Supplemental Digital Content is available in the text
Keywords: diagnosis, internal medicine, lymphoma, prognosis
Abstract
Lymphomas are common malignancies with highly variable clinical presentations and prognosis. Prognostic value of clinical presentation at onset is still questioned. The objective of this study was to compare the disease presentation and the outcome of lymphomas diagnosed in an Internal Medicine Department of a University Hospital to disease presentation and outcome of patients who were referred to the Hematology Department of the same institution by other departments or healthcare facilities.
This retrospective monocentric observational study included 37 patients. They were matched to 73 patients, who were referred to the Hematology Department, according to age, histology, and Ann Arbor stage. The demographics, clinical and biological presentations, overall survival, and progression-free survival were compared.
Patients diagnosed with lymphoma in the Internal Medicine Department were more likely to be febrile (67.5% vs 21.9%; P < .001) and have higher inflammatory markers (mean C-reactive protein 86.6 vs 56.3 mg/L; P = .02). The median overall survival of these patients was poorer (P < .001), even in the subset of patients treated with standard treatment, and remained shorter in multivariable analysis (P = .002). The specific treatment started earlier (20.2 vs 37.5 days; P = .006), but was more frequently palliative (37.8% vs 19.2%; P = .04). There was no significant difference in median progression-free survival.
Lymphomas diagnosed in an Internal Medicine Department had aggressive clinical presentations and a poorer outcome, despite an early start of conventional treatment.
1. Introduction
The World Health Organization (WHO) 2008 classification identifies 74 different entities of lymphomas, ranging from lymphomas with indolent evolution, to the most aggressive forms such as diffuse large B-cell lymphomas (DLBCL).[1] Prognosis is evaluated by risk factors such as age, Ann Arbor staging, lactic dehydrogenase (LDH) levels, and other disease-specific factors grouped in prognostic scoring systems, like International Prognostic Index (IPI)[2] or Hasenclever International Prognostic Score (IPS).[3]
At the Regional University Hospital Centre (CHRU) of Tours, France, lymphomas are more likely to be diagnosed before admission to the Hematology Departments. All the treatments are overseen by hematologists. Diagnoses are made by other physicians, according to their clinical presentations. In the Internal Medicine Department (IMD), some lymphomas are diagnosed with an unusual clinical presentation. In our experience, these lymphomas seem to have a more pejorative prognosis. In the literature, the initial clinical presentation of lymphomas has never been correlated with their prognosis.
The aim of the present study was to compare clinical presentations and outcomes of lymphomas diagnosed in an IMD to lymphomas diagnosed by the other departments and healthcare facilities.
2. Material and methods
2.1. Selection of patients
The study was a monocentric retrospective noninterventional study. Patients with lymphomas were included in the IMD and matched to controls diagnosed by other departments and sent to the Hematology Department of our institution. Inclusion criteria were a primary lymphoma diagnosed during their hospital stay or a few weeks before, without any previous lymphoma-specific treatment. Patients were identified in IMD by interrogation of our electronic records. All admissions between January 1, 2008 and July 31, 2013 were considered. Patients with a previous history of lymphoma were excluded. Hematology patients were identified by interrogating the hospitalisation and consultation reports database. The date of latest patient updates was set at December 1, 2015. For all patients, therapeutic management was proposed by the hematologists in the CHRU of Tours, after a multidisciplinary meeting.
This study was approved by the Ethics Committee of the CHRU of Tours on November 3, 2015. Informed consent of the relatives of deceased patients was waived. Patients who were still alive at the time of data collection were contacted. Their informed consents for this study were collected.
2.2. Data collection and parameters studies
All the following data were collected from each medical record in a standardized form.
Epidemiological data included date of birth, date of consultation or first day of hospitalization, and date of histological diagnosis. The date of onset of symptoms was assessed by patients themselves. Charlson comorbidity score was calculated according to previous work.[4] Clinical signs were noted at the initial examination. The presence of general symptoms and performance status according to the WHO were recorded. The possible presence of lymphadenopathy was mentioned, and also the presence of physical signs (eg, neurological, cardiovascular, pulmonary, gastrointestinal signs). Radiological features included the possible presence of deep lymph nodes, hepatomegaly, or splenomegaly on the staging established by computed tomography (CT) or positron emission coupled to CT (PET-CT).
