Abstract
Background:
Human epididymis protein 4 (HE4), a matrix metalloprotease 2 (MMP2), and a matrix metalloprotease 9 (MMP9) inhibitor, promotes renal fibrosis by inhibiting the degradation of type I collagen. However, the predictive value of HE4 for renal fibrosis remains controversial, even though it has been identified as one of the most upregulated genes in cultured fibrosis-associated myofibroblasts. This systematic review and meta-analysis was conducted to investigate the potential association between circulating HE4 and renal fibrosis.
Methods:
Original and review articles published until January 2017 that analyzed the performance of serum HE4 in renal fibrosis were systematically searched for in PubMed (1966–2017.1), Cochrane Library, Web of Science, EMBASE (1980–2017.1), China National Knowledge Infrastructure, Wanfang Database, and VIP (Weipu Database). The meta-analysis was performed using RevMan 5.3 version. Pertinent studies were reviewed and the standardized mean difference (SMD) with 95% confidence interval was extracted. A total of 5 studies reporting 460 participants were included in the final analysis. Subgroup and sensitivity analyses were performed to explore the potential sources of between-study heterogeneity.
Results:
The results demonstrated that elevated serum HE4 favored the diagnosis of renal fibrosis across all trials (SMD = 1.41; 95% confidence interval, 0.82–2.01; P < .001). The bubble graph indicated statistically robust result. The pooled SMD was similar after removing any single study for sensitivity analysis.
Conclusion:
The present study suggests a positive association between circulating HE4 and renal fibrosis. Further studies are needed to investigate the effects of interventions on HE4, and the value of HE4 as a biomarker.
Keywords: biomarker, Human epididymis protein 4, renal fibrosis
1. Introduction
Chronic kidney disease (CKD) accounts for 12.2 deaths per 100 000 people and is ranked 14th on the list of leading causes of death.[1] The prevalence of CKD is reported to be approximately 11% globally.[2] The final common pathological pathway of most forms of chronic renal disease is renal fibrosis. With the development of renal fibrosis, injured tubular epithelia lose their regenerative capacity and undergo apoptosis leading to tubular atrophy, nonfunctional glomeruli, and eventually the progressive loss of kidney function.[3] The diagnosis of renal fibrosis relies on pathophysiological renal manifestations presented with glomerulosclerosis, tubulointerstitial fibrosis, tubular atrophy and dilation, as well as the rarefaction of glomerular or peritubular capillaries.[4,5] Renal fibrosis significantly affects morbidity and mortality in CKD patients. Thus, early detection would make it possible to intervene as soon as possible and delay the progression of CKD to renal failure.
Histopathological examination is currently considered the gold standard in the diagnosis of renal fibrosis; thus, percutaneous kidney biopsy may be required to establish a diagnosis. However, the lack of noninvasive diagnostic tools for renal fibrosis hinders early diagnosis and treatment. Therefore, identifying sensitive and accurate noninvasive biomarkers for renal fibrosis is imperative to achieve a better prognosis for patients with nephropathy.
Considerable progress has been made in understanding the pathogenesis of renal fibrosis and several novel noninvasive predictors have been proposed. Among a wide spectrum of biomarkers, HE4 is the most promising. During fibrosis, inflammatory cytokines and growth factors lead to the production of extracellular matrix (ECM) that eventually disrupts the normal functioning of the organ. In this process, the myofibroblast is commonly regarded as the predominant effector cell, which acts as the main producer of ECM, crosslinking enzymes and inhibitors of matrix degrading metalloproteinases.[6] In 2013, Le Bleu et al[7] identified HE4 (encoding HE4) as the most upregulated gene in cultured fibrosis-associated myofibroblasts using a transgenic mouse model expressing fluorescent HE4 protein under the control of the α smooth muscle actin promoter, which shed new light on the function of HE4 and indicated that it may be a potential biomarker for renal fibrosis. In 2016, Wan et al reported higher serum concentrations of HE4 in patients with CKD and more severe renal fibrosis. Moreover, the serum HE4 level was significantly increased in the early stages of CKD, and its level in serum correlated with the degree of renal fibrosis,[3] suggesting that the upregulated expression of HE4 may present as one of the initial sign in the onset and progression of renal fibrosis.
