Abstract
Revision total hip arthroplasty (THA) may cause intra- and postoperative massive bleeding. This prospective observational study evaluated if the maximum clot firmness of FIBTEM (MCFFIB) could act as a predictor of perioperative massive bleeding in revision THA.
Fifty-eight adult patients undergoing revision THA were included. Pre- and postoperative MCFFIB, hematological and hemostatic laboratory data, as well as the amount of intra- and postoperative blood loss (IBL and PBL) were obtained.
The change rate (MCFFIB-C) between the pre- and postoperative MCFFIB had a significant correlation with IBL (ρ = 0.431, P = .001). Moreover, PBL had a significant correlation with MCFFIB-C (ρ = 0.292, P = .026). The MCFFIB-C cut-off value of ≥ 29% showed the highest sensitivity and specificity for predicting IBL ≥ 1000 mL or PBL ≥500 mL. The incidence of red blood cell transfusion in the postoperative period was higher in patients showing MCFFIB-C ≥ 29% (34% vs 8%, P = .015).
The change rate between pre- and postoperative MCFFIB values was correlated well with the amount of IBL or PBL. Moreover, particular change rate of MCFFIB could predict massive bleeding in revision THA.
Keywords: blood loss, fibrinogen, FIBTEM, hip arthroplasty, revision
1. Introduction
With recent advancements in health care leading to extension of life span, the number of revision surgeries has been increasing. In the United States, the demand for primary total hip arthroplasty (THA) and revision THA is projected to increase by 174% and 137% by 2030, respectively, compared with the demand in 2005.[1]
Revision THA has 2- to 3-fold higher risk of major complications and mortality than primary THA, and it shows less improvement in postoperative functional status than primary THA.[2,3] In addition, revision THA is associated with substantial blood loss, and thus associated with higher probability of perioperative blood transfusion.[4] It has been documented that perioperative blood loss corresponds to approximately 4 units of red blood cells (RBCs), and a mean volume of RBC transfusion was about 3 units in revision hip arthroplasty.[5,6] Perioperative complications and morality increase as blood loss increases,[7] which could ultimately increase the length of hospital stay and cost.[8] Therefore, it is important to predict the amount of intra- and postoperative blood loss (PBL) and to manage massive bleeding in revision THA appropriately.
Rotational thromboelastometry (ROTEM; Tem International GmbH, Munich, Germany) is one of the point-of-care (POC) tests, which can detect coagulation cascade abnormalities very rapidly and provides comprehensive assessment of the patient's hemostatic status.[9,10] ROTEM test results can be used to guide coagulation therapy.[11,12] FIBTEM is one of the ROTEM parameters and provides information about the change of fibrinolytic system, including fibrinogen concentration and fibrin polymerization.[13] In recent years, a number of articles have reported the usefulness of ROTEM as a POC device in numerous clinical situations such as emergency care, cardiovascular surgery, liver transplantation, trauma surgery, and septic disseminated intravascular coagulation.[11,14–17] FIBTEM is reported as an effective early predictor for massive bleeding and transfusion in orthopedic hip surgery and trauma patients.[16,18,19]
In the present study, we evaluated the association between the maximum clot firmness of FIBTEM (MCFFIB) and perioperative blood loss in patients underwent revision THA for the purpose of finding a role of FIBTEM as one of the predictors to estimate massive bleeding in revision THA.
2. Methods and materials
2.1. Study population
The study was approved by the Institutional Review Board of Seoul National University Bundang Hospital, Seongnam-si, South Korea (protocol B-1502/288-302, March 10, 2015) and registered at the ClinicalTrials.gov (NCT02951741). Written informed consent was obtained from all patients before participation. This study was conducted in Seoul National University Bundang Hospital between March 2015 and February 2017.
This prospective observational study enrolled 58 patients (age range: 20–85 years) with an American Society of Anesthesiologists physical status of 1 to 3, who were scheduled to undergo revision THA (acetabular, femoral, or dual components) under combined spinal-epidural anesthesia or general anesthesia. Preoperative exclusion criteria were as follows: pre-existing hematological disease, recent medications that may interfere with hemostasis (i.e., antiplatelet agent or anticoagulant), and history of transfusion within 1 month.
2.2. Anesthetic care
On arrival in the operating room, the standard monitoring was applied (pulse oximetry, electrocardiogram, and noninvasive arterial blood pressure). Combined spinal-epidural anesthesia was performed in the usual manner. When combined spinal-epidural anesthesia was impossible, general anesthesia was induced.
