43, 95% CI = 0.65–3.16, P = .37; weak III + IV: OR = 3.07, 95% CI = 0.30–30.96, P = .34]), leucopenia (OR = 1.58, 95% CI = 0.44–5.68, P = .49 [Supplementary Fig. 11, leukopenia I + II: OR = 1.90, 95% CI = 0.64–5.63, P = .25; leukopenia III + IV: OR = 0.59, 95% CI = 0.15–2.41, P = .46]), neutropenia (OR = 1.00, 95% CI = 0.34–2.98, P = 1.00 [Supplementary Fig. 12, neutropenia I + II: OR = 1.28, 95% CI = 0.32–5.09, P = .73; neutropenia III + IV: OR = 1.00, 95% CI = 0.13–7.85, P = 1.00]), peripheral neurotoxicity (OR = 1.81, 95% CI = 0.62–5.30, P = .28), cardiotoxicity (OR = 2.16, 95% CI = 0.36–12.84, P = .40), cough (OR = 1.46, 95% CI = 0.43–4.91, P = .54), chest tightness (OR = 4.44, 95% CI = 0.47–42.11, P = .19), pain (OR = 1.22, 95% CI = 0.35–4.24, P = .75), loss of appetite (OR = 2.81, 95% CI = 0.75–10.48, P = .12), and myelosuppression (OR = 0.75, 95% CI = 0.17–3.33, P = .71) did not differ significantly.
Figure 5.
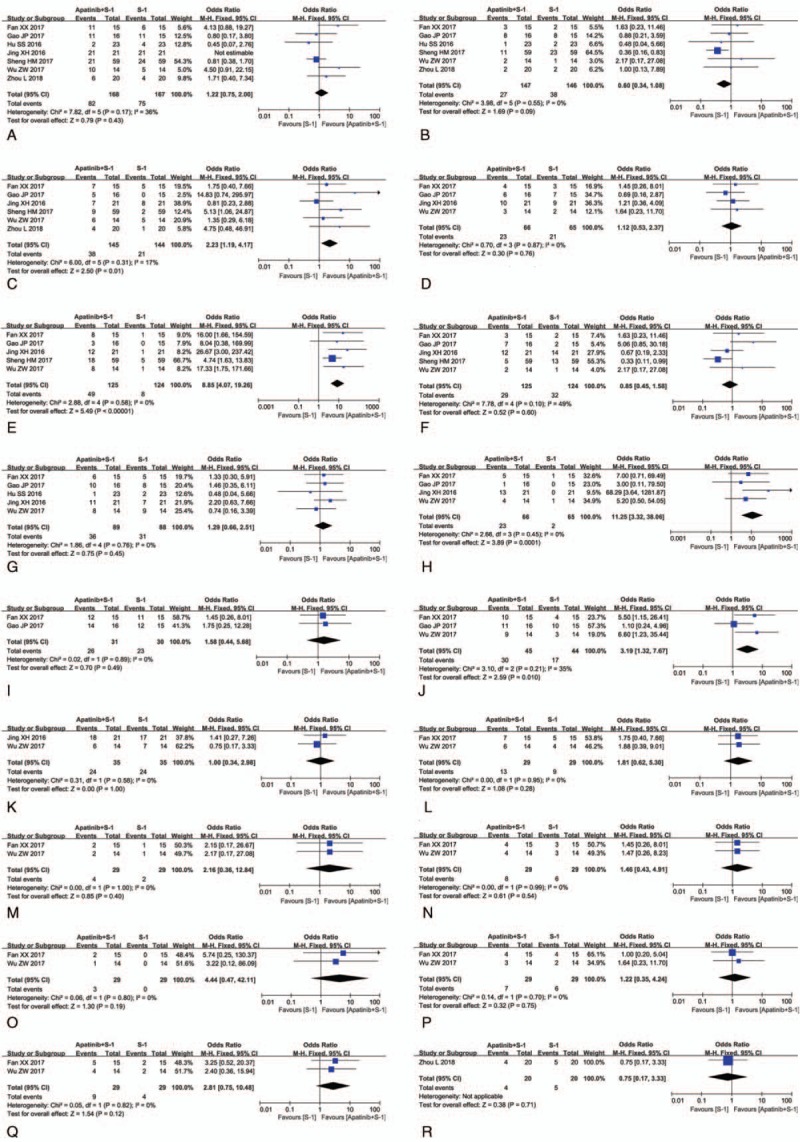
Forest plot of the comparison of adverse effects including nausea and vomiting (A), diarrhea (B), hand-foot syndrome (C), thrombocytopenia (D), hypertension (E), oral mucositis (F), weak (G), albuminuria (H), leucopenia (I), hemoglobin reduction (J), neutropenia (K), peripheral neurotoxicity (L), cardiotoxicity (M), cough (N), chest tightness (O), pain (P), loss of appetite (Q) and myelosuppression (R) between the apatinib + S-1 and S-1 groups. S-1: gimeracil and oteracil porassium capsules. The fixed-effects meta-analysis model (Mantel–Haenszel method) was used.
