Abstract
There is increasing evidence that minimally invasive techniques associated with Enhanced Recovery After Surgery (ERAS) protocols reduce surgery-related stress and promote faster recovery after major colorectal surgery. As a single tertiary referral center for colorectal surgery, our aim was to analyze the effects of our ERAS protocol on a heterogeneous population undergoing laparoscopic colorectal surgery.
Prospectively collected data from 283 patients undergoing laparoscopic colorectal resection at the Division of General and Hepatobiliary Surgery, University of Verona Hospital Trust, between March 2014 and March 2018 were retrospectively analyzed. Patients’ adherence to pre-, intra-, and postoperative ERAS protocol items together with surgical short-term outcomes such as morbidity, mortality, length of hospital stay, and readmission rate was considered.
The study protocol was approved by the Ethics Committee of Azienda Ospedaliera Universitaria Integrata di Verona (CRINF-1034 CESC).
During the study period, 200 patients met the inclusion criteria and were enrolled in the ERAS protocol. In this series, 34% of patients were aged 70 years or older. Rectal resections represented 26% of all cases, with stoma formation performed in 14.5% of patients. Despite such procedural heterogeneity, good short-term results were obtained: by postoperative day (POD) 2, 58.5% of patients had full return of bowel function, while 63.5% and 88% achieved regular soft diet intake and autonomous walking, respectively. Median (range) length of hospital stay was 5.5 days (2–40) with 71% of patients being discharged by POD 6. No postoperative mortality was recorded, and the rate of major complications was 3.5%. During the study period, 6 patients required redo surgery (3%) and 5 patients required rehospitalization within 30 days (2.5%).
This study analyzing the results of the fast-track program in our first 200 cases confirms the feasibility and safety of ERAS protocol application within a heterogeneous population undergoing laparoscopic colonic and rectal resection for benign and malignant diseases.
Keywords: colorectal surgery, ERAS protocol, laparoscopic surgery, minimally invasive surgery, rectal cancer
1. Introduction
Laparoscopy and Enhanced Recovery After Surgery (ERAS) programs represent 2 major recent innovations in colorectal surgery. The ERAS protocol is a model of perioperative care for patients undergoing different types of major surgeries.[1] Such protocols consist of pre-, intra-, and postoperative interventions, with the aim of minimizing surgery-related stress and promoting faster restoration of homeostasis. Several perioperative measures have proven to reduce morbidity and hospital stay in patients undergoing colorectal surgery.[2] ERAS programs streamline such interventions as a perioperative pathway leading to lower complication rates and healthcare cost reduction.[3–6] Recent evidence also shows an association between ERAS item adherence and 5-year survival after colorectal surgery.[7]
In colorectal surgery, the application of minimally invasive techniques together with ERAS programs has also produced an improvement in short-term outcomes.[8–12]
This retrospective observational study analyzed data from the first 200 patients undergoing the ERAS protocol at our center after elective colorectal laparoscopic surgery with the aim of assessing the compliance with the protocol's items and its impact on short-term postoperative outcomes.
2. Methods
2.1. Inclusion criteria and population under study
The ERAS protocol was introduced at the Division of General and Hepatobiliary Surgery, University of Verona Hospital Trust, in March 2014. From that date to March 2018, the ERAS protocol was completed by 200 patients undergoing elective laparoscopic colorectal resections for neoplastic or diverticular diseases aged 18 years or older without age limitations. The exclusion criteria were inflammatory bowel disease (IBD), familial adenomatous polyposis (FAP), palliative surgery, body mass index (BMI) above 35 kg/m2, American Society of Anesthesiologists (ASA) physical status above 3, coagulopathy, impaired kidney function, uncontrolled diabetes, severe cardiovascular impairment or chronic obstructive pulmonary disease (COPD), psychiatric disorders, drug and alcohol addiction, surgery duration of more than 6 hours, and denied consent. The effective reasons of exclusion from the ERAS protocol are shown in Figure 1.
Figure 1.
