Abstract
The study evaluates efficacy and safety of recombinant human parathyroid hormone (1–34) [rhPTH (1–34)] and alendronate (ALN) in the treatment of postmenopausal osteoporosis.
Totally 65 postmenopausal women with osteoporosis were divided into 2 groups. PTH group received daily subcutaneous injection of rhPTH (1–34), and ALN group were treated orally with ALN per week. Bone mineral density (BMD) of lumbar spine (1–4), femoral neck, and total hip, serum levels of calcium, phosphorus, total cholesterol, triglyceride, alkaline phosphatase (ALP), N-terminal propeptide of type I collagen (PINP), and C-telopeptide of type I collagen (CTX) were tested before treatment and at week 24 and 48 after treatment. Serum levels of vascular endothelial growth factor (VEGF) and platelet-derived growth factor-BB (PDGF-BB) were measured before treatment and at week 48 after treatment.
The rhPTH (1–34) increased BMD of lumbar spine (1–4), but decreased BMD of femoral neck and total hip at week 48 after treatment. By contrast, ALN enhanced BMD of lumbar spine (1–4) and femoral neck, but reduced BMD of total hip at week 48 after treatment. In PTH group, serum levels of PINP, ALP, and β-CTX were significantly elevated above baseline at week 24 and 48 after treatment. Treatment with ALN decreased levels of PINP, ALP, and β-CTX compared with baseline at week 24 and 48 after treatment. rhPTH (1–34) and ALN significantly decreased levels of PDGF-BB, but not levels of VEGF. rhPTH (1–34) increased levels of calcium, phosphorus and triglyceride, but decreased levels of total cholesterol. ALN increased levels of calcium and triglyceride, but reduced levels of phosphorus and total cholesterol. rhPTH (1–34) and ALN were safe in the treatment of postmenopausal osteoporosis.
The study demonstrates that efficacy of rhPTH (1–34) on BMD of lumbar spine (1–4) is similar to that of alendronate in the treatment of postmenopausal osteoporosis. The effect of rhPTH (1–34) on BMD of femoral neck or total hip is weaker than that of ALN. In addition, rhPTH (1–34) increases BMD of lumbar spine (1–4) maybe by raising serum levels of VEGF, but reduces BMD of femoral neck and total hip maybe by decreasing serum levels of PDGF-BB.
Keywords: alendronate, bone mineral density, postmenopausal osteoporosis, recombinant human parathyroid hormone
1. Introduction
Osteoporosis, a metabolic bone disease characterized by low bone mass and disruption in bone microarchitecture, can lead to bone fragility and increase fracture risks.[1] Postmenopausal women are more vulnerable to osteoporosis than the rest of the population, partly due to the estrogen deficiency and rapid loss of calcium, that causes excessive bone resorption.[2–4] The medications for postmenopausal osteoporosis primarily include antiresorptive agents and anabolic agents. Antiresorptive agents, such as alendronate, are the most widely used type of drugs for the treatment of osteoporosis. Alendronate reduces the activity of osteoclasts, induces the apoptosis of osteoclasts, restores the balance of bone remodeling, prevents bone loss and increases bone mineral density (BMD), leading to reduced risk for fractures.[5–7] By contrast, recombinant human parathyroid hormone 1–34 [rhPTH (1–34)], an anabolic agent that stimulates the differentiation of bone mesenchymal stem cells and preosteoblasts into osteoblasts, increases the activity of osteoblasts, also inhibits the apoptosis of osteoblasts.[8,9] When patients with osteoporosis are administered once a day by subcutaneous injection, rhPTH (1–34) regulates the balance of bone remodeling, which prevents bone loss, improves BMD and reduces fracture risks.[10,11] Several previous studies have demonstrated that daily small-dose injection of rhPTH (1–34) can improve the BMD in patients with postmenopausal osteoporosis, increase the levels of bone turnover markers, and reduce the risk for fractures.[12,13] In the present study, we investigate the efficacy and safety of rhPTH (1–34) or alendronate in the treatment of osteoporosis in postmenopausal women, and explore the underlying mechanism.
2. Materials and methods
2.1. Patients
A total of 65 patients with postmenopausal osteoporosis (age, 62.8 ± 5.9 years; mean duration of menopause, 14.5 ± 7.5 years) were included in this clinical trial. The patients were recruited at the First Affiliated Hospital of Chongqing Medical University between July 2014 and October 2014. The inclusion criteria were as follows: eligible participants were postmenopausal women ages from 45 to 80 years who had capacity for independent activities, and the women who had menopause for more than 3 years due to natural menopause or surgery (age at surgery was more than 40 years); subjects had T score of –2.5 or less for BMD at lumbar spine or total hip, or had –2.5 < T score < –1.0 for BMD at lumbar spine or total hip and at least 1 postmenopausal fragility fracture and ≥ 3 lumbar vertebrae in L-1 through L-4 region could be measured by dual-energy X-ray absorptiometry (DXA); body mass index (BMI) was between 18 and 30 kg/m2. Exclusion criteria were as follows:
-
1.
Subjects had secondary osteoporosis, such as osteomalacia, rheumatoid arthritis, gout, multiple myeloma, etc.
-
2.
Subjects received treatments of osteoporosis before enrollment by rhPTH; calcitonin, corticosteroids, estrogens, androgens, active vitamin K, or vitamin D analogues within 3 months; bisphosphonates or fluoride for more than 15 days during the previous 6 months; selective estrogen receptor modulators, or strontium within 6 months; or injection of bisphosphonates within 2 years.
-
3.
Subjects had diseases that were known to affect calcium or bone metabolism, such as serious malabsorption syndrome, inflammatory bowel disease, chronic liver disease, cirrhosis of the liver, abnormal thyroid or parathyroid function without adequate substitution therapy, Paget disease of bone, hypercalcemia, hypocalcemia or activity of urolithiasis.