The following laboratory values were obtained: hemoglobin, platelet count, white blood cell count, neutrophil count, lymphocyte count, and monocyte count. The serum LDH, serum beta-2-microglobulin, serum C-reactive protein (CRP), serum albumin, and serum ferritin rates were collected. Lymphocytes-to-monocytes ratio was calculated for all patients. Neutrophils-to-lymphocytes (NtL) and neutrophils/(leukocytes – neutrophils) (NtLN) ratios were calculated only for patients with DLBCL. Pathologic thresholds of these ratios were, respectively, below 2.6 for lymphocytes-to-monocytes ratio,[5] over 3.5 for NtL,[6] and over 4.0 for NtLN.[7]
Type of lymphoma and, in case of DLBCL, MIB-1 values were specified.[8] Low recruitment among T-cell, marginal zone, and mantle cell lymphoma justified the grouping of these patients in the “non-Hodgkin, nonlarge B-cell lymphomas” group (NHNBL). Stage was determined according to Ann Arbor classification.[9] Localized lymphomas gathered stages I and II, and disseminated lymphomas gathered stages III and IV. The prognostic score (IPS or IPI) was calculated according to the current classification for the different types of lymphomas.[2,3]
The date of initiation of corticosteroids or any specific treatments was recorded. Their dates of implementation were defined from the diagnosis date. Overall survival (OS) and progression-free survival (PFS) were calculated from the date of starting treatment, when applicable. Otherwise, they were calculated from the date of diagnosis. Standard treatments and palliative treatments were separated. Palliative treatments included absence of treatment and low-intensity treatments.
2.3. Populations
A 2:1 matching was carried out on patients included in IMD. For the “Hematology” group, the 2 first nonredundant patients corresponding to the eligible criteria of age close to 5 years, types of lymphoma, and Ann Arbor stage with the closest day of diagnosis with the match were selected.
2.4. Statistical analysis
Both populations were compared on the various parameters measured. Comparisons of proportions were calculated using the Fisher exact test. Comparisons of means were determined using the Mann–Whitney test. The OS and PFS curves were performed using the Kaplan–Meier method through the IBM SPSS Version 20.0 software, and comparisons of survival curves with the log-rank test. Multivariate analysis was conducted using the Cox model, taking into account all measured parameters that had a significance level of less than 0.1. The significance level was set at 5%.
3. Results
Forty-two patients with an initial diagnosis of lymphoma in the IMD were identified. Four were excluded from the analysis because of a missing biopsy. One patient refused to participate in the study. One patient with a stage IV blastoid-variant of mantle cell lymphoma was included, but could not be matched because of the absence of a second control. Overall, 37 patients from IMD were matched to 73 patients from Hematology.
3.1. Patient characteristics
Non-Hodgkin, nonlarge B-cell lymphomas in the IMD group included 3 marginal zone lymphomas, 1 digestive mucosa-associated lymphoid tissue (MALT) lymphoma, 1 blastoid-variant of mantle cell lymphoma, 1 lymphocytic lymphoma, 2 angio-immunoblastic T-cell lymphomas, 1 anaplastic T-cell lymphoma, 1 peripheral T-cell lymphoma, 1 lymphoblastic T-cell lymphoma, and 1 T-cell lymphoma with alpha-beta phenotype.
There was no significant difference between the 2 groups on sex, age, type of lymphoma, Ann Arbor stage, and prognostic score (Table 1). There was no significant difference in the Charlson scores between the 2 groups and in the period between the date of onset of symptoms and the date of histological diagnosis.
Table 1.
Patients characteristics.
