Abstract
Rationale:
Medullary thyroid carcinoma (MTC) is an aggressive subtype of thyroid cancer with frequent hematogenous metastasis. While its metastasis is usually observed in the lung, liver, or bone, it rarely migrates to the breast.
Patient concerns:
Here we report 2 cases with a complaint of breast lump after initial treatment of MTC.
Diagnoses:
In both patients, the MTC characteristics of breast nodules were confirmed by pathologic analysis of biopsy specimens.
Interventions:
The genetic mutations within the metastatic breast lesion were evaluated. Wide local excision was thus performed to 1st case, while no therapeutic intervention for another patient due to the wide-spread presence of the disease.
Outcomes:
No sign of relapse or metastasis was found in 1st case during a 14-month follow-up. For 2nd case, the breast nodule grew to 14 mm within 3 months before remaining stable for 10 months.
Lessons:
MTC can be a very indolent disease despite its aggressiveness. Reoperation should be considered for patients with local recurrence or resectable distant metastasis of MTC. The findings for both cases supported serum calcitonin as an important marker for the evaluation of disease. Future studies are needed to advance our understanding of its molecular features and improve strategies for its diagnosis and treatment.
Keywords: breast metastasis, gene analysis, medullary thyroid carcinoma, RET proto-oncogenes
1. Introduction
Medullary thyroid carcinoma (MTC) originates from the parafollicular C-cells of the thyroid gland and constitutes approximately 5% to 8% of all thyroid cancer.[1] Compared with the most common histotype of thyroid cancer, papillary thyroid carcinoma, which accounts for 80% of thyroid malignancies,[2] MTC is usually more aggressive with a higher risk of hematogenous metastasis.[3] Common metastatic sites of MTC include the lung, liver and bones, and secondary tumors in other organs, such as the breast, are rarely reported.
Due to the low incidence of breast metastasis of MTC, its clinical characteristics and prognosis as well as the associated genetic feature are unknown. Here we present 2 cases of MTC with recurrent nodules in the breast, characterize their genetic features by mutational analysis, and review the relevant literature to provide further insight into this rare disease.
2. Case reports
2.1. Case 1
A 29-year-old woman presented with left and isthmus thyroid nodules and palpable left cervical lymph nodes in January 2017. The patient had a 3-year history of diarrhea. Her basal serum calcitonin was 9030 pg/mL and carcinoembryonic antigen (CEA) was 1209 μg/L. Preoperative positron-emission tomography-computed tomography (PET-CT) confirmed a left thyroid nodule with involvement of the left cervical lymph nodes but without systemic metastasis. The patient underwent total thyroidectomy, left cervical lymph node dissection, and right central lymph node dissection. The sternothyroid muscle was partially resected due to tumor invasion. In addition, the enlarged lymph nodes were close to the jugular vein and invaded the anterior scalene muscle. Thus, we completely removed the involved lymph nodes and partially resected the invaded peripheral tissue, leaving the jugular vein intact. Pathologic analysis revealed pT3N1bM0 (stage IVa) medullary carcinoma of the thyroid with multiple lymph node metastases. After surgery, levothyroxine tablets were taken as thyroid replacement. The patient's serum calcitonin level was decreased dramatically to 48 pg/mL at 1 month after surgery.
In March 2017, the patient represented with a left breast mass in the upper inner quadrant. Ultrasound examination revealed a 6.9 mm × 6.8 mm, indistinct, irregular, noncalcified, hypoechoic lesion, which was further evaluated using Breast Imaging Reporting and Data System (BI-RADS) 4a (Fig. 1A). Her calcitonin level had increased slightly to 51.6 pg/mL. Metastatic MTC was confirmed after vacuum-assisted biopsy (Fig. 2). Immunohistochemical staining of the resected specimen showed that the cancer tissue was positive for calcitonin, synapsin (SYN), CgA, CD56, and thyroid transcription factor (TTF)-1, but negative for estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER-2)/Neu, gross cystic disease fluid protein (GCGFP)-15, and thyroglobulin (TG). Wide local excision was thus performed. During a 14-month follow-up, no sign of relapse or metastasis was found, and the calcitonin level decreased and remained stable at 10 to 13 pg/mL. The CEA level gradually decreased within 6 months after surgery and was maintained within the normal level (<5 μg/L) for the remaining 8 months of follow-up.