Table 3.
Comparison of adverse events between the apatinib + S-1 and S-1 groups.
3.6. Publication bias
Funnel plots drawn for the studies on primary outcomes (CR, PR, SD, PD, ORR, DCR, and adverse events) were approximately symmetrical, which indicated generally controlled publication bias and the reliability of our primary conclusions (Figs. 6 and 7 and Supplementary Fig. 13).
Figure 6.
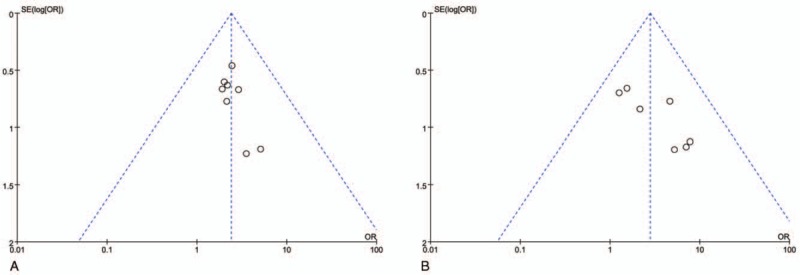
Funnel plot of percentage of overall response rate (ORR, A) and disease control rate (DCR, B).
Figure 7.
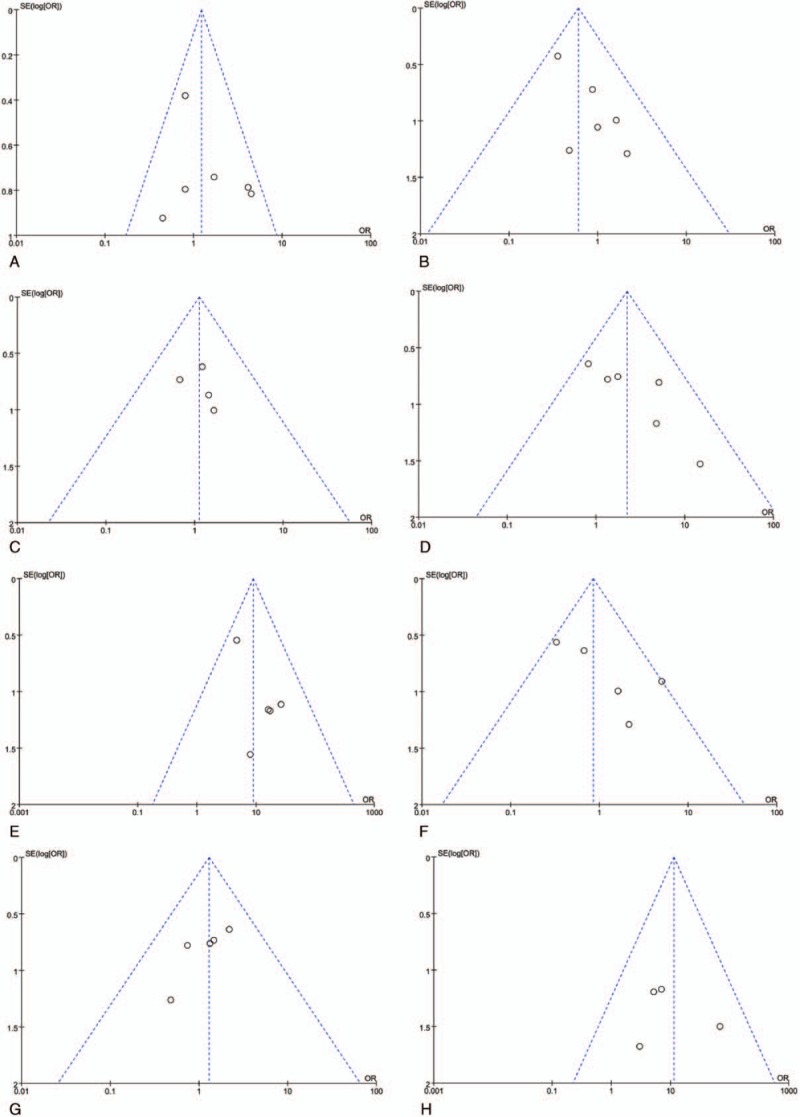
Funnel plot of adverse effects including nausea and vomiting (A), diarrhea (B), hand-foot syndrome (C), thrombocytopenia (D), hypertension (E), oral mucositis (F), weak (G), and albuminuria (H).
