Supplemental Digital Content is available in the text
Keywords: CIN, efficacy, HPV, meta-analysis, Photodynamic therapy, safety
Abstract
Background:
We sought to conduct a systemic review and meta-analysis of randomized clinical trials to assess the efficacy and safety of photodynamic therapy (PDT) in cervical intraepithelial neoplasia (CIN) and cervical human papilloma virus (HPV) infection.
Methods:
The Medline, EMBASE, and Cochrane Central Register databases were searched using relevant keywords for entries up to May 1, 2017, irrespective of year of publication. The language was restricted to English. Randomized clinical trials and qualitative studies comparing PDT and placebo for CIN or HPV-positive patients were included. We assessed the evidence quality using a risk of bias graph in RevMan V5.3 and the Grading of Recommendations Assessment, Development, and Evaluation scoring system.
Results:
Of the 168 studies identified, only 4 RCTs met the inclusion criteria for meta-analysis. In all, 292 and 141 patients received PDT or placebo, respectively. PDT significantly increased the complete remission rate (CRR) among those with CIN (odds ratio [OR]: 2.51 [1.23–5.12]; P = .01) and HPV infection (OR: 3.82 [1.91–7.65]; P = .0002). The adverse events rate (AER) for PDT was greater than that for placebo (OR: 13.32 [4.44, 40.02]; P < .00001). The overall evidence quality was very low. Similarly, in a systematic review including 21 qualitative records, the CRRs for CIN patients with PDT and cervical HPV infection patients with PDT were 82.0% and 77.5%, respectively. The AER for PDT was 31.6%, which was lower than that observed in our meta-analysis (74.6%).
Conclusions:
PDT that targets CIN or cervical HPV infection improves the CRR, but slightly compromises safety. Further studies are necessary to identify the most effective and least toxic photosensitizer.
1. Introduction
Cervical cancer remains one of the most common cancers of the female reproductive system despite advances that have made in its diagnosis and treatment.[1] Cervical intraepithelial neoplasia (CIN) is a premalignant form of cervical cancer, and the risk of cervical cancer in women with CIN is 20-fold greater than that in healthy women.[2,3] Therefore, timely treatment for CIN in the early stages is necessary to avoid progression to invasive cervical cancer.
Cervical human papilloma virus (HPV) infection is detected in more than 99.7% of cases of cervical cancer and is implicated as the main risk factor for CIN.[4–6] HPV genotypes have been classified into 3 categories according to the associated risk of carcinogenesis in the uterine cervix, and HPV genotypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59 have been identified as the high-risk genotypes.[5,7] Given that HPV-positive cervical cancer is prevalent among women of childbearing age and that the possibility of reinfection even after treatment is high,[4] it is necessary to develop effective strategies that minimize the risk of residual disease, malignancy as well as reinfection.
Conventional methods for the treatment of CIN and cervical HPV infection such as diathermocoagulation, cryotherapy, laser evaporation, and laser or electrosurgical excision are invasive. These invasive treatment methods may cause adverse reactions, such as hemorrhages; endometriosis; stenosis of the cervix[8]; and severe complications in subsequent pregnancies, including spontaneous abortion, preterm birth, and low birth weight.[9–11] The traditional methods of radiation and chemotherapy are also used extensively in the management of cervical cancer. However, these methods are likely to affect the patient's fertility. Therefore, it is imperative to develop other efficient alternative methods to treat CIN and cervical HPV without compromising the patient's fertility.
Photodynamic therapy (PDT) is a promising and highly selective therapeutic method that has been employed in the treatment of various non-cancer conditions such as mycosis fungoides and condyloma acuminatum; premalignant dysplasias such as Bowen's disease and CIN; and malignancies such as squamous cell carcinoma and gastrointestinal tumors. PDT involves 2 steps: administration of a photosensitizer and exposure to locally directed light.[12] The key determinants of the success of PDT are oxygen-induced activation of the non-toxic photosensitizer located within the specific tissue, appropriate utilization of visible light, and proper selection of the photosensitizer. All 3 aspects are critical to the therapeutic effect of PDT, which is achieved via the generation of free radicals such as single oxygen[13]; this, in turn, leads to local photo-oxidation, cell damage, and destruction of specific cells.[14] We believe that the characteristics of high tissue selectivity, reduced risk of adverse events compared to conventional methods, and low risk of severe complications may make PDT an effective alternative approach for the treatment of CIN and cervical HPV infection, particularly for young women.[8,15]
Several types of photosensitizers are currently in use: the first generation was photofrin and the second generation was chlorine, (including monoaspartyl chlorine e6, photoclor, tempoporfin, verteporfin, and purlytin), δ-aminolevulinic acid (including PpIX, and hexaminolevulinate), phthalocyanine or naphthol (including AlPcS2), and texaphyrins (including antrin and porphycene).[16] To date, no clinical trial has compared the efficacies of these photosensitizers for treating CIN or cervical HPV infection.
The purpose of this systematic review and meta-analysis was to determine the impact of PDT on patients with CIN and cervical HPV infection in terms of adverse events as well as complete clearance of HPV and remission of CIN. Our hypothesis was that PDT increases the complete remission rate (CRR) of CIN, the clearance rate of HPV, and the adverse events rate (AER).
2. Patients and methods
2.1. Search strategy
This study has been registered in PROSPERO with the registration number CRD42017070722. This review complies with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines for reporting systematic reviews (Supplementary S1).[17] We searched for all relevant articles assessing the efficacy and safety of photodynamic therapy (PDT) in cervical intraepithelial neoplasia (CIN) and cervical human papilloma virus (HPV) infection. Only studies published in English were considered. The search strategy and the search terms used are provided in Supplementary S2. The same terms were also used to retrieve grey literature from Google, ClinicalTrials.gov, Web of Science, National Science and Technology Library (NSTL), and Conference Proceedings Citation Index (CPCI). After contacting the journal editor or reviewers, all articles were accessible at every step.
