Abstract
The aim of the present study is to investigate the value of air bronchogram sign on computed tomography (CT) image in the differential diagnosis of solitary pulmonary consolidation lesions (SPLs).
A total of 105 patients (including 39 cases of lung cancer, 43 cases of tuberculosis, and 23 cases of pneumonia) with SPLs were evaluated for the CT features of air bronchogram sign in this retrospective study. The shape and lumen of the bronchi with air bronchogram sign, the length of the involved bronchus with air bronchogram sign, the length of lesion on the same plane and direction, and the ratio between the length of the involved bronchus and that of the lesion were evaluated.
In total, there were 172 segmental and subsegmental bronchi involved. There were 62 segmental and subsegmental bronchi involved among 39 lung cancer cases, 77 segmental and subsegmental bronchi involved among 43 tuberculosis cases, and 33 segmental and subsegmental bronchi involved among 23 pneumonia cases. The shape of the bronchi with air bronchogram sign was significantly different among lung cancer, tuberculosis, and pneumonia (P < .05). The lumen of the bronchi with air bronchogram sign was also significantly different among the 3 SPLs (P < .05). The length of the involved bronchus with air bronchogram sign and the ratio between the length of the involved bronchus and that of the lesion were significantly different between lung cancer and tuberculosis (P < .05), or between lung cancer and pneumonia (P < .05), but not between tuberculosis and pneumonia (P > .05). No significant difference was found in the length of lesion among the 3 SPLs (P > .05).
The shape and lumen of the bronchi with air bronchogram sign can be used to distinguish lung cancer, tuberculosis, and pneumonia. The length of the involved bronchus with air bronchogram sign and the ratio between the length of the involved bronchus and that of the lesion can be used to distinguish lung cancer from tuberculosis and pneumonia.
Keywords: identification, lung cancer, pneumonia, tuberculosis
1. Introduction
With the development of helical computed tomography (CT), solitary pulmonary consolidation lesions (SPLs) have been increasingly detected in clinical practice.[1] The most common SPLs include lung cancer, tuberculosis, and pneumonia.[2,3] Differentiation of these 3 diseases using noninvasive methods (such as CT scan) is important to avoid the unnecessary treatment for noncancerous lesions.
Some signs on CT can be used to distinguish lung cancer, tuberculosis, and pneumonia when they manifest as SPLs.[3,4] For example, some CT studies focused on morphological changes of the lesion edges, internal characteristics, and the relationship of the lesion to adjacent pulmonary vessels or bronchi.[5] The SPL on CT showed radiolucent bronchi within lesions, manifesting as air bronchogram sign. The air bronchogram sign was first described by Fleischner[6] and named by Felson,[7] to distinguish pulmonary parenchymal lesions from extrapulmonary processes such as pleural effusion. Fleischner[6] demonstrated that airless lung tissue surrounding normal open airways would result in the radiographic effect. The expansion failure of airways and the consolidation processes can induce the air bronchogram sign.[8] Traditionally, the air bronchogram sign is used for diagnosis of alveolar disease with rare exceptions.[3] In our study, the air bronchogram sign was defined as an air-containing bronchus or bronchioles within the SPL in lung cancer, bronchiectasis, and pneumonia. To our knowledge, the length of air bronchogram sign, the length of the lesion, and the ratio of between the length of the involved bronchus and that of the lesion have not been reported.
This study aims to analyze the CT features of the air bronchogram sign and to investigate its role in the differentiation of lung cancer, tuberculosis, and pneumonia. The shape and lumen of the bronchi with air bronchogram sign, the length of the involved bronchus with air bronchogram sign, the length of lesion on the same plane and direction, and the ratio between the length of the involved bronchus and that of the lesion were evaluated.