Combined spinal-epidural anesthesia was performed with the patients in the lateral decubitus position. After identifying the epidural space using epidural needle at L3–4 or L4–5, spinal needle was then passed through the epidural needle into the intrathecal space and the anesthetic drugs were administered (2.0–2.5 mL of 0.75% levobupivacaine with 15–20 μg of fentanyl). And then, an epidural catheter was placed after removing of the spinal needle. General anesthesia was induced with propofol 1.5 mg/kg, continuous infusion of remifentanil with 3.0 ng/mL via a target-controlled infusion pump (Orchestra; Fresenius Vial, France), and rocuronium 0.6 mg/kg intravenously. After confirming sufficient muscle relaxation using a nerve stimulator, endotracheal intubation was performed. General anesthesia was maintained with sevoflurane and air/O2 (total fresh gas flow of 3 L/min, FIO2 0.5).
After the completion of anesthetic induction, a 20-gauge angiocatheter was placed in the radial artery to continuously monitor arterial pressure and to perform blood tests. All patients’ upper trunks were covered with a forced-air warming blanket (Bair Hugger 52200; Arizant Healthcare Inc., 3 M Company, Eden Prairie, MN) to maintain normal body temperature during surgery.
During the operation, a lactated Ringer's solution was infused at 6 mL/kg/h as a maintenance fluid, and any intraoperative blood loss (IBL) was replaced with hydroxyethyl starch solution. We followed our transfusion protocol following massive bleeding (Fig. 1). Transfusion of RBCs was triggered when the hemoglobin level fell below 10 g/dL. After more than 4 units of RBC were administered, fresh frozen plasma (FFP) was transfused. Platelets were provided when the platelet count fell below 50,000/μL. Hypotension (systolic arterial pressure < 80% of baseline or < 90 mm Hg) or bradycardia (heart rate< 45 beats/min) was treated with ephedrine, phenylephrine, or atropine, as appropriate
Figure 1.
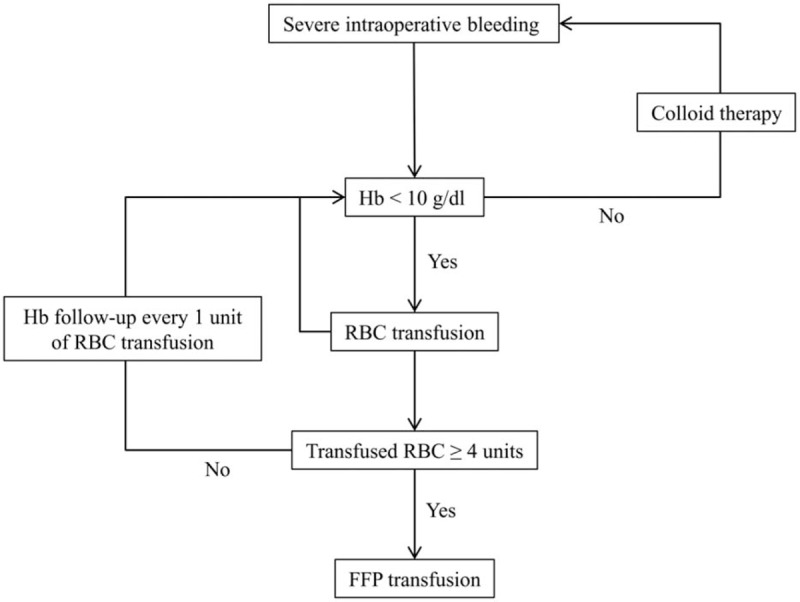
Transfusion protocol. FFP = fresh frozen plasma, Hb = hemoglobin, RBC = red blood cell.
2.3. Hematologic, hemostatic, and FIBTEM analysis
Laboratory tests were performed at 2 time points: before the initiation of surgery (preoperative) and after the end of surgery (postoperative). Blood sample was collected in ethylenediaminetetraacetic acid (EDTA)-containing tubes (Becton Dickinson, Plymouth, UK) to determine the hemoglobin, hematocrit, and platelet counts. It was also put into citrate-containing tubes to determine the international normalized ratio of prothrombin time (PT-INR), activated partial thromboplastin time (aPTT), fibrinogen levels, and maximum clot firmness of FIBTEM (MCFFIB) (Pentapharm, Munich, Germany).
FIBTEM analyses were conducted according to the manufacturer's recommendations. We obtained the MCFFIB value, which offers information of the change of fibrin polymerization, using the recommended reagent (fib-TEM:0.2 M CaCl2 20 μL with cytochalasin D and tissue factor 20 μL). The normal reference range of MCFFIB value is 9 to 25 mm. The change rate of MCFFIB was calculated using the following formula: change rate (%) = (postoperative MCFFIB – preoperative MCFFIB) / postoperative MCFFIB X 100.