2.2. Inclusion criteria for studies
Two reviewers independently screened titles and abstracts of the identified studies and read the full articles for final inclusion. Disagreement between the reviewers was resolved through discussion with a third reviewer. Data were entered into an Excel sheet for comparison. We assessed the results in 2 steps as follows: if both reviewers agreed, the study was entered in the data pool of Endnote, and in case of disagreement, a third experienced researcher was consulted to make the final decision. All these steps were properly documented in Excel (Supplementary S3).
Only randomized controlled trials (RCTs) were included in the meta-analysis, and all other study types were included in the qualitative analysis. Studies meeting the following inclusion criteria were selected for further analysis: all patients had CIN or cervical HPV infection, all patients were treated with PDT or placebo and underwent the same surgical procedure, outcomes included complete remission after 3 months, as confirmed by HPV-DNA, cytology, and histology with colposcopic biopsy, and study was a RCT. Studies were included only if a photosensitizer was used for treatment, irrespective of its dosage or type, and only if the control group received placebo alone.
If the same randomized clinical trial was published more than once, the most recent one was preferred, but any additional data provided in previous publications were collected. We included published abstracts only if we could obtain further details from the reviewers. We excluded qualitative studies and studies in which the diagnosis of CIN was not confirmed by either isolation of HPV-DNA or biopsy.
2.3. Study quality assessment and data extraction
Two reviewers independently extracted the data, and a form was prepared to collect information regarding country of study, author, year, intervention, outcomes, number of patients in both arms, and adverse events.
We used risk of bias assessment tool of the Cochrane handbook[18] to evaluate the methods used for randomization, allocation concealment, and blinding of the assessors and determined whether the study was double blinded, the outcome data were complete, and the reporting of evidence was selective. We used GRADEPRO 3.6.1 software to generate summary of findings table[19] and used GRADE criteria to assess the quality of evidence in terms of design, risk of bias, consistency, directness, precision, and publication bias.
2.4. Data processing and analysis
We used RevMan 5.3 software for statistical analysis. We performed a meta-analysis using the random effects model. We calculated pooled odds ratios (ORs) and 95% confidence intervals (CIs) for binary variables. For qualitative evaluation, we performed a meta-analysis by drawing data into RevMan V5.3. Comparison of PDT and placebo data was reported as OR with 95% CI, which was calculated by the Mantel–Haenszel method and random effects model. The I2 statistic test and χ2 test were used to assess statistically significant heterogeneity. An I2 value of 0% implies that there is no observed heterogeneity, and I2≥50 indicates high heterogeneity, as recommended by the Cochrane Handbook.
2.5. Ethical considerations
All the analyses were based on previously published studies, and no ethical approval or patient consent was required.
3. Results
3.1. Search results
Our electronic search revealed 136 relevant studies published between June 19, 1991 and May 1, 2017 that we retrieved from the Medline, EMBASE, and Cochrane Central Register databases for screening of titles and abstracts. No additional unpublished RCTs were retrieved from the Google search, ClinicalTrials.gov, Web of Science, and National Science and Technology Library (NSTL). However, 3 proceedings records were found in Conference Proceedings Citation Index (CPCI) meeting our qualitative analysis criteria.[20–22] Among the 168 records identified from the Medline, EMBASE, Cochrane Central Register, and CPCI databases, 136 studies were screened after elimination of duplicate articles, and 70 full-text articles were retrieved after screening the title and abstract. After exclusion of 47 articles that were not relevant, 21 qualitative studies and 4 RCTs[23–26] were identified as meeting all the inclusion criteria (Supplementary S4).
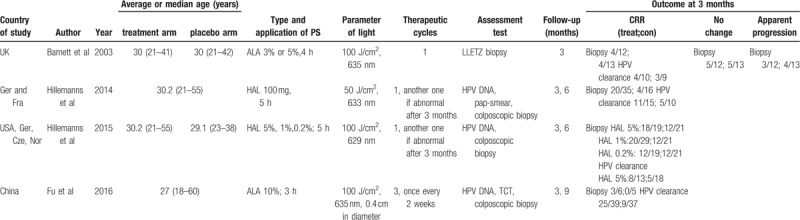
In our meta-analysis, most patients (67.4%) in the included studies received PDT, while the remaining 32.6% of patients were allocated to the placebo group. Other study characteristics, including average age, staging, and follow-up, are presented in Table 1.
Table 1.
Characteristic of included RCTs.
Although there was no high risk of performance, attrition, or reporting bias among the four RCTs, the included studies did show selection and detection bias (Fig. 1). Moreover, the risks of bias for randomization and allocation in three RCTS[24–26] are unclear. The 2015 study by Hillemanns et al[25] represents the largest study with a weight of 48.1% in the meta-analysis of CRR in CIN patients. Fu et al[26] represents the largest study with a weight of 48.6% in the meta-analysis of CRR in cervical HPV. The 2014 study by Hillemanns et al[24] is the largest study with a weight of 86.8% in the meta-analysis of AER in patients receiving PDT. Moreover, the total number of events with each outcome was less than 300. The risk of publication bias was also high because only a small number of included RCTs showed a similar trend of the positive effect of PDT, which implies that there are other studies reporting no effect of PDT and these may be either unpublished or undetected by our search strategy. Hence, the overall quality of evidence in this study is very low, as per the GRADE scoring system (Fig. 2). No funnel plots were produced as the number of studies reporting each outcome was less than 10.