-
4.
Subjects took drugs such as heparin, warfarin, or anticonvulsants (except for benzodiazepines) that affected bone metabolism, or needed chronically or continuously use of digitalis drugs such as digoxin during the test.
-
5.
Subjects had severe lumbar spinal anatomy abnormality that would have influence on the measurement of BMD, such as serious scoliosis.
-
6.
Subjects had severe kidney disease, uncontrolled hypertension (≥150/100 mm Hg), ischemic cardiac disease, cerebral infarction or arteriosclerosis obliterans, malignant neoplasm or other serious underlying diseases.
-
7.
Subjects had abnormal indicators in laboratory tests: serum alkaline phosphatase concentrations > upper normal limit, alanine transaminase, aspartate transaminase or total bilirubin concentration > 2 times of the upper limit, glycosylated hemoglobin ≥ 7%, serum white blood cell count < 3500 mm3 or hemoglobin < 100 g/L or platelet count < 9.0 × 104/mm3, the abnormal levels of parathyroid hormone, the levels of thyroid stimulating hormone > 10.0 μIU/mL, serum creatinine > 1.2 times of the upper limit, serum calcium concentration > upper normal limit.
-
8.
Subjects had esophageal abnormalities which could lead to esophageal emptying delay, gastroesophageal reflux disease, active gastric ulcer, GI bleeding, subtotal gastrectomy before enrollment, or Barrett esophagus.
-
9.
Subjects consumed alcohol > 30 mL per day or consumed cigarette > 10 cigarettes a day, or had psychosis or had no knowledge of himself.
-
10.
Subjects received treatments with other clinical trial, or received operation within 1 month before enrollment, or had participated in our phase II clinical trial. The study was approved by the ethics committee of the First Affiliated Hospital of Chongqing Medical University. Written informed consent was obtained from each participant before the start of screening test according to Declaration of Helsinki.
After a run-in phase of 7 or 9 days, the 65 women were randomly assigned by a computerized program to PTH group (n = 43) and alendronate (ALN) group (n = 22) according to the ratio of 2:1. PTH group received subcutaneous injection of 20 μg rhPTH (1–34) (Genemen Biotechnology Co, Ltd, Suzhou, China) once a day. ALN group received orally of 70 mg alendronate (FOSAMAX; MSD, Kenilworth, NJ) once a week. The treatment duration of the study was 48 weeks. During treatment, both of the treatment groups received Calcichew D3 once a day, which contained 1250 mg of calcium carbonate (providing 500 mg of calcium) and 200 IU of vitamin D3 (General Electric Pharmaceutical Co, Ltd, Boston, MA). Blood was collected before treatment and 16 h after subcutaneous injection of rhPTH (1–34) at week 24 after treatment and at week 48 after treatment. All women were questioned about adverse events at each follow-up visit and were asked to rate the severity of each event as mild, moderate, or severe. In addition, adverse reactions were recorded.
2.2. Collection of general characteristics
Basic clinical data of all subjects were collected, including age, duration of menopause, height, weight, blood pressure, and fracture before the treatment. The BMI was calculated by BMI = weight (kg)/height2 (m2).
2.3. Measurements of biochemical markers
The serum levels of calcium (Ca), phosphorus (P), total cholesterol (TC), triglyceride (TG), alanine transaminase (ALT), aspartate transaminase (AST), urea (Ure), and creatinine (Cr) were measured by MODULA automatic biochemical analyzer (Roche, Basel, Switzerland). Biochemical markers for bone turnover included bone formation markers alkaline phosphatase (ALP) and N-terminal propeptide of type I collagen (PINP), and bone resoption marker C-telopeptide of type I collagen (β-CTX). The levels of serum ALP were measured by radioimmunoassay. Serum PINP and β-CTX levels were determined by electrochemiluminescence (Cobas e601; Roche, Basel, Switzerland).
2.4. Chemiluminescence assay
The levels of serum parathyroid hormone (PTH) were tested by chemiluminescence method (DX1800; Beckman, Brea, CA), with the normal range being 12.0–88.0 pg/mL.
2.5. Enzyme-linked immunosorbent assay (ELISA)
The serum levels of vascular endothelial growth factor (VEGF) and platelet-derived growth factor-BB (PDGF-BB) were determined using ELISA kits according to the manufacturer's manuals (Beijing KYM Science Co, Ltd, Beijing, China).
2.6. Radiography
Areal BMD (g/cm2) was assessed using dual-energy X-ray absorptiometry (Hologic Discovery A; Hologic, Bedford, MA). The coefficient of variation of the DXA scans was 1.7%–1.8% (CV, 0.017–0.018). BMD at lumbar spine (L1–4), total hip and femoral neck of all subjects were measured before treatment, at week 24 and at week 48 after treatment. A vertebral fracture was defined according to the vertebral height on lateral X-ray films. The assessment for vertebral fractures was performed at T4-L4 level.
2.7. Statistical analyses
Statistical analyses were performed using SPSS 19.0 software (IBM, Armonk, NY). Measurement data with normal distribution were expressed as means ± standard deviation. Measurement data with skewed distribution were expressed as median (minimum and maximum). Count data were expressed in rate (%). Baseline normal distribution data were compared between the 2 groups using independent sample t test. Data with skewed distribution were compared between the 2 groups using non-parametric test (rank sum test). Count data were compared using Pearson Chi-squared test. Analysis of variance of repeated measurement data was used to compare differences between 2 treatment groups or before and after treatment within the same group. Pearson Chi-squared test or Fisher exact test were used to examine the rates of adverse events between the 2 treatment groups. Differences with P < .05 were considered statistically significant.