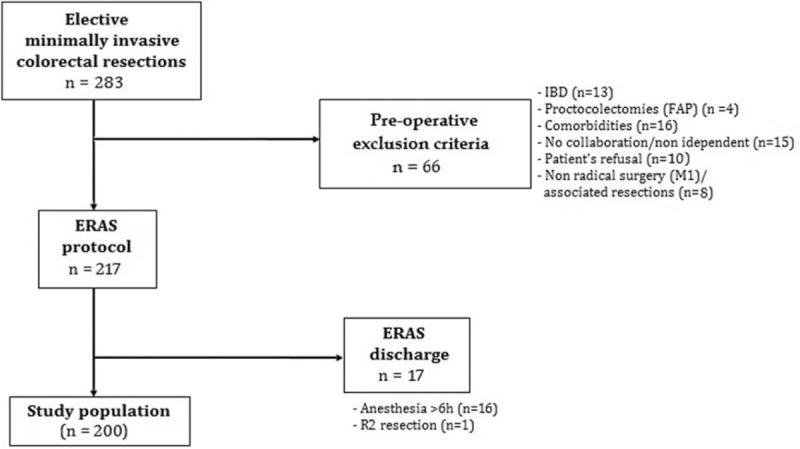
STROBE-compliant flowchart reporting exclusion process from the ERAS protocol for the 283 patients submitted to minimally invasive colorectal resection between March 2014 and March 2018. ERAS = Enhanced Recovery After Surgery.
Informed consent was obtained from all the patients, and the study was approved by the local ethics committee (CRINF-1034 CESC).
2.2. Surgical technique
Surgical technique and anesthesia protocol have already been described in previous publications by our group.[13,14]
Colectomy for lesions located between the cecum and splenic flexure was performed using a 5-port technique, and the specimen was usually extracted through a periumbilical incision obtained by extending the camera port. Anterior resection and sigmoid and left hemicolectomy were performed using a 4-port technique, and the specimen was removed through a suprapubic incision. An additional 11-mm suprapubic trocar was used in low and ultralow anterior resection (LAR) to optimize surgical field exposure. Loop ileostomy was performed after total mesorectal excision (TME). In these cases, the specimen was removed through an incision in the right lower quadrant obtained by extending the 12-mm working port.
2.3. ERAS protocol and postoperative measurements
The protocol was devised in accordance with the recommendations of the ERAS Society[1] and has been previously described.[13,14] The objective of the ERAS program was to provide all the items to all patients as far as possible. ERAS items are shown in Table 1.
Table 1.
ERAS protocol at the Division of General and Hepatobiliary Surgery, University of Verona Hospital Trust.
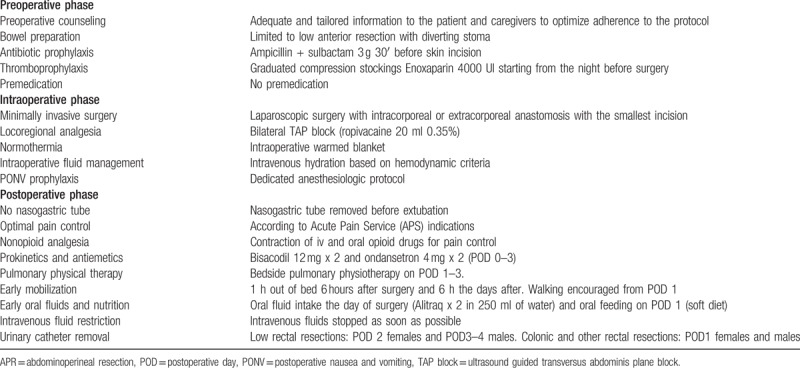
Patients were clinically reviewed at least twice a day by a trained member of the surgical team. The presence of nausea and vomiting, passage of flatus and stools, tolerance to liquid and solid diet, and visual analog score (VAS) score was recorded. Diet was considered tolerated when patient oral intake would be deemed sufficient to avoid starvation and independent of intravenous fluids.