Figure 1.
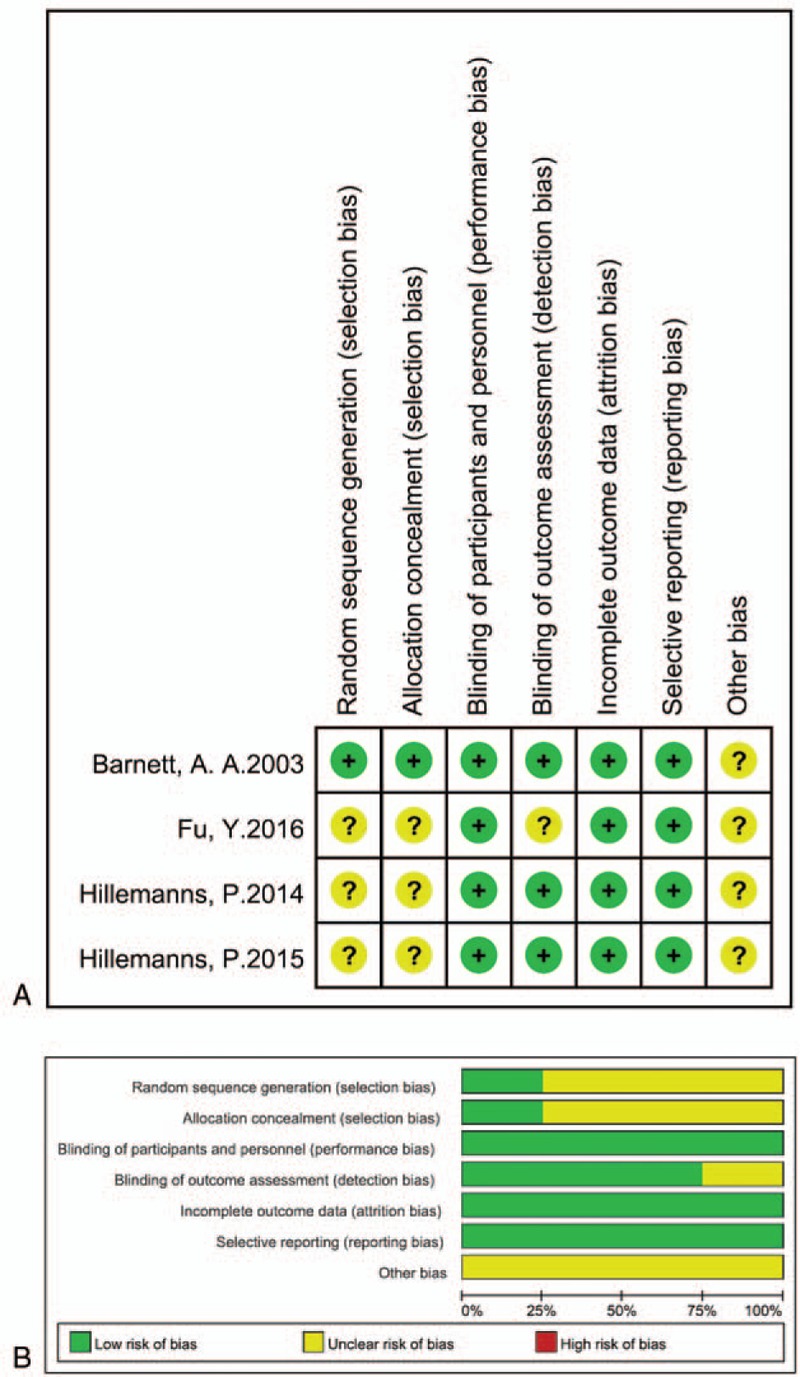
Risk of bias. We evaluated the risk of bias by using RevMan V5.3. A, Risk of bias for individual studies determined using the Cochrane tool for assessment of risk of bias. B, Risk of bias graph: summary of risk of bias is presented as percentage across all included studies.
Figure 2.
