Abstract
Background:
Currently, prophylactic use of drugs to promote a healthy gut microbiota and immune system in preterm infants is hot debated, among which lactoferrin is a promising supplementation. However, the effect and safety of lactoferrin to prevent late-onset sepsis (LOS) and necrotizing enterocolitis (NEC) in preterm infants remains controversial.[1]
Methods:
Databases including Medline, Ovid-Embase, The Cochrane Library, CBM, CNKI, and VIP database of Chinese Journal were searched to collect randomized controlled trials (RCTs) about lactoferrin for preventing LOS and NEC in preterm infants. Languages of included RCTs were restricted to English and Chinese. Meta-analysis was conducted by Rev Man 5.3 software. The Mantel–Haenszel method with random-effects model was used to calculate pooled relative risks (RRs) and 95% confidence intervals (CIs).
Results:
A total of 9 RCTs, involving 1834 patients, were included. Pooled analysis showed that prophylactic lactoferrin could significantly reduce the incidence all culture-proven LOS (41/629 [6.5%] vs 96/659 [15.3%]; RR 0.47; 95% CI 0.33–0.67; P < .01) and NEC (stage II or more) (9/448 [2.0%] vs 26/462 [5.6%]; RR 0.40; 95% CI 0.18–0.86; P < .01). Lactoferrin was also associated with a significantly decreased hospital-acquired infection (16/139 [11.5%] vs 35/140 [25%]; RR 0.47; 95% CI 0.27–0.80; P < .01); and infection-related mortality (4/474 [0.8%] vs 25/505 [4.9%]; RR 0.24; 95% CI 0.04–1.32; P < .01, I = 53%). Lactoferrin could shorten time to reach full enteral feeding (weighted mean difference [WMD] = −2.11, 95% CI −3.12 to −1.10; P < .01) and showed a decreasing trend of duration of hospitalization (WMD = −1.69, 95% CI −6.87 to 3.50; P < .01; I = 95%). Lactoferrin did not have a significant effect on all-cause mortality (22/625 [3.5%] vs 35/647 [5.4%]; RR 0.70; 95% CI 0.38–1.30; P = .16; I = 13%). None of the included trials reported any confirmed adverse effects caused by the supplemented lactoferrin or probiotics.
Conclusion:
Current evidence indicates that lactoferrin could significantly reduce the incidence of NEC and LOS, and decrease the risk of hospital-acquired infection and infection-related mortality in premature infants without obvious adverse effects.
Keywords: lactoferrin, meta-analysis, necrotizing enterocolitis, premature infant, randomized controlled trial, sepsis
1. Introduction
Infectious diseases attribute to most of deaths in neonate.[2] Although modest reductions have been seen for the last decade, late-onset sepsis (LOS) and necrotizing enterocolitis (NEC)—a serious inflammatory gut condition, are still among the leading cause of serious morbidity and mortality in preterm infants. Studies have shown that an increased rate of neonatal infection associated with lower gestational age (GA) and lower birth weight.[3,4] Premature infant are indeed highly prone to infection because of immature immune system, exposure to invasive procedures, and use of broad-spectrum antibiotics.
Studies have demonstrated that very low birth weight (VLBW) infants fed with human milk (HM) develop fewer sepsis, NEC, and cause less neonatal intensive care unit (NICU) costs.[5,6] However, maternal milk is limited for preterm infants, especially VLBW and extremely low birth weight (ELBW), because the production of maternal colostrum is limited after birth or intestinal immaturity hinders full enteral feedings. Various bioactive components in HM can promote the commensal intestinal microbiome, nascent gut development, and host defenses establishment. Lactoferrin, one of the most important protein consumed by breast-fed infants immediately after birth, may be the major milk component responsible for decreasing infection, due to its antimicrobial, antioxidant, antifungal, anti-inflammatory, and immune-modulation properties.[7,8] Therefore, it is considered as a promising supplementation to promote the development of normal intestinal function and reduce the incidence of LOS and NEC in preterm infants.
Currently, the effect and safety of lactoferrin to prevent LOS and NEC in preterm neonates still remains controversial. Recently, many trials have been published investigating the protective effect of lactoferrin in preterm infants. The goal of our meta-analysis is to inform clinicians about the risks and benefits of lactoferrin.
2. Methods
This systematic review and meta-analysis was conducted and reported in adherence to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement[9] and the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions.[10] Because our study was a review of previous published studies, ethical approval or patient consent was not required.
2.1. Search strategies and inclusion criteria
Medline, Ovid-Embase, The Cochrane Library, CBM, CNKI, and VIP were searched for records that compared enteral lactoferrin with or without probiotics to placebo or no intervention in preterm neonates in NICUs from May 1, 2017. The last search was conducted on February 1, 2018. The cited references of retrieved articles and previous reviews were also manually checked to identify any additional eligible trials. All citations were imported into a bibliographic database (End Note X7; Thomson Reuters), and 2 of the authors (YH and LC) independently screened the candidate articles to check their eligibility for inclusion. We searched for the following terms (Table 1).
Table 1.
Search strategy.

