Abstract
Endocrine pancreatic insufficiency secondary to acute pancreatitis (AP) drew increasing attention in the recent years. The aim of the present study was to assess the impact of pancreatic necrosis and organ failure on the risk of developing new-onset diabetes after AP.
The follow-up study was conducted for patients recovered from AP in the treatment center of Jinling Hospital. Endocrine function was evaluated by simplified oral glucose tolerance test (OGTT). Pancreatic necrosis was examined by abdominal contrast-enhanced CT (CECT) scan during hospitalization. The data including APACHE II score, Balthazar's score, organ failure (AKI and ARDS) was also collected from the medical record database. All patients were divided into group diabetes mellitus (DM) and group non-DM according to the endocrine function and group pancreatic necrosis (PN) and persistent organ failure (OF), group PN and non-OF, group non-PN and OF, and group non-PN and non-OF according to the occurrence of pancreatic necrosis and persistent organ failure.
Around 256 patients were included for the final analysis. 154 patients (60.2%) were diagnosed with DM (include impaired glucose tolerance, IGT), while 102 patients (39.8%) were deemed as normal endocrine function. APACHE II score and Balthazar score of the patients in the group DM were significant higher than those in the non-DM group (F = 6.09, P = .01; F = 10.74, P = .001). The incidence of pancreatic necrosis in group DM and group non-DM was, respectively, 64.7% and 53.0% (χ2 = 3.506, P = .06). The patients underwent necrosis debridement by percutaneous catheter drainage (PCD) and/or the operative necrosectomy (ON) were more likely to developed new onset DM than the patients without PCD or ON (χ2 = 2.385, P = .02). The morbidity of new-onset DM after AP gradually increased from group non-PN and non-OF, group non-PN and OF, group PN and non-OF to group PN and OF in order (χ2 = 4.587, P = .03). The value of HOMA-IR of patients at follow-up time was significant higher in group DM than group non-DM (F = 13.414, P = .000).
Patients with both PN and persistent OF may were at increased risk of developing new-onset diabetes after AP. Insulin resistance could be the pivotal mechanism of the development of diabetes.
Keywords: acute pancreatitis (AP), endocrine pancreatic insufficiency (EPI), insulin resistance, new-onset diabetes, organ failure (OF), pancreatic necrosis (PN)
1. Introduction
Endocrine pancreatic insufficiency (EPI) secondary to acute pancreatitis (AP) turns to be known recently,[1,2] although the scholars have different opinions on the risk factors of it. In 2013, a research from New Zealand found that recurrent attacks of AP, obesity, hypertriglyceridemia, age above 45 years, family history of DM were the risk factors. However, it showed no obvious influence of the disease severity on the pancreatic endocrine function.[3] In 2015, Hsiu-Nien Shen from Taiwan found out the risk of diabetes increases by twofold after AP. But the risk of diabetes for mild AP was similar to that for all AP patients.[4] Both of the 2 studies showed no correlation between the severity of AP and EPI which many scholars disagree with.[5–7] As one of the largest sever acute pancreatitis (SAP) treatment centers in China, the patients recovered from AP were included randomly into the follow-up study to verify the role of pancreatic necrosis and organ failure in the new-onset diabetes after AP.
2. Material and methods
2.1. Patients
From January to December 2016, this study was undertaken in the SAP treatment center of Nanjing University, which is one of the largest SAP centers in China. Around 276 discharged patients in the outpatients’ database were randomly invited to participate in the follow-up study by phone or mail. The written informed consent was obtained from each subject. The study was approved by the ethics committee of the Jinling Hospital, Medical School of Nanjing University.
The exclusion criteria were as follows: patients who suffered recurrent AP; patients with chronic pancreatitis; patients with diagnosed DM before AP episodes; patients suffered from chronic diarrhea before AP; patients with intestinal tuberculosis or Crohn's disease; patients with family history of DM; patients with incomplete medical record; and patients who died during hospitalization or after discharge from hospital.
2.2. Assessment methods and data collection
Simplified OGTT[8] was applied to assess the pancreatic endocrine function. The value of fasting plasma glucose (FPG), fasting insulins (FINS), 2-hour postprandial blood glucose (2hPG), HOMA-IR from the test was collected as evaluation indexes. The HOMA-IR that represents the condition of insulin resistance was calculated by the formula of [HOMA-IR = FPG × FINS/22.5].[9,10] The symptoms, diet, exercise, medication, etc., were inquired and recorded. The images of pancreas contrast-enhanced CT (CECT) scan during hospitalization time were collected and pancreatic necrosis was judged by Balthazar's classification through the CECT images.[11] Other major data of each patients during hospitalization such as APACHE II score,[12] persistent organ failure (AKI and ARDS) were also collected.