3.7. Subgroup analysis
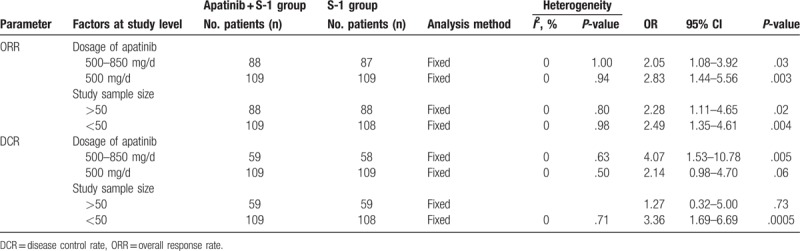
We conducted subgroup analysis to explore the source of heterogeneity in ORR and DCR with respect to apatinib dosages and sample sizes of the included studies. As shown in Table 4, our analysis results showed that apatinib was more effective at higher dosages (500–850 mg/d) and small sample sizes (<50), as indicated by an increased DCR.
Table 4.
Subgroup analyses of ORR and DCR between the apatinib + S-1 and S-1 groups.
4. Discussion
With the development of tumor molecular biology, increasing numbers of drugs have been used for the treatment of malignancies.[3,17] As the important signaling pathway of tumor angiogenesis, VEGF and VEGFRs are closely associated with tumor invasiveness. When it is combined with its receptor, activated VEGF promotes vascular cells proliferation to develop new blood vessels in cancer tissues, ensuring nutrient and oxygen supply and causing tumor growth and metastasis.[18,33] Therefore, targeted anti-VEGFRs agents are a promising treatment option for the treatment of malignant tumors. The VEGFRs family contains VEGFR-1, VEGFR-2, and VEGFR-3.[20] Among these receptors, VEGFR2 plays an essential role in VEGF-mediated tumor angiogenesis.[18,21] Upon binding to VEGF, VEGFR2 dimerization causes intracellular tyrosine kinase domain autophosphorylation, leading to PLC-γ-Raf kinase-MEK-MAP kinase pathway activation and increased endothelial cell proliferation.[18,19] Apatinib is a novel antiangiogenic agent specifically targeting VEGFR2.[21] Several studies have reported that the addition of apatinib could be beneficial to patients with advanced GC.[34,35] Even though there has been statistical analysis of published clinical trials, the exact treatment effects were not systematically investigated and demonstrated because of the variability in sample size among these trials. Moreover, different protocols used by various studies may lead to differences in the therapeutic effects. In the present study, we performed an extensive online search followed by rigorous contrasting and combining data analysis for categorization to provide clear and systematic conclusions.
Our meta-analysis revealed that apatinib-targeted therapy combined with S-1 is associated with a favorable efficacy as compared to that in GC patients receiving S-1 alone. Compared to patients treated with S-1 alone, patients treated with the combined therapy showed markedly increased PR, ORR, and DCR (P < .05). The QoL was also evaluated, but no significant differences were found between groups in this study (P > .05). These results indicated that apatinib-targeted therapy can increase the curative effect of S-1 by inhibiting tumor angiogenesis, but it does not improve patient QoL.
Safety is the top priority of the clinical treatment and is also a key factor for the development of apatinib-targeted therapy. Regarding adverse events and severe toxicities, our analysis showed no significant difference in most adverse event indicators between the 2 groups. The group receiving S-1 plus apatinib-targeted therapy had higher rates of hand-foot syndrome, hypertension, albuminuria, and hemoglobin reduction, which are usually controllable events and do not require permanent discontinuation of therapy.
Some factors may influence the therapeutic effects of apatinib-targeted therapy. In our subgroup analysis, apatinib was more effective at higher dosages (500–850 mg/d) in included studies with small sample sizes (<50). However, recent studies on the impact of these factors on the curative effect of apatinib-targeted therapy remain insufficient and further investigations should be performed.