Figure 1.
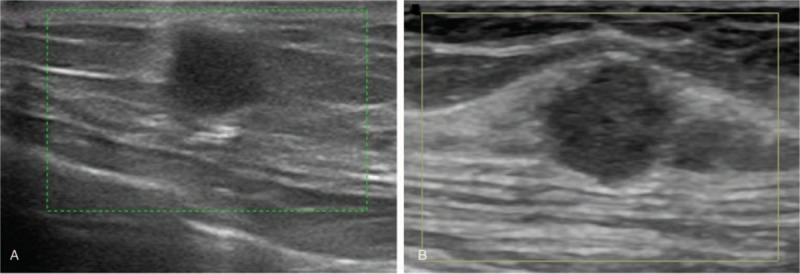
(A) Ultrasound imaging in patient 1 showed an ill-defined, irregular, noncalcified, hypoechoic lesion in the left breast. (B) Ultrasound imaging in patient 2 showed a solid, ill-defined, microlobulated, hypoechoic nodule in the left breast at 11 to 12 o’clock.
Figure 2.
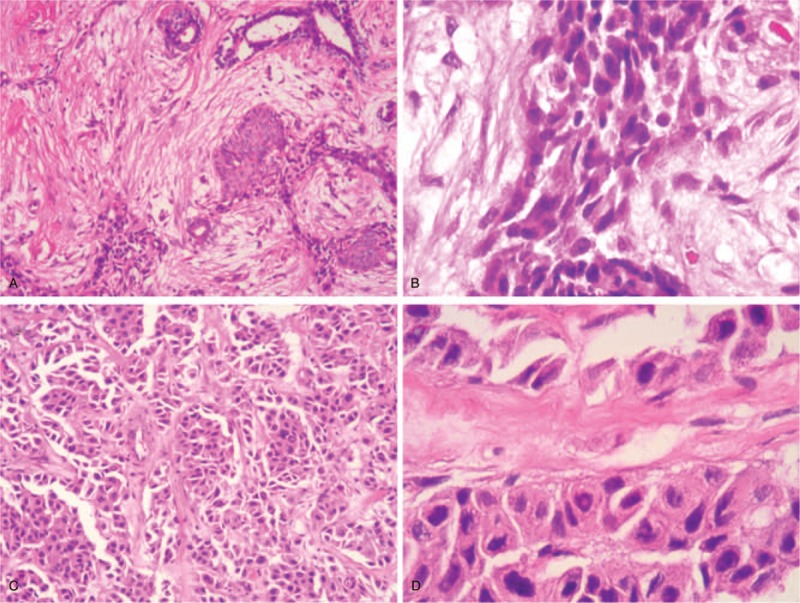
Hematoxylin and eosin staining of the breast biopsy specimen from patient 1 showed a cluster of metastatic cells. (A) 100×. (B) 400×. Core-needle biopsy of the breast from patient 2 showed tumor cells arranged in clusters. (C) 100×. (D) 400×.
2.2. Case 2
A 44-year-old woman was admitted with a left thyroid nodule in December 2013. Total thyroidectomy with left central lymph node dissection was performed and pathologic examination revealed a pT2N0M0 (stage II) medullary carcinoma without lymph node involvement.
In 2015, the patient developed palpable neck nodules. PET-CT scanning showed enlargement of multiple left cervical lymph nodes and cutaneous lesions at the level of the hyoid bone. Modified left cervical lymph node dissection with excision of cutaneous lesions was performed. Recurrence of thyroid medullary carcinoma was confirmed, and external radiation of the neck region was undertaken. In 2016, the patient experienced cutaneous recurrence in the central neck and underwent surgical treatment.