2.4. Other variables
IBL was calculated using the method described by Choi et al[20] (volume in suction bottle – amount of irrigation fluid) + amount of blood on the surgical field + amount of blood on surgical pads (fully soaked: 20 mL; half soaked: 10 mL). The amount of blood drained via a hemovac (ID-VAC; Insung Medical Co., Yangpyung, Korea) during the 48-hour postoperative period was measured as the PBL. Crystalloid, colloid, and blood requirements were evaluated during the intra- and postoperative periods.
2.5. Sample size
In our previous study, the range of correlation coefficient between blood loss and MCFFIB was between -0.297 and -0.475.[18] We assumed that the correlation coefficient between the preoperative MCFFIB and amount of IBL would be approximately -0.37. Accordingly, we estimated that 55 patients were required aiming at a power of 80% and a type-1 error of 5%. Finally, we determined 62 patients with an overall dropout rate of 10%.
2.6. Statistical analysis
The data were presented as the median (interquartile range) or number (proportion). Wilcoxon signed-rank test was performed to compare the pre- and postoperative values. Spearman rank correlation coefficients were calculated between the amount of blood loss and the other clinical covariates.
The area under the receiver-operating characteristic (ROC) curve was calculated with 95% confidence interval (95% CI), and the most optimal cut-off value was determined in accordance with the sensitivity and specificity. Mann–Whitney U test was performed for subgroup analysis. Incidence was analyzed by Chi-square test or Fisher exact test.
All analyses were carried out using IBM SPSS Statistics version 21.0 (IBM Corporation, Armonk, NY) or Sigma Plot 10.0 (Systat Software, Inc., San Jose, CA). P < .05 was considered statistically significant.