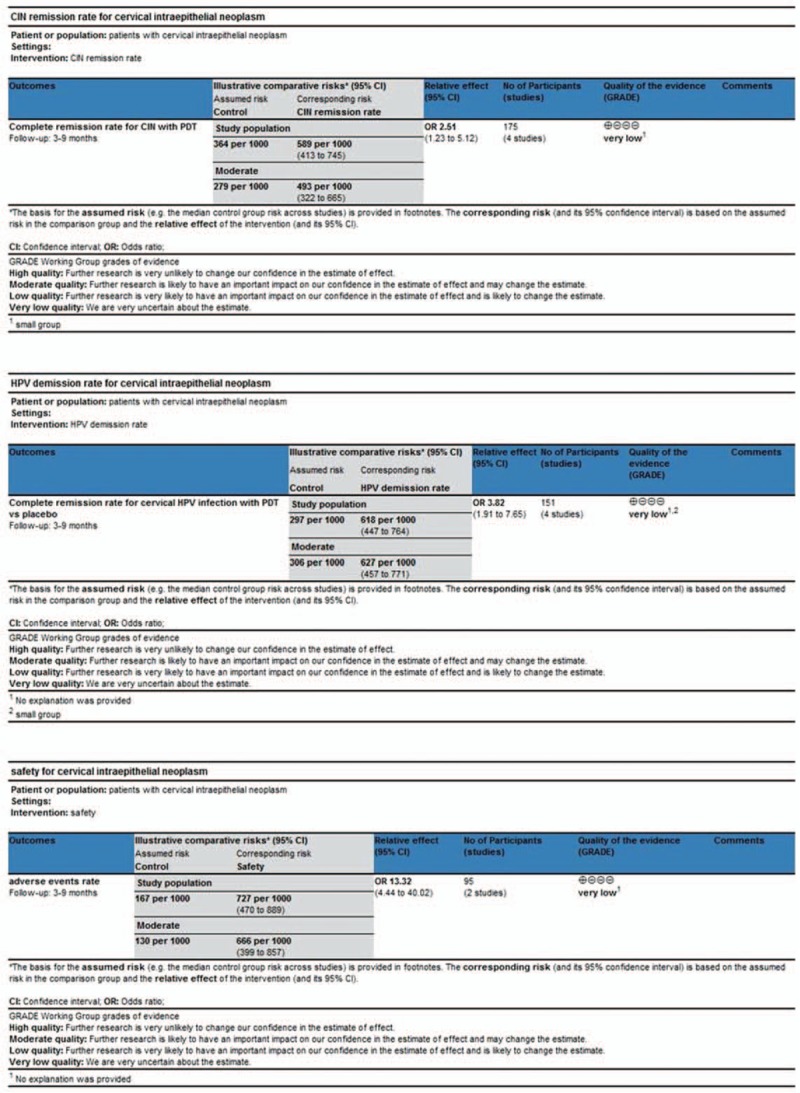
GRADE scores. Three reviewers evaluated the quality of the studies with GRADE profiler 3.6.1 to assess evidence quality. A, GRADE score for CRR of CIN with PDT. B, GRADE score for CRR of cervical HPV infection with PDT. C, GRADE score for AER of CIN or cervical HPV infection with PDT.
3.2. Efficacy of PDT in CIN
3.2.1. PDT increases the CRR of CIN in meta-analysis
In the included studies, the efficacy of PDT was assessed between 3 and 9 months; we chose the 3-month follow-up as the time point for the outcome in this meta-analysis. The rate of spontaneous HPV clearance was 24.26% within 6 months,[27] and this percentage is expected to increase with time; therefore, every RCT included assessment at 3 months to minimize the influence of spontaneous clearance of viral load). In the four RCTs included, CIN of grade I, II, or III was diagnosed in 175 patients by colposcopic biopsy. Of these, 120 patients underwent PDT, while the other 55 patients were prescribed placebo or recommended only follow-up. In all, 77 (64.2%) patients in the PDT group achieved primary complete remission at the end of the 3-month follow-up period, as confirmed by cytology and histology. Furthermore, colposcopic biopsy was the main examination used to assess the efficacy of PDT in patients with CIN. In addition, 20 (36.4%) patients in the placebo arm achieved complete remission. Thus, PDT significantly increased the CRR of CIN, as shown in Figure 3 (OR, 2.51; 95% CI, 1.23–5.12; P = .01 in the overall test).
Figure 3.
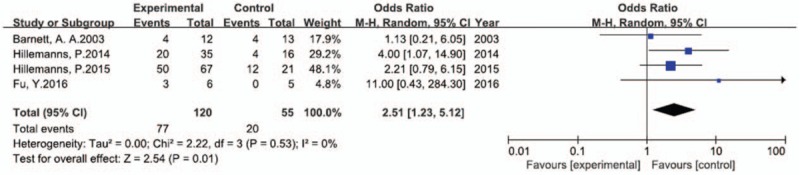
Forest plot of comparison of RCTs: CRR in CIN patients receiving PDT vs. patients receiving placebo. We used RevMan V5.3 for data processing. The data were analyzed by using the Mantel–Haenszel method and random effects model and presented as odds ratio (OR) with 95% confidence interval (CI). I2 = 0 implies that there was no heterogeneity, P < .05 indicates statistical significance.
Moreover, a dosage of 500 mg at 5 hours was found to be the most effective concentration of hexaminolevulinate, with a CRR of 94.7%. However, it remains to be determined whether it is more useful than 5-aminolevulinic acid, since there was no RCT that compared these 2 drugs directly.
3.2.2. PDT treats CIN at a higher CRR in systematic review than in meta-analysis
For the analysis of a systematic review to verify the accuracy of our meta-analysis results, we identified 21[4,8,20–22,28–43] eligible qualitative studies (Table 2) by screening the title and abstract of 16 qualitative articles, 42 reviews (listed in Supplementary S3) and 3 papers from CPCI; then, the information was summarized and data were extracted from these studies. Intriguingly, the CRR indicating PDT efficacy in patients with CIN ranged from 31.3% to 100%. Additionally, 804 of the 980 subjects, that is 82.0% of the patients, had favorable outcomes from PDT. The wide range of CRRs may be attributed to the differences in the follow-up period and the nature of photosensitizers applied in the studies. Further, inclusion of poorly designed studies might also contribute to the finding that CRR in qualitative studies (82.0%) was higher than that recorded in the meta-analysis (64.2%).
Table 2.
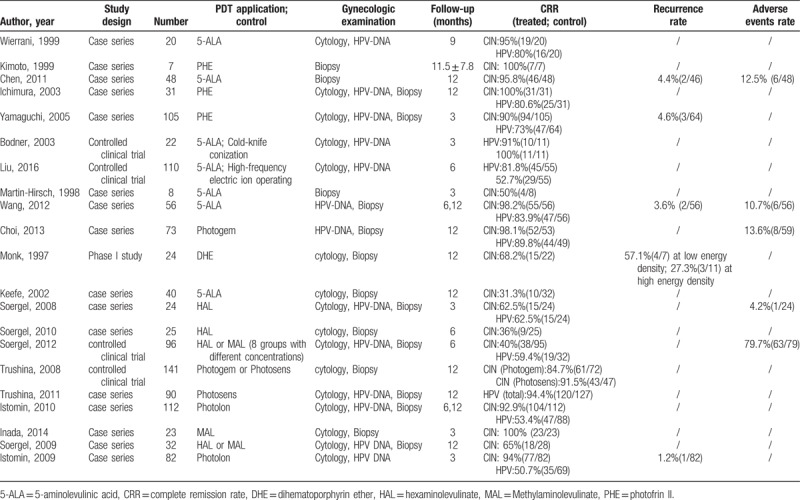
Characteristic of included qualitative studies.
