Abstract
This study is to investigate the effects of slow breathing on heart rate variability (HRV) and arterial baroreflex sensitivity in essential hypertension.
We studied 60 patients with essential hypertension and 60 healthy controls. All subjects underwent controlled breathing at 8 and 16 breaths per minute. Electrocardiogram, respiratory, and blood pressure signals were recorded simultaneously. We studied effects of slow breathing on heart rate, blood pressure and respiratory peak, high-frequency (HF) power, low-frequency (LF) power, and LF/HF ratio of HRV with traditional and corrected spectral analysis. Besides, we tested whether slow breathing was capable of modifying baroreflex sensitivity in hypertensive subjects.
Slow breathing, compared with 16 breaths per minute, decreased the heart rate and blood pressure (all P < .05), and shifted respiratory peak toward left (P < .05). Compared to 16 breaths/minute, traditional spectral analysis showed increased LF power and LF/HF ratio, decreased HF power of HRV at 8 breaths per minute (P < .05). As breathing rate decreased, corrected spectral analysis showed increased HF power, decreased LF power, LF/HF ratio of HRV (P < .05). Compared to controls, resting baroreflex sensitivity decreased in hypertensive subjects. Slow breathing increased baroreflex sensitivity in hypertensive subjects (from 59.48 ± 6.39 to 78.93 ± 5.04 ms/mm Hg, P < .05) and controls (from 88.49 ± 6.01 to 112.91 ± 7.29 ms/mm Hg, P < .05).
Slow breathing can increase HF power and decrease LF power and LF/HF ratio in essential hypertension. Besides, slow breathing increased baroreflex sensitivity in hypertensive subjects. These demonstrate slow breathing is indeed capable of shifting sympatho-vagal balance toward vagal activities and increasing baroreflex sensitivity, suggesting a safe, therapeutic approach for essential hypertension.
Keywords: arterial baroreflex sensitivity, breathing rate, essential hypertension, heart rate variability, spectral analysis
1. Introduction
Hypertension is a chronic disorder with a high prevalence worldwide. Hypertension is a major risk factor for cardiovascular and cerebrovascular diseases.[1] Higher the long-term level of blood pressure, greater the chances of hypertension complications, such as myocardial infarction, renal failure, stroke, and heart failure. Many studies have proposed a causal role of hypertension, such as CaMK4 and GRKs polymorphisms.[2,3] However, the arterial baroreceptor reflex system constitutes one of the most powerful and rapidly acting homeostatic mechanisms for controlling blood pressure. It has attracted the interest of hypertension researchers for many years.[4] Estimate of the again of baroreflex is usually referred as baroreflex sensitivity. Baroreflex sensitivity impairment has a major role in the etiology of hypertension.[5] Several studies have demonstrated the clinical value of baroreflex sensitivity as a prognostic parameter.[6,7] In the previous studies,[8–10] the workers, based on experiments in the animal and clinical laboratory, have concluded that slow breathing rate could increase low-frequency (LF) power, decease high-frequency (HF) power. However, these results are not in accordance with theory of spectral analysis. It could be explained by slow breathing rate-induced respiratory peak shifting. In our previous study,[8] based on healthy people, we have proposed the corrected spectral analysis to correct the effect of slow breathing rate on respiratory peak shifts which in turn influence the spectral analysis. In the present study, we investigate the effect of slow breathing rate on power spectral components of heart rate variability (HRV) with this corrected spectral analysis in essential hypertension. Furthermore, we observed the effect of slow breathing on arterial baroreflex sensitivity in essential hypertension.
2. Methods
2.1. Study population
We studied 60 patients with essential hypertension according to 2013 ESH/ESC guidelines for the management of arterial hypertension[11] and 60 healthy controls in our hospital. As for inclusion criteria, all the subjects were not taking any medication and nonsmokers. Moreover, they were included in the study if no disease (apart from essential hypertension) was found after a clinical examination and routine laboratory tests; none was involved in competitive sport activities. The patients with secondary causes of hypertension, ischemic heart disease, congestive heart failure, chronic atrial fibrillation, renal failure, diabetes mellitus, previous stroke, major organ failure, respiratory diseases, psychiatric disorders, and hearing impairment were excluded from the study. All of the subjects gave their informed consent, and the study was approved by the Local Ethics Committee.