3. Results
Initially, 70 patients were evaluated for eligibility and 58 patients were finally enrolled for this study (Fig. 2). The characteristics of patients, surgery, and anesthesia are provided in Table 1.
Figure 2.
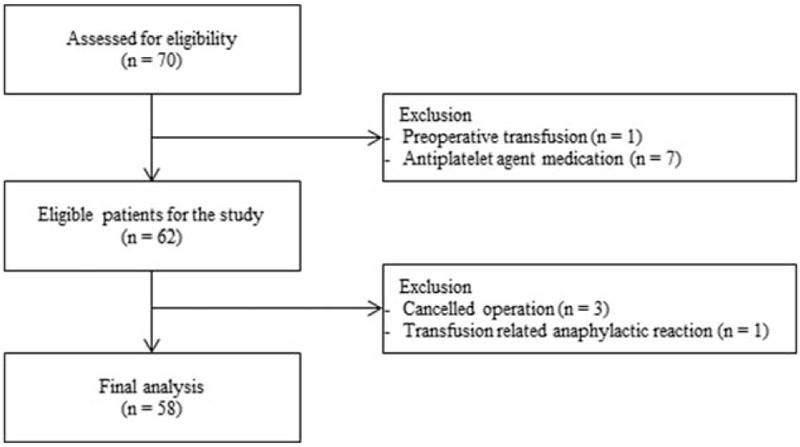
Flow chart.
Table 1.
Characteristics of patients, surgery, and anesthesia.
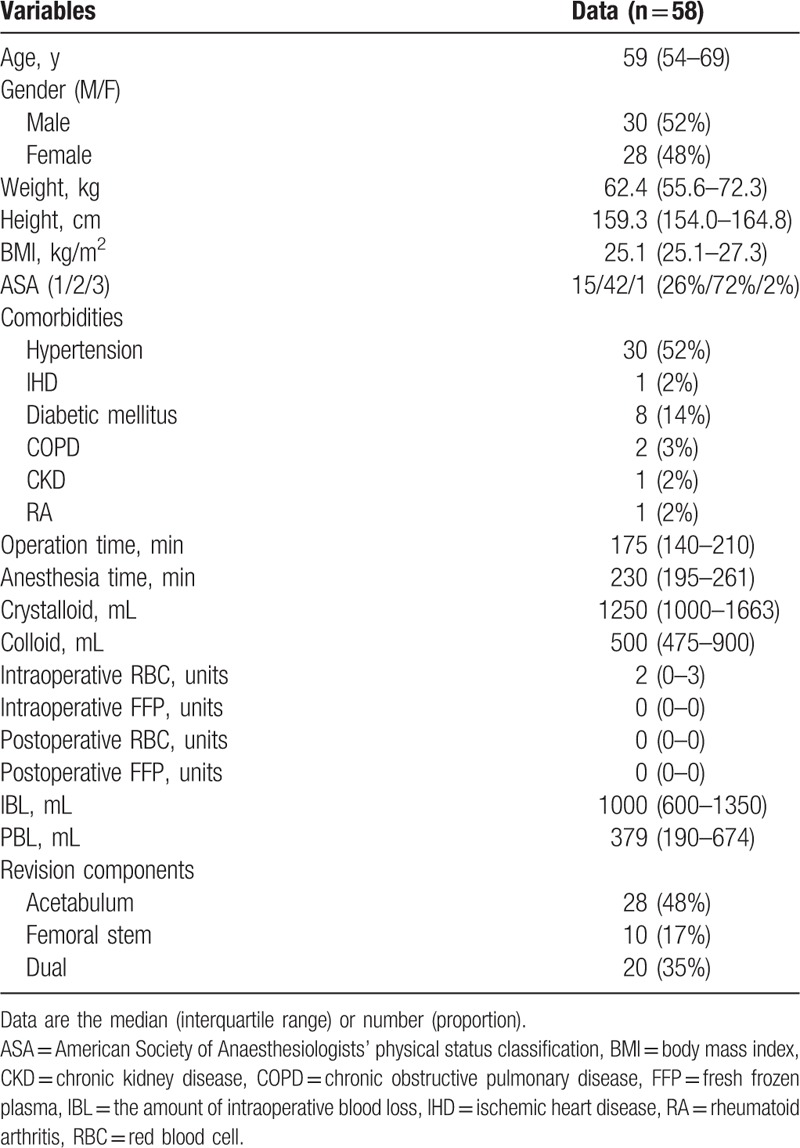
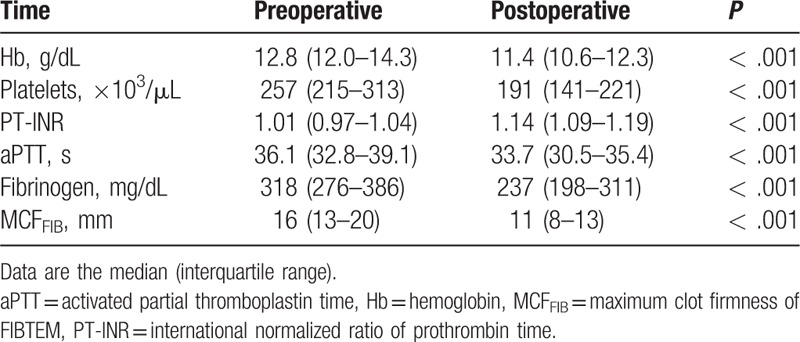
Table 2 summarizes the pre- and postoperative laboratory findings. Postoperative hemoglobin and hematocrit levels, platelets count, fibrinogen level, aPTT, and MCFFIB were significantly decreased compared with the preoperative values (P < .001). Postoperative PT-INR was significantly increased compared with the preoperative one (P < .001).
Table 2.
Laboratory findings.
We could not find any significant correlation between the blood loss and various clinical variables, including age, gender, height, weight, body mass index (BMI), type of surgery, anesthesia time, operation time, amounts of crystalloid and colloid infused, intra- and postoperative RBC/FFP transfused. In addition, no preoperative laboratory findings had a significant correlation with IBL and PBL. Postoperative platelet counts (ρ = -0.303, P = .021), PT-INR (ρ = 0.461, P < .001), fibrinogen level (ρ = -0.304, P = .021), and MCFFIB (ρ = -0.299, P = .023) showed a significant correlation with IBL. Postoperative Hb (ρ = 0.283, P = .032) and platelet counts (ρ = 0.342, P = .009) were correlated with PBL significantly (Table 3).
Table 3.
Spearman rank correlation coefficients between the amount of blood loss and hematologic variables investigated or transfusion (n = 58).