2. Materials and methods
2.1. Patient selection
This is a retrospective study. Patients with SPLs from April 2016 to December 2016 were enrolled in this study. They all underwent CT scan. All patients were diagnosed by a review of pathologic reports and clinical records, surgery, bronchoscopy brushings, biopsy, pleural effusion test, and sputum culture. Inclusion criteria: patients with pathologically or cytologically confirmed lung cancer, tuberculosis, or pneumonia patients; patients with a single solid lesion on CT. Exclusion criteria: patients with multiple solid lesions on CT. Informed consent was obtained from every patient and the study was approved by the ethics review board of Shandong Provincial Chest Hospital.
2.2. CT protocols
All studies were performed using a 64-row multi-slice CT scanner system (Ingenuity Core 128; FHILIPS Medical Systems, Bothell, WA). All images were stored in digital formats. CT parameters were as follow: tube voltage of 120 kV, tube current of 224 mA, matrix of 512 × 512 mm, rotation time of 0.42 seconds, layer thickness of 5 mm, reconstruction thickness of 1 mm, and reconstruction interval of 1 mm.
2.3. Imaging analysis
CT imagines were assessed by 2 radiologists (HQ and JY) blinded to the study design using appropriate window setting to better display the morphology of the air bronchogram sign and the maximum length of the bronchus in the lesion. The evaluated air bronchogram sign included the shape and lumen of the bronchi with air bronchogram sign, the length of the involved bronchus with air bronchogram sign, the length of lesion on the same plane and direction, and the ratio between the length of the involved bronchus and that of the lesion. The shape of bronchi was evaluated by comparing to that of the normal bronchus in the contralateral lung at the same level. The ones with similar shape were defined as normal, those with straight and stiff shape were defined as stiff, and those with twisted shape were defined as tortuous. The patterns of normal, stiff, and tortuous were scored consecutively (normal: 0 point, stiff: 1 point, and tortuous 2 points). The diameter of the bronchus lumen was compared to that of the normal bronchus in the contralateral lung at the same level. The ones with similar lumen diameter were defined as normal, those with expanded lumen diameter were defined as expansion, those with narrowed lumen diameter were defined as stenosis, and those with both narrowed and expanded lumen diameter were defined as stenosis and expansion coexistence. The lumen patterns were scored as follows: normal (0 point), stenosis (1 point), expansion (2 points), and stenosis and expansion coexistence (3 points). At the reconstructed plane, which showed the longest diameter of the affected bronchus, the linear distance between the proximal end of the bronchus and the end point of the bronchus was measured as the length of the involved bronchus with air bronchogram sign. The length of lesion was also measured on the same plane and direction.
2.4. Statistical analysis
SPSS (version 17, IBM Corp, Chicago, IL) software was used for statistical analysis. Chi-squared test was used to analyze the differences in unordered categorical variables. Analysis of variance was performed to analyze differences in continuous categorical variables of different groups. LSD was used for comparison between 2 groups when there was significant difference among 3 groups. P value < .05 was considered statistically significant.
3. Results
3.1. Patients’ characteristics
In total, 105 patients with SPLs were included in this study. There were 39 cases of lung cancer, 43 cases of tuberculosis, and 23 cases of pneumonia. The average age of patients with lung cancer, tuberculosis, and pneumonia was 61.23 ± 7.96 years (range: 42–73 years), 29.02 ± 14.44 years (range: 6–76 years), and 37.13 ± 16.31 years (range: 13–67 years), respectively. There were significant differences in age among patients with different types of diseases (P < .05). The male to female ratio in patients with lung cancer, tuberculosis, and pneumonia was 26/13, 25/18, and with 16/7. Statistically, there were no significant differences in gender among patients with different types of diseases.