Any deviation from the expected postoperative course, even asymptomatic, was considered a complication and recorded together with its management. All adverse events developing within hospitalization or 30 days after surgery were recorded as postoperative morbidity and mortality. Readmission rate was calculated as percentage of patients re-admitted to the hospital within 30 days from discharge, and complications were graded according to the Clavien–Dindo classification. Complications graded as 3 or more were considered as major complications.[15]
The discharge criteria included the following: absence of major complications, resumption of general diet, passage of stool and urine, adequately controlled pain (VAS < 4 with oral analgesics), independent mobilization, and C-reactive protein (CRP) concentration measured on postoperative day (POD) 3 lower than 120 mg/dL.[14] Since the aim of our ERAS protocol was not to pursue very early discharges, these were based on a combination of clinical evaluation and patients’ views on how comfortable they felt with returning home.
2.4. Data collection and analysis
All demographic and clinical data were prospectively collected in a PC data set. Statistical analysis was performed using SPSS software version 21.0. Continuous data were reported as means (standard deviation) or medians (range), while descriptive variables were reported as frequencies. Adherence to the ERAS protocol and clinical outcomes were analyzed as a binary outcome (yes/no), and they were expressed as frequencies.
3. Results
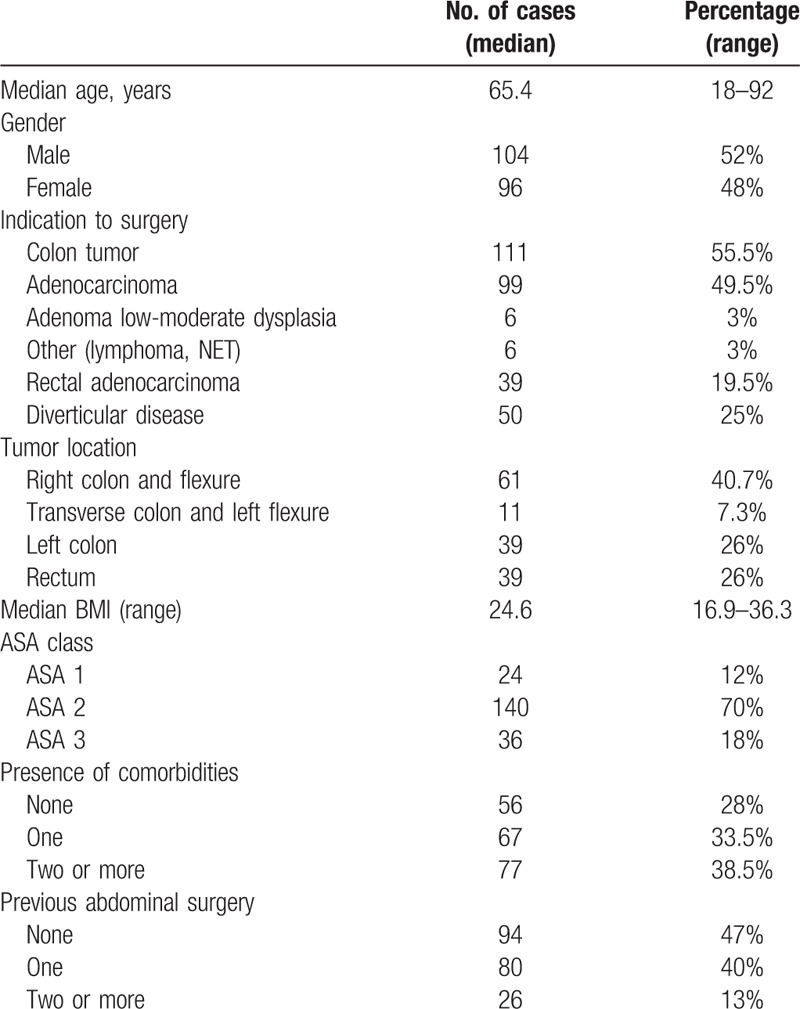
Patients’ demographics and clinical characteristics are presented in Table 2. The most frequent indication to surgery was the presence of a colonic neoplasia (55.5%), although rectal cancer was observed in 19.5% of the patients. Median age was 65.4 (18–92) years with 34% aged ≥70 years (n = 68), 25.5% aged ≥75 years (n = 51), and 15% aged ≥80 years (n = 30). Median BMI was 24.6 (16.9–36.3) kg/m2, and 26.5% of patients (n = 53) were overweight with a BMI ≥28 kg/m2. The percentage of patients classified as ASA 3 was 18%, while 38.5% were affected by 2 or more comorbidities. Most frequent comorbidities were cardiovascular diseases (15.5%), diabetes (9.5%), and pulmonary diseases (6.1%).