In June 2017, the patient represented with a palpable left breast mass. PET-CT scanning demonstrated a left breast nodule and sacrum metastasis, but no lesion in the lung or liver. Ultrasound examination confirmed an 8.8-mm solid tumor in the left breast at 11 to 12 o’ clock. This lesion was ill-defined, microlobulated, and hypoechoic and evaluated as BI-RADS 4b (Fig. 1B). No intermammary disease was detected on mammography. Microscopic examination of a core-needle biopsy specimen from the left breast lesion revealed metastatic MTC (Fig. 2). Immunohistochemical analysis suggested the tumor was positive for calcitonin, SYN, CgA, and TTF-1 but negative for ER, PR, HER-2/Neu, GCGFP-15, and TG. No further treatment was given based on the wide-spread presence of the disease, and the breast nodule grew to 14 mm within 3 months before remaining stable for 10 months. The patient received levothyroxine tablets as thyroid replacement. Her calcitonin and CEA levels were normal during the course of disease.
2.3. Mutational screening
To establish a genetic profile of the metastatic MTC to the breast, the genetic mutations of the breast lesions were examined and are summarized in Table 1. The genomic DNA used in the analysis was extracted from 10-μm sections of formalin-fixed, paraffin-embedded tumor specimens. No M918T mutation of RET or V600E mutation of BRAF was found based on an enriched polymerase chain reaction followed by restriction fragment length polymorphism analysis (PCR-RFLP). Further enriched PCR-RFLP analysis detected no mutations in the KRAS and NRAS genes. Breast lesions in both cases expressed epidermal growth factor receptor (EGFR) on the membrane, which was examined by immunohistochemistry.
Table 1.
Mutations of RET and BRAF and expression levels of EGFR, KRAS, and NRAS in the 2 cases.

3. Discussion
Only a handful cases of breast neoplasms originating from MTC have been reported,[4] and the mutation status of RET has rarely been determined in these metastatic lesions. Activating mutations of the RET oncogene were found in approximately 20% of sporadic MTCs.[5] The somatic RET mutation is associated with the differentiation, proliferation, survival, and motility of cells,[6] and germline mutations in the RET proto-oncogenes have frequently been studied in MTC for their roles in carcinogenesis and their potential as therapeutic targets.[7] The M918T mutation in exon 16 is the most common mutation in sporadic MTC. Patients with somatic RET mutations, especially M918T, have an increased risk of aggressive malignancy[8] and are expected to benefit from RET-targeting agents, such as vandetanib.[9] However, in the 2 presented cases of metastatic MTC, no M918T mutation in the RET gene was detected. The mutation status of RET and its therapeutic value in metastatic MTC remain to be determined in future studies.
In both reported cases of metastatic MTC to the breast, overexpression of EGFR was detected. EGFR has a well-characterized role in the proliferation, migration, and apoptosis of cancer cells. Increased EGFR expression in metastatic MTC lesions was shown to be associated with a poor clinical outcome in Spanish patients independent of RET mutations[10]; however, the underlying mechanisms of MTC metastasis remain to be understood. The high prevalence of EGFR expression in metastatic MTC suggests that anti-EGFR antibodies and small-molecule EGFR kinase inhibitors, which have been shown to inhibit cancer progression,[11] may potentially be useful for this malignancy.
No BRAF or NRAS mutation was found in our patients, in accordance with previous findings.[6] In previous studies, KRAS mutations were found in 4% to 40% of MTCs, whereas no NRAS mutation was reported.[6,12,13] The BRAF V600E mutation was reported in 68.2% of MTC cases in 1 study,[14] while contradictory results were demonstrated in other studies.[6,13] Further mutational screening is required to characterize the genetic features of breast metastasis of MTC.