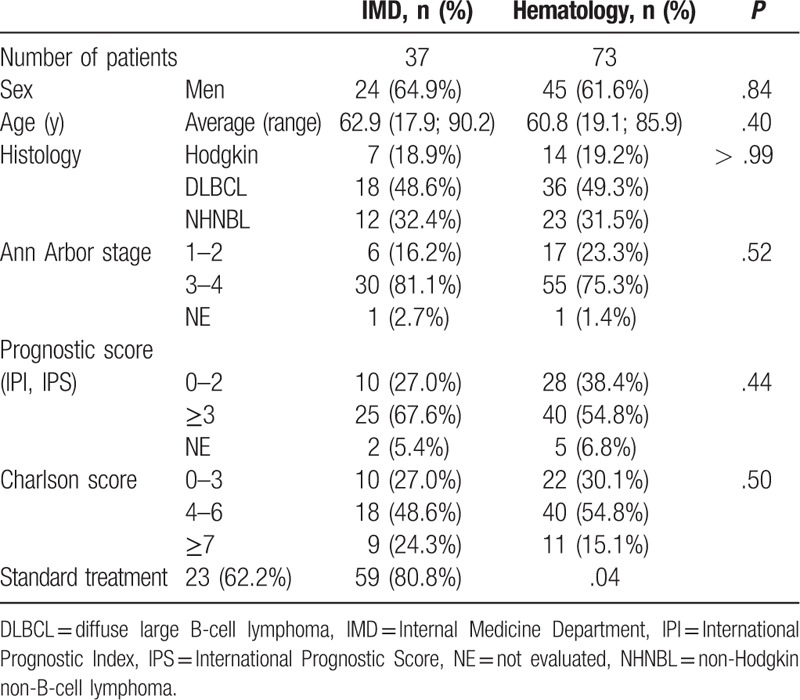
Fever was more frequently present at the first examination in the IMD patients (67.6% vs 21.9%; P < .001) like other B symptoms, except night sweats and pruritus. There was no significant difference in clinical manifestations excepting the presence of cutaneous signs (29.7% vs 12.3%; P = .04) (Supplemental Table 1). Mean performance status was worse in the IMD patients (1.66 vs 1.17; P = .04). The most frequent CT abnormalities in the IMD group were hepatomegaly (43.2% vs 8.2%; P < .001) and splenomegaly (35.1% vs 17.8%; P = .03).
Biological data were not significantly different in the 2 groups (Supplemental Table 2). Only the absolute platelet count and the lymphocytes-to-monocytes ratio were significantly lower in IMD patients (respectively, 222 vs 269 G/L; P = .05, and 2.6 vs 4.5; P = .04). Ferritin levels were not compared between the 2 groups as these data were missing for 50 patients in Hematology and 16 in IMD. CRP mean value tends to be higher in the IMD group (mean CRP 86.6 vs 56.3 mg/L; P = .02). In IMD patients, 3.9 times more liver impairments were observed (27.0% vs 6.8%; P = .007). In DLBCL subgroups, there was no significant difference with NtL or NtLN.
The implementation of an aggressive treatment was less frequent in the IMD group (62.2% vs 80.8%; P = .04) and earlier (20.2 vs 37.5 days; P = .006) in comparison to the Hematology group.
3.2. Overall survival
The median OS was significantly lower in IMD patients (P < .001). The difference remained significant (P = .04) in the subgroup of patients treated with a standard treatment (Fig. 1). This result remained significant (P = .002) after a multivariate analysis (Fig. 2).
Figure 1.