2.2. Experimental protocol
The experiment was performed in the morning in a quiet and light-attenuated room, whose ambient temperature was kept at 20 to 24 °C. All the subjects were asked to have a light breakfast the day of the test and they were asked not to eat chocolates or drink tea, coffee, cola-containing substances, or alcoholic beverages during the previous 24 hours. Just prior to each session, participants were seated in a comfortable chair, and electrocardiogram (ECG) electrodes were pasted to their wrists and left ankle, respiratory transducer (model HKH-11C, made in Electronics Technology Institute, Hefei, China) was tied to their abdomen, and noninvasive blood pressure was acquired via radial artery tonometry (model HK-2000E, made in Electronics Technology Institute). The subjects were asked to remain at rest in a supine position for 10 minutes, and then were instructed to inhale-exhale in synchrony with each tone (at 8 and 16 breaths per minute). The order of breathing rates has been randomized. Each paced breathing period lasted for 5 minutes. The 2 periods were separated from each other by an interval of 15 minutes during which the subjects were allowed to breathe spontaneously. ECG, respiratory, and blood pressure signal were continuously and simultaneously recorded. The arterial baroreflex sensitivity was measured by spectral analysis.
2.3. Data collection and analysis
Model SKY-A4 Bioelectric Signals Processing System (Shanghai SKY Network Technology Company Limited) was used to remove very LF power and to record respiratory, ECG, and blood pressure waveform signals synchronously under different breathing rates (8 and 16 breaths per minute). All signals were acquired continuously on a personal computer and stored on optical disks. Holter was used to identify R peak, and exclude premature ectopic beats and missing beats, then saved as AR files. We opened AR files via BRS&HRV2.0 analysis system and did spectral analysis.
2.4. Observed index
We observed the effect of slow breathing rate on the heart rate, blood pressure, respiration peak shift, HF power, LF power, and LF/HF ratio of HRV and the arterial baroreflex sensitivity in essential hypertension.
2.5. HRV spectral analysis
2.5.1. Traditional spectral analysis
Spectral analysis of HRV was computed by using the fast Fourier transformation (Relatively stable data segment and periods of 256 beats were selected and considered eligible) over 2 frequency bands (Fig. 1A, B): LF (0.04–0.15 Hz) and HF (0.15–0.4 Hz).[12,13] Power spectrum of HRV had many peaks, and the respiration may easily entrain respiration peak, which was tall and narrow in HF band. Recent studies have shown that Central Frequency of Respiration Peak can be used as a quantitative measure of respiration peak (Central Frequency of Respiration Peak is equal to breathing rate divided by the heart rate).[8,10]
Figure 1.
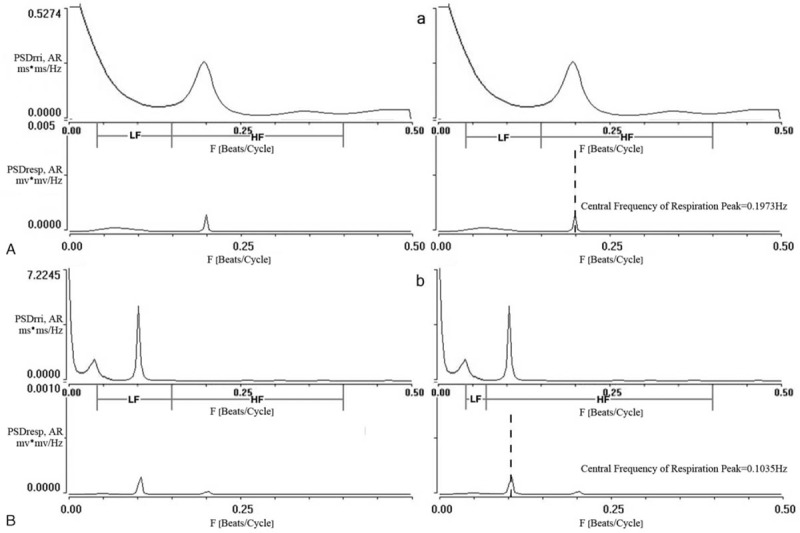
Power spectra of respiratory, RR interval signal during controlled respiration at 16 and 8 breaths per minute in 1 subject. 0.15 Hz is the conjunction between LF band and HF band of HRV of traditional spectral analysis at breathing rate of 16 breaths per minute (A) and 8 breaths per minute (B). We use Central Frequency of Respiration Peak at 0.65 Hz to correct the conjunction between LF band and HF band of HRV of corrected spectral analysis at breathing rate of 16 breaths per minute (a) and 8 breaths per minute (b). F = frequency, HF = high-frequency, HRV = heart rate variability, LF = low-frequency, PSD = power spectrum densities, Resp = respiration, rri = R-R interval.