Although multiple studies have indicated that serum HE4 is correlated with renal fibrosis and so may serve as a potential novel biomarker for renal fibrosis, they have been limited by relatively small study populations. Thus far, no published meta-analysis has addressed this question. In the present study, we undertook a systemic review and meta-analysis of the published literature evaluating the relationship between serum HE4 and renal fibrosis among patients with CKD, to provide better tools for early diagnosis and treatment of renal fibrosis.
2. Methods
This study is a meta-analysis, and ethic statement is not applicable.
2.1. Literature search
Original and review articles published until January 2017 that analyzed the performance of serum HE4 in renal fibrosis were systematically searched for in 4 English language databases: PubMed (1966–2017.1), Cochrane Library, Web of Science, and EMBASE (1980–2017.1). To include as many as possible relevant articles, we first focused on HE4 and fibrosis as search subjects. Subsequently, further eligible articles would be selected within the initial search results if renal fibrosis was confirmed by renal biopsy. Specifically, we used the following medical subject heading terms and words when searching in PubMed: (((Fibroses) OR Cirrhosis) OR “Fibrosis”[Mesh]) AND ((human epidydimal secretory protein E4) OR (EDDM4 protein, human) OR (HE4 protein, human) OR (ESP-H4 protein, human) OR (WAP5 protein, human) OR (WAP 4-disulfide core domain 2 protein, human) OR (human epididymis-specific protein E4) OR (“WFDC2 protein, human” [Supplementary Concept]) OR (human epididymis protein 4) OR (HE4)). We also searched 3 Chinese databases, China National Knowledge Infrastructure, Wangfang Database, and VIP (Weipu Database), using subject heading terms and text words as follows: (“ ” [translation of “human epididymis protein 4”] OR “HE4”) AND (“
” [translation of “human epididymis protein 4”] OR “HE4”) AND (“ ” [translation of “fibrosis”]). Their reference lists were searched manually to find additional relevant publications.
” [translation of “fibrosis”]). Their reference lists were searched manually to find additional relevant publications.
2.2. Inclusion and exclusion criteria
Inclusion criteria were as follows: (1) studies with a case group of patients with a histologically confirmed diagnosis of renal fibrosis, and a control group of healthy people or patients with CKD with histologically confirmed absence of renal fibrosis; (2) serum HE4 concentration was tested; and (3) studies discuss the relationship between serum HE4 and renal fibrosis.
Exclusion criteria were as follows: (1) manuscripts in the format of letters, editorials, case reports, or reviews; (2) studies without complete data; and (3) studies with duplicate data reported in other studies.
2.3. Data extraction
Two reviewers independently selected manuscripts based on the inclusion and exclusion criteria. These reviewers then extracted the following information from the eligible studies: author, year of publication, country of origin, sample size, assay methods. Disagreements on the eligibility of studies were resolved by full-text review and discussion; if a consensus could not be reached, then a third reviewer was consulted. The target condition of this study was renal fibrosis, including glomerulosclerosis, renal interstitial fibrosis, and intrarenal vascular sclerosis.
2.4. Statistical analyses
The meta-analysis was performed using RevMan 5.3 version (The Cochrane Collaboration, Oxford, UK). Group differences in continuous outcomes were analyzed as the pooled SMD in either change from baseline to endpoint (preferred) or endpoint scores [only preferred if change score results were skewed, i.e., Standard deviation [SD] > twice the mean]. We assessed statistical heterogeneity between 2 studies using Cochrane's Q test (significance level of P < .10) and I2 statistics (ranges from 0% to 100% with lower values representing less heterogeneity),[8] and DerSimonian-Laird random-effects models was applied. Subgroup and sensitivity analyses were performed to explore the potential sources of between-study heterogeneity. We extracted the number of participants with data from each study to calculate SMD and corresponding 95% confidence intervals. We conducted a sensitivity analysis by omitting each study in sequence to test the robustness of association. Forest plots of accuracy indexes were also constructed. P < .05 was considered to indicate statistical significance. The bubble graph was obtained using Excel 2010 (Microsoft Inc.).
3. Results
3.1. Literature screening results
As shown in Fig. 1, 96 potentially relevant articles were retrieved through database searching. After removing duplicates, we had 76 articles, of which we excluded 26 that were not relevant to our study based on the type of manuscript. The remaining 50 articles were subjected to a full-text review, and 45 articles were excluded based on study content. Consequently, we obtained 5 publications[3,7,9–11] that met all inclusion and exclusion criteria for this meta-analysis.
Figure 1.