Our analysis had several limitations. First, the numbers of GC patients included in this study were small and the follow-up durations were short. Moreover, our data were partly extracted from published papers rather than original patient records, which meant we were not able to avoid the analytical bias based on the information presented in the articles. Due to these limitations, future studies and generated data will be valuable to further verify the safety and efficacy of apatinib-targeted therapy.
5. Conclusion
In summary, our study confirmed that apatinib-targeted therapy combined with S-1 was an effective treatment for patients with advanced GC. Apatinib markedly enhanced the treatment efficacy of S-1 in advanced GC. However, this combined treatment could lead to a higher incidence of hand-foot syndrome, hypertension, albuminuria, and hemoglobin reduction. Therefore, the benefits and risks should be considered before treatment.
Author contributions
Ruihua Zhang and Yan Liu conceived and designed the experiments. Yan Liu and Changchun Zhou performed the experiments, analyzed the data and drafted the manuscript. All authors reviewed and participated in amending the manuscript before submission of the mutually agreed final version of the manuscript.
Conceptualization: Yan Liu, Yikuan Feng.
Formal analysis: Changchun Zhou.
Writing – original draft: Yan Liu, Changchun Zhou, Ruihua Zhang.
Writing – review & editing: Yan Liu, Kai Zhang, Yikuan Feng.
Supplementary Material
Footnotes
Abbreviations: CR = complete response rates, DCR = disease control rate, GC = gastric cancer, OR = odds ratio, CI = confidence interval, ORR = overall response rate, PD = progressive disease rates, PR = partial response rates, QoL = quality of life, RCTs = randomized controlled trials, SD = stable disease rates, VEGF = vascular endothelial growth factor, VEGFRs = vascular endothelial growth factor receptors.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Chhetri P, Giri A, Shakya S, et al. Current development of anti-cancer drug S-1. J Clin Diagn Res 2016;10:XE01–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kim HJ, Oh SC. Novel systemic therapies for advanced gastric cancer. J Gastric Cancer 2018;18:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Xie S, Zhang H, Wang X, et al. The relative efficacy and safety of targeted agents used in combination with chemotherapy in treating patients with untreated advanced gastric cancer: a network meta-analysis. Oncotarget 2017;8:26959–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chen W, Zheng R, Zeng H, et al. Annual report on status of cancer in China, 2011. Chin J Cancer Res 2015;27:2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mu Y, Wang WH, Xie JP, et al. Efficacy and safety of cord blood-derived dendritic cells plus cytokine-induced killer cells combined with chemotherapy in the treatment of patients with advanced gastric cancer: a randomized Phase II study. Onco Targets Ther 2016;9:4617–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ter Veer E, Mohammad NH, Lodder P, et al. The efficacy and safety of S-1-based regimens in the first-line treatment of advanced gastric cancer: a systematic review and meta-analysis. Gastric Cancer 2016;19:696–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yang J, Zhou Y, Min K, et al. Xu CN: S-1-based vs non-S-1-based chemotherapy in advanced gastric cancer: a meta-analysis. World J Gastroenterol 2014;20:11886–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mori T, Fujiwara Y, Yano M, et al. Experimental study to evaluate the usefulness of S-1 in a model of peritoneal dissemination of gastric cancer. Gastric cancer 2003;6:13–8. [DOI] [PubMed] [Google Scholar]
- [9].Mori T, Fujiwara Y, Yano M, et al. Prevention of peritoneal metastasis of human gastric cancer cells in nude mice by S-1, a novel oral derivative of 5-fluorouracil. Oncology 2003;64:176–82. [DOI] [PubMed] [Google Scholar]
- [10].Fukushima M, Satake H, Uchida J, et al. Preclinical antitumor efficacy of S-1: a new oral formulation of 5-fluorouracil on human tumor xenografts. Int J Oncol 1998;13:693–8. [DOI] [PubMed] [Google Scholar]
- [11].Ajani JA, Faust J, Ikeda K, et al. Phase I pharmacokinetic study of S-1 plus cisplatin in patients with advanced gastric carcinoma. J Clin Oncol 2005;23:6957–65. [DOI] [PubMed] [Google Scholar]