2.5.2. Corrected spectral analysis
Slow breathing rate can shift respiratory peak toward left. In order to correct the effect of slow breathing rate on respiratory peak shift which in turn influence spectral analysis, we used Central Frequency of Respiration Peak to correct HF and LF bands.[8,14] LF area was chosen from 0.04 Hz to Central Frequency of Respiration Peak∗0.65 Hz. HF area was chosen from Central Frequency of Respiration Peak∗0.65 to 0.40 Hz (Fig. 1a, b).
2.6. Statistical analysis
The results were given as mean ± standard deviation (SD). Paired t tests were used to determine the differences of baseline characteristics of hypertensive and control subjects and the effect of slow breathing rate on respiration peak shift, HF power, LF power, and LF/HF ratio of HRV and the arterial baroreflex sensitivity. All statistical analysis was performed using SPSS 16.0. A P-value <.05 was considered significant.
3. Results
3.1. Study population
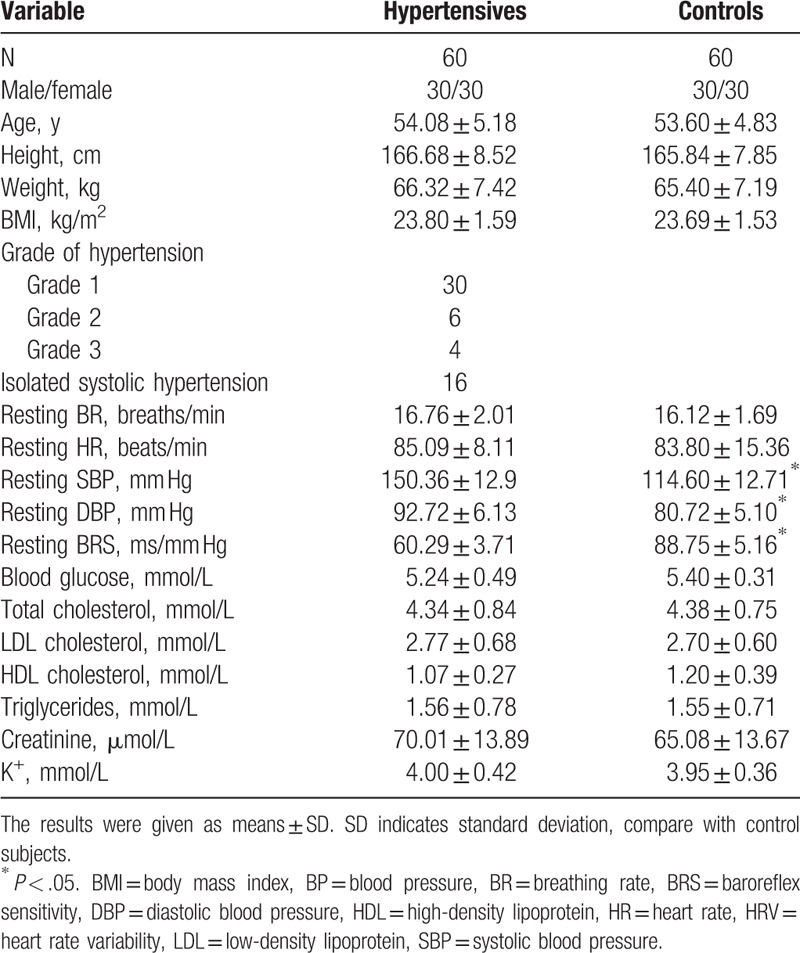
The averaged anthropometric and physiological parameters of the participants were given in Table 1.