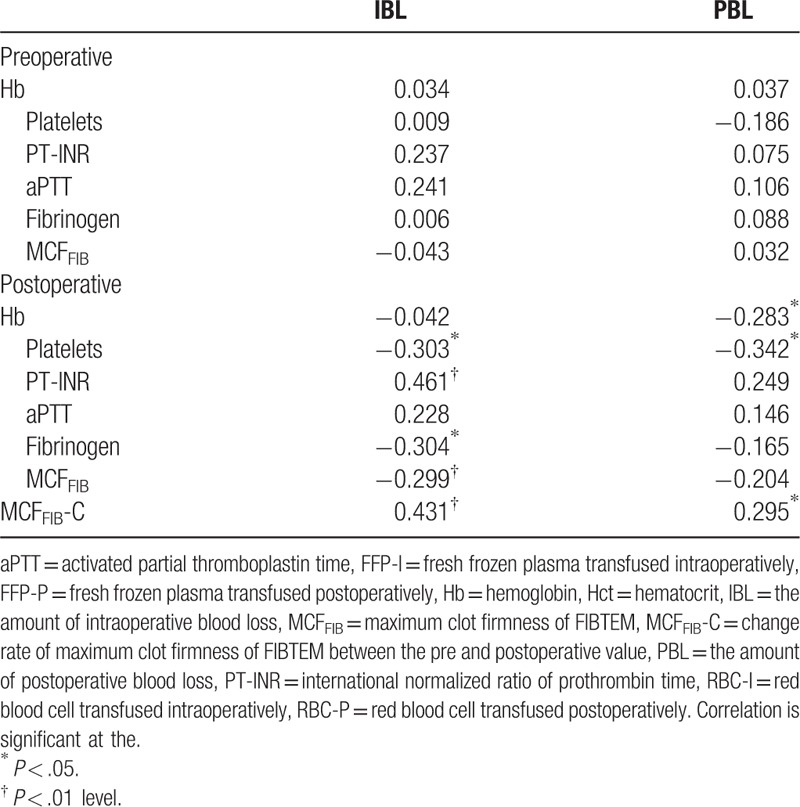
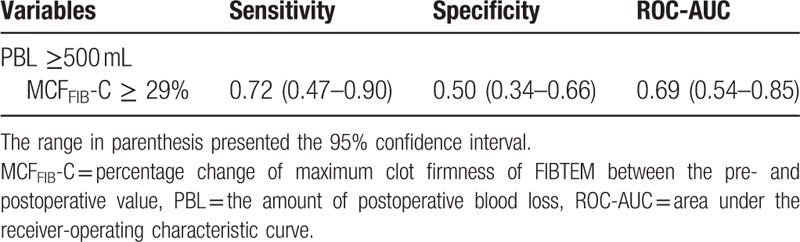
When we calculated the change rate (%) of MCFFIB between the pre- and postoperative periods (MCFFIB-C), it showed significant correlations with IBL (ρ = 0.431, P = .001) and PBL (ρ = 0.292, P = .026) (Table 3). When creating the ROC curves for MCFFIB-C to predict PBL of ≥ 500 mL, the most proper threshold was MCFFIB-C of 29%. The sensitivity and specificity of MCFFIB-C ≥29% for predicting PBL of ≥500 mL were 0.72 and 0.50, respectively (Table 4).
Table 4.
Change rate (%) of maximum clot firmness of FIBTEM and their prediction for the amount of blood loss.
When patients were subdivided in accordance with the MCFFIB-C (low MCFFIB-C: < 29%, high MCFFIB-C: ≥29%), the 2 groups showed significant differences in the postoperative platelets counts (P = .011) and PT-INR (P = .042) (Table 5). IBL was greater in the high MCFFIB-C group than in the low MCFFIB-C group (P = .001) (Table 5). A significantly larger amount of crystalloid (P = .005) and colloid (P < .001) was given during the operation in the high MCFFIB-C group than in the low MCFFIB-C group (Table 5).
Table 5.
Subgroup analysis according to the change rate (%) of MCFFIB.
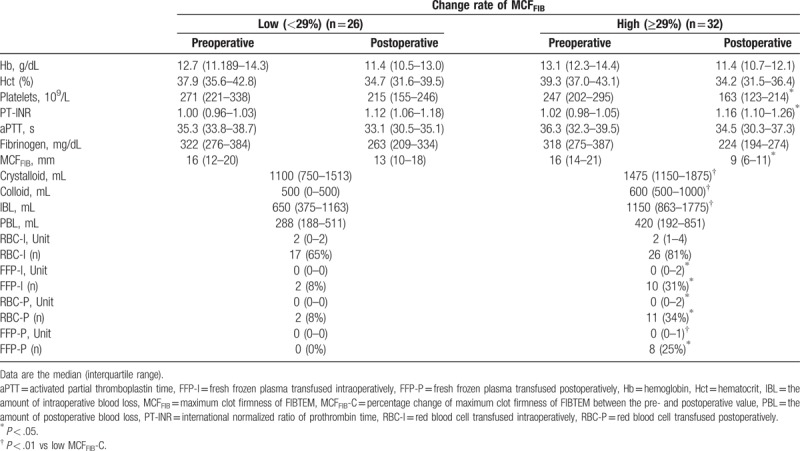
The unit or proportion of intraoperative RBC transfusion did not have a significant difference between the 2 groups; however, both the unit (P = .010) and the proportion (P = .015) of RBC transfusion were significantly greater in the high MCFFIB-C group than in the low MCFFIB-C group during the postoperative period (Table 5). During the intra- and postoperative periods, patients in the high MCFFIB-C group received more FFP both in amount (P = .024 and P = .007 at intra- and postoperative time, respectively) and in proportion (P = .028 and P = .005 at intra- and postoperative time, respectively; Table 5) than those in the low MCFFIB-C group.
