Abstract
Left ventricular hypertrophy (LVH) is associated with increased risk for vascular events and mortality. This study investigated 8-year clinical outcomes of hypertensive patients with LVH who underwent percutaneous coronary intervention (PCI) with drug-eluting stents (DES) compared with hypertensive patients without LVH.
A total of 1704 consecutive hypertensive patients who underwent PCI from 2004 to 2014 were enrolled. We classified them into either the LVH group (n = 406) or the control group (without LVH, n = 1298). LVH was defined by LV mass index > 115 g/m2 in men and > 95 g/m2 in women. After propensity score matched (PSM) analysis, 2 PSM groups (366 pairs, n = 732, c-statistic = 0.629) were generated.
For up to 8 years, the LVH group showed a higher incidence of cardiac death (4.4% vs 1.2%, log-rank P = .023, hazard ratio: 3.371, 95% confidence interval: 1.109–10.25; P = .032) compared with the control group. However, there were no significant differences between the 2 groups in the incidence of total death, myocardial infarction, revascularization, and major adverse cardiac events up to 8 years.
LVH in hypertensive patients who underwent successful PCI with DES was associated with higher incidence of cardiac death up to 8 years of follow-up. More careful managements and clinical follow-up are needed and treatment strategies should specifically focus to target prevention and reversal of LVH in hypertensive patients.
Keywords: coronary artery disease, hypertension, left ventricular hypertrophy, outcome
1. Introduction
Hypertension is a strong, independent risk factor for cardiovascular disease.[1] Left ventricular hypertrophy (LVH) is a manifestation of hypertensive target organ damages and is associated with increased risk for vascular events and mortality.[2] Even though LVH is an adaptive response to a pressure overload on the heart in hypertensive patients, it is a major risk factor for cardiovascular disease for its damaging effects on ventricular function, coronary circulation, and arrhythmia.[3–5] The prevalence of LVH ranges from 5% in patients undergoing angiography to as much as 44% in patients with hypertension.[6] However, there are limited data regarding the impact of LVH on long-term clinical outcomes in hypertensive patients who underwent successful percutaneous coronary intervention (PCI) with drug-eluting stents (DES). The aim of this study is to investigate 8-year clinical outcomes of hypertensive patients with LVH who underwent PCI with DES compared with hypertensive patients without LVH.
2. Methods
The study is a single-center, retrospective, all-comer registry designed to reflect the real-world practice since 2004. Data were collected by trained study coordinators with a standardized case report form. The protocol was approved by an ethics committee and was performed in accordance with the ethical standards established in the 1964 declaration of Helsinki and the subjects gave written informed consent.
2.1. Study population
A total of 4043 consecutive patients who underwent PCI from January 2004 to December 2014 at Cardiovascular Center, Korea University Guro Hospital (KUGH), Seoul, South Korea were enrolled. Among them, patients with these conditions were excluded: death (n = 219), no history of hypertension (n = 1461), bare-metal stent deployment (n = 71), plain old balloon angioplasty (n = 15), not participated (n = 363), and follow-up loss (n = 350). Finally, a total of 1704 eligible hypertensive patients who underwent PCI with DES were enrolled. The patients were classified into either LVH group (n = 406) or control group (without LVH, n = 1298) according to the presence of LVH (Fig. 1). Major clinical outcomes were compared between the 2 groups up to 8 years. To adjust for potential confounders, a propensity score-matched (PSM) analysis was performed using the logistic regression model. After PSM analysis, 2 propensity score-matched groups (366 pairs, n = 732, c-statistic = 0.629) were generated and their baseline characteristics were balanced.
Figure 1.
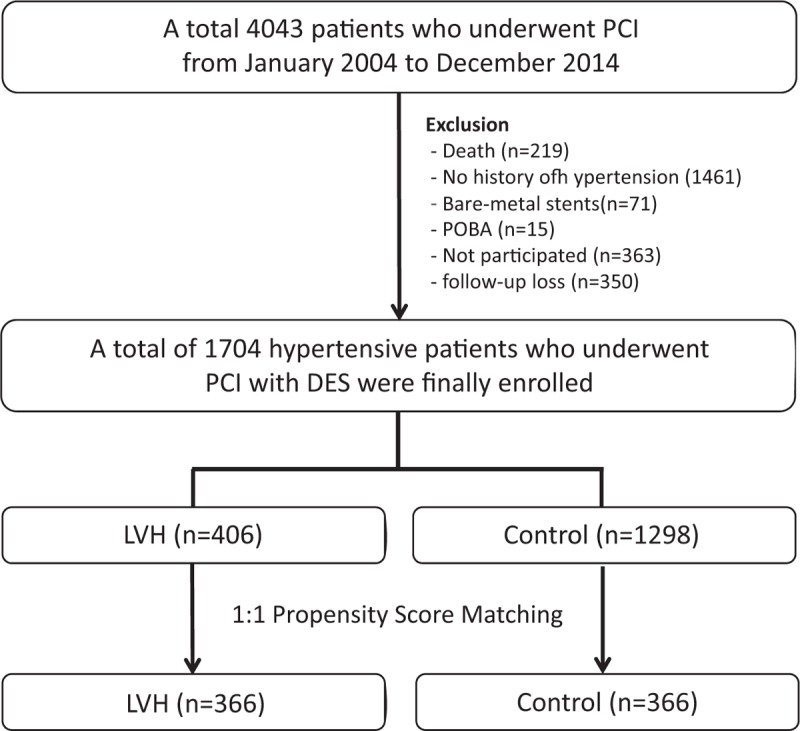
Flow chart of study patients. DES = drug-eluting stent, LVH = left ventricular hypertrophy, PCI = percutaneous coronary intervention, POBA = plain old balloon angioplasty.