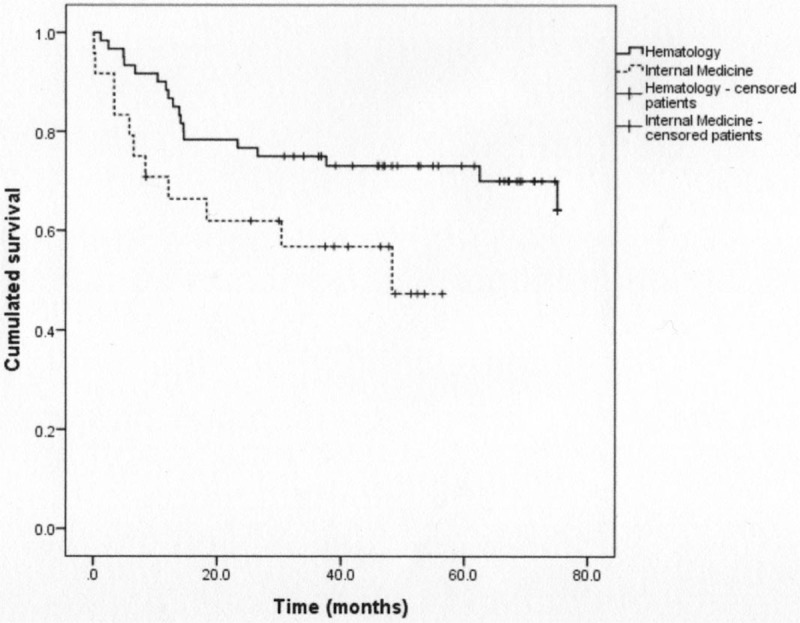
Overall survival curves in the 2 groups with only patients who received standard treatment (P = .04).
Figure 2.
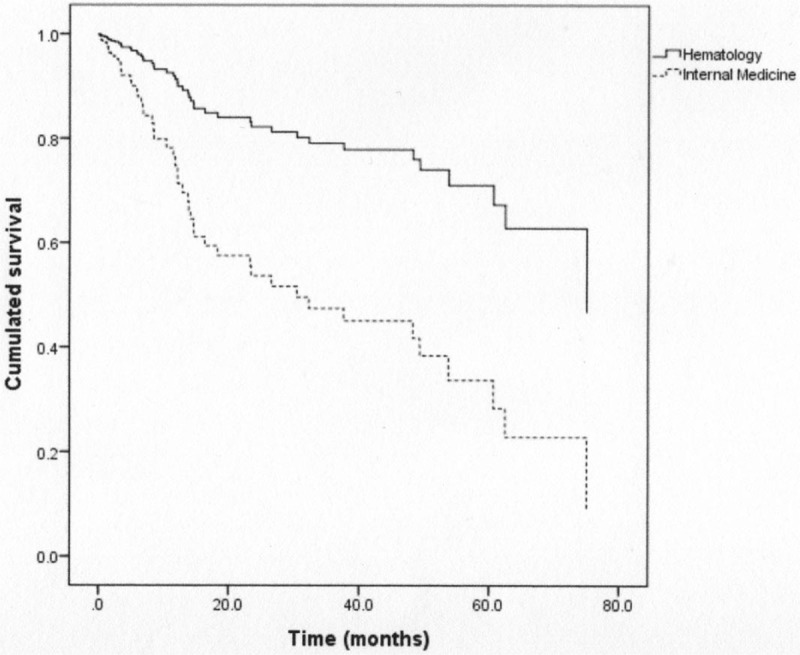
Overall survival curves in the 2 groups from the multivariate analysis (P = .002).
Patients who had a HL seem to have an improved median OS than those with other types of lymphomas, but it was not significant. Biological abnormalities associated with a shorter OS were serum albumin lower than 35 g/L (P < .001), cholestasis (P = .01), serum beta-2-microglobulin over 2.5 mg/L (P = .001), CRP over 10 mg/L (P = .03), and monocytosis over 1 G/L (P = .01). Patients treated with a low-intensity treatment had a poorer survival (P < .001), like patients whose treatment started early, within 21 days of diagnosis (P = .004).
In DLBCL subgroup, NtL ratio higher than 3.5, NtLN ratio equal to or over 4, and MIB-1 value equal to or over 85% were not significantly associated with a lower worse outcome.