We developed a Patient, Intervention, Comparators, Outcome, and Study design approach as the eligibility criteria—Population: preterm infants <37 weeks or birth weight (BW) <2500 g or both; infants with sever congenital anomalies (gastrointestinal problem, suspected congenital infections) were excluded. Intervention: lactoferrin with or without probiotics administered for >7 days; Comparators: placebo or no lactoferrin; Outcome: The primary outcome was NEC stage II or more (defined as clinical signs with the presence of pneumatosis intestinalis on abdominal X-ray, according to the modified Bell criteria[11]) and all culture-proven LOS (defined as a positive blood culture and/or cerebrospinal fluid culture obtained after 72 hours of life in the presence of clinical signs and symptoms of infection[12]). The secondary outcome was hospital-acquired infection (defined as hospital-acquired bacteremia, pneumonia, urinary tract infection, gastroenteritis and NEC[13]), infection-related mortality (defined as death within 5 days after the last positive culture result from any site without other causes), duration of hospitalization, days to achieve full enteral feeding, all-cause mortality, and adverse effects. Study design: only randomized controlled trials (RCTs) were eligible. Discrepancies regarding study inclusion between the 2 authors (YH and LC) were resolved through discussion with the correspondence author (JY), as required. Only published data were used for those studies. For duplicated publications of the same clinical trial, we choose the latest updated data.
2.2. Date extraction and quality assessment
Two of the authors (YH and LC) independently extracted relevant data from each included trials by using a unified data form. Extracted data were entered into a standardized EXCEL file. The items included in the data form were as follows: source (first author), number of preterm infants enrolled, interventions, type of milk (HM or formula milk [FM]), and outcomes of interest. Discrepancies between authors were resolved by consensus. We used the Cochrane Risk-of-Bias Tool to assess the risk of bias for each RCT.[14]
2.3. Statistical analysis
Since the included RCTs were performed in different regions including developing countries and developed countries, we presume that there are variability in races, time of intervention, dosages, lab detection accuracy, and other unknown confounding factors. Owing to the assumption of within- and between-study heterogeneity, the Mantel–Haenszel method with random-effects model was used to calculate pooled relative risks (RRs) and 95% confidence intervals (CIs). Trials with uneven distribution of demographic characteristics and sepsis-related risk factors between study and control groups were not included in our meta-analysis, such as GA, BW, Apgar score, prenatal steroids, antimicrobial drugs, and use of invasive devices. Heterogeneity across studies was tested by using the I2 statistic. Studies with an I2 value of >50% were considered to have significant heterogeneity.[15] Subgroup analyses were conducted according to pathogen of sepsis and birth weight. Sensitivity analyses were conducted by exclusion of any single study to investigate the influence of a single study on the overall pooled RRs. However, we could not assess publication bias by visually inspecting funnel plot because of limited numbers of RCTs. A P value < .01 was considered as statistically significant, except where otherwise specified. All the statistical analyses were performed using Rev Man 5.3 (The Nordic Cochrane Centre, Copenhagen, Denmark).
2.4. Search results
A total of 397 potentially relevant records were identified by our search strategy, of which 268 records remain after duplicates were removed; 247 records were excluded after the screening the titles and abstracts. The remaining 21 articles were assessed for eligibility and 12 articles[16–27] were considered eligible for inclusion after full-text reading. Three duplicate publications[19,20,24] were found. Finally, 9 RCTs were included in the systematic review. The flow diagram of the study selection process is given in Fig. 1. Hence, 9 trials were statistically analyzed. Characteristics of the 9 trials are summarized in Table 2. The quality of the trials assessed by the Cochrane Risk-of-Bias Tool is summarized in Figs. 2 and 3.
Figure 1.
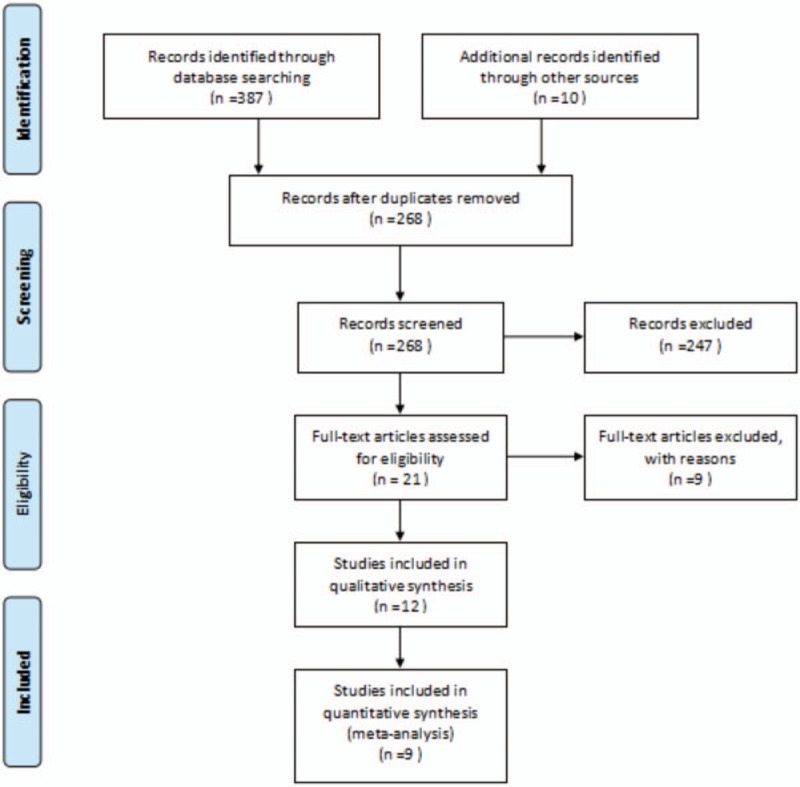
Selection process for the studies included in the meta-analysis.
Table 2.
Characteristics of randomized controlled trials included in the meta-analysis.