2.2. Percutaneous coronary intervention procedure and medical treatment
Diagnostic coronary angiography and PCI were performed through either the femoral or radial artery after an administration of unfractionated heparin (70–100 IU/kg). Patient's activated clotting time was maintained above 250 seconds during the procedure. Revascularization was considered clinically indicated when the patient had angina and/or signs of ischemia and ≥50% diameter stenosis by angiography, or ≥70% diameter stenosis even in the absence of signs and symptoms. The use of cilostazol (Pletaal, Otsuka Pharmaceutical Co., Tokyo, Japan) or platelet glycoprotein IIb/IIIa receptor blockers was left to the operator's discretion. A successful PCI was defined as the achievement of angiographic residual stenosis <30% and final thrombolysis in myocardial infarction blood flow grade of 3. During hospitalization, enrolled patients were administered medications such as beta blockers (BB), angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), calcium channel blockers (CCB), and lipid-lowering agents. After discharge, patients were encouraged to maintain their medication regimen.
2.3. Study definitions and clinical follow-up
The recording of cardiovascular risk factors and medical histories were based on patient's self-report. LVH was considered according to the amount of left ventricular mass (LVM). LVM was calculated by means of Devereux's formula[7] and then the LVM was indexed to body surface area. Finally, LVH was defined by of left ventricular mass index (LVMI), such as >115 g/m2 in men and >95 g/m2 in women based on the American Society of Echocardiography's guidelines.[8] All these analysis of echocardiographic measuring and assessment were done by a core lab of KUGH.
The primary outcome was the incidence of major adverse cardiac events (MACE), which was defined as a composite of total death, cardiac death, nonfatal acute myocardial infarction (AMI), and revascularization rates including target vessel revascularization (TVR) rate and non-TVR. All deaths were classified as cardiac or noncardiac death. Nonfatal AMI was defined as the presence of clinical symptoms, electrocardiographic changes, or abnormal imaging findings of myocardial infarction, combined with an increase in the creatine kinase myocardial band fraction above the upper normal limits or an increase in troponin-T/troponin-I to greater than the 99th percentile of the upper normal limit.[9] TVR was defined as revascularization of the target vessel or any segment of the coronary artery including the target lesion. Non-TVR was defined as revascularization of any segment of the nontarget coronary artery. The primary endpoints were a composite of MACE, composite of death, nonfatal myocardial infarction (MI), and revascularization during an 8-year follow-up period. In this study, all clinical follow-up was done through face-to-face interviews at the outpatient clinic, medical chart reviews, and telephone contacts. Mean follow-up period was 1600 ± 909 days and clinical data from all enrolled patients were accessible for the follow-up.
2.4. Statistical analysis
For continuous variables, differences between the 2 groups were evaluated by the unpaired t test or Mann–Whitney rank test. Data were expressed as mean ± standard deviations. For discrete variables, differences between the groups were expressed as counts and percentages and analyzed by the chi-squared or Fisher exact test. To adjust for potential confounders, PSM analysis was performed using the logistic regression model. We tested all available variables that could be of potential relevance: gender (men), age, systolic blood pressure, diastolic blood pressure, left ventricular ejection fraction, ST-segment elevation myocardial infarction (STEMI), non-ST-segment elevation myocardial infarction (non-STEMI), unstable angina, stable angina, cardiogenic shock, cardiovascular diseases risk factors (diabetes, dyslipidemia, cerebrovascular accident [CVA], peripheral vascular disease [PVD], chronic kidney disease [CKD], history of coronary artery disease (CAD), previous coronary artery bypass graft, previous PCI, previous MI, current smokers, and current alcoholics), laboratory findings (e.g., hemoglobin, CK-MB, troponin T, lipid profiles, apolipoprotein A-1, apolipoprotein B, apolipoprotein C-II, apolipoprotein E, lipoprotein (a), high-sensitivity C-reactive protein, fasting blood glucose, hemoglobin A1c, and serum creatinine), angiographic characteristics (targeted vessels, American College of Cardiology [ACC]/American Heart Association [AHA] B1/B2/C lesions), type of DES, numbers of diseased vessels, left main disease, bifurcation lesion, calcified lesion, procedure time, total doses of unfractionated heparin, final activated clotting time), and post-PCI medications (aspirin, clopidogrel, cilostazol, prasugrel, BB, CCB, ACEI, ARB, diuretics, lipid-lowering agents, and proton pump inhibitors). The logistic model through which propensity scores were estimated showed good predictive value (c-statistic = 0.629). Subjects were matched with a caliper width equal to 0.01. The procedure yielded 366 well-matched pairs. Various clinical outcomes were estimated with the Kaplan–Meier method, and differences between the 2 groups were compared with the log-rank test. For all analyses, a 2-sided P < .05 was considered statistically significant. All data were processed using Statistical Package for the Social Sciences version 20.0 (IBM SPSS, Inc., Chicago, IL).