3. Results
3.1. General characteristics of the subjects
To have an overall understanding of the subjects, general characteristics of all 65 patients were collected. There were no significant differences between ALN and PTH groups at baseline in age, years after menopause, BMI, history of fractures, serum calcium, phosphorus, total cholesterol, triglyceride, alanine transaminase, aspartate transaminase, urea, creatinine, the levels of serum PTH, serum ALP, PINP, β-CTX, BMD of lumbar spine 1–4 (L1–4), femoral neck and total hip (Table 1). Four women in PTH group withdrew from the test because of adverse events and another 4 withdrew due to personal reasons. No one in ALN group withdrew during the study period. Finally, 35 patients in PTH group and 22 patients in ALN group completed the entire study. During the 48-week treatment period, 1 patient in ALN group experienced nonvertebral fracture, whereas no patients in PTH group had fracture. There was no significant difference between the 2 treatment groups.
Table 1.
General characteristics (means ± standard deviation unless noted) of the subjects.
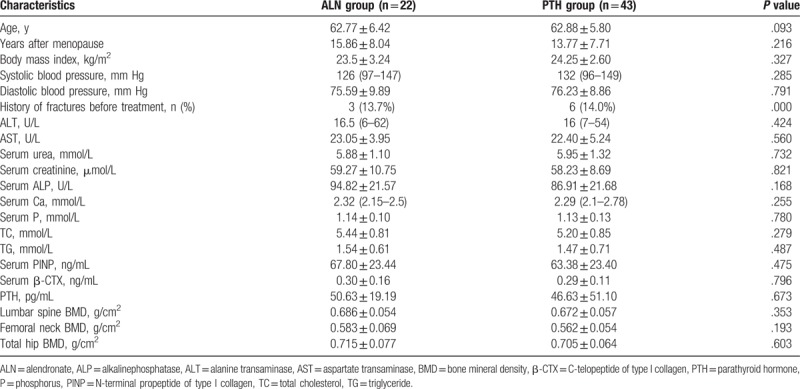
3.2. rhPTH and ALN treatments have different effects on changes in BMD of lumbar spine, femoral neck and total hip
To test BMD, dual-energy X-ray absorptiometry was performed. After 48 weeks of treatment, both of the treatment groups showed significantly increased BMD of lumbar spine (1–4). The BMD of lumbar spine (1–4) was increased by 1.5% at week 24 (P > .05 compared with baseline) and 4.0% at week 48 (P < .001 compared with baseline) in PTH group. The percentage of increase in BMD at lumbar spine (1–4) was 0.9% at week 24 (P > .05 compared with baseline) and 3.5% at week 48 (P < .001 compared with baseline) in ALN group. There were no significant difference in lumbar spine BMD between the 2 groups at week 24 or week 48 (P > .05) (Fig. 1A). The percentage increase in the BMD at femoral neck in ALN group was 1.5% (P > .05 compared with baseline) and 2.4% (P < .05 compared with baseline) at week 24 and week 48, respectively. However, rhPTH treatment decreased femoral neck BMD by 1.2% at week 24 and 0.3% at week 48 (P > .05 compared with baseline). There were significant differences in the changes of femoral neck BMD between the 2 treatment groups at week 24 or week 48 (P < .05) (Fig. 1B). Total hip BMD increased in ALN group was 0.9% at week 24 (P > .05 compared with baseline). However, total hip BMD was decreased by 2.8% in PTH group (P < .001 compared with baseline). There was significant difference in change of total hip BMD between the 2 treatment groups at week 24 (P < .001). Total hip BMD at week 48 was decreased by 0.1% (P > .05 compared with baseline) in ALN group and by 1.8% (P < .01 compared with baseline) in PTH group. There was no significant difference in changes of total hip BMD between the 2 groups at week 48 (P > .05) (Fig. 1C). These results suggest that rhPTH increases the BMD of lumbar spine (1–4), but decreases the BMD of femoral neck and total hip at week 48 after treatment. By contrast, ALN enhances the BMD of lumbar spine (1–4) and femoral neck, but reduces the BMD of total hip at week 48 after treatment.
Figure 1.
Changes of bone mineral density (BMD) before and after treatment at (A) lumbar spine, (B) femoral neck and (C) total hip. The percentages of changes from baseline were measured before treatment and at week 24 and week 48 after treatment. #P < .05 and ∗P < .001 between recombinant human parathyroid hormone (PTH) group and alendronate (ALN) group at the same time point. ALN = alendronate, BMD = bone mineral density, PTH = parathyroid hormone.