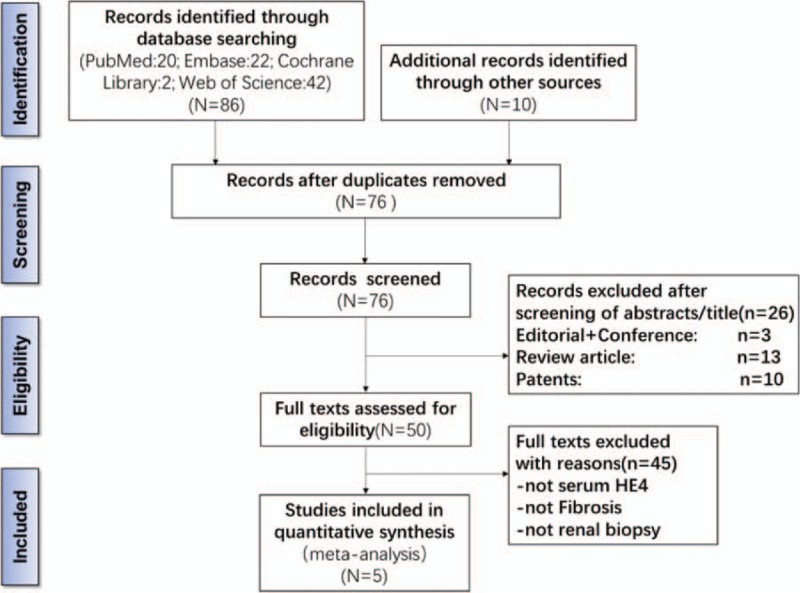
Flowchart depicting the study selection process for this systematic review and meta-analysis. HE4 = human epididymis 4.
3.2. Characteristics of the included studies and quality assessments
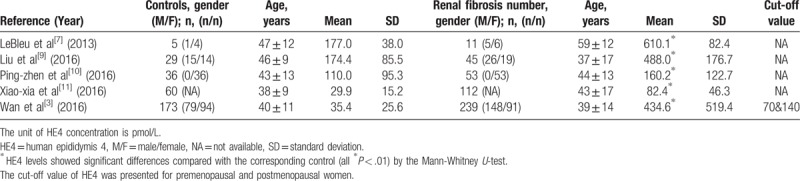
In this meta-analysis, the final set of 5 studies included a total of 460 patients with renal fibrosis and 303 controls (healthy people or patients with kidney diseases and histologically confirmed the absence of renal fibrosis). All the renal fibrosis patients were diagnosed based on histopathological examination. Regarding the origin of the 5 studies, 4 were performed in China,[2,11–13] which highlighted the diagnostic value of serum HE4 for renal fibrosis, and 1 was conducted in the USA,[14] which analyzed the correlation between serum HE4 and renal tissue HE4 in patients with renal fibrosis. Table 1 and Table 2 summarize the main characteristics of the 5 studies. All 5 studies were retrospective database reviews. All the investigations indicated that serum HE4 levels of both men and women were significantly higher in the renal fibrosis group compared with the controls or other patients with CKD (P < .01, Table 2).
Table 1.
Characteristics of the studies included in the meta-analysis.
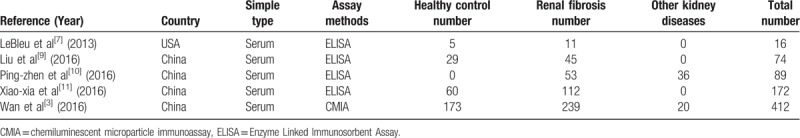
Table 2.
Mean and standard deviation of the serum human epididymis 4 (HE4) values included in the meta-analysis.
3.3. Diagnostic correlation of HE4 and renal fibrosis
Five studies involving 460 patients with renal fibrosis and 303 control individuals were included. In randomized comparisons, serum HE4 was higher in the renal fibrosis group, and SMD in serum HE4 favored renal fibrosis across all trials (1.41; 95% confidence interval, 0.82–2.01, P < .00001) was statistically significant. However, the study showed high heterogeneity and between-study variability (I2 = 89%, P < .00001) as evident in Fig. 2.
Figure 2.
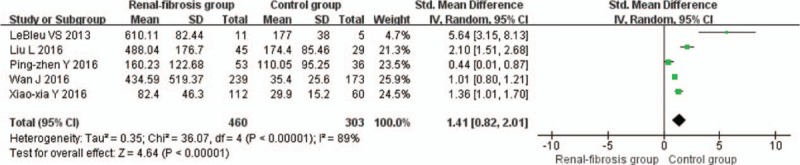
Forest plots of estimated serum Human epididymis 4 (HE4) in the patients with renal fibrosis. CI = confident interval, HE4 = human epididymis 4, SD = standard deviation.