The 10-year overall survival for MTC was reported to be approximately 72%,[15] and 7.7% of patients develop recurrent disease more than 10 years after diagnosis.[16] The longest reported disease-free interval was 25 years.[17] Given the prolonged disease-free period of MTC, care should be taken to differentiate metastatic MTC lesions from those of other origins. Unlike the metastatic lesions in the breast originating from other malignancies, ultrasound examinations of our cases showed firm ill-defined, irregular, hypoechoic nodules, similar to primary breast cancer. In addition, the nodules were not visualized on mammography in this study, although a previous report documented calcifications and irregularity of metastatic MTC nodules on mammography.[18] Therefore, core-needle biopsy and immunohistochemical staining are required for the decisive diagnosis of breast lesions, especially in patients with a previous history of MTC. Fine-needle aspiration cytology can also be an alternative approach to differentiating metastatic MTC from other malignancies.[19]
Serum calcitonin is an important prognostic biomarker for MTC. The basal calcitonin level can predict preoperative tumor stage, and monitoring the postoperative calcitonin level helps predict tumor progression and prognosis. In the present study, elevated calcitonin expression in patient 1 effectively indicated metastasis of the disease, supporting the importance of monitoring the serum calcitonin level, particularly for those patients with an increased preoperative calcitonin level, in the diagnosis of MTC recurrence and metastasis.[20]
Reoperation should be considered for patients with local recurrence or resectable distant metastasis of MTC. For patient 1 in this study, a reduction in the calcitonin level after reoperation was achieved after local dissection of the breast nodule, and no evidence of recurrence was found during the follow-up period. Patient 2 had a more aggressive disease, and repeated operations were performed. The benefit of reoperation for recurrent and persistent MTC is supported by a previous study.[21]
In summary, MTC can be a very indolent disease despite its aggressiveness, and patients may develop widespread disease. However, our knowledge of this malignancy remains limited due to its low incidence. Future studies are needed to advance our understanding of its molecular features and improve strategies for its diagnosis and treatment.
Author contributions
Conceptualization: Kexin Meng, Hailong Chen.
Data curation: Kexin Meng, Wanyuan Chen, Kewang Sun, Hailong Chen.
Methodology: Wei Tian.
Writing – original draft: Kexin Meng, Hailong Chen.
Footnotes
Abbreviations: BI-RADS = Breast Imaging Reporting and Data System, CEA = carcinoembryonic antigen, EGFR = epidermal growth factor receptor, ER = estrogen receptor, GCGFP = gross cystic disease fluid protein, HER-2 = human epidermal growth factor receptor 2, MTC = medullary thyroid carcinoma, PCR-RFLP = restriction fragment length polymorphism analysis, PET-CT = preoperative positron-emission tomography-computed tomography, PR = progesterone receptor, SYN = synapsin, TG = thyroglobulin, TTF = thyroid transcription factor.
This work was supported the National Nature Science Foundation of China (grant no: 81502598).
Written informed consent was obtained from the patients for publication of this case report.
The authors have no conflicts of interest to disclose.