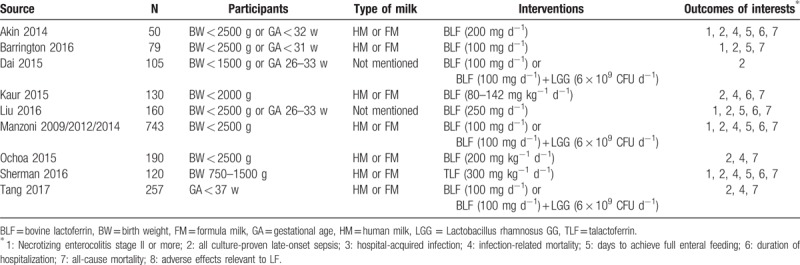
Figure 2.
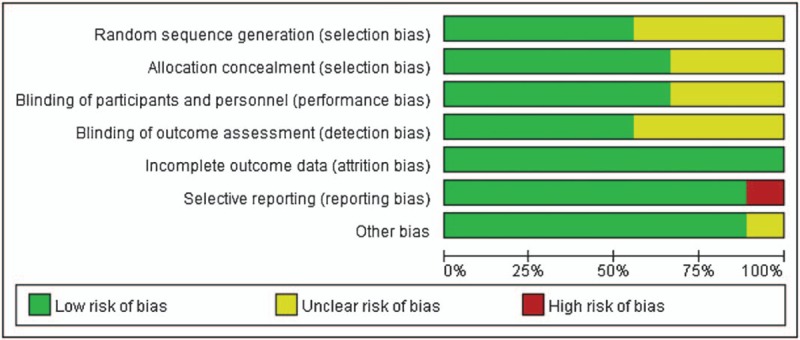
Risk of bias graph.
Figure 3.
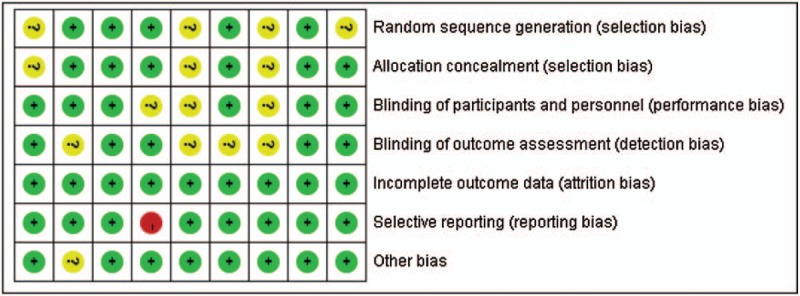
Risk of bias summary.
3. Outcomes
3.1. Comparison 1: Lactoferrin supplementation vs placebo
3.1.1. Outcome 1.1: NEC stage II or more
Out of the 9 included studies, NEC was the primary outcome of interest in 5 studies, whereas the remaining 4 did not mention the outcome. Figure 4 shows the efficacy of lactoferrin in the prevention of NEC in preterm neonates, using a random-effects model. Of the 5 estimates, the incidence of NEC was 9/448 (2.0%) versus 26/462 (5.6%); RR 0.40; 95% CI 0.18–0.86; P < .01; heterogeneity: P = .57, I = 0%. Further exclusion of any single study did not materially alter the overall combined RR, with a range from 0.33 (95% CI 0.14–0.74) to 0.44 (95% CI 0.20–0.98).
Figure 4.
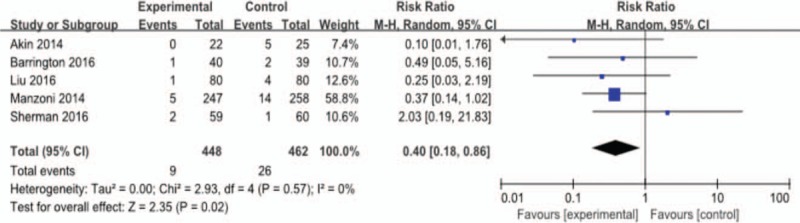
Outcome 1.1: Effect of lactoferrin on necrotizing enterocolitis in preterm neonates.
3.1.2. Outcome 1.2: LOS
Pooled results from 9 RCTs (N = 1834) using random-effects model meta-analysis showed that lactoferrin significantly decreased the risk of all culture-proven LOS (41/629 [6.5%] vs 96/659 [15.3%]; RR 0.47; 95% CI 0.33–0.67; P < .01; heterogeneity: P = .66, I = 0%). Results of all the studies were homogeneous except Ochoa 2015. On sensitivity analysis, the beneficial effects continued to be observed after excluding studies with high risk of bias for random sequence generation and also for allocation concealment. Mazoni 2014 did not report on the result of sepsis, thus the study has high risk of reporting bias. We extracted data of sepsis from the previous study Mazoni 2009, which should minimize the effect of its reporting bias. Furthermore, after excluding the trial with high risk of reporting bias—Mazoni 2014, the RR of LOS was still consistent with the main analysis (RR 0.52, 95% CI 0.35–0.78, P < .01, heterogeneity: P = .68, I = 0%).