2.3. Definition
Diabetes including impaired glucose tolerance (IGT) was defined using the 1999 World Health Organization criteria. Diabetes was diagnosed by typical diabetes symptoms with any of the following items:
-
1.
FPG≥7.0 mmol/L.
-
2.
Random blood glucose≥11.1 mmol/L.
-
3.
FPG<7.0 mmol/L and 2hPG>11.1 mmol/L after a 75-g OGTT.
Diabetes was also diagnosed by any of the following items if without classical diabetes symptom:
-
1.
FPG>7.0 mmol/L for 2 times.
-
2.
2hPG≥11.1 mmol/L for 2 times.
IGT was diagnosed by FPG<7.0 mmol/L and 7.8 mmol/L<2hPG<11.1 mmol/L after a 75-g OGTT.
2.4. Statistics analysis
Statistical analyses were performed using SPSS 22.0 (IBM Co, Armonk, NY). The continuous outcome variable was analyzed by one-way ANOVA and the categorical variable was analyzed by χ2 test between the different groups. Odds ratios (ORs) are expressed with 95% confidence intervals (CIs). The linear trend test was used to analyze the risk of new-onset diabetes base on PN and OF. A P value of < .05 was considered significant.
3. Results
3.1. General information
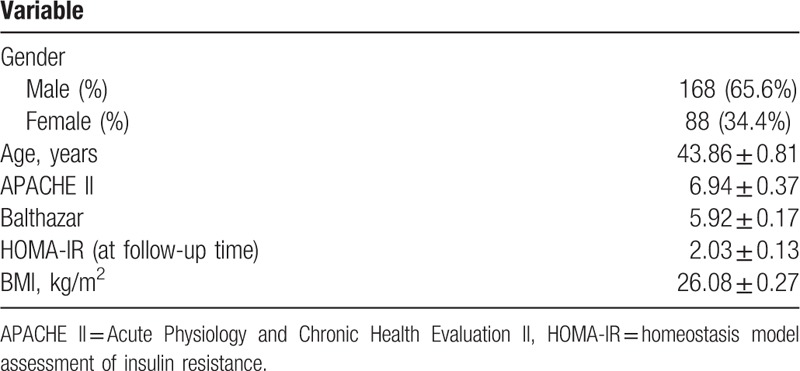
Finally, 256 patients were included and 20 patients were excluded due to meeting the exclusion criteria, change of address, or declining to participate in the study. Among the 20 cases, 14 patients (5.1% in all patients) died during hospitalization or after discharge from hospital due to different reasons, 8 for septic shock, 5 for major bleeding, and 1 died out of hospital for unknown reason. Of the 256 eligible patients, there were 168 males and 88 females with a mean age of 43.86 ± 0.81 years. The shortest time interval from the AP onset to follow-up assessment was 1 month and the longest was 260 months with a mean value of 42.93 ± 4.03 months (median, 30 months). The percent of the patients with the time interval<3 months, 3 months to 5 years and >5 years was, respectively, 7.9%, 66.4%, and 25.7%. For the etiology, the percent of biliary, hyperlipemia, alcoholic, and others were, respectively, 57.5%, 34.5%, 2.7%, and 5.3%. For the severity, 54 patients (21.1%) were classified as mild AP (MAP), 42 patients (16.4%) as moderate severe AP (MSAP), and the remaining 160 patients (62.5%) were all diagnosed as severe AP (SAP). A total of 175 (68.4%) patients with organ failure and/or severe pancreatic infection were admitted into the ICU. The detail data were listed in Table 1.
Table 1.
General characteristics of the patients.
3.2. Morbidity of EPI
Around 154 of 256 patients (60.2%) were diagnosed with new-onset diabetes (include IGT), 102 patients (39.8%) were deemed as normal endocrine function as shown in Figure 1.
Figure 1.