To test the robustness of our findings, we performed a sensitivity analysis to assess the influence of each individual study on the pooled SMD by omitting individual studies. In the bubble graph (Fig. 3), each bubble represents 1 trial and sizes of the bubbles are proportional to the size of each trial. The weight of each included study was balanced except the study of Le Bleu et al.[7] The analysis results suggested that no individual studies significantly affected the pooled SMD, indicating a statistically robust result. The pooled SMD was similar after removing any single study for sensitivity analysis.
Figure 3.
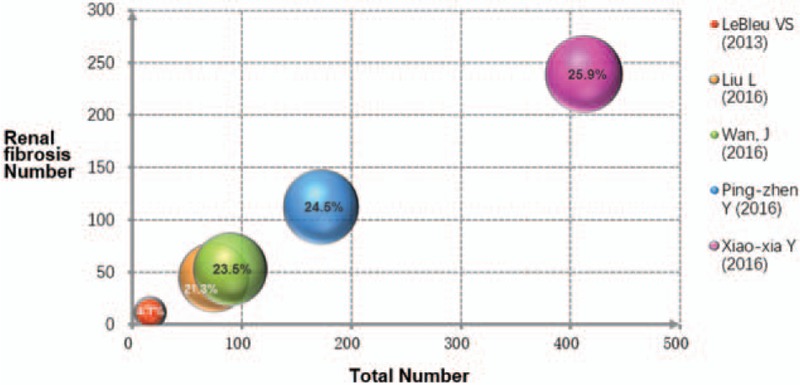
Bubble graph for weighting analysis of renal fibrosis studies. Sensitivity analysis to test the robustness of results. Each bubble represents the weight of a separate study. The weight of each included study was similar except the study of Le Bleu et al and removal of any one trial did not affect the results significantly.
4. Discussion and Conclusions
Our study confirmed that elevated serum HE4 is associated with renal fibrosis. To the best of our knowledge, this study is the first meta-analysis to evaluate serum HE4 in patients with renal fibrosis identified by biopsy. The meta-analysis result indicates that elevated serum HE4 may be a potential novel biomarker for renal fibrosis. We analyzed a total of 5 studies and found serum HE4 was higher in the renal fibrosis group when compared with the control group (N = 763, SMD = 1.41; 95% confidence interval, 0.82–2.01; P < .01). However, these data were not able to identify the diagnostic cut-off value, sensitivity or specificity of serum HE4 for renal fibrosis.
HE4 was initially identified in the epididymis in 1991 and originally predicted to be involved in sperm maturation.[15] HE4, also called WAP 4-disulfide core domain 2 secreted protein (WFDC2), is a member of the whey acidic protein (WAP) family, which may possess anti-protease, anti-inflammatory, and host defense activities.[3,16,17] HE4 expression has been identified in many normal tissues,[18] and in abnormal tissues including ovarian epithelial cancer, pulmonary adenocarcinoma, endometrial cancer, and cystic fibrosis.[18–20] In 2008, serum HE4 was approved by the Food and Drug Administration in the United States as a biomarker to monitor patients with ovarian cancer for disease recurrence.[21] Increased serum HE4 was also found in lung cancer, benign lung diseases, cystic fibrosis, and benign pelvic diseases.[17,19,20] Moreover, serum HE4 can be influenced by various demographic factors, including age, gender, smoking habit, menstrual cycle, menopause, pregnancy, and body mass index (BMI).[19] Although these above factors may affect serum HE4 levels, the 5 included studies had already excluded most patients with known nonrenal factors which can lead to significant changes in serum HE4 levels, and simultaneously carried out single factor or multivariate statistical analysis of the related clinical data. Recently, several studies reported that serum HE4 levels increased with the advanced stage of renal fibrosis, and receiver operating characteristic (ROC) analysis revealed serum HE4 as a suitable biomarker, which was more sensitive than serum creatinine for diagnosis of renal fibrosis in patients with CKD,[3,9] above studies all identified positive correlations with serum creatinine levels reaching statistical significance.[22,23]
The exact physiological and pathological functions of HE4 are poorly understood. For renal fibrosis, upregulated expression of HE4 has previously been identified in fibrosis-associated myofibroblasts.[7] HE4 has been demonstrated to be a pan-serine protease inhibitor, as well as MMP2 and MMP9 inhibitor, which can promote renal fibrosis by inhibiting the degradation of type I collagen.[7] Studies have shown that the balance between active proteases, such as MMPs and serine proteases, protease inhibitors including HE4, and production of extracellular matrix proteins such as collagen and fibronectin, may determine whether the outcome of kidney injury will be wound healing or fibrosis.[24]