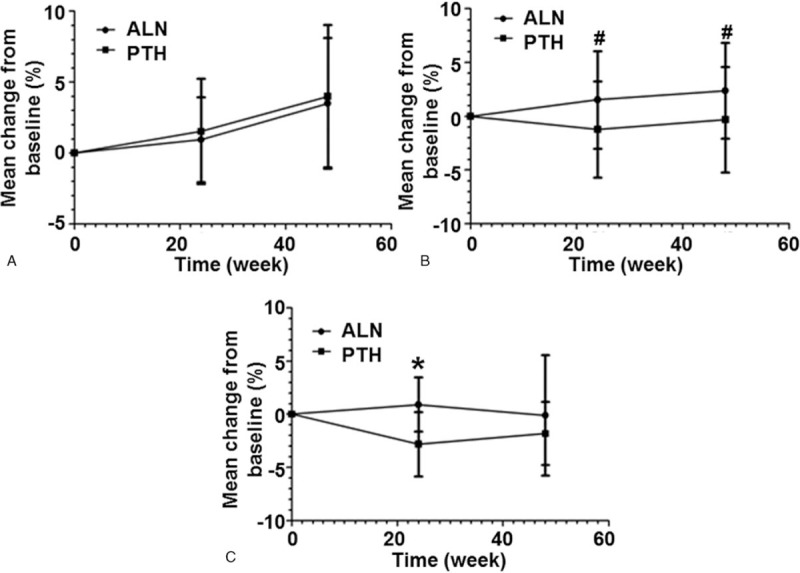
3.3. rhPTH and ALN have opposite effects on serum levels of PINP, ALP, and β-CTX
To investigate bone turnover, serum levels of biochemical markers PINP, ALP, and β-CTX were measured. In PTH group, the serum PINP levels were significantly elevated above baseline at week 24 after treatment (median, 468.5%; P < .001) and remained significantly elevated at week 48 (median, 420.2%; P < .001). In ALN group, the serum PINP levels were decreased by 70.0% compared with baseline values at week 24 (P < .001) and decreased by 69.1% compared with baseline values at week 48 (P < .001). There was significant difference in changes of serum PINP levels between the 2 treatment groups at week 24 and week 48 (P < .001) (Fig. 2A). Moreover, PTH group significantly increased the levels of serum ALP over the 48-week period (increased by 67.8% at week 24 and 78.2% at week 48). ALN group had reduced the levels of serum ALP after 24 and 48 weeks of treatment, compared with baseline values (31.4% and 26.6%, respectively). Differences in serum ALP levels between the 2 groups were significant at both week 24 and week 48 (P < .001) (Fig. 2B). In PTH group, the levels of serum β-CTX were significantly elevated compared to baseline at week 24 (median, 323.2%; P < .001) and remained significantly elevated at week 48 (median, 349.0%; P < .001). Treatment with ALN decreased the concentration of serum β-CTX by 50.6% compared with baseline at week 24 (P < .001) and by 32.6% compared with baseline at week 48 (P < .001). There were significant difference in changes of serum β-CTX levels between the 2 treatment groups at both week 24 and week 48 (P < .001) (Fig. 2C). These results indicate that rhPTH and ALN have opposite effects on serum levels of PINP, ALP, and β-CTX.
Figure 2.
Changes of (A) PINP, (B) ALP and (C) β-CTX levels in serum before and after treatment. The percentages of changes from baseline were measured before treatment and at week 24 and week 48 after treatment. ∗P < .001 between recombinant human parathyroid hormone (PTH) group and alendronate (ALN) group at the same time point. ALN = alendronate, ALP = alkalinephosphatase, β-CTX = C-telopeptide of type I collagen, PINP = N-terminal propeptide of type I collagen, PTH = parathyroid hormone.
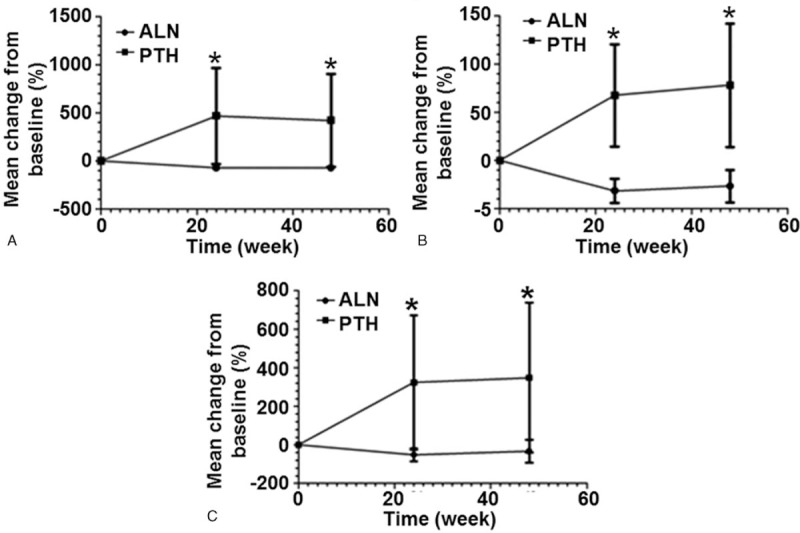
3.4. Both rhPTH and ALN treatments significantly decrease the serum levels of PDGF-BB, but not the levels of VEGF
To determine the serum levels of VEGF and PDGF-BB, ELISA was carried out. Compared with treatment before, the serum levels of VEGF were increased by 23.4% in PTH group at week 48. The percentages of increases in VEGF levels in ALN group were 20.4% at week 48 compared with baseline. In both of the PTH and ALN groups, the levels of serum VEGF at week 48 were not significantly different from that before treatment (P > 0.05). In addition, there were no significant differences in changes of VEGF levels between the 2 treatment groups at week 48 (P > .05) (Fig. 3A). By contrast, both PTH and ALN significantly decreased the levels of PDGF-BB at week 48 compared with those before treatment (P < .001). However, no statistically significant difference was detected in the levels of serum PDGF-BB between the 2 treatment groups at week 48 (Fig. 3B). These results suggest that both rhPTH and ALN treatments significantly decrease the serum levels of PDGF-BB, but not the levels of VEGF.
Figure 3.
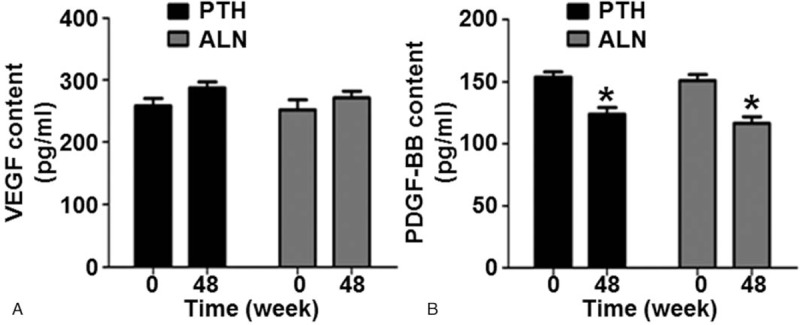
Serum levels of (A) vascular endothelial growth factor and (B) platelet-derived growth factor-BB in PTH group and ALN group before and after treatment. ∗P < .001 compared with baseline within the same treatment group. ALN = alendronate, PTH = parathyroid hormone.