Table 1.
Baseline characteristics of hypertensive and control subjects.
3.2. Effects of slow breathing rate on heart rate, blood pressure, and respiration peak shift
Compared with 16 breaths per minute, the heart rate and the blood pressure simultaneously decreased (all P < .05) at 8 breaths per minute in essential hypertension (Table 2). Central Frequency of Respiration Peak of HRV and respiratory located at the same frequency. Breathing rate at 16 breaths per minute, both of them located at HF band. Breathing rate at 8 breaths per minute, Central Frequency of Respiration Peak was located at LF band (P < .05, see Fig. 1A, B, Table 2).
Table 2.
Effects of slow breathing rate on heart rate, blood pressure, and Central Frequency of Respiration Peak of HRV in essential hypertension.

3.3. Effects of slow breathing rate on HF, LF power, and LF/HF ratio of HRV according to traditional spectral analysis
Compared to 16 breaths per minute, traditional spectral analysis showed increased LF power and LF/HF ratio, decreased HF power of HRV at 8 breaths/minute (P < .05, see Table 3).
Table 3.
Effects of slow breathing rate on HRV in essential hypertension (traditional and corrected spectral analysis).

3.4. Effects of slow breathing rate on HF, LF power, and LF/HF ratio of HRV according to corrected spectral analysis
Compared to 16 breaths per minute, corrected spectral analysis showed increased HF power, decreased LF power and LF/HF ratio of HRV (P < .05, see Table 3).
3.5. Effects of slow breathing rate on arterial baroreflex sensitivity
Compared to controls, resting baroreflex sensitivity decreased in hypertensive subjects (P < .05, Table 1). Compared to 16 breaths per minute, breathing at 8 breaths/minute significantly increased the baroreflex sensitivity in hypertensive subjects (from 59.48 ± 6.39 to 78.93 ± 5.04 ms/mm Hg, P < .05) and controls (from 88.49 ± 6.01 to 112.91 ± 7.29 ms/mm Hg, P < .05).
4. Discussion
In our previous study,[8] based on healthy people, we have proposed the corrected spectral analysis to correct the effect of slow breathing rate on respiratory peak shifts which in turn influence the spectral analysis. In this present study, compared with 16 breaths per minute, corrected spectral analysis[8] showed increased HF power, decreased LF power, and LF/HF ratio of HRV in 8 breaths per minute in essential hypertension (Table 3). These demonstrated slow breathing was indeed capable of increasing vagal activities and shifting sympatho-vagal balance toward vagal activities in essential hypertension.
The physiological function of the arterial baroreflex is to normally alter heart rate and blood pressure due to arterial wall tension changes.[4] Many evidences[4,15] have showed that the arterial baroreflex baroreceptors sensed the systemic blood pressure through baroreceptor terminals innervating aortic arch and carotid sinus, and then transmitted the arterial baroreceptor afferent discharge into the dorsal medial nucleus tractus solitarii. As a result, peripheral vascular resistance, heart rate, and arterial blood pressure were subsequently decreased. Carotid and aortic baroreceptors sense the intraarterial blood pressure and modulate the sympathetic tone toward the opposite direction, that is, high blood pressure results in reduced sympathetic tone through baroreceptor activation, while enhanced sympathetic tone compensates for low blood pressure.[4]
In this present study, in Table 1, we showed baroreflex sensitivity has been decreased in hypertensive subjects, which was consistent with the established literature.[16–19] Blunt peripheral pressure sensors passivation and central cardiovascular regulatory dysfunction could be responsible for the attenuated baroreflex sensitivity. Stiffening and thickening of the aortic wall and of the carotid sinus wall is likely to reduce the baroreceptor afferent impulses, leading to decreasing the sensitivity of aortic and carotid baroreceptors.[20,21]. The central mechanism of the decreased arterial baroreflex might be related to dysfunction of integration center. This change may result in exaggerated sympathetic activity due to impaired sympathetic inhibition.[22] Decreased arterial baroreflex baroreceptors is an independent predictor of malignant arrhythmia and sudden cardiac death.[23,24] Pharmacological therapies remain the mainstay of antihypertensive treatments to improve baroreflex sensitivity. Yet, it has been estimated that less than 50% of hypertensive patients achieve their target effects while on medications.[25] It is, therefore, not surprising that greater interest is focusing on the role of nonpharmacological interventions. Our data showed that slow-breathing induced increase in HF power and reduction in the LF power and the LF/HF ratio of HRV, which is benefit for decreasing sympathetic activity and increasing vagal activities. This may be account for the breathing of 8 breaths/minute increased baroreflex sensitivity in essential hypertension.