3.2. Imaging features of the air bronchogram sign
To differentiate between lung cancer, tuberculosis, and pneumonia, the bronchial signs in single solid lesion of lung were measured. Statistical analysis showed that the shape of the bronchi in the lesion was different among patients with lung cancer, tuberculosis, and pneumonia (P < .05) (Table 1). There were 25 cases (64.10%) of lung cancers with the air bronchogram sign presenting as stiff (Fig. 1), 14 cases (32.56%) of tuberculosis with the air bronchogram sign presenting normal, 18 cases (41.86%) of tuberculosis with the air Bronchogram sign presenting stiff, and 14 cases (60.87%) of pneumonia with the air Bronchogram sign presenting normal (Table 1).
Table 1.
Shapes of bronchi in different subgroups.
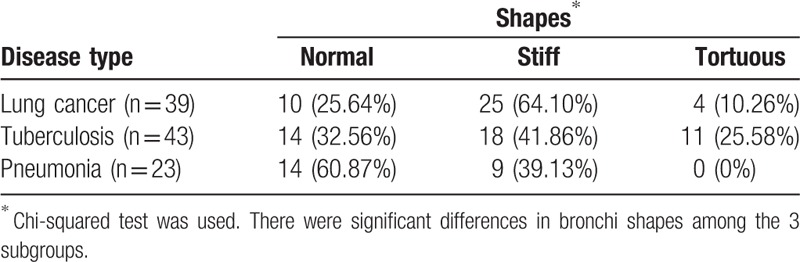
Figure 1.
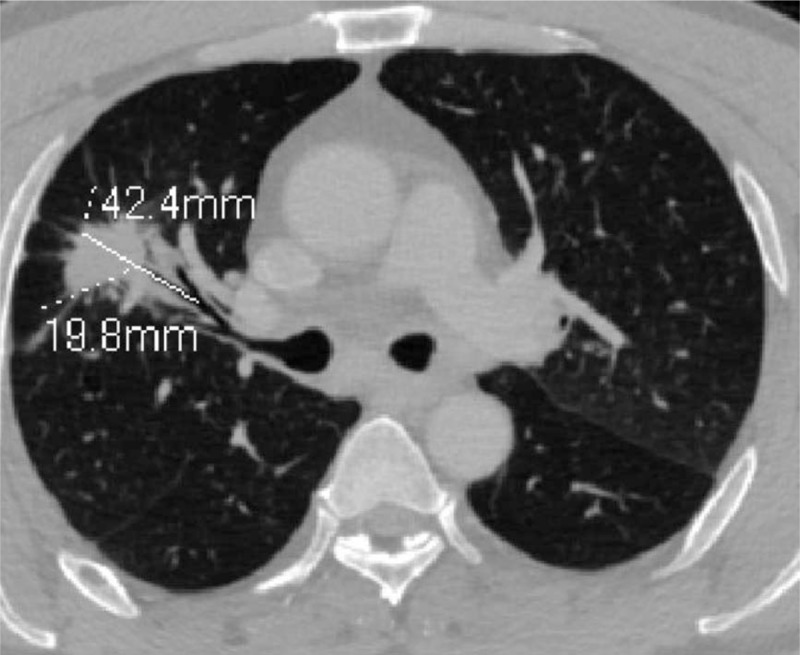
MPR shows the bronchus presenting stiff and stenosis in a lung cancer patient. In this image, the length of the involved bronchus with air bronchogram sign was 19.8 mm. The length of lesion on the same plane and direction was 42.4 mm. MPR = multi-planner reformation.
Statistical analysis showed that the lumen of the bronchi in the lesion was significantly different among patients with lung cancer, tuberculosis, and pneumonia (P < .05) (Table 2). There were 32 cases (82.05%) of lung cancers with the air bronchogram sign presenting stenosis in lumen, 19 cases (44.19%) of tuberculosis with the air bronchogram sign presenting expansion in lumen (Fig. 2), and 14 cases (60.87%) of pneumonia with the air bronchogram sign presenting normal in lumen (Fig. 3; Table 2).
Table 2.
The lumen of bronchi in different subgroups.