3.5. rhPTH increases the serum levels of calcium, phosphorus, and triglyceride and decreases the serum level of total cholesterol, whereas ALN increases the serum levels of calcium and triglyceride and decreases the serum levels of phosphorus and total cholesterol at week 48
The serum levels of calcium, phosphorus, total cholesterol, and triglyceride were measured by Roche MODULA automatic biochemical analyzer. In PTH group, serum calcium levels were increased by 4.22% at week 48 compared with that before treatment (P < .01). However, the percentage increase of serum calcium levels in ALN group was only 0.11% at week 48 (P > .05). There were significant differences in changes of serum calcium levels between the 2 treatment groups at week 24 and week 48 (P < .01) (Fig. 4A). The levels of serum phosphorus were increased by 7.07% in PTH group at week 48 compared with that before treatment (P < .05). In ALN group, the serum phosphorus levels were decreased by 4.22% at week 48 compared with that before treatment (P > .05). There were significant differences in changes of serum phosphorus levels between the 2 groups at week 24 and week 48 (P < .01) (Fig. 4B). For PTH group, the serum levels of total cholesterol were decreased below baseline at week 48 (median, 3.4%; P < .05 compared with baseline). In ALN group, the serum levels of total cholesterol were decreased by 10.1% compared with the baseline value at week 48 (P < .01). There was no significant difference in changes of total cholesterol levels between the 2 groups at week 48 (P > .05) (Fig. 4C). The serum levels of triglyceride in PTH group were increased by 15.5% at week 48 (P < .05 compared with baseline), and that in ALN group were increased by 20.0% at week 48 (P > .05 compared with baseline). There was no significant difference in changes of the serum levels of triglyceride between the 2 groups at week 48 (P > .05) (Fig. 4D). These results indicate that rhPTH increases the serum levels of calcium, phosphorus and triglyceride, but decreases the serum level of total cholesterol. ALN increases the serum levels of calcium and triglyceride, but decreases the serum levels of phosphorus and total cholesterol at week 48.
Figure 4.
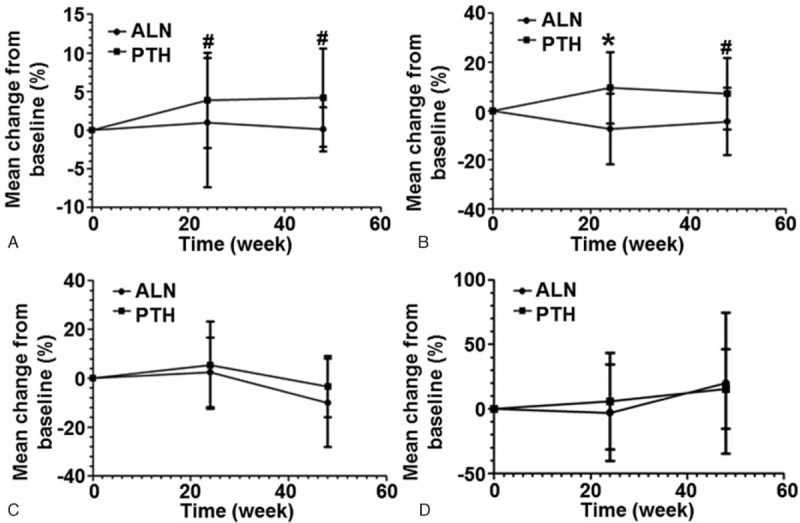
Changes of levels of (A) calcium, (B) phosphorus, (C) total cholesterol, and (D) triglyceride before and after treatment. The percentages of changes from baseline were measured before treatment and at week 24 and week 48 after treatment. #P < .05 and ∗P < .001 between recombinant human parathyroid hormone (PTH) group and alendronate (ALN) group at the same time point. ALN = alendronate, PTH = parathyroid hormone.
3.6. rhPTH and ALN are safe in the treatment of postmenopausal osteoporosis
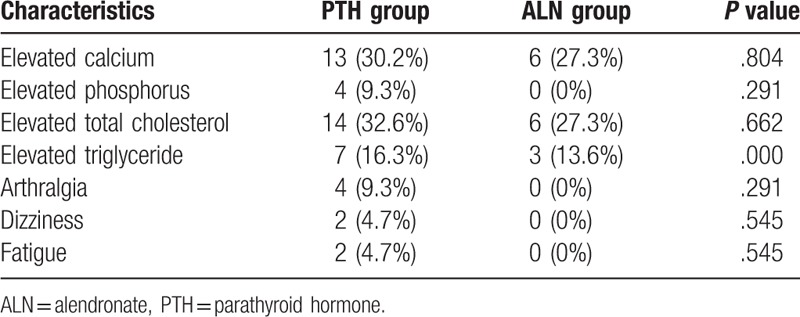
To evaluate the safety of rhPTH and ALN adverse events were monitored. The incidence of drug-related adverse events was 58.1% in PTH group, and 27.3% in ALN group. There was no significant difference between the 2 treatment groups with regard to the incidence of adverse reaction (P > .05). There was no severe drug-related adverse reaction in either treatment group. The main adverse events in PTH group through 48 weeks were elevated calcium, elevated phosphorus, elevated total cholesterol, elevated triglyceride, arthralgia, dizziness, and fatigue. The main adverse events in ALN group included elevated calcium, elevated total cholesterol and elevated triglyceride. The most common adverse event was elevated calcium in either group. The difference in the incidence of elevated calcium between PTH and ALN groups was not statistically significant (P = .804) (Table 2). The results indicate that PTH and ALN are safe in the treatment of postmenopausal osteoporosis.