4. Discussion
In this prospective observational study, we found that the postoperative platelets count and change rate of MCFFIB correlated well with the amount of PBL in revision THA. Moreover, a specific change rate of MCFFIB could be used as a reference value to identify any increased likelihood of postoperative transfusion. Although several postoperative laboratory values showed a correlation with IBL, they could not act as meaningful factors for predicting IBL.
Revision THA frequently requires RBC transfusion at higher rates than the other orthopedic surgeries.[8,21] For this reason, various methods have been attempted to reduce the allogenic blood transfusion requirements with its associated adverse effects.[22] The following are ways to conserve blood and avoid transfusion: pre-donation of autologous blood, erythropoietin treatment, normovolemic hemodilution, controlled hypotension, fibrin tissue sealants, antifibrinolytic agents, as well as intraoperative and postoperative autologous RBC salvage.[4,6,23,24] Despite recent improvements in surgical and anesthetic techniques, revision THA is still associated with significant blood loss and subsequent blood transfusion during the perioperative period. Therefore, it is important to be able to predict the massive bleeding for proper management.
During a major orthopedic surgery, coagulation factors and platelets are consumed due to bleeding, and volume replacement therapy using crystalloid and colloid cause dilutional coagulopathy via an impairment of fibrinogen/fibrin polymerization.[25] Therefore, it is important to assess not only the status of coagulation factors but also the condition of fibrinogen and fibrin polymerizations.
Our previous retrospective study revealed that MCFFIB could be a predictor of massive bleeding in primary THA.[18] We designed this study to evaluate whether MCFFIB could also be a predictive factor for massive bleeding and transfusion in revision THA. Unfortunately, we did not find any laboratory parameter to sufficiently predict massive IBL; the main reason, we believe, is due to the variety of revision components, including acetabular, femoral stem, or dual component revision. Revision THA for both acetabular and femoral stems, concurrently, is known to cause more substantial bleeding than a single-component revision THA.[7,26]
In this study, 2 postoperative laboratory factors, postoperative platelets count and the change rate of MCFFIB, showed a significant correlation with PBL. One drawback, however, is that these factors showed a relatively low correlation coefficient; therefore, it cannot be a good correlation model. Furthermore, although the postoperative platelets count correlated with PBL, it was within the normal range and no additional platelets transfusion was required to reduce bleeding after surgery. The frequency of RBC and FFP transfusion was much higher when the change rate of MCFFIB was greater than 29%. RBC and FFP transfusion incidences were increased by about 4- and over 25-fold, respectively, in the high MCFFIB-C group postoperatively. This cut-off value could be a good standard for predicting the need for transfusion after revision THA compared with the absolute values, and the results of this study demonstrate the key role of fibrinogen in the coagulation cascade and hemostasis.
It is already known that plasma fibrinogen level plays an important role in guiding blood transfusion clinically.[12,27] According to the guidelines from the European Society of Anesthesiology for management of severe perioperative bleeding, treatment with fibrinogen is recommended when significant bleeding is accompanied with a low fibrinogen concentration or functional decline of fibrinogen.[28] In addition, the ROTEM analysis is recently recommended for the management of perioperative bleeding as a POC tool in various guidelines.[28–30] Especially, fibrinogen replacement therapy can be performed on the basis of the FIBTEM value, which has a strong correlation with the plasma fibrinogen level.[31,32]
In our transfusion protocol, the Hb level of 10 g/dL was used as a trigger of RBC transfusion. When performing RBC transfusion, it is important to consider the condition of patients. However, there are still controversies which transfusion technique is superior between the liberal and the restrictive transfusion in the mortality or morbidity.[33,34] In addition, RBC:FFP transfusion ratio of 1:1 is recommended as the standard care of massive transfusion.[35] Further study is needed to evaluate the efficacy of transfusion protocol incorporating FIBTEM parameter on the patients’ outcomes after revision THA.
There are limitations to be considered in the present study. First, this study targeted the revision THA, which included both single-component (either acetabular or femoral stem) and dual-component revision. Therefore, the range of blood loss was very broad from 500 to 4500 mL. This may blunt the strength of correlation between the MCFFIB and blood loss. It is necessary to study the effectiveness of MCFFIB in more specific target of revision THA, such as dual-component revision THA. Second, this study did not address the policy of fibrinogen treatment. It will need to be confirmed in the future whether fibrinogen treatment is really necessary and how much fibrinogen should be given in cases of massive bleeding during the revision THA.