3.3. Efficacy of PDT in cervical HPV infection
3.3.1. PDT enhances the HPV clearance rate in meta-analysis
One hundred fifty-one patients with cervical HPV infection were included in the 4 RCTs. Of these, 77 patients received PDT, while 48 (62.3%) showed complete remission in terms of HPV-DNA isolation at the 3-month follow-up. However, complete remission was also achieved in 22 (29.7%) of the 74 patients who were managed with placebo or follow-up alone. Therefore, PDT also facilitated HPV clearance (OR, 3.82; 95% CI, 1.91–7.65; P = .00002 in the overall test; Fig. 4).
Figure 4.
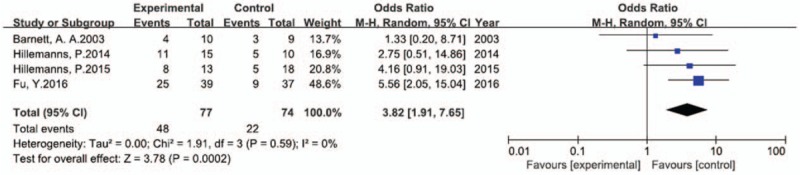
Forest plot of comparison of RCTs: CRR in cervical HPV infection patients receiving PDT vs. patients receiving placebo. Data processing was performed using RevMan V5.3. The data were analyzed by Mantel–Haenszel method and random effect model and presented as odds ratio (OR) with 95% confidence interval (CI). I2 = 0 indicates the absence of heterogeneity, P < .05 indicates statistical significance.
Only one RCT specifically described the CRR of high-risk HPV genotypes 16 and 18. The CRRs for the PDT and placebo groups were 64.1% and 24.3%, respectively. Further examinations are necessary to explore whether PDT is more useful in eradicating high-risk HPV genotypes than low-risk genotypes.
3.3.2. PDT treats cervical HPV infection at a higher CRR in systematic review
The CRRs indicating PDT efficacy in patients with cervical HPV infection ranged from 53.4% to 94.4%. In all, 520 (80.4%) of the 647 patients with cervical HPV infection were deemed free of the infection at the last follow-up. The differences between the studies in the follow-up period and use of photosensitizers and poor study design may be factors contributing to the wide range of CRRs with respect to HPV eradication and the higher CRR (80.4%) percentage in qualitative studies as compared to the percentage (62.3%) determined in the meta-analysis.
3.4. Safety of PDT
3.4.1. PDT increases AER in meta-analysis
Only 2 of the RCTs provided details of the adverse events in both arms separately. In all, 95 subjects were included in these 2 studies. Adverse reactions were recorded in 44 (74.6%) of the 59 patients in the PDT arm and in 6 (16.7%) of the 36 patients in the placebo arm. These data show that PDT resulted in a considerably higher rate of adverse events than placebo (OR, 13.32; 95% CI, 4.44–40.02; P < .00001 in the overall test; Fig. 5). No recurrence rate was reported.
Figure 5.

Forest plot of comparison of RCTs: AER in patients receiving PDT vs. patients receiving placebo. We performed data processing through RevMan V5.3. Data were analyzed by Mantel-Haenszel method and random effects model and presented as odds ratio (OR) with 95% confidence interval (CI). I2 = 0 indicates the absence of heterogeneity, P < .05 indicates statistical significance.
Most of the adverse reactions recorded were local discomfort, burning sensation, and vaginal discharge with mild-to-moderate severity, and the seven serious adverse events that were observed were unrelated to PDT treatment. None of the patients were required to abort treatment due to adverse events, and no instances of skin phototoxicity, vital signs change, and systemic response to photosensitizers were reported in any of the studies.
None of the patients were pregnant at the time of starting PDT, and 6 patients conceived within 3 months of discontinuing PDT and were able to deliver full-term, healthy infants. This suggests that PDT may be an effective method for patients with CIN or cervical HPV infection, without any major adverse effect on fertility.
3.4.2. AER caused by PDT was lower in the systematic review than in the meta-analysis
Qualitative studies reported recurrence rates ranging from 3.6% to 38.9% with an overall relative risk (RR) of 7.6%. The AER in the systematic analysis was 31.6%, based on a range from 4.2% to 79.7%, which was lower than that observed in the meta-analysis (74.6%). As expected, there were no systemic symptoms or severe complications reported in the qualitative studies.
4. Discussion
In this meta-analysis, we sought to evaluate the efficacy and safety of PDT in the eradication of premalignant lesions and HPV infection. Our data were assessed in terms of complete remission of CIN (PDT 64.2%, placebo 36.4%) and HPV clearance (PDT 62.3%, placebo 29.7%). Significantly greater anti-malignancy efficacy was observed in the PDT arm (292 patients) than in the placebo arm (141 patients) (OR, 2.51; 95% CI, 1.23–5.12; P = .01), as well as obvious anti-viral efficacy (OR, 3.82; 95% CI, 1.91–7.65; P = .0002), as assessed by RevMan V5.3. Adverse events in both arms were transient and mostly of mild-to-moderate severity; however, PDT resulted in more adverse reactions than placebo, with an OR of 13.32 (95% CI, 4.44–40.02; P < .00001).