- [12].Fujii T, Horiguchi J, Yanagita Y, et al. Phase II study of S-1 plus trastuzumab for HER2-positive metastatic breast cancer (GBCCSG-01). Anticancer research 2018;38:905–9. [DOI] [PubMed] [Google Scholar]
- [13].Ikezawa Y, Asahina H, Oizumi S, et al. A randomized phase II trial of erlotinib vs. S-1 as a third- or fourth-line therapy for patients with wild-type EGFR non-small cell lung cancer (HOT1002). Cancer Chemother Pharmacol 2017;80:955–63. [DOI] [PubMed] [Google Scholar]
- [14].Okabe H, Hata H, Ueda S, et al. A phase II study of neoadjuvant chemotherapy with S-1 and cisplatin for stage III gastric cancer: KUGC03. J Surg Oncol 2016;113:36–41. [DOI] [PubMed] [Google Scholar]
- [15].Bando H, Yamada Y, Tanabe S, et al. Efficacy and safety of S-1 and oxaliplatin combination therapy in elderly patients with advanced gastric cancer. Gastric Cancer 2016;19:919–26. [DOI] [PubMed] [Google Scholar]
- [16].Ito S, Ohashi Y, Sasako M. Survival after recurrence in patients with gastric cancer who receive S-1 adjuvant chemotherapy: exploratory analysis of the ACTS-GC trial. BMC Cancer 2018;18:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Davidson M, Okines AF, Starling N. Current and future therapies for advanced gastric cancer. Clin Colorectal Cancer 2015;14:239–50. [DOI] [PubMed] [Google Scholar]
- [18].Zhao J, Zhang X, Gong C, et al. Targeted therapy with apatinib in a patient with relapsed small cell lung cancer: a case report and literature review. Medicine 2017;96:e9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fontanella C, Ongaro E, Bolzonello S, et al. Clinical advances in the development of novel VEGFR2 inhibitors. Ann Transl Med 2014;2:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003;9:669–76. [DOI] [PubMed] [Google Scholar]
- [21].Roviello G, Ravelli A, Polom K, et al. Apatinib: a novel receptor tyrosine kinase inhibitor for the treatment of gastric cancer. Cancer Lett 2016;372:187–91. [DOI] [PubMed] [Google Scholar]
- [22].Zhang H. Apatinib for molecular targeted therapy in tumor. Drug Des Devel Ther 2015;9:6075–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fan XX, Lv HF, Chen BB, et al. Clinical study of apatinib combined with S-1 in the treatment of patients with advanced gastric cancer and review of literature. Chin J Front Med Sci 2017;9:63–7. [Google Scholar]
- [24].Gao JP, Han T, Piao Y, et al. Apatinib combined tegafur in treatment for elderly or emaciated patients with advanced gastric cancer. Clin J Med Officers 2017;45:9–12. [Google Scholar]
- [25].Hu SS, Xiang M, Lu P, et al. Clinical effect analysis of apatinib plus S-1 in the treatment of elderly patients with advanced gastric cancer. Clin Med 2016;36:33. [Google Scholar]
- [26].Jing XH. Effect of apatinib combined with S-1 as first-line therapy on elderly patients with advanced gastric cancer. Chin J Pract Med 2016;43:37–9. [Google Scholar]
- [27].Sheng HM, Wu C, Deng LC, et al. The clinical effect comparison between apatinib and S-1 as the second-line treatment for advanced gastric cancer. Oncol Progress 2017;15:1436–8. [Google Scholar]
- [28].Wang DP, Song SS, Wang K. Clinical effect analysis of apatinib mesylate combined with chemotherapy in the treatment of patients with advanced gastric cancer. World Latest Med Inform 2016;16:130–1. [Google Scholar]
- [29].Wu ZW, Cai J, Lu WK, et al. Clinical study of Apatinib combined with Tegafur as second line treating advanced gastric cancer. China Mod Med 2017;24:69–71. [Google Scholar]
- [30].Zhou L, Sun YS, Wang XL, et al. Clinical observation of Apatinib combined with Tegafur in treatment of advanced gastric cancer patients after failure of second-line or beyond treatment. Contemp Med 2018;24:52–4. [Google Scholar]
- [31].Zeng X, Zhang Y, Kwong JS, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med 2015;8:2–10. [DOI] [PubMed] [Google Scholar]
- [32].Jackson D, White IR, Riley RD. Quantifying the impact of between-study heterogeneity in multivariate meta-analyses. Stat Med 2012;31:3805–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kerbel RS. Tumor angiogenesis. N Eng J Med 2008;358:2039–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Li J, Qin S, Xu J, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol 2016;34:1448–54. [DOI] [PubMed] [Google Scholar]
- [35].Huang L, Wei Y, Shen S, et al. Therapeutic effect of apatinib on overall survival is mediated by prolonged progression-free survival in advanced gastric cancer patients. Oncotarget 2017;8:29346–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