Subgroup analysis of the pathogen type of sepsis suggests that the beneficial effect of lactoferrin apply to all kinds of sepsis: Gram (−) sepsis (6 studies, 21/474[4.4%] vs 44/505 [8.7%]; RR 0.51; 95% CI 0.30–0.87; P = .01; heterogeneity: P = .55, I = 0%), Gram (+) sepsis (6 studies, 13/474 [2.7%] vs 51/505 [10.1%]; RR 0.28; 95% CI 0.16–0.51; P < .01; heterogeneity: P = 1.0, I = 0%), bacterial sepsis (6 studies, 34/474 [7.2%] vs 95/505 [18.8%]; RR 0.39; 95% CI 0.27–0.56; P < .01; heterogeneity: P = .77, I = 0%), and fugal sepsis (4 studies, 1/320 [0.3%] vs 22/350 [6.3%]; RR 0.13; 95% CI 0.03–0.47; P < .01; heterogeneity: P = .89, I = 0%). Although the distribution of pathogens by type was not significantly different in the treated and control groups, lactoferrin tends to have better prevention efficacy in fungal sepsis compared to bacterial sepsis (RR 0.13 vs 0.39) and in Gram (+) sepsis compared to Gram (−) sepsis (RR 0.28 vs 0.51). It also detects a decrease in coagulase-negative staphylococci sepsis among preterm infants with the supplementation of lactoferrin, which is the most prevalent Gram (+) pathogen causing neonatal sepsis after GBS (2 studies, 7/99 [7.1%] vs 17/99 [17.2%]; RR 0.43; 95% CI 0.18–1.01; P = .04; heterogeneity: not applicable).
The benefit of lactoferrin is significant for VLBW (infants with BW < 1500 g) (4 studies, 22/297 [7.4%] vs 64/296 [21.6%]; RR 0.38; 95% CI 0.24–0.59; P < .01; heterogeneity: P = .65, I = 0%). For low birth weight (LBW) (infants with BW 1500–2500 g), the efficacy of lactoferrin is not statistically significant (2 studies, 6/86 [7.0%] vs 9/92 [9.8%]; RR 0.71; 95% CI 0.26–1.90; P = .49; heterogeneity: not applicable).
The results of lactoferrin on LOS are shown in Fig. 5.
Figure 5.
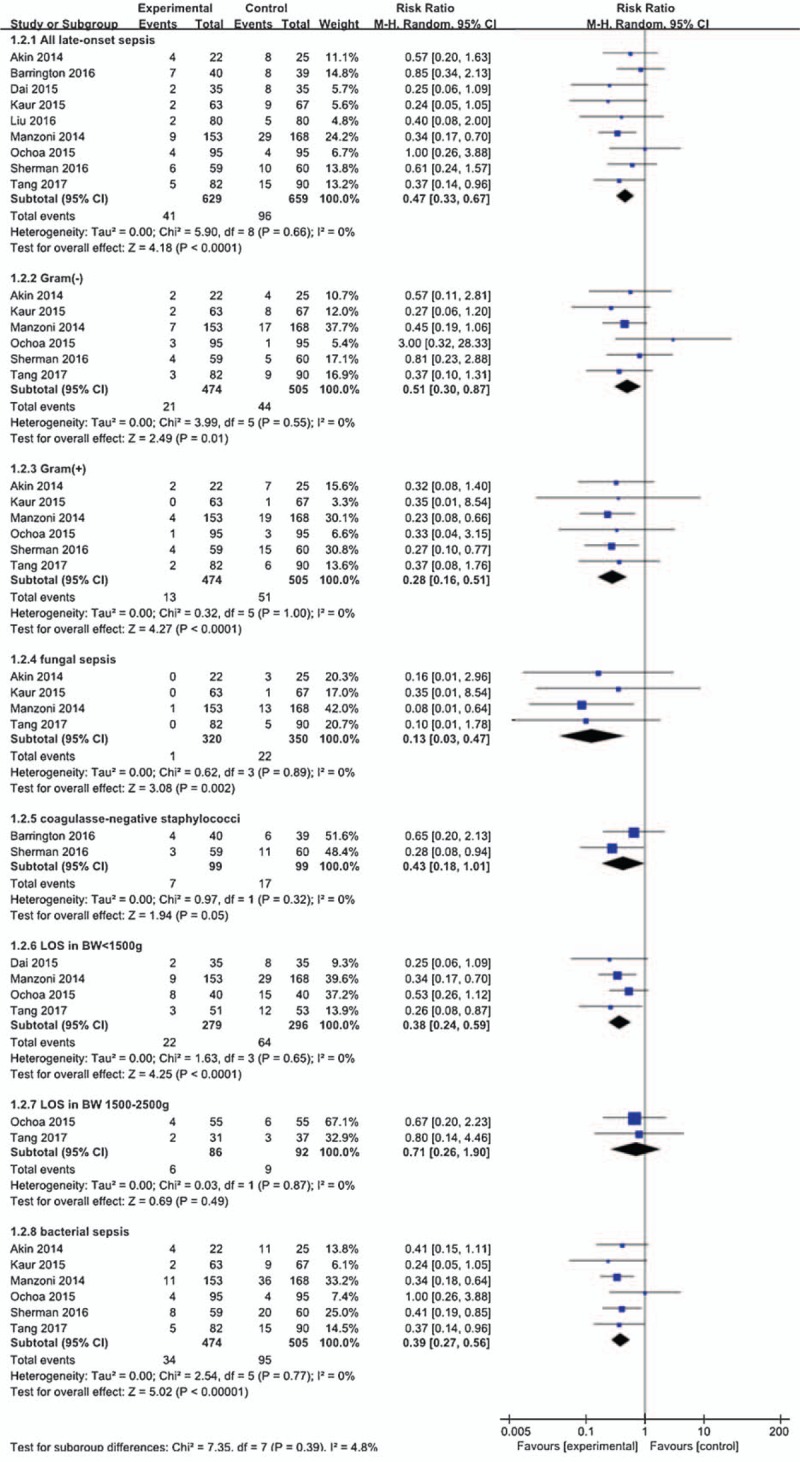
Outcome 1.2: Effect of lactoferrin on late-onset sepsis in preterm neonates.