Table 2.
Demographic and clinical characteristics for the 200 patients under study.
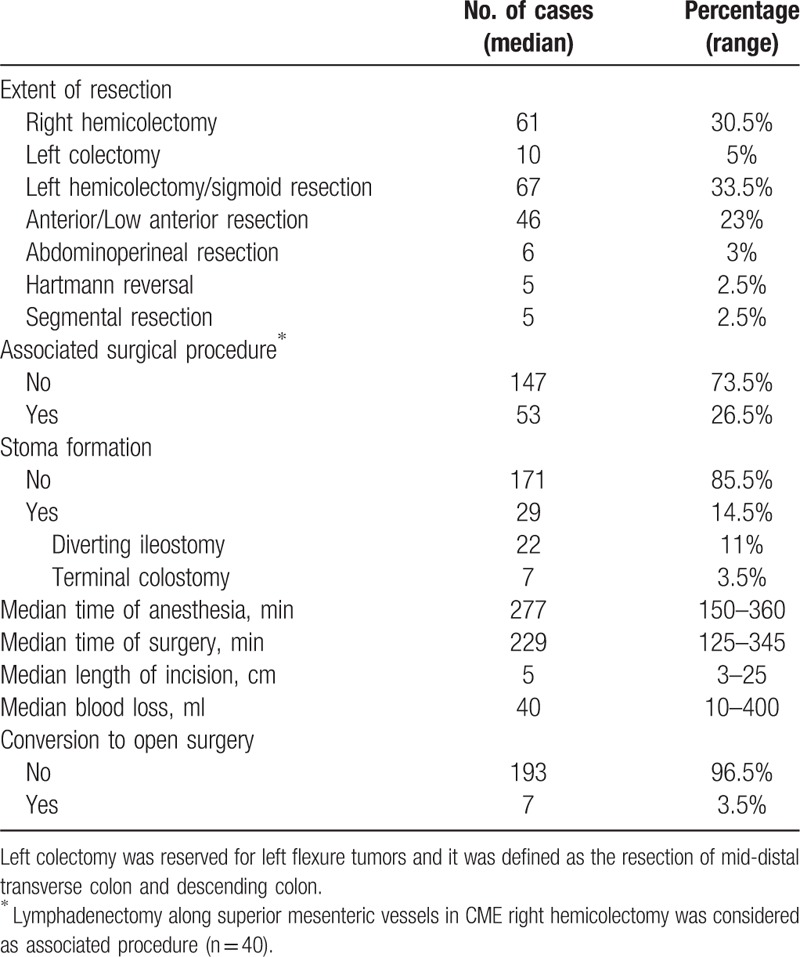
Surgical procedure data are presented in Table 3. The most frequently performed surgical procedures were right hemicolectomy (30.5%) and sigmoid resection/left hemicolectomy (33.5%), followed by rectal resection (26%). Twenty-nine patients (14.5%) required stoma formation, this being either a terminal colostomy (3.5%) after abdominoperineal resection or a diverting ileostomy (11%) following TME with low colorectal (n = 16) or coloanal anastomosis (n = 6). No diverting ileostomy was performed after anterior resection or other colonic resections.
Table 3.
Data of surgical procedure for the 200 patients under study.
Postoperative outcomes are shown in Table 4. No mortality was observed during the study period. Median length of hospital stay (LOS) was 5.5 days (2–40) with no significant difference between colonic (5.5, 2–20 days) and rectal resections (6, 4–40 days) (P = 0.073). Among the 200 patients, 71% were discharged by POD 6 (n = 142), 50% by POD 5 (n = 100), and 27.5% by POD 4 (n = 55). Re-admission within 30 days after discharge was required for 5 patients (2.5%).
Table 4.
Postoperative outcome data for the 200 patients under study.