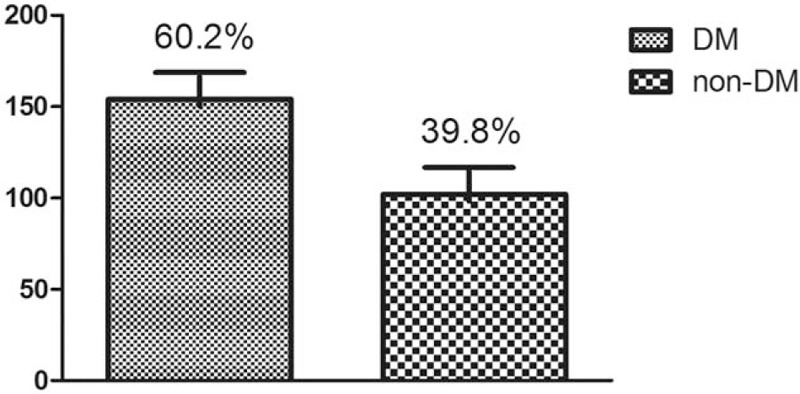
Morbidity of endocrine pancreatic insufficiency (EPI). EPI = endocrine pancreatic insufficiency.
3.3. Comparison of disease severity and metabolism indexes between group DM and group non-DM
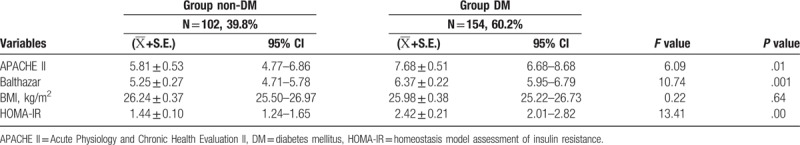
APACHE II score and Balthazar score of the patients in group DM were significant higher than group non-DM (F = 6.09, P = .01; F = 10.74, P = .001). The value of body mass index (BMI) before AP in the 2 groups showed no significant different (F = 0.219, P = .64). The value of HOMA-IR at follow-up time in group DM was significant higher than group non-DM (F = 13.41, P = .000) as listed in Table 2.
Table 2.
Comparison of disease severity and metabolism indexes between group DM and group non-DM.
3.4. Risk of new-onset diabetes based on PN and persistent OF
The ratio of PN in group DM and group non-DM was, respectively, 64.7% and 53.0% (χ2 = 3.506, P = .06). The morbidity of ARDS and AKI in the 2 groups showed no significant difference, respectively (χ2 = 3.136, P = .07; χ2 = 1.595, P = .21). Around 184 (71.7%) patients underwent necrosis debridement by percutaneous catheter drainage (PCD) with or without operative necrosectomy (ON) during admission named group debridement. Around 72 (28.3%) patients conducted neither PCD nor ON named group non-debridement. The patients in group debridement easily developed new onset DM than group non-debridement (χ2 = 2.385, P = .02) as listed in Table 3. The morbidity of DM after AP gradually increased from the following 4 groups in order, group non-PN and non-OF, group non-PN and OF, group PN and non-OF and group PN and OF in linear trend test (χ2 = 4.587, P = .03) as shown in Figure 2.
Table 3.
Comparison of the PN and OF between group DM and group non-DM.
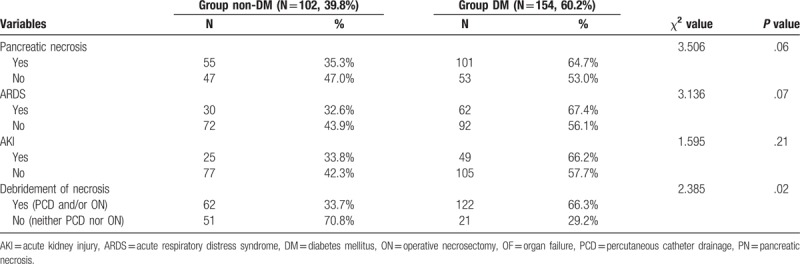
Figure 2.
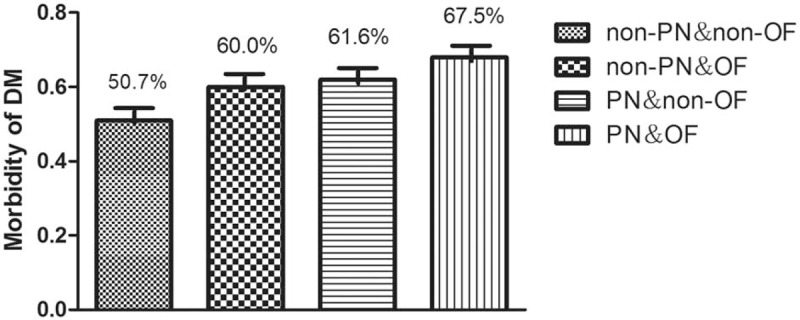
Risk of new-onset diabetes after AP based on PN and OF. AP = acute pancreatitis, OF = organ failure, PN = pancreatic necrosis.