In conclusion, the change rate between the pre- and postoperative MCFFIB value was correlated well with the amounts of PBL. In addition, a particular value of change rate of MCFFIB could offer the predictive standard for the possibility of postoperative transfusion in revision THA. This would allow early and effective management of patients with blood replacement and hemostatic treatment. However, to get more strong predictable values of FIBTEM, further study is required in patients undergoing specific type of surgery (i.e., dual components revision THA).
Author contributions
Conceptualization: Hyo-Seok Na.
Data curation: Byung-Hun Min.
Formal analysis: Hyun-Jung Shin.
Funding acquisition: Hyo-Seok Na.
Investigation: Hyun-Jung Shin, Byung-Hun Min.
Methodology: Hyun-Jung Shin, Byung-Hun Min.
Supervision: Hyo-Seok Na.
Writing – original draft: Hyun-Jung Shin, Hyo-Seok Na.
Writing – review & editing: Hyo-Seok Na.
Footnotes
Abbreviations: α-angle = alpha angle, CFT = clot formation time, CT = clotting time, IBL = intraoperative blood loss, MCF = maximum clot firmness, MCFFIB = maximum clot firmness of FIBTEM, PBL = postoperative blood loss, THA = revision total hip arthroplasty.
Funding/support: The ROTEM reagents were provided by Dae Ryun Instruments Co., Ltd. in South Korea.
The authors declare that they have no conflict of interests.
References
- [1].Patel A, Pavlou G, Mujica-Mota RE, et al. The epidemiology of revision total knee and hip arthroplasty in England and Wales: a comparative analysis with projections for the United States. A study using the National Joint Registry dataset. Bone Joint J 2015;97-B:1076–81. [DOI] [PubMed] [Google Scholar]
- [2].Mahomed NN, Barrett JA, Katz JN, et al. Rates and outcomes of primary and revision total hip replacement in the United States medicare population. J Bone Joint Surg Am 2003;85-A:27–32. [DOI] [PubMed] [Google Scholar]
- [3].Katz JN, Losina E, Barrett J, et al. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States medicare population. J Bone Joint Surg Am 2001;83-A:1622–9. [DOI] [PubMed] [Google Scholar]
- [4].Bridgens JP, Evans CR, Dobson PM, et al. Intraoperative red blood-cell salvage in revision hip surgery. A case-matched study. J Bone Joint Surg Am 2007;89:270–5. [DOI] [PubMed] [Google Scholar]
- [5].Callaghan JJ, O’Rourke MR, Liu SS. Blood management: issues and options. J Arthroplasty 2005;20:51–4. [DOI] [PubMed] [Google Scholar]
- [6].Noordin S, Waters TS, Garbuz DS, et al. Tranexamic acid reduces allogenic transfusion in revision hip arthroplasty. Clin Orthop Relat Res 2011;469:541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mahadevan D, Challand C, Keenan J. Revision total hip replacement: predictors of blood loss, transfusion requirements, and length of hospitalisation. J Orthop Traumatol 2010;11:159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Spahn DR. Anemia and patient blood management in hip and knee surgery: a systematic review of the literature. Anesthesiology 2010;113:482–95. [DOI] [PubMed] [Google Scholar]
- [9].Johansson PI, Stensballe J. Effect of haemostatic control resuscitation on mortality in massively bleeding patients: a before and after study. Vox Sang 2009;96:111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Carroll RC, Craft RM, Langdon RJ, et al. Early evaluation of acute traumatic coagulopathy by thrombelastography. Transl Res 2009;154:34–9. [DOI] [PubMed] [Google Scholar]
- [11].Schochl H, Nienaber U, Hofer G, et al. Goal-directed coagulation management of major trauma patients using thromboelastometry (ROTEM)-guided administration of fibrinogen concentrate and prothrombin complex concentrate. Crit Care 2010;14:R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rahe-Meyer N, Solomon C, Winterhalter M, et al. Thromboelastometry-guided administration of fibrinogen concentrate for the treatment of excessive intraoperative bleeding in thoracoabdominal aortic aneurysm surgery. J Thorac Cardiovasc Surg 2009;138:694–702. [DOI] [PubMed] [Google Scholar]
- [13].Biolik G, Kokot M, Sznapka M, et al. Platelet reactivity in thromboelastometry. Revision of the FIBTEM test: a basic study. Scand J Clin Lab Invest 2017;77:216–22. [DOI] [PubMed] [Google Scholar]
- [14].Momeni M, Carlier C, Baele P, et al. Fibrinogen concentration significantly decreases after on-pump versus off-pump coronary artery bypass surgery: a systematic point-of-care ROTEM analysis. J Cardiothorac Vasc Anesth 2013;27:5–11. [DOI] [PubMed] [Google Scholar]