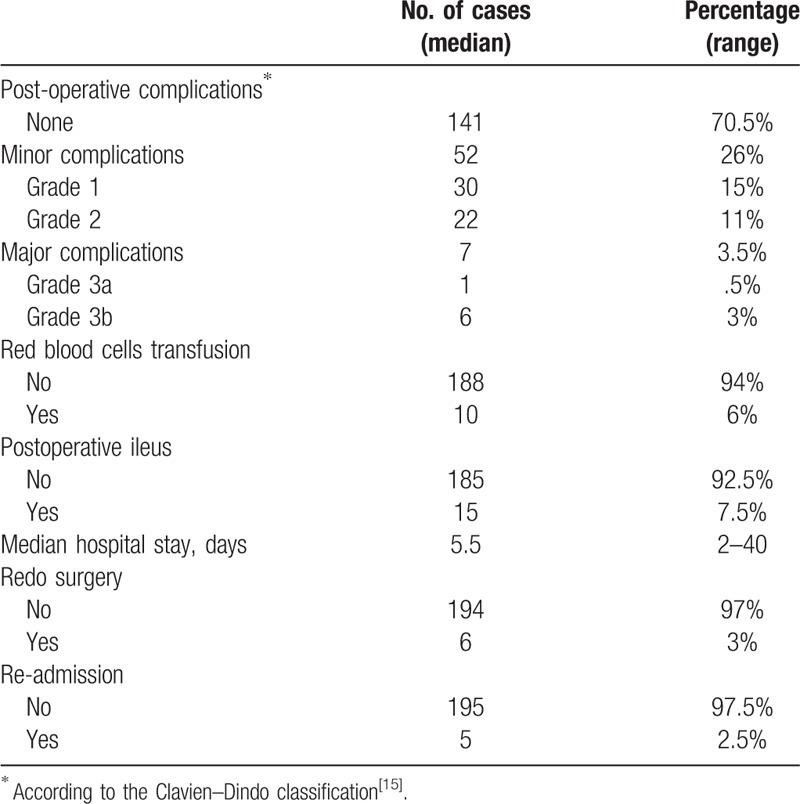
Overall complication rate was 29.5%. Most complications were classified as Clavien–Dindo grades 1 and 2 (26%), while major complications developed in 3.5% of the patients. Redo surgery was required in 3% of the patients. The causes were anastomotic leak and mechanical bowel obstruction with 3 cases each. Primary postoperative ileus was observed in 7.5% of the patients (n = 15). Red blood cell transfusion was required in 10 patients (6%).
Adherence to ERAS items and postoperative outcomes is shown in Table 5.
Table 5.
Adherence to ERAS protocol items for the 200 patients under study.
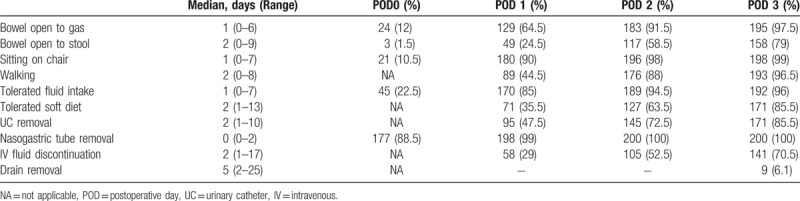
All patients received multidisciplinary counseling, antibiotics, antithrombotic prophylaxis, and intraoperative active warming. A nasogastric tube was placed upon induction of general anesthesia and generally removed at the end of the procedure (88.5%) or on POD 1 for procedures completed late in the evening (10.5%). Nasogastric tube re-insertion rate was 6.5%.
Most patients (96.5%) adhered to respiratory physiokinetic therapy in the perioperative period; 90% mobilized from bed to chair on POD 1 for at least 4 hours, and 88% walked independently by POD 2. Opioid drugs were prescribed quite frequently in the early postoperative phase (42%), and good pain control (VAS<4) was achieved in the majority of patients (86.5%) by POD 3. Routine prokinetics and antiemetic drugs were administered in 94.5%. Nevertheless, postoperative nausea and vomiting (PONV) rate during the first 3 PODs was recorded at 36.5%. As a result, intravenous fluid therapy was discontinued only in 29% of patients on POD 1 and 52.5% of patients on POD 2. Around 129 patients (64.5%) passed flatus on POD 1, while stool passage was recorded in 58.5% and 79% of patients on PODs 2 and 3, respectively.