4. Discussion
The new-onset diabetes after AP was often seen and attracted more attention recently. Our study showed 60.2% patients developed DM after AP episodes including IGT which was higher than that in previous studies.[3,4] Several studies had been published regarding the prevention and treatment of new-onset diabetes after AP, but the risk factors remain controversial.[5–7] Some researchers considered it was related with recurrent attacks, alcohol intake, sex and age, but no relation with the severity of AP.[13,14] Others suggested that new-onset diabetes after AP was determined majorly by the severity of AP.[5,6]
The disease severity of AP mainly depends on the occurrence and extent of PN which reflects the pancreas local circumstance and the organs dysfunction which represents the systemic situation. In our study, we found the ratio of PN in group DM was higher than that in group non-DM (64.7% to 53.0%, χ2 = 3.506, P = .06, likely attributed to type II error), which indicated the role of PN on the new-onset diabetes after AP. Connor et al[15] reviewed the clinical outcomes of 88 patients who underwent pancreatic necrosectomy and found that 33% patients without prior diabetes mellitus developed endocrine insufficiency. Umapathy et al[16] reported the natural history after acute necrotizing pancreatitis and showed new-onset diabetes, oral pancreatic enzyme replacement therapy and disability were noted in 45%, 25%, and 53%, respectively, in eligible patients. Bavare et al[17] found that necrotizing pancreatitis affects pancreatic exocrine or endocrine function in more than half of the patients in the follow-up study for the patients receiving necrosectomy. Tsiotos et al[18] also found that necrotizing pancreatitis had prominent effects on long-term pancreatic exocrine and endocrine function in half of the patients. In a long-term outcomes observation after AP, Winter Gasparoto et al[19] found that endocrine dysfunction was observed in half of the cases, morphological changes were frequent (62.5%) and more prevalent in those who faced extensive necrosis. Boreham and Ammori[20] found that the development of exocrine insufficiency correlated strongly with the extent of pancreatic necrosis (r = −0.754, P < .001), and the severity of EPI (n = 4, r = −0.453, P = .03) in the prospective evaluation of pancreatic exocrine function in patients with AP. Busse and Ainsworth[21] reported 10 years of experience with transgastric necrosectomy for wall-of necrosis (WON) in AP and found that endocrine and exocrine insufficiency was often seen at follow-up. 18 (45%) patients developed late complications defined as endocrine and/or exocrine malfunction of the pancreas (diabetes (n = 10), exocrine insufficiency (n = 4), both diabetes and exocrine insufficiency (n = 4)). Chandrasekaran et al[22] compared the long term outcomes in patients with SAP managed by operative and non-operative measures and found patients undergoing necrosectomy had higher incidence of endocrine dysfunction (61.9% in surgery and 28.5% in non-operative group [P = .05]).
Hence, PN could play an important role in developing DM. Extensive PN would lead to the atrophy or absence of the pancreas tissue which could decrease the number of normal islet β cells and the amount of secreted insulin.[23] The phenomenon that patients in the group debridement suffered higher incidence of new onset DM than group non-debridement (χ2 = 2.385, P = .02) reflected the same facts. It is very similar to the pathogenesis of pancreatogenic diabetes after pancreatectomy.[24–26] The development of DM after pancreatic resection is quite common, with different types of resections conveying different risks for disease progression. Compare to distal pancreatectomy (DP), central pancreatectomy (CP) had a higher postoperative morbidity rate and a higher incidence of pancreatic fistula, but a lower risk of endocrine insufficiency (RR 0.22, 95% CI 0.14 to 0.35; P < .001).[27] DP places patients at a greater risk for the development of new-onset diabetes.[28,29] Oh et al assessed the patients’ glucose metabolism and CT scan 1 year after the pancreaticoduodenectomy (PD) and found that the atrophy of the remaining pancreas increases the risk of pancreatogenic DM after PD.[30,31]
On the other hand, as to the complication of persistent OF, there was no significant difference in the morbidity of ARDS or AKI between the 2 groups. But fortunately, we also observed that the morbidity of developing DM after AP gradually increased in the following 4 groups in order, group non-PN and non-OF, group non-PN and OF, group PN and non-OF and group PN and OF (χ2 = 4.587, P = .03). The role of PN combined with OF on the EPI after AP was therefore verified.