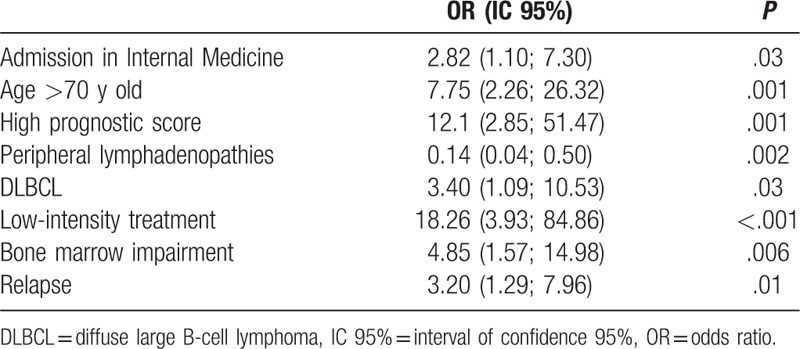
A multivariate analysis was performed, including patients with complete data (67 patients including 22 patients from IMD). In this analysis, the factors that were independently associated with poorer median OS were initially admission in IMD, age over 70 years, high prognostic score, DLBCL, palliative treatment, bone marrow impairment, and a relapse (Table 2). Presence of peripheral lymphadenopathies was associated with a better OS.
Table 2.
Multivariate analysis for overall survival.
The causes of death were the evolution of the lymphoma (13 patients in IMD vs 13 in Hematology), the infectious complications such as severe sepsis or septic shock (7 vs 2 patients), another etiology (1 vs 4 patients), or an unknown origin (3 vs 11 patients). The difference was significant between the 2 groups (P = .03). Other causes included 1 glioblastoma in the IMD group, the evolution of prostate cancer in 2 cases, 1 myelodysplasia secondary to treatment of lymphoma, and 1 sudden cardiovascular death in the Hematology group.
3.3. Progression-free survival
The median PFS was not significantly different in the 2 groups (P = .07). There was still no significant difference after adjusting for the average of the following factors: age, Charlson score, presence of asthenia, monocytosis, and liver impairment. In multivariate analysis, only the presence of Charlson score over 7 (odds ratio [OR] 1.99, with an interval of confidence between 1.19 and 3.35, P = .009) remained significantly associated with the occurrence of relapses.
4. Discussion
The present study indicates that patients with lymphoma diagnosed in an IMD had a poorer OS than the control group of patients in Hematology, especially in the first 24 months after diagnosis. This difference between the 2 groups exists, despite the initiation of specific therapy in the IMD group. This result raises questions about potential prognostic differences between the 2 groups. In the literature, the prognosis of lymphomas has been evaluated in several works which have validated scoring systems based on clinical and biological features of patients at diagnosis. The originality of our study is the finding that the clinical presentations of lymphomas at onset may be associated to the prognosis of the disease.
The adjustment for age, Ann Arbor stage, and type of lymphoma enabled to overcome validated prognostic factors. Both groups were comparable regarding the Charlson score, and also had similar comorbidities. In the overall population, factors associated with a shorter OS have already been described in the literature, that is, prognostic scores, bone marrow impairment, or age.[2,3] Biological data, like serum beta-2-microglobulin, do not explain the significant difference of OS between the 2 groups, and also mitotic activity (MIB-1). A PET scan was not systematically performed, which meant that we could not compare glucose avidity of lymphomas. We noted that inflammatory parameters were higher in the IMD group, which suggests that inflammation could account for the prognostic difference. In the literature, some associations of inflammatory parameters with a poorer prognosis have been identified such as tumor necrosis factor (TNF)-alpha and interleukin (IL)-6 with all types of lymphomas,[10] IL-10, TNF-alpha, and tumor growth factor-beta with DLBCL,[11] IL-2R-alpha, IL-8, and MIP-1-beta with mantle cell lymphomas,[12] IL-6 and IL-2R in HL.[13] A prospective study would help to correlate a poorer clinical presentation to higher cytokines levels, to include them into prognostic scores, as suggested by Uskudar Teke et al[10] recently.