Full oral fluid intake on POD 0 was rarely achieved (22.5%). However, this goal was more consistently achieved by POD 1 (85%). Soft diet was tolerated by PODs 1 and 2 in 35.5% and 63.5% of the patients, respectively.
4. Discussion
This study reports the initial results of our experience with an enhanced recovery pathway in a cohort of 200 patients without age limitations undergoing elective colonic and rectal surgery.
In the last decades, ERAS pathways have been associated with a significant reduction in both morbidity and LOS following open and laparoscopic colorectal surgery.[8–10,16] ERAS interventions aim to reduce surgical stress which alters patients’ homeostasis, causing prolonged recovery and postoperative complications. The faster restoration of patients’ homeostasis derives from the synergistic effect of ERAS intervention and minimally invasive approaches. Such a combination has been shown to promote better outcomes and shorter hospital stay with no increase in morbidity, mortality, and 30-day readmission rates.[17,18]
ERAS protocols were initially considered adequate for younger and fit patients. However, a growing amount of evidences show that benefits of ERAS extend to less selected populations. There is also consensus that age selection or tailored ERAS protocols in elderly people are unnecessary.[9,18,19] We did not consider more advanced ages in the exclusion criteria and have involved 68 patients older than 70 years of age (34%) and 30 patients older than 80 years (15%). The advanced age of our patient population was related to high comorbidity rates with 13% of the total population presenting 2 or more comorbidities and 18% being classified as ASA 3. Among the 283 patients who underwent minimally invasive colorectal surgery within the study period, we excluded patients with severe or uncontrolled comorbidities (5.5%) and those who were unable to reliably comply with the ERAS interventions. We therefore excluded patients who were greatly dependent on caregivers for daily activities and those with psychoneurological impairment or substance (mostly alcohol) addiction (5.3%). Among the excluded patients, 10 refused to adhere to the ERAS protocol (3.5%). Among these, 6 patients were observed in the first year of protocol application. Better patients’ information and team familiarity with the protocol may have acted as motivators toward patients’ agreement in participating in the study in the following years.
We offered the ERAS protocol to patients undergoing rectal cancer surgery who represent a consistent part of our population (19.5%). Similarly, the ERAS protocol was applied to patients who underwent new stoma formation (14.5%), both definitive colostomy for abdominoperineal resection and protective ileostomy for high-risk rectal resections and TME. ERAS items were tailored upon specific needs of different patients or procedures: for example, urinary catheter removal after LAR was considered from POD 2 in women and PODs 3–4 in men to avoid urinary retention. Despite some item variations, patients undergoing surgery for rectal cancer showed similar results in terms of both protocol adherence and outcomes when compared to other colonic resections. Unlike those described in other studies,[20] our results show that median LOS was 6 days for both laparoscopic rectal and colonic resections.
During the first 4 years of protocol application, we have obtained good surgical outcomes such as low major morbidity (3.5%), redo surgery (3%), and re-admission rates (2.5%). Re-admission rate in our cohort was notably lower compared to recent reports where LOS was even shorter than ours[21] and in line with the results of the PeriOperative Italian Society (POIS) Registry.[18]
Adherence to items such as respiratory physiokinetic therapy (96.5%) and routine prokinetics/antiemetic drug administration (94.5%) was notably high. Other ERAS goals, such as out-of-bed mobilization, walking, early fluid and diet intake, and intravenous fluid administration interruption, were generally achieved with a delay of about one day, highlighting room for further improvement. Passage of flatus occurred on POD 1 in 64.5% of patients, whereas complete return to bowel function occurred in 58.5% of patients by POD 2. The aim of our ERAS protocol was to not achieve exceedingly early discharges but promote early restoration of patient's homeostasis. We consider our median LOS of 5.5 days a good result for such a heterogeneous cohort of patients. Excluding 29 patients with stoma formation whose LOS was influenced by the time necessary for stoma education (PODs 3–6), among the remaining 171 patients, we registered a “possible POD 3 discharge ratio” of 82% (140 patients). This would be the total number of patients that could be potentially discharged on POD 3 based on the following criteria: bowel open to gas, well tolerated semiliquid diet, independent ambulation, urinary catheter removal, and adequate pain control (VAS≤4). Within the population of “POD 3 potentially dischargeable patients,” only 1 would have been subject to early readmission (<1%).