The most comprehensive studies investigating the molecular and biologic mechanisms of HE4 are the studies on ovarian cancer. Two conflicting hypotheses have been generated. HE4 may be an ovarian epithelial tumor promoter, associated with cancer cell adhesion, migration, and tumor growth via the EGFR-MAPK signaling pathway.[25,26,27] However, several studies conversely suggest that HE4 plays a protective role in epithelial ovarian cancer, as overexpression of HE4 led to significant inhibition of cell proliferation, migration, and invasiveness in vitro.[25,28] This protective role may be via inhibition of cell proliferation through regulation of the mitogen-activated protein kinase and phosphoinositide 3-kinase/AKT signal transduction pathways.[29] Furthermore, in cystic fibrosis, HE4 may be a component of the innate immune defenses of the lung, nasal, and oral cavities.[20] Therefore, the detailed functional mechanism of HE4 in CKD or renal fibrosis and other diseases remains to be elucidated.
Several studies have explored the relationship between HE4 and renal fibrosis. Due to the small sample size of each investigation and the inconsistent results among these studies, no reliable conclusions were drawn. Therefore, to evaluate the relationship between HE4 and renal fibrosis, we conducted this meta-analysis to mitigate sample size problems of the individual studies and enhance the statistical power.
Heterogeneity is a potential problem when interpreting results of any meta-analysis.[30] In our study, we performed a subgroup analysis of race and ethnicity to explore the potential origin of heterogeneity, however high heterogeneity was still shown (data not shown). Therefore, we conducted a sensitivity analysis to determine the influences of each individual study or the statistical methods (random-effects model vs fixed-effects model). The results suggested that no individual study significantly affected the pooled SMD, indicating a statistically robust result. Moreover, we speculate that the inclusion of patients at different stages of CKD, different methods of laboratory testing, as well as the various sources of reagents could all contribute to heterogeneity. However, with the limited number of eligible studies, we could not further elucidate the origin of heterogeneity.
In addition to heterogeneity, our study has other limitations. After rigorous literature searching, we found only 5 studies meeting our inclusion and exclusion criteria. However, no study is a randomized control trial and we did not define the criteria for renal fibrosis due to data limitations of the included papers. Additionally, only articles published in English or Chinese were included in this meta-analysis, which could introduce bias. Furthermore, each individual study has its own limitations.
In the study of Xiao-xia et al,[11] renal fibrosis was defined as an area of tubulointerstitial fibrosis ≥ 5% of the whole specimen area, which was the only study among the 5 which conforms to the scoring criteria of the Interstitial Fibrosis/Tubular Atrophy (IF/TA,2007 Banff classification).[31] Furthermore, the control group ruled out patients with malignant tumors. In the study of Ping-zhen and Hong-ling,[10] all participants were female patients at different stages of CKD diagnosed by renal biopsy. The control group consisted of female patients with histologically confirmed absence of renal fibrosis.
Wan et al[3] conducted the first large-scale clinical study demonstrating that high levels of serum HE4 are associated with CKD and renal fibrosis in patients. They tested serum HE4 concentrations by chemiluminescent microparticle immunoassay (CMIA) on the fully automated ARCHITECT instrument (Abbott, Abbott Park, IL), which is different from the 4 remaining studies which all used an enzyme-linked immunosorbent assay (ELISA). Using different testing methods may cause variations in the results of serum HE4 concentration; however, the CMIA showed suitable analytical performance characteristics with respect to good precision and linearity.[32] Moreover, it was a study design with relatively more patients with end-stage CKD. Thus, a study with more patients with early-stage CKD is required to determine the importance of serum HE4 in early renal fibrosis.[3] Notably, during the statistical analysis, we could not determine the mean value and standard deviation (SD) of serum HE4 concentration directly, and so we used a formula[33] to calculate the mean and SD from the median and interquartile range.