3.1.3. Outcome 1.3: Hospital-acquired infection
The pooled meta-analysis showed that lactoferrin supplementation resulted in a statistically significant reduction in the incidence of hospital-acquired infection (2 studies, 16/139 [11.5%] vs 35/140[25%]; RR 0.47; 95% CI 0.27–0.80; P < .01; heterogeneity: not applicable). The results of lactoferrin are shown in Fig. 6.
Figure 6.
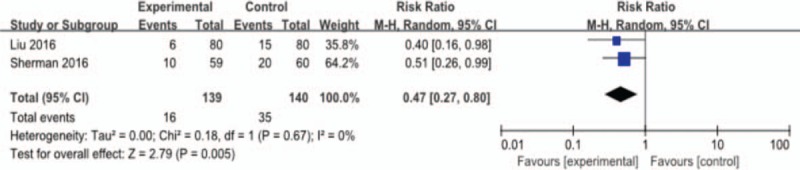
Outcome 1.3: Effect of lactoferrin on hospital-acquired infection in preterm neonates.
3.1.4. Outcome 1.4: Infection-related mortality
Figure 7 reveals that lactoferrin could reduce infection-related mortality, with statistical significance; however, the result is of high heterogeneity (6 studies, 4/474 [0.8%] vs 25/505 [4.9%]; RR 0.24; 95% CI 0.04–1.32; P < .01, heterogeneity: P = .08, I = 53%). After sensitivity analysis, we find that Ochoa 2015 led to the high heterogeneity. In this study, it should be noted that the treatment only began when oral or tube feeding started and 6 of 33 sepsis episodes occurred before starting the intervention. The author also reported that they have enrolled babies of high risk (500–1000 g). However, since they did not give explicit reasons of the death, we cannot find out if it is related to the timing of intervention and high risky basis of the babies. This may explain for the high heterogeneity of this study. The results were significant with low heterogeneity after excluding this study (5 studies, 0/379 [0%] vs 23/410 [5.6%]; RR 0.10; 95% CI 0.02–0.44; P < .01; heterogeneity: P = .81, I = 0%).
Figure 7.
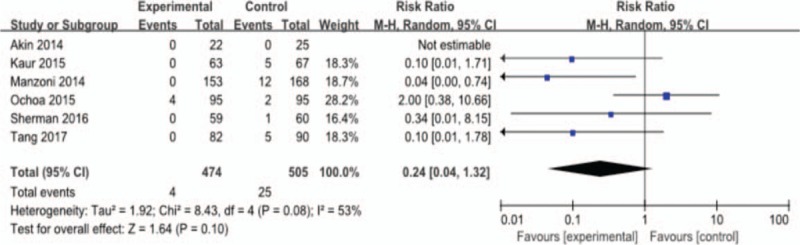
Outcome 1.4: Effect of lactoferrin on infection-related mortality in preterm neonates.
3.1.5. Outcome 1.5: Days to achieve full enteral feeding
The mean difference for days to achieve full enteral feeding in preterm infants was −2.19, with statistical significance (4 studies, weighted mean difference [WMD] = −2.19, 95% CI −2.97 to −1.42; P < .01; heterogeneity: P = .37, I = 4%). Lactoferrin slightly shortened the time to achieve full enteral feeding as is shown in Fig. 8.
Figure 8.
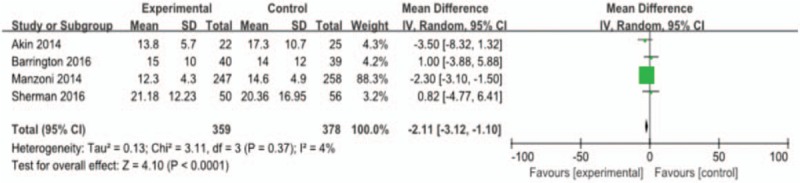
Outcome 1.5: Effect of lactoferrin on days to achieve full enteral feeding in preterm neonates.
3.1.6. Outcome 1.6: Duration of hospitalization
The mean difference for duration of hospitalization was −1.02 (5 studies, WMD = −2.11, 95% CI −3.12 to −1.10; P < .01; heterogeneity: P < .1, I = 95%). Considering the different discharge requirement in China, which leads to longer hospitalization, the high heterogeneity of Liu 2016 with other studies is easy to explain. After exclusion the study Liu 2016 with high heterogeneity, the result is still significant (4 studies, WMD = −0.85, 95% CI −1.09 to −0.60; P < .01; heterogeneity: P = .44, I = 0%).
3.1.7. Outcome 1.7: All-cause mortality
We noted no statistically significant difference in all-cause mortality (7 studies, 22/625 [3.5%] vs 35/647 [5.4%]; RR 0.70; 95% CI 0.38–1.30; P = .11; heterogeneity: P = .33, I = 13%). The result was not statistically significant.
3.1.8. Outcome 1.8: Adverse effects related to lactoferrin
None of the included trials reported any confirmed adverse effects, treatment death, or feeding intolerance caused by the supplemented lactoferrin or probiotics. Only 1 study Sherman 2016 reported possibly related adverse effects of study drug (5/59 [8.4%] in talactoferrin [TLF] group and 7/60 [11.7%] in control group), which detects no statistically significant difference in 2 groups (P = .57). They reported that gastrointestinal (76%), blood and lymphatic (60%), nutrition and metabolism (72%), and respiratory disorders (72%) were the most common treatment emergent adverse effects.
3.2. Comparison 2: Lactoferrin supplementation combining with LGG vs placebo
3.2.1. Outcome 2.1: NEC stage II or more
Lactoferrin supplementation with Lactobacillus rhamnosus GG (LGG) decreased the incidence of NEC stage II or III and the result is not statistically significant (1 study, 0/238 [0%] vs 14/258 [5.4%]; RR 0.04; 95% CI 0.00–0.62; P = .02; heterogeneity: not applicable).