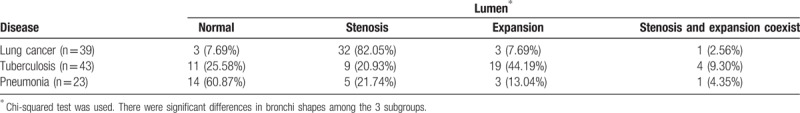
Figure 2.
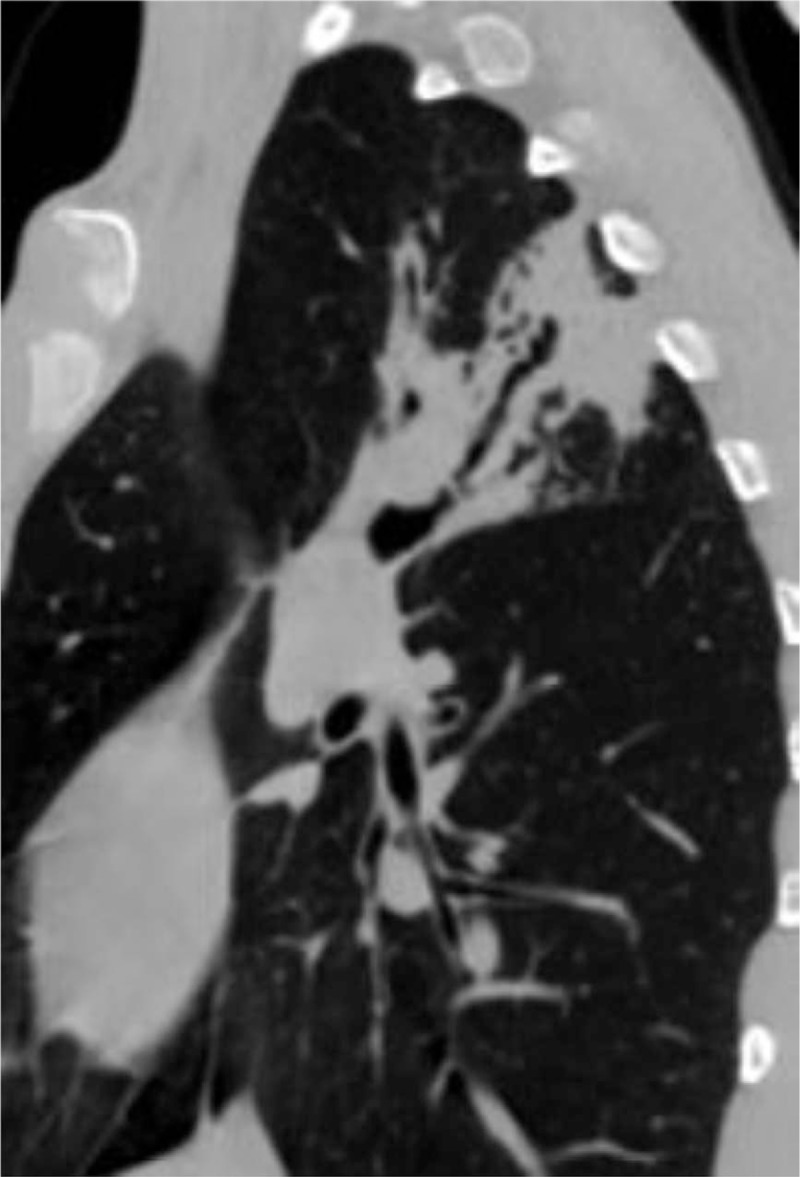
MPR shows the bronchus presenting stiff and expansion in a tuberculosis patient.
Figure 3.