3. Results
3.1. Clinical and laboratory characteristics
After PSM analysis, 2 propensity score-matched groups (366 pairs, n = 732, c-statistic = .629) were generated. Their baseline characteristics and laboratory findings are summarized in Table 1. In the unmatched population, the mean age was 65.2 ± 10.2 years for the LVH group, and 64.9 ± 10.9 years for the control group (P = .690) and the LVM was 244 ± 60 g for the LVH group, and 172 ± 52 g for the control group (P < .001). The LVMI was 147 ± 35 g/m2 for the LVH group and 97 ± 28 g/m2 for the control group (P < .001). Also, other echocardiographic parameters such as LV end diastolic dimension (53 ± 5 vs 47 ± 4 mm, P < .001), LV end diastolic volume index (56 ± 12 vs 49 ± 14 mL/m2, P < .001) were significantly larger in the LVH group compared with the control group. But, their LV ejection fractions between these 2 groups were similar before (54 ± 11% vs 54 ± 10%, P = .429) and after (54 ± 12% vs 54 ± 10%, P = .543) PSM processing.
Table 1.
Clinical characteristics and laboratory findings.
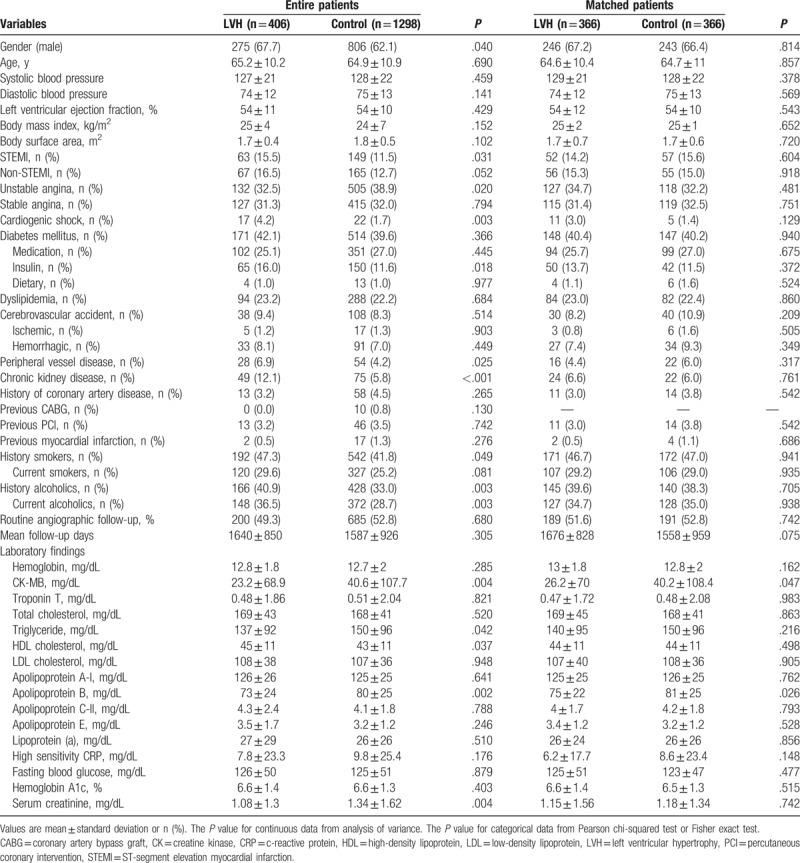
The LVH group had higher numbers of STEMI, cardiogenic shock, PVD, CKD, smokers, and alcoholic patients compared with the control group. However, the control group had higher serum level of CK-MB, triglyceride, apolipoprotein B, and serum creatinine level than the LVH group. The types of treated vessels, ACC/AHA lesion type, numbers of treated vessels, left main disease, bifurcation lesions, calcified lesion, and total procedure times were not notably different between the 2 groups (Table 2). Only Zotarolimus-eluting stent (Resolute, Medtronic Inc, Santa Rosa, CA) was more frequently deployed in the LVH group (38.7% vs 32.7%, P = .026). Periprocedural complications are also shown in Table 2. In the unmatched population, major hematoma (>4 cm) was more common in the control group than in the LVH group (3.8% vs 0.7%, P = .002), but transfusion was done more frequently in the LVH group (10.8% vs 7.2%, P = .020) (Table 3). During hospitalization, diuretics were more commonly used in the LVH group than in the control group (29.6% vs 22.3%, P = .003). After discharge, lipid-lowering agents were prescribed much more frequently in the control group (86.4% vs 81.8%, P = .023, Table 4). However, this bias was abolished after PSM processing. During hospitalization, the incidences of heart failure (HF) and CVA were similar between the 2 groups before and after PSM processing (Table 3).