Within certain health systems, the actual time of discharge often does not reflect the clinical readiness to discharge. This is due to the fact that certain patients, especially the elderly, may have home support requirements, the logistics of which often delays discharge. In certain circumstances, validated indicators of short-term recovery such as “time to readiness for discharge” should be used against ERAS efficacy.[22]
Our results can be compared to those in the recent literature where a good ERAS adherence is associated with both a reduction of LOS and overall morbidity and improvements in surgical outcomes.[23] We included in this study only patients treated with a minimally invasive approach, since its effect on recovery after major surgery is well reported and it increases adherence to postoperative ERAS protocol items as recently reported by the PeriOperative Italian Society.[20] Since the best postoperative outcomes are achieved combining ERAS protocol and laparoscopy, we considered the laparoscopic approach as a standard item of the ERAS protocol when indicated.
5. Conclusions
Our experience with 200 patients confirms the feasibility and safety of ERAS protocol application within a heterogeneous population undergoing laparoscopic colorectal resection. Moreover, the laparoscopic approach should be considered part of the enhanced recovery pathway also in elderly people and rectal resections.
Author contributions
Conceptualization: Corrado Pedrazzani, Nicola Menestrina, Alfredo Guglielmi.
Data curation: Cristian Conti, Guido Mantovani, Giulia Turri, Enrico Lazzarini.
Formal analysis: Corrado Pedrazzani, Giulia Turri.
Investigation: Corrado Pedrazzani.
Methodology: Corrado Pedrazzani, Andrea Ruzzenente.
Supervision: Alfredo Guglielmi.
Validation: Nicola Menestrina.
Visualization: Andrea Ruzzenente.
Writing – original draft: Corrado Pedrazzani, Cristian Conti, Guido Mantovani, Eduardo Fernandes.
Writing – review & editing: Nicola Menestrina, Alfredo Guglielmi.
Footnotes
Abbreviations: ASA = American Society of Anesthesiologists, BMI = body mass index, COPD = chronic obstructive pulmonary disease, ERAS = Enhanced Recovery After Surgery, FAP = familial adenomatous polyposis, IBD = inflammatory bowel disease, LAR = low anterior resection, LOS = length of hospital stay, POD = postoperative day, POIS = PeriOperative Italian Society, PONV = postoperative nausea and vomiting, TME = total mesorectal excision, VAS = visual analog score.
The authors have no funding and no conflicts of interest to disclose.
References
- [1].Gustafsson UO, Scott MJ, Schwenk W, et al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. World J Surg 2013;37:259–84. [DOI] [PubMed] [Google Scholar]
- [2].Delaney CP, Zutshi M, Senagore AJ, et al. Prospective, randomized, controlled trial between a pathway of controlled rehabilitation with early ambulation and diet and traditional postoperative care after laparotomy and intestinal resection. Dis Colon Rectum 2003;46:851–9. [DOI] [PubMed] [Google Scholar]
- [3].Nicholson A, Lowe MC, Parker J, et al. Systematic review and meta-analysis of enhanced recovery programmes in surgical patients. Br J Surg 2014;101:172–88. [DOI] [PubMed] [Google Scholar]
- [4].Bakker N, Cakir H, Doodeman HJ, et al. Eight years of experience with Enhanced Recovery After Surgery in patients with colon cancer: Impact of measures to improve adherence. Surgery 2015;157:1130–6. [DOI] [PubMed] [Google Scholar]
- [5].Lassen K, Soop M, Nygren J, et al. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg 2009;144:961–9. [DOI] [PubMed] [Google Scholar]
- [6].Aarts MA, Okrainec A, Glicksman A, et al. Adoption of enhanced recovery after surgery (ERAS) strategies for colorectal surgery at academic teaching hospitals and impact on total length of hospital stay. Surg Endosc 2012;26:442–50. [DOI] [PubMed] [Google Scholar]