3.2.2. Outcome 2.2: LOS
Our results suggest a beneficial effect of lactoferrin with LGG in all kinds of sepsis: All LOS (3 studies, 12/271 [4.4%] vs 52/293 [17.7%]; RR 0.25; 95% CI 0.14–0.47; P < .01; heterogeneity: P = .77, I = 0%); bacterial sepsis (2 studies, 11/236 [4.7%] vs 51/258 [19.8%]; RR 0.24; 95% CI 0.13–0.45; P < .01; heterogeneity: not applicable); Gram (−) sepsis (2 studies, 8/236 [3.4%] vs 26/258 [10.1%]; RR 0.34; 95% CI 0.16–0.73; P < .01; heterogeneity: not applicable); Gram (+) (2 studies, 3/236 [1.3%] vs 25/258 [9.7%]; RR 0.13; 95% CI 0.04–0.44; P < .01; heterogeneity: not applicable); fugal (2 studies, 4/236 [1.7%] vs 18/258 [7.0%]; RR 0.24; 95% CI 0.08–0.71; P = .01; heterogeneity: not applicable).
3.2.3. Outcome 2.3: Infection-related mortality
A reduction of infection-related mortality can be detected in infants supplemented with lactoferrin combined with LGG, with statistical significance (2 studies, 2/236 [0.8%] vs 13/258 [5.0%]; RR 0.17; 95% CI 0.04–0.75; P = .02; heterogeneity: not applicable).
3.2.4. Outcome 2.4: Time to achieve full enteral feeding
Only 1 study assessed the outcome and lactoferrin supplementation with LGG shortened time to achieve full enteral feeding (1 study, WMD = −1.40, 95% CI −2.27 to −0.53; P < .01; heterogeneity: not applicable).
3.2.5. Outcome 2.5: All-cause mortality
Our results detect no significant difference of all-cause mortality in 2 groups (2 studies, 13/323 [4.0%] vs 24/348 [6.9%]; RR 0.58; 95% CI 0.30–1.13; P = .11; heterogeneity: not applicable).
4. Discussion
4.1. Comparison with previous study
In summary, our meta-analysis identified 9 trials that enrolled 1834 preterm infants to evaluate the safety and efficacy of lactoferrin to prevent LOS and NEC in preterm neonates. Current evidence revealed a significant reduction in the incidence of LOS and NEC without sever adverse effects, indicating a benefit of prophylactic lactoferrin supplementation in preterm infants.
Differences between our study and one latest Cochrane meta-analysis should be noted. The meta-analysis by Pammi and Suresh[28] included 6 trials involving 1041 subjects and concluded that lactoferrin decreased NEC stage II or III (RR 0.40, 95% CI 0.18–0.86) and LOS (RR 0.59, 95% CI 0.40–0.87). After the previous meta-analysis, several studies investigating the effects of lactoferrin in preterm infants were published. Our updated meta-analysis included maximum number of completed studies. Overall, our results are generally consistent with the previous study, with lower heterogeneity. In contrast with the previous study, the current one detects that lactoferrin supplementation not only decreased the incidence of LOS and NEC (low-quality evidence), but also significantly reduced the incidence of hospital-acquired infection and infection-related mortality (low- to very low-quality evidence). Additionally, lactoferrin also has benefit in the general condition of preterm infants by reducing the duration of hospitalization and the time to achieve full enteral feeding (low- to very low-quality evidence). The benefit for general conditions seems minor, probably because premature infants generally have feeding issues such as poor suck, gastroesophageal reflux, immature gut motility, and thus requires longer NICU care than term babies. We detect no significant difference in all-cause mortality between lactoferrin supplementation group and control group. Of interest, comparing to LBW, the protective effect of lactoferrin for LOS seems to be more distinct in ELBW and VLBW, with lower RR for LOS (RR 0.36 vs 0.71) and statistical significance. The possible reason for this difference is that ELBW and VLBW infants received higher per-kilogram lactoferrin doses. Because only 3 trials[16,21,22] administered lactoferrin based on weight, the pooled results of these 3 trials were under-powered to detect a greater benefit of weight-adjust dosage. Comparing neonates under the intervention of lactoferrin with and without LGG, the latter have lower RR for NEC, LOS, and infection-related mortality. This suggests a possible greater benefit of lactoferrin combined with probiotics LGG. However, whether probiotics could enhance the protective effect of lactoferrin is hard to detect under current evidence. One reason was that current studies were not adequately powered to reveal its beneficial effect because of sample size.
4.2. Quality assessment
We assume the quality of evidence to be low using the GRADE method mainly because of unclear risk of bias and limited objectives (Fig. 9).
Figure 9.