Table 2.
Angiographic characteristics.
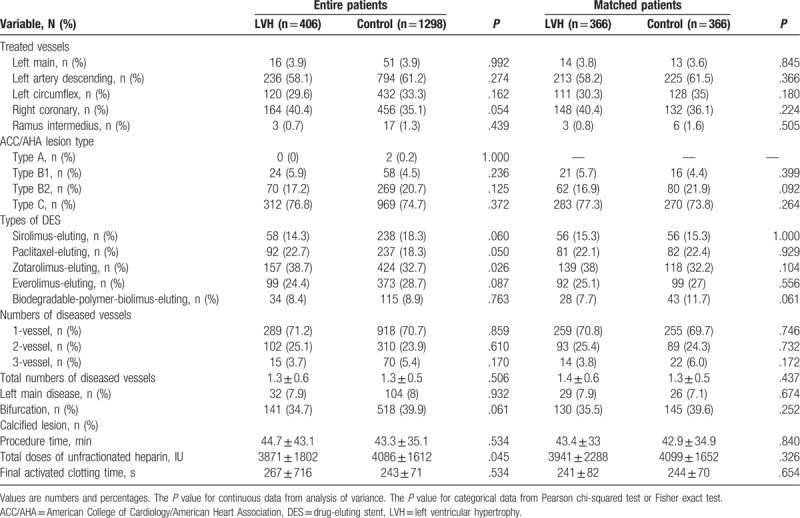
Table 3.
Periprocedural complications.
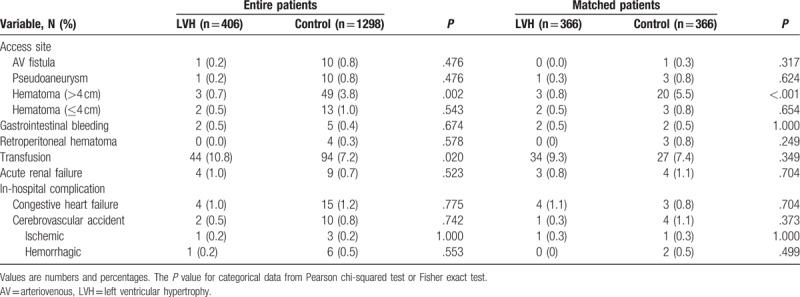
Table 4.
Types of medications.
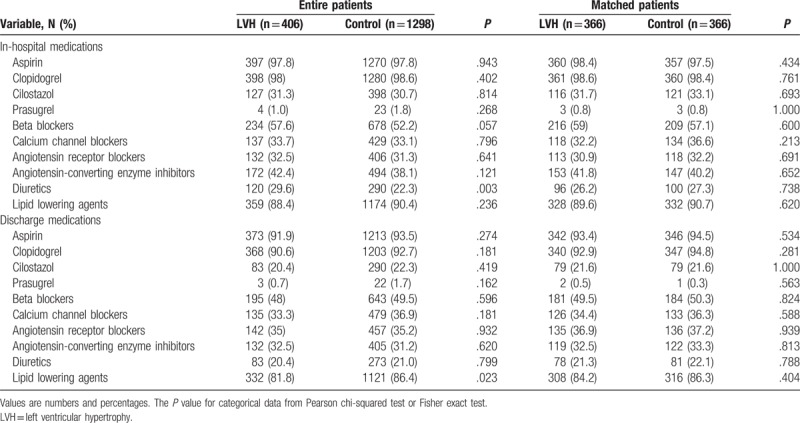
3.2. Clinical outcomes
The cumulative clinical outcomes up to 8 years between the LVH group and the control group are shown in Table 5. After PSM, the incidence of cardiac death (4.4% vs 1.2%, log-rank P = .023, hazard ratio [HR], 3.371; 95% confidence interval [CI], 1.109–10.25; P = .032) was higher in the LVH group compared with the control group. However, the incidence of total death (7.9% vs 5.4%, log-rank P = .117, HR, 1.653; 95% CI, 0.875–3.122; P = .121), MI (7.9% vs 4.9%, log-rank P = .230, HR, 1.523; 95% CI, 0.762–3.043; P = .234), revascularization (19.1% vs 17.0%, log-rank P = .733, HR, 1.072; 95% CI, 0.720–1.594; P = .733), and MACE (25.1% vs 20.5%, log-rank P = .219, HR, 1.239; 95% CI, 0.879–1.747; P = .220) were not significantly different between the 2 groups. Overall, Kaplan–Meier analysis of cumulative clinical outcomes up to 8 years between the LVH group and control group are shown in Fig. 2.