References
- [1].Somnay YR, Schneider D, Mazeh H. Thyroid: Medullary Carcinoma. Atlas of genetics and cytogenetics in oncology and haematology 2013;17:291–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nikiforov YE. Thyroid carcinoma: molecular pathways and therapeutic targets. Mod Pathol 2008;21:S37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ricciato MP, Lombardi CP, Raffaelli M, et al. Metastatic breast involvement from medullary thyroid carcinoma: a clue to consider the need of early diagnosis and adequate surgical strategy. Thyroid 2010;20:831–2. [DOI] [PubMed] [Google Scholar]
- [4].Mandanas S, Margaritidou E, Christoforidou V, et al. Breast metastasis from medullary thyroid carcinoma in a male patient: case report and review of the literature. Rare Tumors 2015;7:5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hofstra RM, Landsvater RM, Ceccherini I, et al. A mutation in the RET proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature 1994;367:375–6. [DOI] [PubMed] [Google Scholar]
- [6].Boichard A, Croux L, Al Ghuzlan A, et al. Somatic RAS mutations occur in a large proportion of sporadic RET-negative medullary thyroid carcinomas and extend to a previously unidentified exon. J Clin Endocrinol Metab 2012;97:E2031–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Schlumberger M, Carlomagno F, Baudin E, et al. New therapeutic approaches to treat medullary thyroid carcinoma. Nature clinical practice. Endocrinol Metab 2008;4:22–32. [DOI] [PubMed] [Google Scholar]
- [8].Cote GJ, Evers C, Hu MI, et al. Prognostic significance of circulating RET M918T mutated tumor DNA in patients with advanced medullary thyroid carcinoma. J Clin Endocrinol Metab 2017;102:3591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wells SA, Jr, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol 2012;30:134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rodriguez-Antona C, Pallares J, Montero-Conde C, et al. Overexpression and activation of EGFR and VEGFR2 in medullary thyroid carcinomas is related to metastasis. Endocr Relat Cancer 2010;17:7–16. [DOI] [PubMed] [Google Scholar]
- [11].Bergstrom JD, Westermark B, Heldin NE. Epidermal growth factor receptor signaling activates met in human anaplastic thyroid carcinoma cells. Exp Cell Res 2000;259:293–9. [DOI] [PubMed] [Google Scholar]
- [12].Moura MM, Cavaco BM, Pinto AE, et al. High prevalence of RAS mutations in RET-negative sporadic medullary thyroid carcinomas. J Clin Endocrinol Metab 2011;96:E863–8. [DOI] [PubMed] [Google Scholar]
- [13].Schulten HJ, Al-Maghrabi J, Al-Ghamdi K, et al. Mutational screening of RET, HRAS, KRAS, NRAS, BRAF, AKT1, and CTNNB1 in medullary thyroid carcinoma. Anticancer Res 2011;31:4179–83. [PubMed] [Google Scholar]
- [14].Goutas N, Vlachodimitropoulos D, Bouka M, et al. BRAF and K-RAS mutation in a Greek papillary and medullary thyroid carcinoma cohort. Anticancer Res 2008;28:305–8. [PubMed] [Google Scholar]
- [15].Randle RW, Balentine CJ, Leverson GE, et al. Trends in the presentation, treatment, and survival of patients with medullary thyroid cancer over the past 30 years. Surgery 2017;161:137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Saad MF, Ordonez NG, Rashid RK, et al. Medullary carcinoma of the thyroid. A study of the clinical features and prognostic factors in 161 patients. Medicine 1984;63:319–42. [PubMed] [Google Scholar]
- [17].Ahuja S, Ernst H. Dormant metastases in medullary thyroid carcinoma. A case report. Exp Clin Endocrinol 1991;98:37–41. [DOI] [PubMed] [Google Scholar]
- [18].Soo MS, Williford ME, Elenberger CD. Medullary thyroid carcinoma metastatic to the breast: mammographic appearance. AJR Am J Roentgenol 1995;165:65–6. [DOI] [PubMed] [Google Scholar]
- [19].Andreiuolo F, Suciu V, Bayou EH, et al. Bilateral breast lesions in a patient with medullary thyroid carcinoma. Cytopathology 2009;20:403–5. [DOI] [PubMed] [Google Scholar]
- [20].Lips CJ, Hoppener JW, Thijssen JH. Medullary thyroid carcinoma: role of genetic testing and calcitonin measurement. Ann Clin Biochem 2001;38:168–79. [DOI] [PubMed] [Google Scholar]
- [21].Fialkowski E, DeBenedetti M, Moley J. Long-term outcome of reoperations for medullary thyroid carcinoma. World J Surg 2008;32:754–65. [DOI] [PubMed] [Google Scholar]