- [7].Gustafsson UO, Oppelstrup H, Thorell A, et al. Adherence to the ERAS protocol is associated with 5-year survival after colorectal cancer surgery: a retrospective cohort study. World J Surg 2016;40:1741–7. [DOI] [PubMed] [Google Scholar]
- [8].Roulin D, Donadini A, Gander S, et al. Cost-effectiveness of the implementation of an enhanced recovery protocol for colorectal surgery. Br J Surg 2013;100:1108–14. [DOI] [PubMed] [Google Scholar]
- [9].Slieker J, Frauche P, Jurt J, et al. Enhanced recovery ERAS for elderly: a safe and beneficial pathway in colorectal surgery. Int J Colorectal Dis 2017;32:215–21. [DOI] [PubMed] [Google Scholar]
- [10].Vlug MS, Wind J, Hollmann MW, et al. Laparoscopy in combination with fast track multimodal management is the best perioperative strategy in patients undergoing colonic surgery: a randomized clinical trial (LAFA-study). Ann Surg 2011;254:868–75. [DOI] [PubMed] [Google Scholar]
- [11].Zhuang CL, Ye XZ, Zhang XD, et al. Enhanced recovery after surgery programs versus traditional care for colorectal surgery: a meta-analysis of randomized controlled trials. Dis Colon Rectum 2013;56:667–78. [DOI] [PubMed] [Google Scholar]
- [12].Spanjersberg WR, van Sambeeck JD, Bremers A, et al. Systematic review and meta-analysis for laparoscopic versus open colon surgery with or without an ERAS programme. Surg Endosc 2015;29:3443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pedrazzani C, Menestrina N, Moro M, et al. Local wound infiltration plus transversus abdominis plane (TAP) block versus local wound infiltration in laparoscopic colorectal surgery and ERAS program. Surg Endosc 2016;30:5117–25. [DOI] [PubMed] [Google Scholar]
- [14].Pedrazzani C, Moro M, Mantovani G, et al. C-reactive protein as early predictor of complications after minimally invasive colorectal resection. J Surg Res 2017;210:261–8. [DOI] [PubMed] [Google Scholar]
- [15].Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Braga M, Pecorelli N, Scatizzi M, et al. Enhanced recovery program in high-risk patients undergoing colorectal surgery: results from the PeriOperative Italian Society Registry. World J Surg 2017;41:860–7. [DOI] [PubMed] [Google Scholar]
- [17].Greco M, Capretti G, Beretta L, et al. Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg 2014;38:1531–41. [DOI] [PubMed] [Google Scholar]
- [18].Pędziwiatr M, Kisialeuski M, Wierdak M, et al. Early implementation of Enhanced Recovery After Surgery (ERAS() protocol. Compliance improves outcomes: a prospective cohort study. Int J Surg 2015;21:75–81. [DOI] [PubMed] [Google Scholar]
- [19].Fiore JF, Faragher IG, Bialocerkowski A, et al. Time to readiness for discharge is a valid and reliable measure of short term recovery after colorectal surgery. World J Surg 2013;37:2927–34. [DOI] [PubMed] [Google Scholar]
- [20].Braga M, Borghi F, Scatizzi M, et al. Impact of laparoscopy on adherence to an enhanced recovery pathway and readiness for discharge in elective colorectal surgery: results from the PeriOperative Italian Society registry. Surg Endosc 2017;31:4393–9. [DOI] [PubMed] [Google Scholar]
- [21].Pecorelli N, Hershorn O, Baldini G, et al. Impact of adherence to care pathway interventions on recovery following bowel resection within an established enhanced recovery program. Surg Endosc 2017;31:1760–71. [DOI] [PubMed] [Google Scholar]
- [22].Gonzalez-Ayora S, Pastor C, Guadalajara H, et al. Enhanced recovery care after colorectal surgery in elderly patients. Compliance and outcomes of a multicenter study from the Spanish working group on ERAS. Int J Colorectal Dis 2016;31:1625–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pędziwiatr M, Pisarska M, Kisielewski M, et al. ERAS protocol in laparoscopic surgery for colonic versus rectal carcinoma: are there differences in short-term outcomes? Med Oncol 2016;33:56. [DOI] [PMC free article] [PubMed] [Google Scholar]