The main limitation of this study is the lack of data for individual patients, which prevents useful summarizations for specific CIN stages. Another concern is that we did not observe even a single case of relapse, which is possibly because the 3-month follow-up period was too early for recurrence. Hence, further investigations are necessary to explore whether would patients would develop HPV reinfection at a later time point or develop CIN again. The level of evidence is also very low because of study limitations, imprecision due to the small number of patients in each study, and high publication bias as mentioned above in search results.
We compared the findings of relevant studies with those of ours regarding the efficacy outcome, CRR, and the safety outcome, AER. Our systematic review revealed that the CRRs for PDT with CIN and cervical HPV infection were 81.0% (range, 31.3%–100%) and 80.4% (range, 53.4%–94.4%), respectively. Similarly, the systematic review by Tao et al reported the CRR with PDT for CIN at 0% to 100% and for HPV eradication at 53.4% to 80.0%, which strongly supports our hypothesis that PDT is an effective therapy for treating patients with CIN and cervical HPV infection. Liu et al[34] suggested that PDT with δ-aminolevulinic acid was more efficient than high-frequency electric-ion operation for CIN I patients. They observed CRRs at the 6-month follow-up examination of 81.8% in the PDT arm and 52.7% in the positive control arm (high-frequency electric-ion operation). The CRR in their study was higher than that in ours, partly because their follow-up time was longer than ours.
With respect to the safety of PDT in terms of the adverse events, including cutaneous photosensitvity, Choi et al[37] reported AER of 13.6% (8/59), which was lower than that in both our meta-analysis (PDT 74.6%, placebo 16.7%) and systematic review (31.6%; range, 4.2%–79.7%). The lower AER in the study by Choi et al[37] could be explained by the fact that they reviewed only 73 patients who received PDT, which is a much lower number than the number in our study, and they did not include a placebo group. Additionally, the incidences of local inflammatory reactions, hyperemia, burning or stinging sensations, local necrosis, sloughing, scarring, pruritus vulvae, and vaginal discharge were not clearly reported in that study.
Bodner et al[33] showed that HPV infection could be eradicated by both cold-knife conization and PDT with application of topical δ-aminolevulinic acid in 73% of their patients at the end of the 3-month follow-up period. As expected, even at the end of 12 months, 100% and 91% of the patients who received conization and PDT, respectively, remained disease free. Furthermore, they also showed that cold-knife conization was more effective than PDT. These data suggest that long-term follow-up is mandatory to assess the CRR after PDT. We recommend that patients should be followed up for at least 1 year.
In the light of the highly selective and non-surgical nature of PDT, Ahn et al[44] developed a new method, namely, concurrent chemo-photodynamic therapy (CCPDT), to treat uterine cervical cancer at stages 1B1 and 1B2, especially in women of the childbearing age who wished to preserve their fertility. Two of the 3 patients in their study went on to successfully deliver full-term infants via cesarean section 16 months after discontinuing CCPDT. The remaining patient delivered twins 45 months after CCPDT through cesarean section. Furthermore, there were no instances of recurrence or relapse of cancer at the end of the 60-month follow-up period. Thus, the study by Ahn et al[44] was a successful trial indicating the value of PDT in the treatment of uterine cervical cancer. However, further clinical investigations are warranted to verify the efficacy of PDT in early-stage invasive cancer.
With regard to patients’ fertility status after PDT, Istomin et al[8] reported that 15 of their patients were able to conceive after treatment. Six of these patients delivered full-term infants, with 2 of them requiring cesarean section and 1 of them delivering a stillborn child. In 4 of their patients who conceived within 3 months of discontinuing PDT, the pregnancy was terminated due to incomplete healing of the cervix. Two of their patients requested termination of pregnancy for personal reasons. However, in our study, all 6 patients who conceived within 3 months of discontinuing PDT delivered full-term, healthy infants. The discrepancy between the number of successful deliveries in our and their studies may be attributed to the fewer number of pregnancies in our study.
In clinical practice, PDT has been increasingly being applied to treat cervical HPV infection and CIN due to its modest selectivity, organ-preserving and non-surgical features. With respect to basic research, we found that differences in photosensitizers and duration of exposure affected the CRR of PDT therapy. Hence, future investigations seem to be necessary to determine the precise use of PDT.
In women with CIN and cervical HPV infection, PTD seems to increase the CRR and AER. However, the quality of evidence was very low for all outcomes. Thus, our results should be interpreted with caution. For a more thorough understanding, the obstetric outcomes and rate of recurrence of CIN or HPV infection after PDT need to be evaluated over the long term in future studies.
Author contributions
Conceptualization: Wenjia Zhang, Aijia Zhang, Wende Sun, Ying Yue, Hong Li.
Data curation: Wenjia Zhang, Aijia Zhang, Wende Sun, Ying Yue, Hong Li.
Formal analysis: Wenjia Zhang, Aijia Zhang, Wende Sun, Ying Yue, Hong Li.
Investigation: Wenjia Zhang, Aijia Zhang, Wende Sun, Ying Yue, Hong Li.
Methodology: Wenjia Zhang, Aijia Zhang, Wende Sun, Ying Yue, Hong Li.
Project administration: Wenjia Zhang, Aijia Zhang, Wende Sun, Ying Yue, Hong Li.
Resources: Wenjia Zhang, Aijia Zhang, Wende Sun, Ying Yue, Hong Li.
Software: Wenjia Zhang, Aijia Zhang, Wende Sun, Ying Yue, Hong Li.