The second important finding of this study is that lymphomas diagnosed in an IMD seem to have a specific clinical presentation. The prevalence of B symptoms was higher. The initial presence of fever remains associated, firstly, with a higher prognostic score (IPI greater than 2 in 64.5% of cases vs 22.2% in the absence of fever) and, secondly, to a poorer OS.[14]
A higher proportion of DLBCL was diagnosed (48.6%), which is consistent with epidemiological studies.[15] The proportion of HL and T-cell lymphomas, especially in our population, is larger than previously described. Indeed, they accounted for 13% and 5% of all lymphomas in the literature, respectively.[16] This singular recruitment is first explained by an incidence peak of HL, anaplastic anaplastic lymphoma kinase (ALK)-negative T-cell lymphoma, or DLBCL over 55 years old.[17–19] Moreover, the high prevalence of systemic symptoms among these patients explains their admission in IMD. Thus, in HL or in DLBCL, 33% patients have general symptoms.[19,20] Finally, their clinical presentation may be more varied. From 28% to 40% of DLBCL have an extranodular impairment,[19,21] which is also the case for 20% of anaplastic ALK-negative T-cell lymphomas.[18]
Biological data were also comparable between the 2 groups, apart from a significantly lower platelets count and a significantly lower lymphocytes-to-monocytes ratio in IMD patients. Thrombocytopenia was 2 times more common in patients who have a febrile way of revealing of their lymphoma (P = .02), and also leukopenia (P = .009) and lymphopenia (P < .001).[14] However, the presence of an autoimmune thrombocytopenia is not associated with a poorer median OS.[22,23]
The third interest of the present study is the fact that treatments in IMD were often palliative. The relatively small proportion of “treated” patients is likely to be linked with, firstly, the advanced age of the population, and secondly, to a clinical condition which was too poor to tolerate intensive treatments. In the literature, the percentage of patients treated for DLBCL above 80 years is 77.1%,[21] and it decreases to 65.5% beyond 90 years for aggressive lymphomas.[24] Obviously, palliative therapy is independently associated with a worse OS. Several studies have shown that a treatment including rituximab[25] or anthracyclines[26] leads to a better outcome in older or in younger patients. But anthracyclines increase the risk of death and side effects, notably cardiac impairment. In Carson et al's[27] study, 18% of patients died or discontinued treatment after a single dose of doxorubicin. The high prevalence of palliative treatment in IMD patients included in this study can be explained by the reluctance of the practitioners to prescribe intensive treatment which could result in serious side effects. In a single-arm phase II study of the efficacy and tolerance of rituximab combined with reduced-intensity Cyclophosphamide, Hydroxyadriamycin, Vincristine (O for ONCOVIN (R)) and Prednisone, in 150 patients with DLBCL over 80 years, the median OS was 29 months, which underlines the fact that older patients may tolerate attenuated conventional treatments.[28] Another explanation is the fact that performance status was evaluated at the diagnosis. A prephase of corticosteroid therapy for 7 days improves the outcome of patients after the first cycle of chemotherapies.[29] It is important to note that the evaluation of performance status after this prephase could increase the number of patients eligible for standard therapies. However, Bowcock et al[30] treated patients with a poor general condition (performance status 3 or 4): OS rose with intensity of treatment from 0% to 64% at 1 year.
The median PFS in the IMD group was not significantly different from median PFS of patients in Hematology. The prevalence of palliative treatment could participate in the absence of difference in PFS. Charlson score as a factor of poorer outcome was already described.[31] The absolute monocyte and lymphocyte prognostic index greater than 1 and monocytosis do not appear significant in our study.[32,33]
The limitations of our study are its retrospective and monocentric design. The relative low number of included patients is a weakness. A larger and multicentric study would confirm the data of this pilot study. However, our results have challenged the management of lymphomas in our IMD. We estimate that some patients were undertreated and were not offered anthracyclines or rituximab because of a poor physical condition. Through a careful evaluation of patient fitness, particularly after a prephase and with a closer collaboration between internists and hematologists, OS of patients with lymphoma may be improved.
5. Conclusions
Patients with lymphomas diagnosed in IMD have a worse OS, associated with a specific clinical inflammatory presentation, a poorer general condition, and a high prevalence of palliative treatments. A closer collaboration between internists and hematologists might help to improve their prognosis.
Author contributions
Conceptualization: Benoit Pernot, Emmanuel Gyan, François Maillot, Nicole Ferreira-Maldent.
Data curation: Benoit Pernot.
Formal analysis: Benoit Pernot, Emmanuel Gyan.
Methodology: Nicole Ferreira-Maldent.