Table 5.
Cumulative clinical outcomes up to 8 years between the LVH group and the control group.
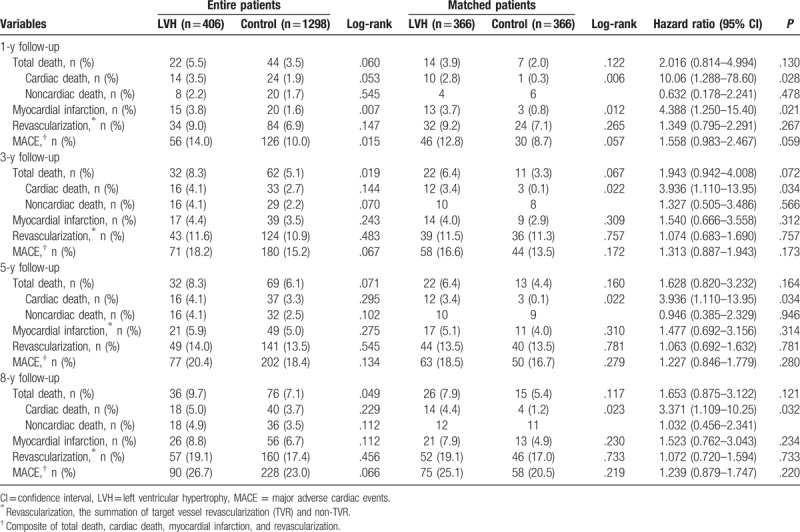
Figure 2.
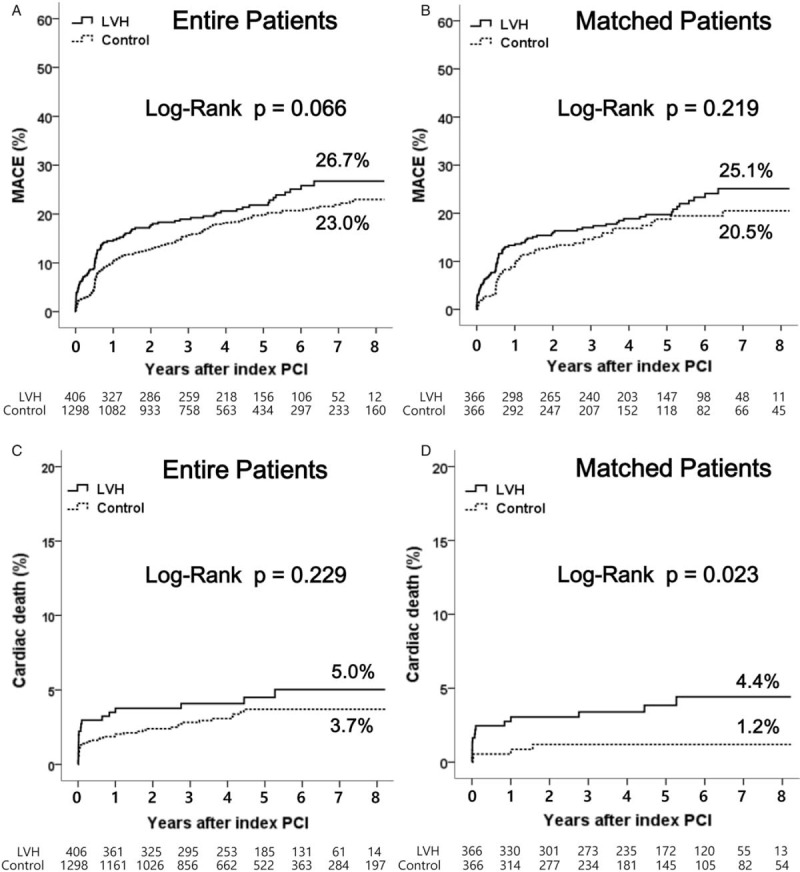
Kaplan–Meier analysis of major adverse cardiac events and cardiac death up to 8 years between the left ventricular hypertrophy group and the control group. LVH = left ventricular hypertrophy, MACE = major adverse cardiac events, PCI = percutaneous coronary intervention.
4. Discussion
The main findings of this study is that hypertensive patients with LVH underwent PCI with DES were associated with higher cardiac mortality up to 8 years. However, there were no significant differences between the 2 groups in the incidence of total death, MI, revascularization, and MACE up to 8 years.
4.1. Molecular factors of LVH
LVH was associated with increased mortality through several mechanisms. For example, renin–angiotensin system was related to the LVH and may promote the progression of atherosclerosis by the effects of angiotensin II on vascular tone, coagulation system, and vascular smooth muscle cell proliferation.[10] Some other potential molecular mechanisms are also related to LVH.[11–14] Urotensin II consist of 11-aminoacid peptide and has effects of vasoconstriction and vasodilation on vascular beds.[11] Urotensin II detected in human vascular smooth muscle, cardiac myocytes, and endothelial cells and induce cardiac myocyte hypertrophy.[12] CCN2/connective tissue growth factor is a kind of matricellular protein and is related to the extracellular matrix synthesis, cell proliferation, and angiogenesis, and suggested to play an important role in cardiac remodeling.[13] Wang et al[14] reported that Toll-Like Receptor 2 (TLR2) on leukocytes was a meaningful mediator of LV systolic, diastolic dysfunction, and LV fibrosis under sustained pressure overload.