In the study of Liu et al,[9] the “other” medical history of the included participants and the renal fibrosis score were not given. Therefore, we only included patients with focal segmental glomerulosclerosis or hypertensive nephrosclerosis, which must contain renal fibrosis by definition.
In summary, the current study provides a comprehensive analysis of the available evidence concerning the association between serum HE4 and renal fibrosis. Our meta-analysis suggests that the existing literature on the relationship between serum HE4 and renal fibrosis is limited and heterogeneous. The heterogeneity can be partially attributed to a combination of several confounding factors, including different study designs, different ethnicities, different underlying medical history, and unclear cut-off values of the serum HE4 assays. Despite the use of the random effect model to strengthen the validity of the results, they should be interpreted cautiously. Therefore, randomized controlled trial studies with larger sample sizes taking these factors into account need to be performed. Our findings support that serum HE4 is elevated in patients with renal fibrosis and is a potential early diagnostic marker of renal fibrosis among patients with CKD.
Acknowledgments
The authors are very grateful for many helpful comments by reviewers and editors on an earlier version of this manuscript.
They also thank Jun-Yu Long PhD and Qian-Ling Liu MD for their discussion and help.
Footnotes
Abbreviations: αSMA = α smooth muscle actin, BMI = body mass index, CKD = chronic kidney disease, CMIA = chemiluminescent microparticle immunoassay, ELISA = enzyme-linked immunosorbent assay, HE4 = human epididymis protein 4, IF/TA = Interstitial Fibrosis/Tubular Atrophy, MMP2 = matrix metalloprotease 2, MMP9 = matrix metalloprotease 9, ROC = receiver operating characteristic, SD = standard deviation, SMD = standardised mean difference, WAP = whey acidic protein, WFDC2 = WAP 4-disulfide core domain 2 secreted protein.
CPP and YQ equally contributed to this study.
Funding: This work was supported by Peking Union Medical College Youth Fund (3332016012), the National Natural Science Foundation of China (81100545) and Beijing Municipal Science and Technology Commission (D131100004713007, D09050704310901).
The authors have no conflicts of interest to disclose.
References
- [1].Mortality and global health estimates: Causes of death; Projections for 2015 2030; Projection of death rates. Available at: http://apps.who.int/gho/data/node.main.PROJRATEWORLD?lang=en Accessed 29 June, 2017. [Google Scholar]
- [2].Webster AC, Nagler EV, Morton RL, et al. Chronic Kidney Disease. Lancet 2017;389:1238–52. [DOI] [PubMed] [Google Scholar]
- [3].Wan J, Wang Y, Cai G, et al. Elevated serum concentrations of HE4 as a novel biomarker of disease severity and renal fibrosis in kidney disease. Oncotarget 2016;7:67748–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Boor P, Ostendorf T, Floege J. Renal fibrosis: novel insights into mechanisms and therapeutic targets. Nat Rev Nephrol 2010;6:643–56. [DOI] [PubMed] [Google Scholar]
- [5].Sun YB, Qu X, Caruana G, et al. The origin of renal fibroblasts/myofibroblasts and the signals that trigger fibrosis. Differentiation 2016;92:102–7. [DOI] [PubMed] [Google Scholar]
- [6].Falke LL, Gholizadeh S, Goldschmeding R, et al. Diverse origins of the myofibroblast—implications for kidney fibrosis. Nat Rev Nephrol 2015;11:233–44. [DOI] [PubMed] [Google Scholar]
- [7].Le Bleu VS, Teng Y, O’Connell JT, et al. Identification of human epididymis protein-4 as a fibroblast-derived mediator of fibrosis. Nat Med 2013;19:227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Liu LM, Ning XX, Bai M, et al. Prognostic and diagnostic value of HE4 expression in patients with chronic kidney diseases. Int J Clin Exp Pathol 2016;9:7930–40. [Google Scholar]
- [10].Ping-zhen Y, Hong-ling H. Changes of serum human epididymis protein 4 concentrations in chronic kidney disease and the relationship between serum HE4 and renal fibrosis. J Clin Nephrol 2016;16:92–5. [Google Scholar]
- [11].Xiao-xia Y, Ming B, Liu L, et al. Significance of serum human epididymis protein 4 levels in patients with chronic kidney disease. Chin J Nephrol Dial Transplant 2016;25:128–33. [Google Scholar]