Resources: Emmanuel Gyan.
Supervision: Nicole Ferreira-Maldent.
Validation: François Maillot, Penelope Hodges, Marjan Ertault, Nicole Ferreira-Maldent.
Writing – original draft: Benoit Pernot.
Writing – review & editing: Benoit Pernot, François Maillot, Nicole Ferreira-Maldent.
Supplementary Material
Footnotes
Abbreviations: CHRU = Regional University Hospital Center, CRP = C-reactive protein, CT = computed tomography, DLCBL = diffuse large B-cell lymphomas, IMD = Internal Medicine Department, IPI = International Prognostic Index, IPS = International Prognostic Score, LDH = lactic dehydrogenase, MALT = mucosa-associated lymphoid tissue, NHNBL = non-Hodgkin nonlarge B-cell lymphomas, NtL = neutrophils-to-lymphocytes ratio, NtLN = neutrophils/(leukocytes – neutrophils) ratio, OS = overall survival, PET-CT = positron emission coupled to computed tomography, PFS = progression-free survival, WHO = World Health Organization.
The authors of this work have nothing to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Campo E, Swerdlow SH, Harris NL, et al. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood 2011;117:5019–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med 1993; 329:987–994. [DOI] [PubMed] [Google Scholar]
- [3].Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin's disease. International Prognostic Factors Project on Advanced Hodgkin's Disease. N Engl J Med 1998;339:1506–14. [DOI] [PubMed] [Google Scholar]
- [4].Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- [5].Li Z-M, Huang J-J, Xia Y, et al. Blood lymphocyte-to-monocyte ratio identifies high-risk patients in diffuse large B-cell lymphoma treated with R-CHOP. PloS One 2012;7:e41658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Porrata LF, Ristow K, Habermann T, et al. Predicting survival for diffuse large B-cell lymphoma patients using baseline neutrophil/lymphocyte ratio. Am J Hematol nov 2010;85:896–9. [DOI] [PubMed] [Google Scholar]
- [7].Troppan K, Deutsch A, Gerger A, et al. The derived neutrophil to lymphocyte ratio is an independent prognostic factor in patients with diffuse large B-cell lymphoma. Br J Cancer 2014;110:369–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gerdes J, Dallenbach F, Lennert K, et al. Growth fractions in malignant non-Hodgkin's lymphomas (NHL) as determined in situ with the monoclonal antibody Ki-67. Hematol Oncol 1984;2:365–71. [DOI] [PubMed] [Google Scholar]
- [9].Carbone PP, Kaplan HS, Musshoff K, et al. Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Res 1971;31:1860–1. [PubMed] [Google Scholar]
- [10].Uskudar Teke H, Gulbas Z, Bal C. Serum levels of cytokines and prevalence of autoantibodies in lymphoma patients and their prognostic value. J BUON 2014;19:191–7. [PubMed] [Google Scholar]
- [11].Tarabar O, Cikota-Aleksić B, Tukić L, et al. Association of interleukin-10, tumor necrosis factor-α and transforming growth factor-β gene polymorphisms with the outcome of diffuse large B-cell lymphomas. Int J Clin Oncol 2014;19:186–92. [DOI] [PubMed] [Google Scholar]
- [12].Sonbol MB, Maurer MJ, Stenson MJ, et al. Elevated soluble IL-2Rα, IL-8, and MIP-1β levels are associated with inferior outcome and are independent of MIPI score in patients with mantle cell lymphoma. Am J Hematol 2014;89:E223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Marri PR, Hodge LS, Maurer MJ, et al. Prognostic significance of pretreatment serum cytokines in classical Hodgkin lymphoma. Clin Cancer Res 2013;19:6812–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhang J, Chen B, Xu X, et al. Clinical features of 66 lymphoma patients presenting with a fever of unknown origin. Intern Med 2012;51:2529–36. [DOI] [PubMed] [Google Scholar]