4.2. Clinical implications of left ventricular hypertrophy
LVH can contribute to increased rates of cardiovascular events through its effects on ventricular function, coronary circulation, and arrhythmogenesis.[3] The adjusted risk of future cardiovascular morbidity associated with baseline LVH ranged from 1.5 to 3.5.[2] Some possible mechanisms concerning the relationship between LVH and increased mortality have been reported. Activation of the renin–angiotensin system leads to LVH and may promote the progression of atherosclerosis by the effects of angiotensin II on vasomotor tone, coagulation, and vascular smooth muscle cell proliferation.[15,16] LVH can cause increased prevalence of sudden cardiac death (SCD). Several studies have demonstrated that an increased risk of complex ventricular ectopic activity and risk of SCD[17] and regression of LVH may be associated with reduced risk of SCD.[18] Coronary flow reserve is markedly reduced in patients with LVH due to hypertension or aortic stenosis. It means the coronary arteries’ ability to increase blood flow when stress is reduced.[19]
4.3. Left ventricular hypertrophy and prognosis
Evaluation of risk factors related with long-term prognosis in patients with CAD is an important component in initial determination of appropriate therapy. As previously mentioned, although LVH is an important risk factor of cardiovascular mortality,[20,21] data are limited regarding the impact of LVH on long-term prognosis in patients with CAD undergoing PCI especially in the DES era. Park et al[22] reported that LVH was associated with increased rate of adverse clinical outcomes in 30-day survivors after STEMI who underwent successful PCI. Although they analyzed only STEMI patients, we included all consecutive hypertensive patients who successfully underwent PCI with DES between January 2004 and December 2014, so our study results may have a meaningful message regarding long-term clinical outcomes in hypertensive patients after index PCI with DES with relatively larger, all-comer study population. LVH can cause anatomical changes in the intramyocardial coronary artery and these changes increase in the vascular resistance, which finally leads to mismatch between myocardial oxygen demand, and supply.[23] In this study, 14 cardiac deaths occurred in the LVH group during the 8-year follow-up period after PSM matching. In these patients, 5 deaths were due to STEMI, 4 due to non-STEMI, 2 due to SCD, and 3 due to HF. These results mean that LVH can cause MI, SCD, and HF in hypertensive patients with CAD following successful PCI with DES. Similarly, 2 patients died of STEMI and another 2 due to non-STEMI in the control group (Table 4). In this study, the majority cardiac deaths occurred in the first year of enrollment. Thereafter, slope of the cardiac death was not steep. During this period cardiac death more frequently occurred in the LVH group (10/14, 71.4%) compared with noncardiac death. In the control group, noncardiac death was more common than cardiac death. The incidences of nonfatal myocardial infarction were also higher in the LVH group (3.7% vs 0.8%, log-rank P = .012, HR, 4.388; 95% CI, 1.250–15.40; P = .021, Table 5) compared with the control group. According these results, we can guess that LVH may be related to increased incidences of cardiac death and nonfatal myocardial infarction after successful index PCI in hypertensive patients. These increased incidences of cardiac deaths in the LVH group compared with the control group were sustained during 8-year follow-up periods. The authors think that these patterns of cardiac deaths were very important and meaningful and show us the causative relationship between LVH and cardiac death in hypertensive patients after successful PCI with DES.
East et al[24] reported echocardiographic LVH to be independently associated with a 56% increase in the risk of 3-year mortality among patients with CAD, but only 37% of LVH patients underwent revascularization. In Heart and Soul Study,[4] although all patients had CAD, only 62% with LVH had been vascularized. Recently, Brown et al[25] reported that LVH was found not to be an independent predictor of mortality (HR, 0.93; 95% CI, 0.68–1.28; P = .67). He analyzed 4284 patients with CAD following PCI median 3-year follow-up periods. However, the primary endpoint of his study was not cardiac death, but all-cause mortality following discharge from the hospital for the index PCI. In our study, during 8-year follow-up period, total death was also not significantly different between the 2 groups (7.9% vs 5.4%, log-rank P = .117, HR, 1.653; 95% CI, 0.875–3.122; P = .121), but the incidence of cardiac death (4.4% vs 1.2%, log-rank P = .023, HR, 3.371; 95% CI, 1.109–10.25; P = .032, 1D) was higher in the LVH group compared with the control group after PSM. According to David's report, the mortality at a median follow-up of 3 years for patients with LVH was 14% versus 8.9% in patients without LVH (log-rank P < .001) before adjustment. In our study, the mortality rates during the 8-year follow-up period between the 2 groups were 8.9% versus 5.9% (log-rank P = .033) in entire patients and 7.9% versus 5.4% (log-rank P = .117) in PSM patients (Table 5). David's study populations were collected between January 1, 1998 and October 1, 1999 before the introduction of DES. Bare metal stents would have impact on these results. Other possible explanations are advances in PCI device technology and improvements in medical therapy. These factors have possibly reduced the morality rates after PCI.