Insulin signaling in the target tissues is mediated by stress kinases such as p38 mitogen-activated protein kinase, c-Jun NH2-terminal kinase, inhibitor of NF-kB kinase complex β (IKKβ), AMP-activated protein kinase, and RNA-activated protein kinase.[32,33] This has been one of the key mechanisms observed in the tissues that are implicated in insulin resistance especially in type 2 diabetes mellitus (T2-DM).[34] In our study, we found that the value of HOMA-IR at follow-up time in group DM was higher than that in group non-DM (2.42 ± 0.21 to 1.44 ± 0.10, F = 13.41, P = .000). Therefore insulin resistance may play an pivotal role in developing DM. Balzano et al[35] found that T3cDM appeared to be associated with classical risk factors for type 2 diabetes (i.e., age, sex, family history of diabetes, and BMI), and both β-cell dysfunction and insulin resistance appeared relevant determinants. T3cDM is a heterogeneous entity strongly overlapped with type 2 diabetes. However, the value of HOMA-IR in the study is obtained during follow-up phase, not the hospitalization time. We need further studies to confirm that the insulin resistance after AP was caused by the AP itself or the potential metabolism status proneness of T2-DM.
5. Conclusion
Patients with both PN and persistent OF may were at increased risk of developing new-onset diabetes after AP. Insulin resistance could be the pivotal mechanism of the development of diabetes.
Acknowledgments
The authors are indebted to all doctors for the follow-up assessment and data collection during the study from the severe acute pancreatitis care center of Jinling Hospital, Medical School of Nanjing University. The authors would also like to thank Professor Hanqing He from the Center for Disease Control, Zhejiang Province, China for his help with the statistical analysis.
Author contributions
Study concept and design: Jianfeng Tu, Lu Ke.
Statistical analysis: Yangqi, Guotao Lu, Jingzhu Zhang.
Acquisition of data; analysis and interpretation of data: Yue Yang, Baiqiang Li.
Drafting of the manuscript: Jianfeng Tu.
Critical revision of the manuscript for important intellectual content: Weiqin Li.
Administrative, technical, or material support: Lu Ke & Zhihui Tong.
Study supervision: Weiqin Li & Jieshou Li.
Conceptualization: Zhihui Tong.
Data curation: Yue Yang.
Investigation: Jingzhu Zhang, Baiqiang Li.
Methodology: Qi Yang.
Project administration: Jianfeng Tu, Weiqin Li.
Software: Guotao Lu.
Supervision: Weiqin Li, Jieshou Li.
Writing – review & editing: Lu Ke.
Footnotes
Abbreviations: 2hPG = 2-hour postprandial blood glucose, AKI = acute kidney injury, AP = acute pancreatitis, ARDS = acute respiratory distress syndrome, BMI = body mass index, CECT = contrast-enhanced computed tomography, DM = diabetes mellitus, EPI = endocrine pancreatic insufficiency, FBG = fasting blood-glucose, FINS = fasting insulin, HOMA-IR = homeostasis model assessment of insulin resistance, IGT = impaired glucose tolerance, MAP = mild AP, MSAP = moderate severe AP, OF = organ failure, OGTT = oral glucose tolerance test, PN = pancreatic necrosis, SAP = sever acute pancreatitis, WON = wall-off necrosis.
JT, YY, and JZ are the co-first authors and contributed equally to this work.
Funding: This study was supported by National Natural Science Foundation of China (No.81670588, No.81570584), Science and Technology Foundation of Zhejiang Province, China (No. 2013C37022), and Natural Science Foundation of Zhejiang Provincial, China (No. LY18H150005).
Declarations: Ethics approval and consent to participate: The study was approved by the ethics committee of the Jinling Hospital, Medical School of Nanjing University. All participants consented to participate in the study and the written informed consent was obtained from each subject.
Competing interests: The authors declare that they have no competing interests.