5. Study limitations
In the present study, we only observed short-term effects of slow breathing. Further studies are needed to assess whether longer-term practice will lead to stable modifications of HRV and of the baroreflex sensitivity.
6. Conclusion
Effects of slow breathing rate on respiratory peak shift should be corrected when we performed HRV spectral analysis. Corrected spectral analysis demonstrated slow respiration can increase HF power and decrease LF power and LF/HF ratio in essential hypertension. Slow breathing showed the potential to be a simple and inexpensive method to improve autonomic balance and increase the baroreflex sensitivity in hypertensive patients. This opens a new area of future research in the better management of patients with essential hypertension.
Acknowledgments
The authors thank all the people who participated in the study.
Author contributions
Conceptualization: Changjun Li, Wenshu Chai.
Data curation: Changjun Li, Qinghua Chang, Jia Zhang, Wenshu Chai.
Formal analysis: Changjun Li, Qinghua Chang, Jia Zhang, Wenshu Chai.
Investigation: Changjun Li, Qinghua Chang, Jia Zhang, Wenshu Chai.
Methodology: Changjun Li, Qinghua Chang, Wenshu Chai.
Project administration: Changjun Li, Qinghua Chang, Wenshu Chai.
Resources: Changjun Li, Qinghua Chang, Wenshu Chai.
Software: Changjun Li, Qinghua Chang, Jia Zhang, Wenshu Chai.
Supervision: Changjun Li, Qinghua Chang, Wenshu Chai.
Validation: Changjun Li, Qinghua Chang, Wenshu Chai.
Visualization: Changjun Li, Qinghua Chang, Wenshu Chai.
Writing – original draft: Changjun Li, Qinghua Chang, Wenshu Chai.
Writing – review & editing: Changjun Li, Qinghua Chang, Jia Zhang, Wenshu Chai.
Footnotes
Abbreviations: ECG = electrocardiogram, HF = high-frequency, HRV = heart rate variability, LF = low-frequency.
CL is the first author.
Ethics approval: The study was approved by the Local Ethics Committee of the First Affiliated Hospital of Jinzhou Medical University.
All authors wrote and revised the manuscript, and approved the final version submitted for publication.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Aslanger E, Sezer M, Umman S. High blood pressure: an obscuring misnomer? Anatol J Cardiol 2016;16:713–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Santulli G, Cipolletta E, Sorriento D, et al. CaMK4 gene deletion induces hypertension. J Am Heart Assoc 2012;1:e001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Santulli G, Trimarco B, Iaccarino G. G-protein-coupled receptor kinase 2 and hypertension: molecular insights and pathophysiological mechanisms. High Blood Press Cardiovasc Prev 2013;20:5–12. [DOI] [PubMed] [Google Scholar]
- [4].Lee CK, Park KH, Baik SK, et al. Decreased excitability and voltage-gated sodium currents in aortic baroreceptor neurons contribute to the impairment of arterial baroreflex in cirrhotic rats. Am J Physiol Regul Integr Comp Physiol 2016;310:R1088–101. [DOI] [PubMed] [Google Scholar]
- [5].Del Colle S, Milan A, Caserta M, et al. Baroreflex sensitivity is impaired in essential hypertensives with central obesity. J Hum Hypertens 2007;21:473–8. [DOI] [PubMed] [Google Scholar]
- [6].Zhang D, Liu J, Tu H, et al. In vivo transfection of manganese superoxide dismutase gene or nuclear factor (B shRNA in nodose ganglia improves aortic baroreceptor function in heart failure rats. Hypertension 2014;63:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gademan MG, Sun Y, Han L, et al. Rehabilitation: periodic somatosensory stimulation increases arterial baroreflex sensitivity in chronic heart failure patients. Int J Cardiol 2011;152:237–41. [DOI] [PubMed] [Google Scholar]