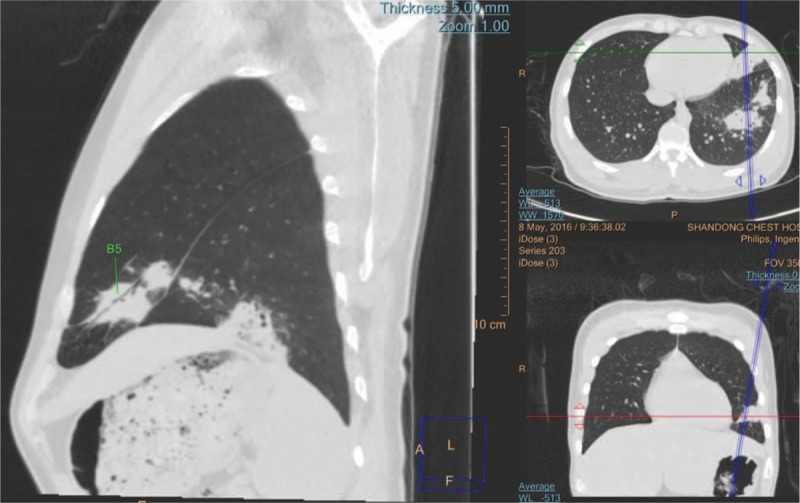
MPR shows the bronchus presenting normal in a pneumonia patient. The bronchus in the lower segment of the left upper lobe was affected.
LSD analysis showed that the length of the involved bronchus with air bronchogram sign was significantly different from tuberculosis and pneumonia (P < .05) (Table 3). The length of the involved bronchus with air bronchogram sign in pneumonia was largest when compared with tuberculosis and lung cancer, showing no statistically significance (P > .05). There was no significant difference in the length of lesion between the 3 subgroups (P > .05) (data not shown). The ratio between the length of the involved bronchus and that of the lesion in lung cancer was significantly different from that in tuberculosis and pneumonia (P < .05) (Table 4), but the difference between tuberculosis and pneumonia was not statistically significant.
Table 3.
The length of the involved bronchus with air bronchogram sign in different subgroups.
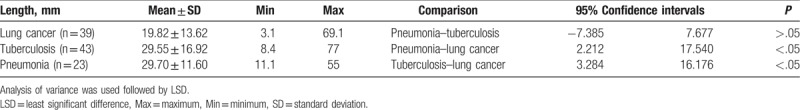
Table 4.
The ratio between the length of the involved bronchus and that of the lesion length in different subgroups.

4. Discussion
The SPL is defined as a single round or ovoid lesion within the lung parenchyma in the absence of adenopathy, atelectasis, or pneumonia.[9] The sharp or blurry borders are located completely in pulmonary tissue. CT image shows thoracic abnormal presentations in SPL lesions.
The morphology of pulmonary lesions has been analyzed in the past decade.[3,10] In particular, in many studies, radiographic lesion density, lesion diameter, lesion margin characteristics, relations between lesions and surrounding structures, and enhancement have been investigated.[2,4] The 3D CT image of lesions and anatomical structures are used to assess the applicability of CT to the diagnosis of SPLs.[11] Although the CT examination is indispensable for the detection or exclusion of intrapulmonary lesions, as well as the location, size and extension of SPLs, SPLs can seldom be diagnosed by CT image alone.[12] Unless fairly conclusive evidence is obtained from medical history, physical examination, laboratory studies, endoscopy, fluoroscopy, and/or angiocardiography, exploratory thoracotomy is necessary for diagnosis except unusual conditions.[12]
CT can detect air bronchogram sign both in SPLs and pulmonary nodules.[13,14] Gaeta et al[13] reported that the frequency of the air bronchogram sign in SPL on thin section CT was 66.7%. Kuriyama et al[15] reported that in a group of SPLs presenting as 40 nodules on CT with 1.5 to 2.0 mm of diameter, 14 had air bronchogram (35.0%). Kui et al[16] showed that of 132 cases of SPLs, 34 had air bronchogram sign (27.5%). The above studies show that more than 25% of SPLs may have air bronchogram sign on conventional or thin section CT, and that various sized nodules and masses may also have air bronchogram sign. The morphology of the air bronchogram within an SPL may provide information on the pathogenicity of the lesion. Previous authors have noted the morphology of aerated bronchi in the air bronchogram sign.