Funding: The article processing charge was funded by the Natural Science Foundation of China (No. 81570584, 81670588). The collection, analysis, and interpretation of data were funded by the Science and Technology Foundation of Zhejiang Province, China (No. 2013C37022) and the Natural Science Foundation of Zhejiang Provincial, China (No. LY18H150005).
The authors have no conflicts of interest to disclose.
References
- [1].Ewald N, Kaufmann C, Raspe A, et al. Prevalence of diabetes mellitus secondary to pancreatic diseases (type 3c). Diabetes Metab Res Rev 2012;28:338–42. [DOI] [PubMed] [Google Scholar]
- [2].Nils Ewald, Reinhard G, Bretzel Diabetes mellitus secondary to pancreatic diseases (Type 3c)—are we neglecting an important disease? Eur J Intern Med 2013;24:203–6. [DOI] [PubMed] [Google Scholar]
- [3].Das SL, Singh PP, Phillips AR, et al. Newly diagnosed diabetes mellitus after acute pancreatitis: a systematic review and meta-analysis. Gut 2013;0:1–4. [DOI] [PubMed] [Google Scholar]
- [4].Hsiu-Nien Shen, Chun-Chieh Yang, Ya-Hui Chang, et al. Risk of diabetes mellitus after first-attack acute pancreatitis: a National Population-Based Study. Am J Gastroenterol 2015;110:1698–706. [DOI] [PubMed] [Google Scholar]
- [5].Vipperla K, Papachristou GI, Slivka A, et al. Risk of new-onset diabetes is determined by severity of acute pancreatitis. Pancreas 2016;45:e14–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Uomo G, Gallucci F, Madrid E, et al. Pancreatic functional impairment following acute necrotizing pancreatitis: long-term outcome of a non-surgically treated series. Dig Liver Dis 2010;42:149–52. [DOI] [PubMed] [Google Scholar]
- [7].Symersky T, Van Hoom B, Masclee AA. The outcome of a long-term follow-up of pancreatic function after recovery from acute pancreatitis. JOP 2006;7:447–53. [PubMed] [Google Scholar]
- [8].Rämö JT, Kaye SM, Jukarainen S, et al. Liver fat and insulin sensitivity define metabolite profiles during a glucose tolerance test in young adult twins. J Clin Endocrinol Metab 2016;3:jc20153512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ha CH, Swearingin B, Jeon YK. Relationship of visfatin level to pancreatic endocrine hormone level, HOMA-IR index, and HOMA β-cell index in overweight women who performed hydraulic resistance exercise. J Phys Ther Sci 2015;27:2965–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Peplies J, Börnhorst C, Günther K, et al. Longitudinal associations of lifestyle factors and weight status with insulin resistance (HOMA-IR) in preadolescent children: the large prospective cohort study IDEFICS. Int J Behav Nutr Phys Act 2016;13:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Raghuwanshi S, Gupta R, Vyas MM, et al. CT evaluation of acute pancreatitis and its prognostic correlation with CT severity index. J Clin Diagn Res 2016;10:TC06–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Saito N, Kawasaki A, Kim A. APACHE II score and at III activity on admission relates to mortality in ICU patients. Crit Care Med 2016;44(12 suppl 1):354. [Google Scholar]
- [13].Ho TW, Wu JM, Kuo TC. Change of both endocrine and exocrine insufficiencies after acute pancreatitis in non-diabetic patients: A Nationwide Population-Based Study. Medicine (Baltimore) 2015;94:e1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nikkola J, Laukkarinen J, Lahtela J, et al. The long-term prospective follow-up of pancreatic function after the first episode of acute alcoholic pancreatitis: recurrence predisposes one to pancreatic dysfunction and pancreatogenic diabetes. J Clin Gastroenterol 2017;51:183–90. [DOI] [PubMed] [Google Scholar]
- [15].Connor S, Alexakis N, Raraty MG, et al. Early and late complications after pancreatic necrosectomy. Surgery 2005;137:499–505. [DOI] [PubMed] [Google Scholar]
- [16].Umapathy C, Raina A, Saligram S, et al. Natural history after acute necrotizing pancreatitis: a large US tertiary care experience. J Gastrointest Surg 2016;20:1844–53. [DOI] [PubMed] [Google Scholar]