- [8].Chang Q, Liu R, Shen Z. Effects of slow breathing rate on blood pressure and heart rate variabilities. Int J Cardiol 2013;169:e6–8. [DOI] [PubMed] [Google Scholar]
- [9].Brown TE, Beightol LA, Koh J, et al. Important influence of respiration on human R-R interval power spectra is largely ignored. J Appl Physiol 1993;75:2310–7. [DOI] [PubMed] [Google Scholar]
- [10].Chen HB, Liu RG, Shen ZY. The effect of breathing rate and tidal volume on rabbit heart rate variability. Pacing Electrocardiol J China 2011;25:258–62. [Google Scholar]
- [11].Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013;34:2159–219. [DOI] [PubMed] [Google Scholar]
- [12].Guasti L, Simoni C, Mainardi L, et al. Global link between heart rate and blood pressure oscillations at rest and during mental arousal in normotensive and hypertensive subjects. Auton Neurosci 2005;120:80–7. [DOI] [PubMed] [Google Scholar]
- [13].Bartels MN, Jelic S, Ngai P, et al. The effect of ventilation on spectral analysis of heart rate and blood pressure variability during exercise. Respir Physiol Neurobiol 2004;144:91–8. [DOI] [PubMed] [Google Scholar]
- [14].Aysin B, Aysin E. Effect of respiration in heart rate variability (HRV) analysis. Conf Proc IEEE Eng Med Biol Soc 2006;1:1776–9. [DOI] [PubMed] [Google Scholar]
- [15].Li YL. Elevated angiotensin II in rat nodose ganglia primes diabetes-blunted arterial baroreflex sensitivity: involvement of NADPH oxidase-derived superoxide. J Diabetes Metab 2011;2:1000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Celovska D, Kruzliak P, Rodrigo L, et al. Effect of low dose atorvastatin therapy on baroreflex sensitivity in hypertensives. High Blood Press Cardiovasc Prev 2016;23:133–40. [DOI] [PubMed] [Google Scholar]
- [17].Rossi NF, Maliszewska-Scislo M, Chen H, et al. Neuronal nitric oxide synthase within paraventricular nucleus: blood pressure and baroreflex in two-kidney, one-clip hypertensive rats. Exp Physiol 2010;95:845–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yemane H, Busauskas M, Burris SK, et al. Neurohumoral mechanisms in deoxycorticosterone acetate (DOCA)-salt hypertension in rats. Exp Physiol 2010;95:51–5. [DOI] [PubMed] [Google Scholar]
- [19].Xing-Sheng Y, Yong-Zhi L, Jie-Xin L, et al. Genetic influence on baroreflex sensitivity in normotensive young men. Am J Hypertens 2010;23:655–9. [DOI] [PubMed] [Google Scholar]
- [20].Pierce GL, Harris SA, Seals DR, et al. Estimated aortic stiffness is independently associated with cardiac baroreflex sensitivity in humans: role of ageing and habitual endurance exercise. J Hum Hypertens 2016;30:513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lábrová R, Honzíková N, Maderová E, et al. Age-dependent relationship between the carotid intima-media thickness, baroreflex sensitivity, and the inter-beat interval in normotensive and hypertensive subjects. Physiol Res 2005;54:593–600. [PubMed] [Google Scholar]
- [22].Klippel BF, Duemke LB, Leal MA, et al. Effects of kefir on the cardiac autonomic tones and baroreflex sensitivity in spontaneously hypertensive rats. Front Physiol 2016;7:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Huang D, Zhou J, Su D, et al. Variations of perioperative baroreflex sensitivity in hypertensive and normotensive patients. Clin Exp Hypertens 2017;39:74–9. [DOI] [PubMed] [Google Scholar]
- [24].Nováková Z. From the first spectral analysis of blood pressure variability in the world to the present time: contribution of the Department of Physiology of the Faculty of Medicine, Masaryk University, Brno. Physiol Res 2013;62:341–50. [DOI] [PubMed] [Google Scholar]
- [25].Wang TJ, Vasan RS. Epidemiology of uncontrolled hypertension in the United States. Circulation 2005;112:1651–62. [DOI] [PubMed] [Google Scholar]