- [15].Lowry L, Linch D. Non-Hodgkin's lymphoma. Medicine (Baltimore) 2013;41:282–9. [Google Scholar]
- [16].Morton LM. Dissecting lymphoma incidence to inform epidemiologic and clinical research. Leuk Lymphoma 2013;54:1575–6. [DOI] [PubMed] [Google Scholar]
- [17].Marchesi F, Cenfra N, Altomare L, et al. A retrospective study on 73 elderly patients (≥75years) with aggressive B-cell non Hodgkin lymphoma: clinical significance of treatment intensity and comprehensive geriatric assessment. J Geriatr Oncol 2013;4:242–8. [DOI] [PubMed] [Google Scholar]
- [18].Ferreri AJM, Govi S, Pileri SA, et al. Anaplastic large cell lymphoma, ALK-negative. Crit Rev Oncol Hematol 2013;85:206–15. [DOI] [PubMed] [Google Scholar]
- [19].Martelli M, Ferreri AJM, Agostinelli C, et al. Diffuse large B-cell lymphoma. Crit Rev Oncol Hematol 2013;87:146–71. [DOI] [PubMed] [Google Scholar]
- [20].Gobbi PG, Ferreri AJM, Ponzoni M, et al. Hodgkin lymphoma. Crit Rev Oncol Hematol 2013;85:216–37. [DOI] [PubMed] [Google Scholar]
- [21].Griffiths R, Gleeson M, Knopf K, et al. Racial differences in treatment and survival in older patients with diffuse large B-cell lymphoma (DLBCL). BMC Cancer 2010;10:625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hauswirth AW, Skrabs C, Schützinger C, et al. Autoimmune thrombocytopenia in non-Hodgkin's lymphomas. Haematologica 2008;93:447–50. [DOI] [PubMed] [Google Scholar]
- [23].Fink K, Al-Mondhiry H. Idiopathic thrombocytopenic purpura in lymphoma. Cancer 1976;37:1999–2004. [DOI] [PubMed] [Google Scholar]
- [24].Trebouet A, Marchand T, Lemal R, et al. Lymphoma occurring in patients over 90 years of age: characteristics, outcomes, and prognostic factors. A retrospective analysis of 234 cases from the LYSA. Ann Oncol 2013;24:2612–8. [DOI] [PubMed] [Google Scholar]
- [25].Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 2002;346:235–42. [DOI] [PubMed] [Google Scholar]
- [26].Link BK, Brooks J, Wright K, et al. Diffuse large B-cell lymphoma in the elderly: diffusion of treatment with rituximab and survival advances with and without anthracyclines. Leuk Lymphoma 2011;52:994–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Carson KR, Riedell P, Lynch R, et al. Comparative effectiveness of anthracycline-containing chemotherapy in United States veterans age 80 and older with diffuse large B-cell lymphoma. J Geriatr Oncol 2015;6:211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Peyrade F, Jardin F, Thieblemont C, et al. Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: a multicentre, single-arm, phase 2 trial. Lancet Oncol 2011;12:460–8. [DOI] [PubMed] [Google Scholar]
- [29].Pfreundschuh M. How I treat elderly patients with diffuse large B-cell lymphoma. Blood 2010;116:5103–10. [DOI] [PubMed] [Google Scholar]
- [30].Bowcock SJ, Fontana V, Patrick HE. Very poor performance status elderly patients with aggressive B cell lymphomas can benefit from intensive chemotherapy. Br J Haematol 2012;157:391–3. [DOI] [PubMed] [Google Scholar]
- [31].Wildes TM, Augustin KM, Sempek D, et al. Comorbidities, not age, impact outcomes in autologous stem cell transplant for relapsed non-Hodgkin lymphoma. Biol Blood Marrow Transplant 2008;14:840–6. [DOI] [PubMed] [Google Scholar]
- [32].Batty N, Ghonimi E, Feng L, et al. The absolute monocyte and lymphocyte prognostic index for patients with diffuse large B-cell lymphoma who receive R-CHOP. Clin Lymphoma Myeloma Leuk 2013;13:15–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Huang J-J, Li Y-J, Xia Y, et al. Prognostic significance of peripheral monocyte count in patients with extranodal natural killer/T-cell lymphoma. BMC Cancer 2013;13:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