[17,18] Gaete et al[17] reported that thin-section CT was a valuable tool for demonstrating the type of tumor–bronchi relationship and predicting the results of transbronchial forceps biopsy and bronchial brushing in solitary pulmonary nodule associated with a positive bronchus sign. Lee et al[19] studied tubular constriction of the airways due to bronchial encasement and constriction by the malignant cells and the author concluded that the air bronchogram sign may be helpful in the diagnosis of neoplasms. Kui et al[16] showed that the encased bronchi exhibited 4 morphologic patterns and that when evaluating a solitary pulmonary nodule with CT, attention should be paid to the presence of the air bronchogram sign and the morphology of the bronchi for differential diagnosis.[16]
In our study, we assessed the role of air bronchogram sign in the diagnosis of the SPL lesions. The bronchus may remain unchanged in a tubular shape, or may be altered and become stiff and tortuous.[16,18] The lumen of bronchi may also remain normal diameter, or may present stenosis, expansion, stenosis, and co-existing expansion.[16,18] In this study, the stiff and stenosis types appeared to be associated with lung cancer, while the normal morphology was seen in pneumonia. In addition to the morphology of the aerated bronchi in the SPL, we also recorded the length of the bronchi involvement and the length of lesions at the same level of reconstruction, and then calculated their ratio. We found that the length of the air bronchogram sign was shorter in lung cancer than that of tuberculosis and pneumonia. If the tumor simply surrounds the bronchi, the bronchial shape may not change.[20] Once the tumor invades the bronchial wall, especially the cartilaginous or elastic layer, the bronchi may present not only as cut-off due to strangulation and obliteration, but also as tortuous and ecstatic.[20] On the other hand, benign lesions usually have a capsule or contain structure-less materials like necrosis.[4] These factors may prevent the benign lesions from integrating the patent bronchus.[4]
There are some limitations. First, there were only 3 types of pulmonary parenchymal diseases involved in our study and the disease type was relatively small. Second, the pathological types of lung cancer, the disease course of TB, and different types of infections in pneumonia were not differentiated. Third, the sample size was relatively small. Further studies are warranted.
In conclusion, radiology plays a crucial role in the evaluation of SPLs. The air bronchogram sign in an SPL is well shown on CT. This study found that the length of air bronchogram signs, the length of the lesion, and the length ratios could be used to differentiate lung cancer, tuberculosis, and pneumonia from each other. A diagnostic procedure based on the air bronchogram sign should be developed and standardized in the future.
Author contributions
Formal analysis: Guangbin Wang.
Investigation: Huifang Qu.
Resources: Wenchao Zhang.
Software: Huifang Qu.
Supervision: Jisheng Yang.
Validation: Shouqin Jia.
Writing – original draft: Huifang Qu.
Writing – review & editing: Guangbin Wang.