- [12].Earley A, Miskulin D, Lamb EJ, et al. Estimating equations for glomerular filtration rate in the era of creatinine standardization: a systematic review. Ann Intern Med 2012;156:785–95. [DOI] [PubMed] [Google Scholar]
- [13].Hoste L, Deiteren K, Pottel H, et al. Routine serum creatinine measurements: how well do we perform? BMC Nephrol 2015;16:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Levey AS, Becker C, Inker LA. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: a systematic review. JAMA 2015;313:837–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kirchhoff C, Habben I, Ivell R, et al. A major human epididymis-specific cDNA encodes a protein with sequence homology to extracellular proteinase inhibitors. Biol Reprod 1991;45:350–7. [DOI] [PubMed] [Google Scholar]
- [16].Lindquist JA, Mertens PR. Myofibroblasts, regeneration or renal fibrosis—is there a decisive hint? Nephrol Dial Transplant 2013;28:2678–81. [DOI] [PubMed] [Google Scholar]
- [17].Nagy B, Jr, Nagy B, Fila L, et al. Human epididymis protein 4: a novel serum inflammatory biomarker in cystic fibrosis. Chest 2016;150:661–72. [DOI] [PubMed] [Google Scholar]
- [18].Galgano MT, Hampton GM, Frierson HF., Jr Comprehensive analysis of HE4 expression in normal and malignant human tissues. Mod Pathol 2006;19:847–53. [DOI] [PubMed] [Google Scholar]
- [19].Zheng H, Gao Y. Serum HE4 as a useful biomarker in discriminating ovarian cancer from benign pelvic disease. Int J Gynecol Cancer 2012;22:1000–5. [DOI] [PubMed] [Google Scholar]
- [20].Bingle L, Cross SS, High AS, et al. WFDC2 (HE4): a potential role in the innate immunity of the oral cavity and respiratory tract and the development of adenocarcinomas of the lung. Respir Res 2006;7:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cheng D, Sun Y, He H. The diagnostic accuracy of HE4 in lung cancer: a meta-analysis. Dis Markers 2015;2015:352670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Escudero JM, Auge JM, Filella X, et al. Comparison of serum human epididymis protein 4 with cancer antigen 125 as a tumor marker in patients with malignant and nonmalignant diseases. Clin Chem 2011;57:1534–44. [DOI] [PubMed] [Google Scholar]
- [23].Nagy B, Jr, Krasznai ZT, Balla H, et al. Elevated human epididymis protein 4 concentrations in chronic kidney disease. Ann Clin Biochem 2012;49(pt 4):377–80. [DOI] [PubMed] [Google Scholar]
- [24].Lindquist JA, Mertens PR. Myofibroblasts, regeneration or renal fibrosisuis there a decisive hint? Nephrol Dial Transpl 2013;28:2678–81. [DOI] [PubMed] [Google Scholar]
- [25].Jia LT, Zhang YC, Li J, et al. The role of human epididymis protein 4 in the diagnosis of epithelial ovarian cancer. Clin Transl Oncol 2016;18:233–9. [DOI] [PubMed] [Google Scholar]
- [26].Lu R, Sun X, Xiao R, et al. Human epididymis protein 4 (HE4) plays a key role in ovarian cancer cell adhesion and motility. Biochem Biophys Res Commun 2012;419:274–80. [DOI] [PubMed] [Google Scholar]
- [27].Moore RG, Hill EK, Horan T, et al. HE4 (WFDC2) gene overexpression promotes ovarian tumor growth. Sci Rep 2014;4:3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gao L, Cheng HY, Dong L, et al. The role of HE4 in ovarian cancer: inhibiting tumour cell proliferation and metastasis. J Int Med Res 2011;39:1645–60. [DOI] [PubMed] [Google Scholar]
- [29].Kong X, Chang X, Cheng H, et al. Human epididymis protein 4 inhibits proliferation of human ovarian cancer cells via the mitogen-activated protein kinase and phosphoinositide 3-kinase/AKT pathways. Int J Gynecol Cancer 2014;24:427–36. [DOI] [PubMed] [Google Scholar]
- [30].Coory MD. Comment on: heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol 2010;39:932. [DOI] [PubMed] [Google Scholar]
- [31].Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant 2008;8:753–60. [DOI] [PubMed] [Google Scholar]
- [32].Ruggeri G, Bandiera E, Zanotti L, et al. HE4 and epithelial ovarian cancer: comparison and clinical evaluation of two immunoassays and a combination algorithm. Clin Chim Acta 2011;412:1447–53. [DOI] [PubMed] [Google Scholar]
- [33].Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]