It is possible that certain elements of medication for secondary prevention following index PCI, such as BB, ACEI, ARB, and lipid-lowering agents, may have reduced the regression of LVH which in turn may have reduced the mortality rate. However, these biases were corrected after PSM in this study.
As shown in our study, LVH was an important prognostic factor for cardiac death in hypertensive patients who underwent successful PCI with DES. As a result, more attentions focused on LVH should be needed during treatment of hypertensive patients.
This study has some limitations. First, it is single-center study with a nonrandomized design. Furthermore, like every “real-world” registry, there may have been some underreporting and/or missing data. However, this manner has great advantages as it reflects real and routine hospital clinical practices. Second, because we have not enrolled the patients with aortic stenosis in our study, it may act as another weak point of this study. Third, in our study, we examined only baseline significance of LVH and we did not estimate the impact of LVH on their serial changes during 8-year follow-up period because the progression or regression of LVH might affect these clinical outcomes. Fourth, although we have strictly emphasized regular medication after index PCI during follow-up period, drug compliance can influence the end results of our study. In addition, because we did not have full information for the types and doses of secondary medications, this can act as bias in this study. Therefore, large, randomized, and controlled clinical trials will be required for a more definitive conclusion.
In conclusion, LVH in hypertensive patients who underwent successful PCI with DES was associated with higher incidence of cardiac death up to 8 years of follow-up. More careful management and clinical follow-up is needed and treatment strategies should specifically focus to target prevention and reversal of LVH in hypertensive patients.
Author contributions
Conceptualization: Yong Hoon Kim, Ae-Young Her, Seung-Woon Rha.
Data curation: Yong Hoon Kim, Ae-Young Her, Byoung Geol Choi, Se Yeon Choi, Jae Kyeong Byun, Man Jong Baek, Yang Gi Ryu, Yoonjee Park, Ahmed Mashaly, Won Young Jang, Woohyeun Kim, Jah Yeon Choi, Eun Jin Park, Jin Oh Na, Cheol Ung Choi, Hong Euy Lim, Eung Ju Kim, Chang Gyu Park, Hong Seog Seo, Seung-Woon Rha.
Formal analysis: Yong Hoon Kim, Ae-Young Her, Byoung Geol Choi, Seung-Woon Rha.
Investigation: Yong Hoon Kim, Ae-Young Her, Seung-Woon Rha.
Methodology: Yong Hoon Kim, Ae-Young Her, Byoung Geol Choi, Cheol Ung Choi.
Project administration: Yong Hoon Kim, Ae-Young Her, Seung-Woon Rha.
Resources: Cheol Ung Choi.
Software: Se Yeon Choi, Man Jong Baek, Yang Gi Ryu, Ahmed Mashaly, Cheol Ung Choi.
Supervision: Seung-Woon Rha.
Validation: Yong Hoon Kim, Ae-Young Her, Seung-Woon Rha.
Visualization: Yong Hoon Kim, Ae-Young Her, Seung-Woon Rha.
Writing – original draft: Yong Hoon Kim, Ae-Young Her.
Writing – review & editing: Yong Hoon Kim, Ae-Young Her, Seung-Woon Rha.
Footnotes
Abbreviations: ACC = American College of Cardiology, ACEI = angiotensin-converting enzyme inhibitors, AHA = American Heart Association, AMI = acute myocardial infarction, ARB = angiotensin receptor blockers, BB = beta blockers, CAD = coronary artery disease, CCB = calcium channel blockers, CFR = coronary flow reserve, CI = confidence interval, CKD = chronic kidney disease, CVA = cerebrovascular accident, HF = heart failure, HR = hazard ratio, KUGH = Korea University Guro Hospital, LVM = left ventricular mass, LVMI = left ventricular mass index, MI = myocardial infarction, PSM = propensity score matched, PVD = peripheral vascular disease, SCD = sudden cardiac death, STEMI = ST-segment elevation myocardial infarction, TVR = target vessel revascularization.