- [15].Koami H, Sakamoto Y, Ohta M, et al. Can rotational thromboelastometry predict septic disseminated intravascular coagulation? Blood Coagul Fibrinolysis 2015;26:778–83. [DOI] [PubMed] [Google Scholar]
- [16].Schochl H, Cotton B, Inaba K, et al. FIBTEM provides early prediction of massive transfusion in trauma. Crit Care 2011;15:R265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Alamo JM, Leon A, Mellado P, et al. Is “intra-operating room” thromboelastometry useful in liver transplantation? A case-control study in 303 patients. Transplant Proc 2013;45:3637–9. [DOI] [PubMed] [Google Scholar]
- [18].Na HS, Shin HJ, Do SH. FIBTEM provides prediction of massive bleeding in total hip replacement arthroplasty. Blood Coagul Fibrinolysis 2016;27:340–6. [DOI] [PubMed] [Google Scholar]
- [19].Veigas PV, Callum J, Rizoli S, et al. A systematic review on the rotational thrombelastometry (ROTEM(R)) values for the diagnosis of coagulopathy, prediction and guidance of blood transfusion and prediction of mortality in trauma patients. Scand J Trauma Resusc Emerg Med 2016;24:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Choi SJ, Ahn HJ, Chung SS, et al. Hemostatic and electrolyte effects of hydroxyethyl starches in patients undergoing posterior lumbar interbody fusion using pedicle screws and cages. Spine (Phila Pa 1976) 2010;35:829–34. [DOI] [PubMed] [Google Scholar]
- [21].Saleh E, McClelland DB, Hay A, et al. Prevalence of anaemia before major joint arthroplasty and the potential impact of preoperative investigation and correction on perioperative blood transfusions. Br J Anaesth 2007;99:801–8. [DOI] [PubMed] [Google Scholar]
- [22].Tenholder M, Cushner FD. Intraoperative blood management in joint replacement surgery. Orthopedics 2004;27:s663–8. [DOI] [PubMed] [Google Scholar]
- [23].Lemaire R. Strategies for blood management in orthopaedic and trauma surgery. J Bone Joint Surg Br 2008;90:1128–36. [DOI] [PubMed] [Google Scholar]
- [24].Ghoz A, Al-Khateeb H, Rajkumar S, et al. Use of a thrombin fibrin sealant in reducing blood loss in revision hip arthroplasty. Open Orthop J 2015;9:511–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mittermayr M, Streif W, Haas T, et al. Hemostatic changes after crystalloid or colloid fluid administration during major orthopedic surgery: the role of fibrinogen administration. Anesth Analg 2007;105:905–17. table of contents. [DOI] [PubMed] [Google Scholar]
- [26].Zarin J, Grosvenor D, Schurman D, et al. Efficacy of intraoperative blood collection and reinfusion in revision total hip arthroplasty. J Bone Joint Surg Am 2003;85-A:2147–51. [DOI] [PubMed] [Google Scholar]
- [27].Ketchum L, Hess JR, Hiippala S. Indications for early fresh frozen plasma, cryoprecipitate, and platelet transfusion in trauma. J Trauma 2006;60:S51–8. [DOI] [PubMed] [Google Scholar]
- [28].Kozek-Langenecker SA, Afshari A, Albaladejo P, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol 2013;30:270–382. [DOI] [PubMed] [Google Scholar]
- [29].Rossaint R, Bouillon B, Cerny V, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fourth edition. Crit Care 2016;20:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Force ASoAT. Practice guidelines for perioperative blood management: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Management∗. Anesthesiology 2015;122:241–75. [DOI] [PubMed] [Google Scholar]
- [31].Huissoud C, Carrabin N, Audibert F, et al. Bedside assessment of fibrinogen level in postpartum haemorrhage by thrombelastometry. BJOG 2009;116:1097–102. [DOI] [PubMed] [Google Scholar]
- [32].Rugeri L, Levrat A, David JS, et al. Diagnosis of early coagulation abnormalities in trauma patients by rotation thrombelastography. J Thromb Haemost 2007;5:289–95. [DOI] [PubMed] [Google Scholar]
- [33].Carson JL, Sieber F, Cook DR, et al. Liberal versus restrictive blood transfusion strategy: 3-year survival and cause of death results from the FOCUS randomised controlled trial. Lancet 2015;385:1183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Murphy GJ, Pike K, Rogers CA, et al. Liberal or restrictive transfusion after cardiac surgery. N Engl J Med 2015;372:997–1008. [DOI] [PubMed] [Google Scholar]
- [35].Pham HP, Shaz BH. Update on massive transfusion. Br J Anaesth 2013;111(Suppl 1):i71–82. [DOI] [PubMed] [Google Scholar]