Table 2.
Adverse events during treatment in parathyroid hormone (PTH) group and alendronate (ALN) group (n, %).
4. Discussion
Parathyroid hormone is a polypeptide hormone that plays an important role in the regulation of calcium, phosphorus and bone metabolism. Low-dose injection of parathyroid hormone not only stimulates bone formation, but also has effects on bone resorption.[14,15] Alendronate is currently recommended for the treatment of postmenopausal osteoporosis, male osteoporosis and glucocorticoid-induced osteoporosis in clinical practice.[16–18]
In this study, we have shown that daily subcutaneous injection of 20 μg rhPTH (1–34) or oral intake of 70 mg alendronate once a week causes significantly increased BMD at lumbar spine in postmenopausal women with osteoporosis after 48 weeks of treatment. The effect of rhPTH (1–34) on the BMD of lumbar spine (1–4) is greater than that of alendronate. McClung et al [19] indicated that 203 postmenopausal women with osteoporosis were treated with 20 μg of injectable teriparatide and oral placebo (n = 102) or 10 mg of oral alendronate and injectable placebo (n = 101) for 18 months, and treatment with alendronate increases the BMD of lumbar spine in postmenopausal women with osteoporosis by 2.8%, whereas treatment with teriparatide has gained additional 7.3% increase in BMD of lumbar spine. The increase in the BMD of lumbar spine observed in the present study is consistent with the previous research, suggesting that low-dose of rhPTH (1–34) treatment increases the BMD of lumbar spine in postmenopausal women with osteoporosis. The percentage increase in the BMD of femoral neck in ALN group at week 48 was 2.4% compared with the value before treatment. By contrast, the BMD of total hip was decreased by 1.8% at week 48 in ALN group. However, rhPTH (1–34) treatment has decreased the BMD of femoral neck by 0.3% and the BMD of total hip by 1.8% at week 48 compared with baseline. McClung et al[19] have shown that teriparatide therapy and alendronate therapy were associated with similarly significant increases from baseline in BMD at femoral neck after 18 months. A meta-analysis of comparison between parathyroid hormone and bisphosphonate treatments on BMD in osteoporosis therapy [20] has revealed that the increases in the BMD of femoral neck are not significant between rhPTH and bisphosphonate treatments (WMD = 2.24; 95% CI: 20.48–4.97; P = .11). Miyauch et al [21] have indicated that teriparatide significantly increases the BMD of total hip in Japanese postmenopausal women with osteoporosis who are treated with teriparatide or placebo-teriparatide for 12 months. There are differences in the BMD of femoral neck and total hip between the present study and the previous study. One reason may be that rhPTH (1–34) is powder-injection, whereas teriparatide is special filling agent. When the patients received subcutaneous injections of them, there is dose difference between them. Another reason may be that the follow-up visit of the present study is short.
The rhPTH (1–34) and alendronate have different influences on BMD by different mechanisms, which are reflected by changes in bone turnover markers. Our study shows that the levels of serum PINP, ALP, and β-CTX are significantly increased at week 24 and week 48 in rhPTH group. By contrast, the serum levels of PINP, ALP, and β-CTX in ALN group are significantly decreased at week 24 and week 48. Body et al[22] have revealed that teriparatide increases the BMD of lumbar spine and total hip in postmenopausal women with osteoporosis by increasing bone ALP and NTX levels. In addition, alendronate increases the BMD of lumbar spine and total hip in postmenopausal women with osteoporosis by reducing bone ALP and NTX levels. Therefore, we speculate that rhPTH (1–34) increases the serum levels of PINP, ALP and β-CTX, leading to increased BMD of lumbar spine. However, alendronate suppresses the serum levels of PINP, ALP and β-CTX, leading to increases in the BMD of lumbar spine and femoral neck.
The rhPTH (1–34) and alendronate have different influences on BMD. This may be more importantly reflected by some cytokines that are associated with bone metabolism. Both VEGF and PDGF-BB are important cytokines that regulate bone metabolism. High VEGF level is found in osteoblasts and osteoclasts.[23–25] VEGF not only has important role in stimulating angiogenesis in fracture repair and the regulation of bone remodeling, but also significantly induces osteoblast differentiation, proliferation, and mineralization, and improves BMD of lumbar spine.[26–29] Recently, intermittent of rhPTH (1–34) treatment has been shown to induce production and gene expression of VEGF in bone marrow osteoprogenitor cells. rhPTH (1–34) treatment also increases VEGF mRNA expression and secretion of VEGF by human osteoblasts in trabecular bone, induce osteoblast proliferation and mineralization, and stimulate bone formation.[30–32] The present study shows that the percentage increase in serum VEGF levels in rhPTH (1–34) group at week 48 is 23.4% higher than the values before treatment. The study indicates that rhPTH (1–34) stimulates bone formation and increases BMD may be by raising the serum levels of VEGF. PDGF-BB is secreted by pre-osteoclasts as well as mature osteoclasts, induces mesenchymal stem cell or osteoblast proliferation and migration, promotes periosteal bone formation.[33,34] The serum levels of PDGF-BB in both bone marrow and peripheral blood are significantly lower in ovariectomized mice than in sham-operation mic.[33] Increasing the maturation of osteoclasts from preosteoclasts reduces PDGF-BB abundance in ovariectomized mice, probably having an influence on bone formation.[33] The finding in the present study shows that the percentage decrease in serum PDGF-BB levels in rhPTH (1–34) group at week 48 is 16.6% compared with the values before treatment. We speculate that rhPTH (1–34) reduces the BMD of femoral neck and total hip may be by decreasing the serum levels of PDGF-BB. However, more researches are needed to confirm this idea.