Supervision: Wenjia Zhang, Aijia Zhang, Wende Sun, Ying Yue, Hong Li.
Validation: Wenjia Zhang, Aijia Zhang, Wende Sun, Ying Yue, Hong Li.
Visualization: Wenjia Zhang, Aijia Zhang, Wende Sun, Ying Yue, Hong Li.
Writing – original draft: Wenjia Zhang, Aijia Zhang, Wende Sun, Ying Yue, Hong Li.
Writing – review & editing: Wenjia Zhang, Aijia Zhang, Wende Sun, Ying Yue, Hong Li.
Supplementary Material
Footnotes
Abbreviations: AER = adverse events rate, CIN = cervical intraepithelial neoplasia, CPCI = Conference Proceedings Citation Index, CRR = complete remission rate, HPV = human papilloma virus, PDT = photodynamic therapy.
The authors declare no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Lowy D. Human papillomavirus, cervical cancer prevention, and more. Vaccine 2008;26(Suppl 10):iii–v. [DOI] [PubMed] [Google Scholar]
- [3].Sasieni P, Castanon A, Cuzick J. Effectiveness of cervical screening with age: population based case-control study of prospectively recorded data. BMJ 2009;339:b2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Trushina OI, Novikova EG, Sokolov VV, et al. Photodynamic therapy of virus-associated precancer and early stages cancer of cervix uteri. Photodiagnosis Photodyn Ther 2008;5:256–9. [DOI] [PubMed] [Google Scholar]
- [5].Bosch FX, Sanjose SD, Castellsagué X. Epidemiology of genitoanal HPV infections and associated cancer. 2011;Berlin Heidelberg: Springer, 427–439. [Google Scholar]
- [6].Graham SV. The human papillomavirus replication cycle, and its links to cancer progression: a comprehensive review. Clin Sci (Lond) 2017;131:2201–21. [DOI] [PubMed] [Google Scholar]
- [7].Kajitani N, Satsuka A, Kawate A, et al. Productive lifecycle of human papillomaviruses that depends upon squamous epithelial differentiation. Front Microbiol 2012;3:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Istomin YP, Lapzevich TP, Chalau VN, et al. Photodynamic therapy of cervical intraepithelial neoplasia grades II and III with Photolon. Photodiagnosis Photodyn Ther 2010;7:144–51. [DOI] [PubMed] [Google Scholar]
- [9].Hillemanns P, Soergel P. HAL/MAL photodynamic therapy for CIN. Photodiagnosis Photodyn Ther 2011;8:169–169. [DOI] [PubMed] [Google Scholar]
- [10].Nene BM, Hiremath PS, Kane S, et al. Effectiveness, safety, and acceptability of cryotherapy by midwives for cervical intraepithelial neoplasia in Maharashtra, India. Int J Gynaecol Obstet 2008;103:232–6. [DOI] [PubMed] [Google Scholar]
- [11].Rema P, Suchetha S, Thara S, et al. Effectiveness and safety of loop electrosurgical excision procedure in a low-resource setting. Int J Gynaecol Obstet 2008;103:105–10. [DOI] [PubMed] [Google Scholar]
- [12].van Straten D, Mashayekhi V, de Bruijn HS, et al. Oncologic photodynamic therapy: basic principles, current clinical status and future directions. Cancers (Basel) 2017;9:E19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Szpringer E, Lutnicki K, Marciniak A. Photodynamic therapy: mechanism and employment. Ann Univ Mariae Curie Sklodowska Med 2004;59:498–502. [PubMed] [Google Scholar]
- [14].Agostinis P, Berg K, Cengel KA, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin 2011;61:250–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hillemanns P, Einstein MH, Iversen OE. Topical hexaminolevulinate photodynamic therapy for the treatment of persistent human papilloma virus infections and cervical intraepithelial neoplasia. Expert Opin Investig Drugs 2015;24:273–81. [DOI] [PubMed] [Google Scholar]
- [16].Jin shi MA. Development of second generational porphyrin-based photosensitizers. Photograp Sci Photochem 2002;20:131–48. [Google Scholar]
- [17].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jpt H, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol 2011;5:S38. [Google Scholar]
- [19].Shen XP, Chen YL, Wang Q. The agreement for grading the quality of evidence using GRADE System. Cochrane Collab 2012. [Google Scholar]
- [20].Istomin Y P, Kaplan M A, Shliakhtsin S V, et al. Immediate and long-term efficacy and safety of photodynamic therapy with Photolon (Fotolon): a seven-year clinical experience. 12th World Congress of the International Photodynamic Association 2009;SPIE. [Google Scholar]
- [21].Soergel P, Staboulidou I, Hertel H, et al. Photodynamic therapy of cervical intraepithelial neoplasia using hexaminolevulinate and methylaminolevulinate. 12th World Congress of the International Photodynamic Association 2009;SPIE. [Google Scholar]
- [22].Inada NM, Lombardi W, Leite MFM, et al. Photodynamic therapy of cervical intraepithelial neoplasia. SPIE BiOS 2014;SPIE. [Google Scholar]
- [23].Barnett AA, Haller JC, Cairnduff F, et al. A randomised, double-blind, placebo-controlled trial of photodynamic therapy using 5-aminolaevulinic acid for the treatment of cervical intraepithelial neoplasia. Int J Cancer 2003;103:829–32. [DOI] [PubMed] [Google Scholar]