Footnotes
Abbreviations: CT = computed tomography, SPLs = solitary pulmonary consolidation lesions.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Kikano GE. Evaluation of the solitary pulmonary nodule. Am Fam Physician 2015;92:1084–91. [PubMed] [Google Scholar]
- [2].Totanarungroj K, Chaopotong S, Tongdee T. Distinguishing small primary lung cancer from pulmonary tuberculoma using 64-slices multidetector CT. J Med Assoc Thai 2012;95:574–82. [PubMed] [Google Scholar]
- [3].Imaad-ur-Rehman, Memon W, Husen Y, et al. Accuracy of computed tomography in diagnosing malignancy in solitary pulmonary lesions. J Pak Med Assoc 2011;61:48–51. [PubMed] [Google Scholar]
- [4].Seemann MD, Seemann O, Luboldt W, et al. Differentiation of malignant from benign solitary pulmonary lesions using chest radiography, spiral CT and HRCT. Lung Cancer 2000;29:105–24. [DOI] [PubMed] [Google Scholar]
- [5].Choi JA, Kim JH, Hong KT, et al. CT bronchus sign in malignant solitary pulmonary lesions: value in the prediction of cell type. Eur Radiol 2000;10:1304–9. [DOI] [PubMed] [Google Scholar]
- [6].Fleischner FG. The visible bronchial tree. A roentgen sign in pneumonic and other pulmonary consolidations. Radiology 1948;50:184–9. [DOI] [PubMed] [Google Scholar]
- [7].Felson B. Chest Roentgenology. 2nd ed.1973;Philadelphia, PA: WB Saunder, 64–70. [Google Scholar]
- [8].Reed JC, Madewell JE. The air bronchogram in interstitial disease of the lungs. Radiology 1975;116:1–9. [DOI] [PubMed] [Google Scholar]
- [9].Khouri NF, Meziane MA, Zerhouni EA, et al. The solitary pulmonary nodule: assessment, diagnosis, and management. Chest 1987;91:128–33. [DOI] [PubMed] [Google Scholar]
- [10].Shi Z, Wang Y, He X. Differential diagnosis of solitary pulmonary nodules with dual-source spiral computed tomography. Exp Ther Med 2016;12:1750–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mori K, Saito Y, Tominaga K, et al. Three-dimensional computed tomography image of small pulmonary lesions. Jpn J Chin Oncol 1992;22:159–63. [PubMed] [Google Scholar]
- [12].Carillo GA, Vázquez JE, Villar AF. Prevalence of benign pulmonary lesions excised for suspicion of malignancy: could it reflect a quality management index of indeterminate lung lesions? Korean J Thorac Cardiovasc Surg 2014;47:458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gaeta M, Pandolfo I, Volta S, et al. Bronchus sign on CT in peripheral carcinoma of the lung: value in predicting results of transbronchial biopsy. AJR Am J Roentgenol 1991;157:1181–5. [DOI] [PubMed] [Google Scholar]
- [14].Zwirewich CV, Vedal S, Miller RR, et al. Solitary pulmonary nodule: high-resolution CT and radiologic-pathologic correlation. Radiology 1991;179:469–76. [DOI] [PubMed] [Google Scholar]
- [15].Kuriyama K, Tateishi R, Doi O, et al. Prevalence of air bronchograms in small peripheral carcinomas of the lung on thin-section CT: comparison with benign tumors. AJR Am J Roentgenol 1991;156:921–4. [DOI] [PubMed] [Google Scholar]
- [16].Kui M, Templeton PA, White CS, et al. Evaluation of the air bronchogram sign on CT in solitary pulmonary lesions. J Comput Assist Tomogr 1996;20:983–6. [DOI] [PubMed] [Google Scholar]
- [17].Gaete M, Barone M, Russi EG. Carcinomatous solitary pulmonary nodules: evaluation of the tumor-bronchi relationship with thin-section CT. Radiology 1993;187:535–9. [DOI] [PubMed] [Google Scholar]
- [18].Sun PF, Xiao XS, Liu SY. Evaluation of bronchial changes of solitary pulmonary lesion using multi-slice CT. Radiol Practice 2007;22:150–3. [Google Scholar]
- [19].Lee WA, Hruban RH, Kuhlman JE, et al. HRCT of inflation-fixed lungs: pathologicradiologic correlation of pulmonary lesions in patients with leukemia, lymphoma, or other hematopoietic proliferative disorders. Clin Imag 1992;16:15–24. [DOI] [PubMed] [Google Scholar]
- [20].Zheng B, Zhou X, Chen J, et al. A modified model for preoperatively predicting malignancy of solitary pulmonary nodules: an Asia cohort study. Ann Thorac Surg 2015;100:288–94. [DOI] [PubMed] [Google Scholar]