Yong Hoon Kim and Ae-Young Her have contributed equally to the writing of this article.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360:1903–13. [DOI] [PubMed] [Google Scholar]
- [2].Vakili BA, Okin PM, Devereux RB. Prognostic implications of left ventricular hypertrophy. Am Heart J 2001;141:334–41. [DOI] [PubMed] [Google Scholar]
- [3].Clement DL, De Buyzere M, Duprez D. Left ventricular function and regression of left ventricular hypertrophy in essential hypertension. Am J Hypertens 1993;6:14s–9s. [DOI] [PubMed] [Google Scholar]
- [4].Turakhia MP, Schiller NB, Whooley MA. Prognostic significance of increased left ventricular mass index to mortality and sudden death in patients with stable coronary heart disease (from the Heart and Soul Study). Am J Cardiol 2008;102:1131–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Westerhout CM, Lauer MS, James S, et al. Electrocardiographic left ventricular hypertrophy in GUSTO IV ACS: an important risk marker of mortality in women. Eur Heart J 2007;28:2064–9. [DOI] [PubMed] [Google Scholar]
- [6].Sullivan JM, Vander Zwaag RV, el-Zeky F, et al. Left ventricular hypertrophy: effect on survival. J Am Coll Cardiol 1993;22:508–13. [DOI] [PubMed] [Google Scholar]
- [7].Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 1986;57:450–8. [DOI] [PubMed] [Google Scholar]
- [8].Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18:1440–63. [DOI] [PubMed] [Google Scholar]
- [9].The Joint European Society of Cardiology/American College of Cardiology Committee. Myocardial infarction redefined—a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. Eur Heart J 2000;21:1502–13. [DOI] [PubMed] [Google Scholar]
- [10].Lyons D, Webster J, Benjamin N. Angiotensin II: adrenergic sympathetic constrictor actions in humans. Circulation 1995;91:1457–60. [DOI] [PubMed] [Google Scholar]
- [11].Cirillo P, De Rosa S, Pacileo M, et al. Human urotensin II induces tissue factor and cellular adhesion molecules expression in human coronary endothelial cells: an emerging role for urotensin II in cardiovascular disease. J Thromb Haemost 2008;6:726–36. [DOI] [PubMed] [Google Scholar]
- [12].Zou Y, Nagai R, Yam Azaki T. Urotensin II induces hypertrophic response s in cultured cardiomyocytes from neonatal rats. FEBS Lett 2001;508:57–60. [DOI] [PubMed] [Google Scholar]
- [13].Ritschel V, Shetelig C, Seljeflot I, et al. Evaluation of circulating levels of CCN2/connective tissue growth factor in patients with ST-elevation myocardial infarction. Sci Rep 2017;7:11945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang JW, Fontes MSC, Wang X, et al. Leukocytic toll-like receptor 2 deficiency preserves cardiac function and reduces fibrosis in sustained pressure overload. Sci Rep 2017;7:9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Goldsmith SR, Hasking GJ, Miller E. Angiotensin II and sympathetic activity in patients with congestive heart failure. J Am Coll Cardiol 1993;21:1107–13. [DOI] [PubMed] [Google Scholar]
- [16].Vaughan DE, Lazos SA, Tong K. Angiotensin II regulates the expression of plasminogen activator inhibitor-1 in cultured endothelial cells. A potential link between the renin-angiotensin system and thrombosis. J Clin Invest 1995;95:995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Haider AW, Larson MG, Benjamin EJ, et al. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol 1998;32:1454–9. [DOI] [PubMed] [Google Scholar]
- [18].Wachtell K, Okin PM, Olsen MH, et al. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive therapy and reduction in sudden cardiac death: the LIFE study. Circulation 2007;116:700–5. [DOI] [PubMed] [Google Scholar]
- [19].Artham SM, Lavie CJ, Milani RV, et al. Clinical impact of left ventricular hypertrophy and implications for regression. Prog Cardiovasc Dis 2009;52:153–67. [DOI] [PubMed] [Google Scholar]
- [20].Schillaci G, Verdecchia P, Porcellati C, et al. Continuous relation between left ventricular mass and cardiovascular risk in essential hypertension. Hypertension 2000;35:580–6. [DOI] [PubMed] [Google Scholar]
- [21].Liao Y, Cooper RS, McGee DL, et al. The relative effects of left ventricular hypertrophy, coronary artery disease, and ventricular dysfunction on survival among black adults. JAMA 1995;273:1592–7. [PubMed] [Google Scholar]
- [22].Park JS, Shin JS, Lee YH, et al. Left ventricular hypertrophy on long-term cardiovascular outcomes in patients with ST-elevation myocardial infarction. Clin Exp Hypertens 2015;37:674–9. [DOI] [PubMed] [Google Scholar]
- [23].Camici PG, Olivotto I, Rimoldi OE. The coronary circulation and blood flow in left ventricular hypertrophy. J Mol Cell Cardiol 2012;52:857–64. [DOI] [PubMed] [Google Scholar]
- [24].East MA, Jollis JG, Nelson CL, et al. The influence of left ventricular hypertrophy on survival in patients with coronary artery disease: do race and gender matter? J Am Coll Cardiol 2003;41:949–54. [DOI] [PubMed] [Google Scholar]
- [25].Brown DL. Effect of left ventricular hypertrophy on long-term survival of patients with coronary artery disease following percutaneous coronary intervention. Heart Int 2009;4:e9. [DOI] [PMC free article] [PubMed] [Google Scholar]