- [17].Bavare C, Prabhu R, Supe A. Early morphological and functional changes in pancreas following necrosectomy for acute severe necrotizing pancreatitis. Indian J Gastroenterol 2004;23:203–5. [PubMed] [Google Scholar]
- [18].Tsiotos GG, Luque-de León E, Sarr MG. Long-term outcome of necrotizing pancreatitis treated by necrosectomy. Br J Surg 1998;85:1650–3. [DOI] [PubMed] [Google Scholar]
- [19].Winter Gasparoto RC, Racy Mde C, De Campos T. Long-term outcomes after acute necrotizing pancreatitis: what happens to the pancreas and to the patient? JOP 2015;16:159–66. [DOI] [PubMed] [Google Scholar]
- [20].Boreham B, Ammori BJ. A prospective evaluation of pancreatic exocrine function in patients with acute pancreatitis: correlation with extent of necrosis and pancreatic endocrine insufficiency. Pancreatology 2003;3:303–8. [DOI] [PubMed] [Google Scholar]
- [21].Busse MJ, Ainsworth AP. Ten years of experience with transgastric necrosectomy for walled-off necrosis in acute pancreatitis. Dan Med J 2015;62:A5131. [PubMed] [Google Scholar]
- [22].Chandrasekaran P, Gupta R, Shenvi S, et al. Prospective comparison of long term outcomes in patients with severe acute pancreatitis managed by operative and non operative measures. Pancreatology 2015;15:478–84. [DOI] [PubMed] [Google Scholar]
- [23].Gupta R, Wig JD, Bhasin DK, et al. Severe acute pancreatitis: the life after. J Gastrointest Surg 2009;13:1328–36. [DOI] [PubMed] [Google Scholar]
- [24].Kapoor VK. Complications of pancreato-duodenectomy. Rozhl Chir 2016;95:53–9. [PubMed] [Google Scholar]
- [25].Kahl S, Malfertheiner P. Exocrine and endocrine pancreatic insufficiency after pancreatic surgery. Best Pract Res Clin Gastroenterol 2004;18:947–55. [DOI] [PubMed] [Google Scholar]
- [26].Roeyen G, Jansen M, Chapelle T, et al. Diabetes mellitus and pre-diabetes are frequently undiagnosed and underreported in patients referred for pancreatic surgery. A prospective observational study. Pancreatology 2016;16:671–6. [DOI] [PubMed] [Google Scholar]
- [27].Iacono C, Verlato G, Ruzzenente A, et al. Systematic review of central pancreatectomy and meta-analysis of central versus distalpancreatectomy. Br J Surg 2013;100:873–85. [DOI] [PubMed] [Google Scholar]
- [28].Burkhart RA, Gerber SM, Tholey RM, et al. Incidence and severity of pancreatogenic diabetes after pancreatic resection. J Gastrointest Surg 2015;19:217–25. [DOI] [PubMed] [Google Scholar]
- [29].Xu SB, Zhu YP, Zhou W, et al. Patients get more long-term benefit from central pancreatectomy than distal resection: a meta-analysis. Eur J Surg Oncol 2013;39:567–74. [DOI] [PubMed] [Google Scholar]
- [30].Oh HM, Yoon YS, Han HS, et al. Risk factors for pancreatogenic diabetes after pancreaticoduodenectomy. Korean J Hepatobiliary Pancreat Surg 2012;16:167–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Garip G, Sarandöl E, Kaya E. Effects of disease severity and necrosis on pancreatic dysfunction after acute pancreatitis. World J Gastroenterol 2013;19:8065–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nandipati KC, Subramanian S, Agrawal DK. Protein kinases: mechanisms and downstream targets in inflammation-mediated obesity and insulin resistance. Mol Cell Biochem 2017;426:27–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gluvic Z, Zaric B, Resanovic I, et al. Link between metabolic syndrome and insulin resistance. Curr Vasc Pharmacol 2017;15:30–9. [DOI] [PubMed] [Google Scholar]
- [34].Tang Q, Li X, Song P, et al. Optimal cut-off values for the homeostasis model assessment of insulin resistance (HOMA-IR) and pre-diabetes screening: developments in research and prospects for the future. Drug Discov Ther 2015;9:380–5. [DOI] [PubMed] [Google Scholar]
- [35].Balzano G, Dugnani E, Pasquale V, et al. Clinical signature and pathogenetic factors of diabetes associated with pancreas disease (T3cDM): a prospective observational study in surgical patients. Acta Diabetol 2014;51:801–11. [DOI] [PubMed] [Google Scholar]