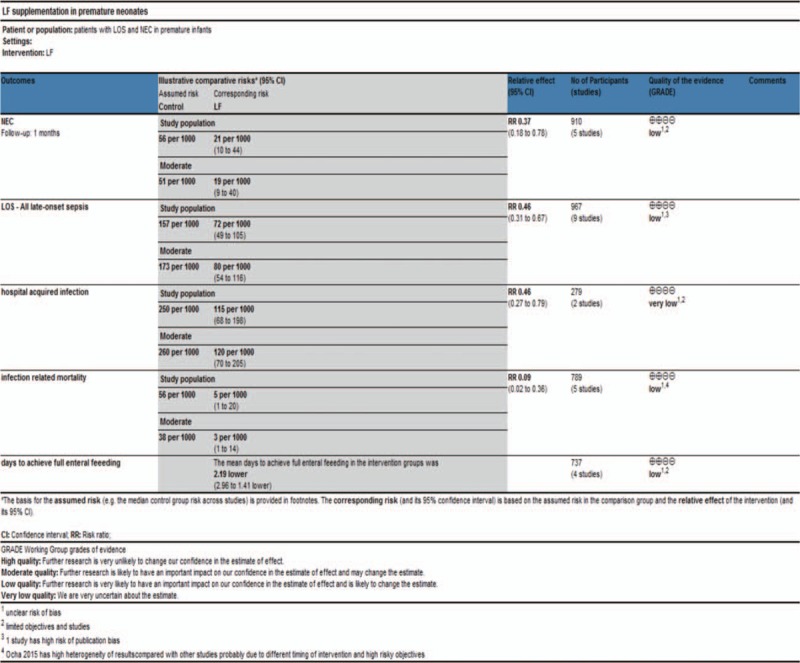
Quality of evidence using GRADE method.
Current available studies had obvious risk of bias and incomplete data extraction that might have an influence on the final results and conclusion. Out of the 9 included trials, only 5 studies reported explicit randomization and 6 studies reported explicit allocation concealment and blinding. In Dai 2015 and Liu 2016, the investigator did not give explicit description of the selection and blinding process, which led to unclear risk of bias and downgraded the quality of evidence. In Manzoni 2014, the researchers only reported the incidence of NEC, causing high risk of selective reporting bias in LOS.
Besides, several potential limitations should also be taken into consideration when interpreting the results. First, the included RCTs were performed in different regions including developing countries and developed countries. Lower detection rates of culture proven sepsis and higher risk of sepsis attack in lower income countries can cause high heterogeneity of data. However, we detect low heterogeneity of the data when analyzing the benefit effect of lactoferrin in preventing LOS, NEC, and infection-related mortality. The heterogeneity of duration of hospitalization is high but it is most likely to be related to different discharge standards. Second, the initial time of intervention was different among studies. The concentration of lactoferrin is high in colostrum and relatively low in mature milk. It is proved that lactoferrin interacts with gut cells differently in a concentration-dependent manner in vitro studies, manifesting as lactoferrin-driven gut cell proliferation and maturation in high concentration and intestinal differentiation in lower concentration.[29] To mimic the property of lactoferrin in colostrum, the initial time of supplementing lactoferrin in preterm infants should be in the very first days of life.[30] Ochoa 2015 did not started the treatment until the start of oral or tube feeding on average at 4.0 ± 1.4 days of life. When analyzing the data, we do detect high heterogeneity of this study, which gives enhanced evidence about the timing of starting lactoferrin supplementation. Our further analysis of data indicates no significant different incidence of LOS when treatment initiated between the 1st and 3rd of life. Finally, HM lactoferrin may act as a confounding factor in our analysis, but since the distribution of feeding patterns were even in all the treated and untreated group, we assumed breastfeeding had little influence on the result. Hence, no subgroup analysis of HM and FM feeding was done. Besides, for infants fed with exclusive maternal milk the benefit of lactoferrin is still significant and the incidence of LOS in untreated infants was similar between HM and FM feeding.[13,18] This suggests that maternal milk alone does not confer the benefit of lactoferrin supplementation and premature infants need additional lactoferrin, specifically to prevent LOS in preterm infants. Although colostrum contains the highest lactoferrin content, the necessity for supplementing additional lactoferrin still matters considering that it can take 2 to 3 weeks until VLBW infants to receive full-volume enteral feedings and full amounts of protective components in HM.[31]
The efficacy of lactoferrin for preventing LOS and NEC still needs more high-quality RCTs to demonstrate. The long-term effects of lactoferrin also need to follow up. Current evidence is under-powered to detect. Future study should focus on the optimal duration, dosage, type (TLF or bovine lactoferrin), and long-term effects of lactoferrin.
5. Conclusion
In conclusion, the 9 included trials indicated that lactoferrin supplementation in preterm neonates is safe without obvious adverse effects. The results of our meta-analysis demonstrated that prophylactic supplementation of lactoferrin could significantly reduce the incidence of NEC, LOS, and hospital-acquired infection in preterm neonates. It also detects a trend of decreased infection-related mortality, with statistical significance. Our study also revealed that lactoferrin could slightly reduce the time to achieve full enteral feeding and duration of hospitalization of preterm infants, with statistical significance.
In addition, lactoferrin with LGG seems to have greater benefits in the prevention of NEC and LOS (very low evidence). However, there are not enough high-quality trials to determine whether the beneficial effects of lactoferrin could be enhanced by combining with probiotics.
Acknowledgment
The authors thank Bing Peng, PhD, for his technical assistance and evaluation.
Author contributions
Formal analysis: Yi He, Luying Cao.
Software: Yi He, Luying Cao.
Supervision: Jialin Yu.
Writing – original draft: Yi He.
Writing – review and editing: Yi He, Jialin Yu, Luying Cao.
Footnotes
Abbreviations: BW = birth weight, CI = confidence interval, ELBW = extremely low birth weight, FM = formula milk, GA = gestational age, HM = human milk, LBW = low birth weight, LGG = Lactobacillus rhamnosus GG, LOS = late-onset sepsis, NEC = necrotizing enterocolitis, NICU = neonatal intensive care unit, RCT = randomized controlled trial, RR = relative risk, TLF = talactoferrin, VLBW = very low birth weight, WMD = weighted mean difference.
This study was supported by the National Natural Science Foundation of China (No.81571483), State key clinic discipline project (No.2011-873), Clinical Research Foundation of Children's Hospital of Chongqing Medical University.
The authors have no conflicts of interest to disclose.