The main drug-related adverse events in rhPTH group during 48-week treatment are elevated calcium, elevated phosphorus, elevated total cholesterol, elevated triglyceride, arthralgia, dizziness, and fatigue. However, the adverse events in ALN group are elevated calcium, elevated total cholesterol and elevated triglyceride. Previous studies have indicated that daily continuous administration of teriparatide increases the serum levels of calcium, and gives rise to hypercalcemia.[35,36] Postmenopausal women with osteoporosis experience an increase in mean whole body fat percentage compared with that before treatment in teriparatide or alendronate group.[37,38] However, lipid parameters are not affected by teriparatide treatment or by alendronate treatment.[37,38] Therefore, we speculate that rhPTH may have influence on lipid metabolism. Therefore, the levels of calcium, total cholesterol, and triglyceride should be measured closely when postmenopausal women are treated by rhPTH (1–34). In conclusion, the present study confirms that the efficacy of rhPTH (1–34) on the BMD of lumbar spine (1–4) is similar to that of alendronate in the treatment of postmenopausal osteoporosis. The effect of rhPTH (1–34) on the BMD of femoral neck or total hip is weaker than that of alendronate. rhPTH (1–34) increases the serum levels of PINP, ALP, and β-CTX and hence, increasing the BMD of lumbar spine. rhPTH (1–34) increases the BMD of lumbar spine (1–4) may be by raising the serum levels of VEGF. rhPTH (1–34) reduces the BMD of femoral neck and total hip may be by decreasing the serum levels of PDGF-BB.
Author contributions
Conceptualization: Zhengping Feng.
Data curation: Yue Li.
Formal analysis: Tingting Pan.
Funding acquisition: Zhengping Feng.
Software: Changhong Zhao.
Supervision: Zhengping Feng, Qifu Li.
Writing – original draft: Jing Deng.
Footnotes
Abbreviations: ALN = alendronate, ALP = alkaline phosphatase, ALT = alanine transaminase, AST = aspartate transaminase, BMD = bone mineral density, Ca = calcium, Cr = creatinine, CTX = C-telopeptide of type I collagen, DXA = dual-energy X-ray absorptiometry, ELISA = enzyme-linked immunosorbent assay, P = phosphorus, PDGF-BB = platelet-derived growth factor-BB, PINP = propeptide of type I collagen, PTH = parathyroid hormone, rhPTH = recombinant human parathyroid hormone, TC = total cholesterol, TG = triglyceride, Ure = urea, VEGF = vascular endothelial growth factor.
The study was supported by the National Key Clinical Specialties Construction Program of China and the Science Foundation of Chongqing Municipal Heath Bureau (no: 2012-2-041).
The authors have no conflicts of interest to disclose.
References
- [1].Hintze G, Graf D. Osteoporosis [in German]. Med Monatsschr Pharm 2016;39:228–34. [PubMed] [Google Scholar]
- [2].Jackson RD, Mysiw WJ. Insights into the epidemiology of postmenopausal osteoporosis: the women's health initiative. Semin Reprod Med 2014;32:454–62. [DOI] [PubMed] [Google Scholar]
- [3].Stauffer M, Baylink D, Wergedal J, et al. Decreased bone formation, mineralization, and enhanced resorption in calcium-deficient rats. Am J Physiol 1973;225:269–76. [DOI] [PubMed] [Google Scholar]
- [4].Sissons HA, Kelman GJ, Marotti G. Mechanisms of bone resorption in calcium-deficiet rats. Calcif Tissue Int 1984;36:711–21. [DOI] [PubMed] [Google Scholar]
- [5].Rogers MJ, Crockett JC, Coxon FP, et al. Biochemical and molecular mechanisms of action of bisphosphonates. Bone 2011;49:34–41. [DOI] [PubMed] [Google Scholar]
- [6].Xu Z. Alendronate for the treatment of osteoporosis in men: a meta-analysis of randomized controlled trials. Am J Ther 2017;24:e130–8. [DOI] [PubMed] [Google Scholar]
- [7].Yu T, Witten PE, Huysseune A, et al. Live imaging of osteoclast inhibition by bisphosphonates in a medaka osteoporosis model. Dis Model Mech 2016;9:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Canalis E, Giustina A, Bilezikian JP. Mechanisms of anabolic therapies for osteoporosis. N Engl J Med 2007;357:905–16. [DOI] [PubMed] [Google Scholar]
- [9].Augustine M, Horwitz MJ. Parathyroid hormone and parathyroid hormone-related protein analogs as therapies for osteoporosis. Curr Osteoporos Rep 2013;11:400–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Huang TW, Chuang PY, Lin SJ, et al. Teriparatide improves fracture healing and early functional recovery in treatment of osteoporotic intertrochanteric fractures. Medicine 2016;95:e3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Song J, Jin Z, Chang F, et al. Single and combined use of human parathyroid hormone (PTH) (1–34) on areal bone mineral density (aBMD) in postmenopausal women with osteoporosis: evidence based on 9 RCTs. Med Sci Monit 2014;20:2624–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yang Y, Zhang XJ, Zhu XJ, et al. Comparison between recombinant human parathyroid hormone (1–34) and elcatonin in treatment of primary osteoporosis. Asian Pac J Trop Med 2015;8:79–84. [DOI] [PubMed] [Google Scholar]
- [13].Iwamotoa J, Konob H, Uzawab M. Comparative effect of alendronate and teriparatide on bone mineral density and bone turnover among Japanese postmenopausal women with history of fragility fractures: A clinical practice-based observational study. Osteoporos Sarcopenia 2015;1:63–9. [Google Scholar]