- [24].Hillemanns P, Petry KU, Soergel P, et al. Efficacy and safety of hexaminolevulinate photodynamic therapy in patients with low-grade cervical intraepithelial neoplasia. Lasers Surg Med 2014;46:456–61. [DOI] [PubMed] [Google Scholar]
- [25].Hillemanns P, Garcia F, Petry KU, et al. A randomized study of hexaminolevulinate photodynamic therapy in patients with cervical intraepithelial neoplasia 1/2. Am J Obstet Gynecol 2015;212:465e461–e467. [DOI] [PubMed] [Google Scholar]
- [26].Fu Y, Bao Y, Hui Y, et al. Topical photodynamic therapy with 5-aminolevulinic acid for cervical high-risk HPV infection. Photodiagnosis Photodyn Ther 2016;13:29–33. [DOI] [PubMed] [Google Scholar]
- [27].Liu Y, Rong X, Zhou YQ, et al. A prospective study on natural history of low-grade cervical intraepithelial neoplasia. China Cancer 2010;19:372–276. [Google Scholar]
- [28].Wierrani F, Kubin A, Jindra R, et al. 5-aminolevulinic acid-mediated photodynamic therapy of intraepithelial neoplasia and human papillomavirus of the uterine cervix: a new experimental approach. Cancer Detect Prev 1999;23:351–5. [DOI] [PubMed] [Google Scholar]
- [29].Kasai T, Kubota K, Iwasaki H, et al. Experimental studies on photoradiation therapy by hematoporphyrin derivative (HpD) and argon dye laser. Nihon Sanka Fujinka Gakkai Zasshi 1984;36:2533–41. [PubMed] [Google Scholar]
- [30].Chen MK, Luo DQ, Zhou H, et al. 5-aminolevulinic acid-mediated photodynamic therapy on cervical condylomata acuminata. Photomed Laser Surg 2011;29:339–43. [DOI] [PubMed] [Google Scholar]
- [31].Ichimura H, Yamaguchi S, Kojima A, et al. Eradication and reinfection of human papillomavirus after photodynamic therapy for cervical intraepithelial neoplasia. Int J Clin Oncol 2003;8:322–5. [DOI] [PubMed] [Google Scholar]
- [32].Yamaguchi S, Tsuda H, Takemori M, et al. Photodynamic therapy for cervical intraepithelial neoplasia. Oncology 2005;69:110–6. [DOI] [PubMed] [Google Scholar]
- [33].Bodner K, Bodner-Adler B, Wierrani F, et al. Cold-knife conization versus photodynamic therapy with topical 5-aminolevulinic acid (5-ALA) in cervical intraepithelial neoplasia (CIN) II with associated human papillomavirus infection: a comparison of preliminary results. Anticancer Res 2003;23:1785–8. [PubMed] [Google Scholar]
- [34].Liu Z, Zheng H, Chen X, et al. Comparison of the efficacy of ALA and high-frequency electric ion operating on cervical intraepithelial neoplasia grade I. Int J Clin Exp Med 2016;9:16782–6. [Google Scholar]
- [35].Martin-Hirsch PL, Whitehurst C, Buckley CH, et al. Photodynamic treatment for lower genital tract intraepithelial neoplasia. Lancet 1998;351:1403. [DOI] [PubMed] [Google Scholar]
- [36].Wang HW, Zhang LL, Miao F, et al. Treatment of HPV infection-associated cervical condylomata acuminata with 5-aminolevulinic acid-mediated photodynamic therapy. Photochem Photobiol 2012;88:565–9. [DOI] [PubMed] [Google Scholar]
- [37].Choi MC, Jung SG, Park H, et al. Photodynamic therapy for management of cervical intraepithelial neoplasia II and III in young patients and obstetric outcomes. Lasers Surg Med 2013;45:564–72. [DOI] [PubMed] [Google Scholar]
- [38].Monk BJ, Brewer C, VanNostrand K, et al. Photodynamic therapy using topically applied dihematoporphyrin ether in the treatment of cervical intraepithelial neoplasia. Gynecol Oncol 1997;64:70–5. [DOI] [PubMed] [Google Scholar]
- [39].Keefe KA, Tadir Y, Tromberg B, et al. Photodynamic therapy of high-grade cervical intraepithelial neoplasia with 5-aminolevulinic acid. Lasers Surg Med 2002;31:289–93. [DOI] [PubMed] [Google Scholar]
- [40].Soergel P, Wang X, Stepp H, et al. Photodynamic therapy of cervical intraepithelial neoplasia with hexaminolevulinate. Lasers Surg Med 2008;40:611–5. [DOI] [PubMed] [Google Scholar]
- [41].Soergel P, Loehr-Schulz R, Hillemanns M, et al. Effects of photodynamic therapy using topical applied hexylaminolevulinate and methylaminolevulinate upon the integrity of cervical epithelium. Lasers Surg Med 2010;42:624–30. [DOI] [PubMed] [Google Scholar]
- [42].Soergel P, Dahl GF, Onsrud M, et al. Photodynamic therapy of cervical intraepithelial neoplasia 1-3 and human papilloma virus (HMV) infection with methylaminolevulinate and hexaminolevulinate–a double-blind, dose-finding study. Lasers Surg Med 2012;44:468–74. [DOI] [PubMed] [Google Scholar]
- [43].Trushina O, Novikova E, Filonenko E, et al. Photosens PDT at the treatment of virus-associated precancer and non-invasive cervical cancer. Photodiagnosis Photodyn Ther 2011;8:217. [DOI] [PubMed] [Google Scholar]
- [44].Ahn TG, Han SJ. The clinical experiences of Concurrent Chemo-Photodynamic Therapy (CCPDT) in the uterine cervical cancer staged 1B1 and 1B2, especially young women desiring fertility. Photodiagnosis Photodyn Ther 2011;8:217. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