References
- [1].Vongbhavit K, Underwood MA. Prevention of necrotizing enterocolitis through manipulation of the intestinal microbiota of the premature infant. Clin Ther 2016;38:716–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 2012;379:2151–61. [DOI] [PubMed] [Google Scholar]
- [3].Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 2002;110:285–91. [DOI] [PubMed] [Google Scholar]
- [4].Stoll BJ, Hansen NI, Bell EF, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. Jama 2015;314:1039–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Patel AL, Johnson TJ, Engstrom JL, et al. Impact of early human milk on sepsis and health-care costs in very low birth weight infants. J Perinatol 2013;33:514–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Johnson TJ, Patel AL, Bigger HR, et al. Cost savings of human milk as a strategy to reduce the incidence of necrotizing enterocolitis in very low birth weight infants. Neonatology 2015;107:271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Underwood MA. Missed opportunities: the cost of suboptimal breast milk feeding in the neonatal intensive care unit. J Pediatr 2016;175:12–4. [DOI] [PubMed] [Google Scholar]
- [8].Sherman MP, Miller MM, Sherman J, et al. Lactoferrin and necrotizing enterocolitis. Curr Opin Pediatr 2014;26:146–50. [DOI] [PubMed] [Google Scholar]
- [9].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009;151:W65–94. [DOI] [PubMed] [Google Scholar]
- [10].Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from: www.cochrane-handbook.org. [Google Scholar]
- [11].Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am 1986;33:179–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Haque KN. Definitions of bloodstream infection in the newborn. Pediatr Critic Care Med 2005;6(suppl):S45–9. [DOI] [PubMed] [Google Scholar]
- [13].McKibben L, Horan T, Tokars JI, et al. Guidance on public reporting of healthcare-associated infections: recommendations of the Healthcare Infection Control Practices Advisory Committee. Am J Infect Control 2005;33:217–26. [DOI] [PubMed] [Google Scholar]
- [14].Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clin Res ed) 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ (Clin Res ed) 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Akin IM, Atasay B, Dogu F, et al. Oral lactoferrin to prevent nosocomial sepsis and necrotizing enterocolitis of premature neonates and effect on T-regulatory cells. Am J Perinatol 2014;31:1111–20. [DOI] [PubMed] [Google Scholar]
- [17].Barrington KJ, Assaad MA, Janvier A. The Lacuna Trial: a double-blind randomized controlled pilot trial of lactoferrin supplementation in the very preterm infant. J Perinatol 2016;36:666–9. [DOI] [PubMed] [Google Scholar]
- [18].Dai JZ, Xie C. The effect of lactoferrin supplementation combining lactobacillus rhamnosus for prevention of late-onset sepsis in premature neonates. China Pract Med 2015;10:98–100. [Google Scholar]
- [19].Kaur G, Gathwala G. Efficacy of bovine lactoferrin supplementation in preventing late-onset sepsis in low birth weight neonates: a randomized placebo-controlled clinical trial. J Trop Pediatr 2015;61:370–6. [DOI] [PubMed] [Google Scholar]
- [20].Liu YH, Guan HS, Liang GJ, et al. The effect of lactoferrin on low birth weight neonates during hospitalization. MCH Care China 2016;31:4464–5. [Google Scholar]
- [21].Manzoni P, Rinaldi M, Cattani S, et al. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: a randomized trial. Jama 2009;302:1421–8. [DOI] [PubMed] [Google Scholar]
- [22].Manzoni P, Stolfi I, Messner H, et al. Bovine lactoferrin prevents invasive fungal infections in very low birth weight infants: a randomized controlled trial. Pediatrics 2012;129:116–23. [DOI] [PubMed] [Google Scholar]
- [23].Manzoni P, Meyer M, Stolfi I, et al. Bovine lactoferrin supplementation for prevention of necrotizing enterocolitis in very-low-birth-weight neonates: a randomized clinical trial. Early Hum Dev 2014;90(suppl 1):S60–5. [DOI] [PubMed] [Google Scholar]
- [24].Ochoa TJ, Zegarra J, Cam L, et al. Randomized controlled trial of lactoferrin for prevention of sepsis in peruvian neonates less than 2500 g. Pediatr Infect Dis J 2015;34:571–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sherman MP, Adamkin DH, Niklas V, et al. Randomized controlled trial of talactoferrin oral solution in preterm infants. J Pediatr 2016;175:68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sherman MP, Sherman J, Arcinue R, et al. Randomized control trial of human recombinant lactoferrin: a substudy reveals effects on the fecal microbiome of very low birth weight infants. J Pediatr 2016;173(suppl):S37–42. [DOI] [PubMed] [Google Scholar]
- [27].Tang JP, Sun HQ, Zheng YH, et al. Randomized control trial of lactoferrin for prevention of late onset sepsis in premature infants. MCH Care China 2017;32:1223–5. [Google Scholar]
- [28].Pammi M, Suresh G. Enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Datab Syst Rev 2017;6:CD007137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Buccigrossi V, de Marco G, Bruzzese E, et al. Lactoferrin induces concentration-dependent functional modulation of intestinal proliferation and differentiation. Pediatr Res 2007;61:410–4. [DOI] [PubMed] [Google Scholar]
- [30].Manzoni P, Mostert M, Stronati M. Lactoferrin for prevention of neonatal infections. Curr Opin Infect Dis 2011;24:177–82. [DOI] [PubMed] [Google Scholar]
- [31].Kaufman DA. Lactoferrin supplementation to prevent nosocomial infections in preterm infants. Jama 2009;302:1467–8. [DOI] [PubMed] [Google Scholar]