- [14].Isogai Y, Takao-Kawabata R, Takakura A, et al. Early effects of single and low-frequency repeated administration of teriparatide, hpth (1–34), on bone formation and resorption in ovariectomized rats. Calcif Tissue Int 2015;97:412–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sugiyama T, Torio T, Sato T, et al. Improvement of skeletal fragility by teriparatide in adult osteoporosis patients: a novel mechanostat-based hypothesis for bone quality. Front Endocrinol (Lausanne) 2015;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gatti D, Adami S, Viapiana O, et al. The use of bisphosphonates in women: when to use and when to stop. Expert Opin Pharmacother 2015;16:2409–21. [DOI] [PubMed] [Google Scholar]
- [17].Giusti A GB. Treatment of primary osteoporosis in men. Clin Interv Aging 2014;10:105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rossini M, Orsolini G, Viapiana O, et al. Bisphosphonates in the treatment of glucocorticoid-induced osteoporosis: pros. Endocrine 2015;49:620–7. [DOI] [PubMed] [Google Scholar]
- [19].McClung MR, San Martin J, Miller PD, et al. Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med 2005;165:1762–8. [DOI] [PubMed] [Google Scholar]
- [20].Shen L, Xie X, Su Y, et al. Parathyroid hormone versus bisphosphonate treatment on bone mineral density in osteoporosis therapy: a meta-analysis of randomized controlled trials. PLoS One 2011;6:e26267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Miyauchi A, Matsumoto T, Sugimoto T, et al. Effects of teriparatide on bone mineral density and bone turnover markers in Japanese subjects with osteoporosis at high risk of fracture in a 24-month clinical study: 12-month, randomized, placebo-controlled, double-blind and 12-month open-label phases. Bone 2010;47:493–502. [DOI] [PubMed] [Google Scholar]
- [22].Body JJ, Gaich GA, Scheele WH, et al. A randomized double-blind trial to compare the efficacy of teriparatide [recombinant human parathyroid hormone (1–34)] with alendronate in postmenopausal women with osteoporosis. J Clin Endocrinol Metab 2002;87:4528–35. [DOI] [PubMed] [Google Scholar]
- [23].Horner A, Bord S, Kelsall AW, et al. Tie2 ligands angiopoietin-1 and angiopoietin-2 are coexpressed with vascular endothelial cell growth factor in growing human bone. Bone 2001;28:65–71. [DOI] [PubMed] [Google Scholar]
- [24].Huang H, Ma L, Kyrkanides S. Effects of vascular endothelial growth factor on osteoblasts and osteoclasts. Am J Orthod Dentofacial Orthop 2016;149:366–73. [DOI] [PubMed] [Google Scholar]
- [25].Li J, Wang Y, Bao XM, et al. Study on RhBMP-2 induced osteoporosis rat BMSCs in vitro osteogenesis and VEGF expression [in Chinese]. Zhongguo Gu Shang 2015;28:446–9. [PubMed] [Google Scholar]
- [26].Hu K, B RO. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone 2016;91:30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jabalee J, Franz-Odendaal TA. Vascular endothelial growth factor signaling affects both angiogenesis and osteogenesis during the development of scleral ossicles. Dev Biol 2015;406:52–62. [DOI] [PubMed] [Google Scholar]
- [28].Hu K, Olsen BR. Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J Clin Invest 2016;126:509–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Costa N, Paramanathan S, Mac Donald D, et al. Factors regulating circulating vascular endothelial growth factor (VEGF): association with bone mineral density (BMD) in post-menopausal osteoporosis. Cytokine 2009;46:376–81. [DOI] [PubMed] [Google Scholar]
- [30].Esbrit P, Alvarez-Arroyo MV, De Miguel F, et al. C-terminal parathyroid hormone-related protein increases vascular endothelial growth factor in human osteoblastic cells. J Am Soc Nephrol JASN 2000;11:1085–92. [DOI] [PubMed] [Google Scholar]
- [31].Drake MT, Srinivasan B, Modder UI, et al. Effects of intermittent parathyroid hormone treatment on osteoprogenitor cells in postmenopausal women. Bone 2011;49:349-–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Alonso V, de Gortazar AR, Ardura JA, et al. Parathyroid hormone-related protein (107-139) increases human osteoblastic cell survival by activation of vascular endothelial growth factor receptor-2. J Cell Physiol 2008;217:717–27. [DOI] [PubMed] [Google Scholar]
- [33].Xie H, Cui Z, Wang L, et al. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat Med 2014;20:1270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rahman MM, Matsuoka K, Takeshita S, et al. Secretion of PDGF isoforms during osteoclastogenesis and its modulation by anti-osteoclast drugs. BiochBioem Biophys Res Commun 2015;462:159–64. [DOI] [PubMed] [Google Scholar]
- [35].Migliore A, Broccoli S, Massafra U, et al. Mixed-treatment comparison of anabolic (teriparatide and PTH 1-84) therapies in women with severe osteoporosis. Curr Med Res Opin 2012;28:467–73. [DOI] [PubMed] [Google Scholar]
- [36].Ponnapakkam T, Katikaneni R, Sakon J, et al. Treating osteoporosis by targeting parathyroid hormone to bone. Drug Discov Today 2014;19:204–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Passeri E, Dozio E, Mendola M, et al. Treatment with teriparatide might be associated with cardiometabolic changes in postmenopausal severe osteoporotic women. J Biol Regul Homeost Agents 2015;29:931–40. [PubMed] [Google Scholar]
- [38].Schafer AL, Sellmeyer DE, Schwartz AV, et al. Change in undercarboxylated osteocalcin is associated with changes in body weight, fat mass, and adiponectin: parathyroid hormone (1–84) or alendronate therapy in postmenopausal women with osteoporosis (the PaTH study). J Clin Endocrinol Metab 2011;96:E1982–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


